Abstract
Twenty years after the discovery of the causal CAG repeat expansion mutation in the HTT gene, Huntington’s disease remains an incurable devastating disorder. However, using disease models, and studies in human patients, great progress has been made in understanding the pathophysiology of HD. Research has led to the development of the first gene therapy approaches with promising results in HD model systems. This raises hopes that HD, a monogenetic fully penetrant autosomal dominant disorder, may be a model for novel therapeutics in neurodegenerative disorders. In addition, thanks to the efforts of the HD community – families, clinicians, health professionals, and researchers – standards of care are improving patients’ and families’ quality of life. HD networks (HSG, EHDN, RLAH), and their observational studies, have further laid the groundwork for conducting clinical trials of high quality on a global stage. This includes collaboration with clinical trial sponsors in designing and conducting clinical trials, preparing and training investigators, and developing the right assessment tools. The time seems right for the clinical trials of the future that hopefully will change our treatment options to relieve the plight of all those affected by HD.
Disclosure
The author reports no conflict of interest.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Predictive testing
- Symptomatic treatment
- Movement disorder
- Behavioural phenotype
- Irritability
- Apathy
- Pathophysiology
- REGISTRY
- COHORT
- ENROLL
Introduction
George Huntington, in a family from New England, gave a detailed account of the phenotype of an inherited movement disorder with cognitive impairment and behavioral problems that progresses relentlessly until death [1]. This disorder now bears his name, Huntington’s disease (HD). HD is the most common inherited cause of chorea. The genetic mutation causing HD was first mapped to chromosome 4 in 1983 [2] with the gene and its mutation identified as a CAG repeat expansion in the HTT gene in 1993 [3]. This enabled the establishment of disease models, most of them in rodents, fruit flies, or worms [4] but recently also in large animals such as sheep or nonhuman primates. These models have contributed greatly to our understanding of the pathophysiology of HD and are suitable to explore therapeutic interventions. New treatment approaches, e.g., gene silencing, are promising; however, 20 years after the identification of the disease-causing gene mutation, there is still no causal treatment. There is rightfully hope that this will change in the foreseeable future; in fact, HD could be a model disease and the first neurodegenerative disease in which novel therapeutic approaches prove successful. However, until then HD treatments remain symptomatic and supportive. This can without doubt improve quality of life of HD patients and their families and carers. Nonetheless, until causal treatment will be available, HD will continue to wreck lives and cause suffering for those affected and their families.
The HD community has made great efforts to advance knowledge and improve standards of care of HD. To this end, networks including clinicians, scientists, and family members have formed in Europe (EHDN; www.euro-hd.net), North America and Australia (Huntington Study Group; www.huntington-study-group.org), and Latin America (Red Latinoamericana de Huntington; www.rlah.net) with the ultimate goal to improve quality of life of those affected by HD. The networks also provide the platforms on which systematic efforts to study and treat HD can build. Good examples are the observational natural history studies of HD, REGISTRY in Europe [5] and COHORT in North America and Australia [6]. These now merge into a global effort called ENROLL-HD that also includes Latin American sites and potentially sites from other regions, e.g., Asia (www.enroll-hd.org). The CHDI Foundation, Inc. (www.chdifoundation.org), a not-for-profit organization, supports in a collaborative way research into HD, with a particular emphasis on developing treatments.
The following chapter will give an overview of the epidemiology, pathophysiology, genetic diagnosis, phenotype, and management of HD. It closes with an outlook towards novel therapeutics that will hopefully change the course of this devastating disease for the better.
Epidemiology
The prevalence of manifest Huntington’s disease in North America and Europe is about 10 per 100,000 inhabitants (for a meta-analysis see [7]). More precise estimates can be difficult given the challenges of ascertainment that may result in bias with subsequent over-inflation or underestimation of prevalence rates; for instance, more genetically confirmed patients may attend multidisciplinary specialist interest clinics and research programs so that prevalence estimates in regions with such services can be higher. In addition, because of improved clinical care, patients may survive for longer and, considering the increasing overall life expectancy, more individuals may develop late-onset HD. Thus, the prevalence of HD may be much higher. HD is prevalent worldwide. Compared with North America and Europe, studies in Japan and Africa have shown lower prevalence rates; among the lowest are found in black South Africans with 0.01 in 100,000 [8, 9]. However, in the absence of epidemiological studies, estimating the prevalence in other geographical regions, and other ethnicities, is more difficult.
Notably in Latin America (e.g., Peru, Colombia, Venezuela) HD also occurs in clusters with the Venezuelan cluster around Lake Maracaibo one of several where the prevalence of HD far exceeds that seen in Europe or North America [10]. Given its dominant inheritance for every person with manifest HD, about five persons live at risk of having also inherited the HD gene mutation. This means that in Europe, North America, and Latin America, there are probably about 100,000 individuals with manifest HD with a further about 500,000 individuals at risk. If one includes the genetically unaffected family as impacted by HD, the number of those impacted by HD is even larger. This suggests that HD imposes a substantial burden on health-care systems and societies.
Genetics
HD is an autosomal dominant disorder. The HD gene, HTT, resides on the short arm of chromosome 4 [3]. HTT contains at its N-terminus a trinucleotide (CAG) repeat in exon 1 that codes for a polyglutamine repeat within the huntingtin protein. Healthy humans have up to 27 CAG repeats; 40 and more CAG repeats invariably (full penetrance) lead to clinical manifestations of HD, while individuals with 36–39 CAG repeats may or may not suffer from HD in their lifetime (reduced penetrance). It is possible that with increasing life expectancy more people in the reduced penetrance range will develop signs of HD so that the lower end of the complete penetrance range may actually be lower. Alleles with 28–35 CAG repeats (intermediate range) also do not cause HD in their carriers; however, these expansions are unstable so that the number of repeats may differ in the subsequent generation. Most commonly, the number of repeats increases, especially when the mutation is inherited from the father suggesting that during spermatogenesis the expanded CAG stretch is more unstable than during oogenesis. This phenomenon is called anticipation; it can explain the occurrence of apparently de novo HD in a family without any other family member affected by HD. In addition, in juvenile HD most commonly the mutation was passed on from an affected father [11, 12]. The risk of a large increase of CAG repeat length depends on the size of the parental CAG repeat. The risk is higher if the parental CAG repeat expansion is already large.
The analytical methods to measure CAG repeat length involve a PCR analysis of the region containing the CAG repeat followed by capillary gel electrophoresis to determine the size of the fragment. This should allow the separation of alleles that differ by one repeat [13, 14] (Fig. 5.1). It is critical that laboratories adhere to published guidelines for genotyping in particular since a considerable proportion of genotyping results are still outside acceptable error limits [15, 16].
HTT genetic test result using PCR capillary electrophoresis. A fragment containing the CAG repeat section within the first exon of the HTT gene is amplified by PCR and labeled with a fluorescent dye. The labeled fragments are then separated according to size using capillary electrophoresis. The highest fluorescent peak is called automatically. A standardized marker indicates the size in base pairs (bp, red triangles). Because the number of triplets of the amplified fragment flanking the CAG repeat section in exon one is known, it is known that the first peak in the controls is at 18 CAG repeats. One can then calculate the difference in bp between the second peak and the first peak, which is divided by three results in the number of CAG repeats of the second allele. In control 1 (healthy control) there is only one peak. In control 2 (disease control) this is 43 CAG repeats; in the patient the CAG repeat size is 42. Note that for technical reasons there is more than one peak. This is why the result is given as 42 ± 1 CAG repeats
Predictive and Diagnostic Genetic Testing
There are two main reasons for requesting HTT genetic testing. If the diagnosis of HD is suspected on clinical grounds, most commonly when clinical signs of the typical movement disorder and a family history for HD are present, genetic testing is requested by a clinician to confirm the clinical diagnosis. A diagnostic test may also be requested to rule out HD in a progressive neuropsychiatric disorder of unknown cause. From when the possibility of HD is considered in the clinically affected relative, family members should be prepared for the potential implications to their own risk in case the diagnostic test confirms the diagnosis of HD. If it does, family members should be offered genetic counseling about their own risk of having inherited the HTT mutation.
Anyone who is healthy but has a parent with genetically confirmed HD is at risk. Genetic testing in this context is predictive of whether an individual can expect HD to manifest some time later in life. This form of genetic testing is called predictive testing. The decision of an at-risk individual to undergo predictive testing is very personal. As long as there is no causal treatment available, that decision is not objectively right or wrong. Appropriate genetic counseling should follow international guidelines so that the participant can make an informed decision and has the necessary support in coping with the predictive testing procedure and the test result [17, 18]. The genetic test for the HTT mutation is deceptively simple. However, the process of undergoing genetic counseling, making a decision about predictive testing and coping with the test result can be very complex. The interested reader is referred to the appropriate literature for in-depth information (e.g., [18]). In brief, it is very important that the person seeking counseling for predictive testing has a clear understanding about what the test results mean for many life decisions. Each participant undergoing genetic counseling has to make an autonomous decision. This requires that the participant is mature and independent enough; this is an important reason why minors should not be tested. In addition, participants should not be coerced to have the test by any third party – such as family members, insurance companies, or employers.
Counseling should consist of three appointments with at least 4 weeks between them. At the first appointment the participant’s motivation for seeking advice should be established. This includes taking a careful family history and verifying that indeed there is a confirmed diagnosis of HD in the family. The individual’s knowledge of HD and predictive testing should be probed as well as the personal experience with HD in the family and the current life circumstances. This helps in providing the participant and his partner and/or family with information about what is known about the genetics, HD phenotype(s), and disease evolution, as well as the current treatment and management options. It is very important that the participant understands that with very few exceptions (intermediate allele, reduced penetrance range; see above) genetic testing can give a clear answer to the question whether the participant has inherited the mutant allele and will thus in his lifetime develop HD. While there is an association of CAG repeat length with age-at-onset, a substantial percentage of the variability of age-at-onset (about 40 %) cannot be explained by the CAG repeat [10]. This means that in a given participant it is not possible to predict accurately when exactly HD will manifest. It is also not possible to make predictions about how HD will manifest, i.e., what the phenotype of a gene carrier’s future HD will be, or how HD will develop once a clinical diagnosis of manifest HD is made. Fortunately, in recent years, information from reliable sources has become available on the Internet about HD and HD research using language and a format that is suitable for laypersons and young people (www.hdbuzz.net; www.hdyo.org; www.predictivetestingforhd.com). At the second appointment, the participant has the opportunity for further counseling. If the decision is to have the test, it is now important to prepare for the disclosure of the test result and the implications this may have for the participant. This includes a detailed assessment of the participant’s risk of not coping well with the test result and his support by family and friends, and professionals, e.g., a psychologist. It is important to acknowledge that the predictive test result can negatively impact on mood so it is paramount to carefully assess the participant for depressive symptoms [19]. The participant needs to consider insurance issues since some insurance policies can only be taken out without knowledge of the HTT gene status. The laws regulating disclosure of genetic test results may differ between countries. In some countries the implications of the disclosure of HTT gene status are such that they may deter people from having the test. This is probably one explanation for the fairly low number of people undergoing predictive testing.
Following the disclosure of the test result, it is very important to arrange for a formal follow-up. This should consist of a telephone call within a few days of the disclosure, another visit to the clinic a few weeks later, and the offer for regular follow-up once a year or on demand to provide a port of call for all questions and concerns the participant may have. This can include counseling regarding the reproductive options, which, e.g., consist of having children without testing, prenatal diagnosis, and preimplantation genetic diagnosis (PIGD) [20, 21]. Such a post-predictive testing follow-up can take place within a specialist HD clinic preferably on a clinic day dedicated to people affected by HD but without signs of manifest HD.
Pathophysiology
Huntingtin (HTT) is a very large soluble acidic protein that consists of 3,144 amino acids. HTT is expressed in every tissue. Its N-terminus contains a polyglutamine stretch and a poly-proline domain; it has nuclear import and export signals and harbors so-called HEAT repeats, about 40 amino acid sequences that are present several times. These repeats are composed of two antiparallel α-helices with helical hairpin configuration [22, 23]. HTT has hundreds of binding partners [24, 25]. In addition, it is extensively modified posttranslationally including phosphorylation, ubiquitination, sumoylation, acetylation, and palmitoylation [26].
HTT is predominantly localized in the cytosol where it can associate with cell membranes such as those of the endoplasmatic reticulum or Golgi [27]. In addition, HTT can shuttle into the nucleus where it may contribute to the regulation of transcriptional activity [27]. Since HTT loss of function is lethal during early mouse development, it is likely that HTT plays an important role in tissue differentiation [28]. HTT is also important for neuronal health, at least in mice, since inactivation of mouse huntingtin in adult mice causes neurodegeneration [29]. Evolutionary, CAG repeat length in the normal range in the N-terminus increases from simple organisms to the human being. Thus, HTT with its longer CAG repeat stretch in humans could have contributed to the development of more complex nervous systems [30]. In addition to regulating transcription, HTT may serve as a scaffolding protein, contribute to vesicular transport, and aid synapse function [24]. However, the cellular functions of normal HTT remain little understood.
The CAG repeat expansion mutation in the HTT gene translates to an expanded polyglutamine stretch in the HTT protein. There is good evidence from HD model systems and human HD to suggest that mutant HTT confers a toxic gain of function [4]. A pathological hallmark of HD is abnormal conformation of mutant HTT giving rise to various forms of mutant HTT including soluble, intermediate, monomeric, and oligomeric forms that in themselves may be toxic. Eventually, this could result in intranuclear and cytoplasmic inclusions composed of aggregated N-terminal HTT fragments and a variety of ubiquitinated proteins [4, 31]. Recent evidence in mouse models (both R 6/2 exon 1 HTT transgenes and HdhQ150 knock-in mice) and human HD suggests that abnormal splicing of exon 1 HTT results in N-terminal exon 1 HTT protein fragments [32]. Thus, exon 1 HTT protein fragments may be a common important denominator in HD pathogenesis [32]. Normal huntingtin can be caught up in these inclusions resulting in an additional loss of function [33]. The inclusions challenge the cell’s clearance systems, in particular the ubiquitin-proteasome system and autophagy pathways [26, 34, 35]. It remains an open question if inclusions themselves are toxic or a response of the cell to protect itself. However, promoting clearance of mutant HTT would be expected to be beneficial for patients. Hyper-acetylation, e.g., by inhibiting deacetylation with histone deacetylase (HDAC) inhibitors, can help targeting the mutant HTT protein to autophagosomes to facilitate its degradation and thus clearance [36]. For a more in-depth review of the pathophysiology, the reader is referred to reviews dedicated to this topic [4, 26].
Much of what we know about the pathophysiology of HD comes from model systems. This certainly has contributed substantially to our understanding of HD pathophysiology. However, hypotheses derived from model systems, in particular those relevant for novel therapies, need to be tested in humans with the disease. The HD gene mutation can be identified reliably; thus the molecular changes underlying the pathophysiology of HD can be investigated in manifest HD patients but also in expansion mutation carriers many years before they develop unequivocal signs of HD. Understanding the evolution of HD biology independent of, and in conjunction with, the clinical phenotype can help identify targets for treatments that may prevent or delay the emergence of HD signs and slow disease progression. Such changes can serve as biomarkers of the activity of HD biology and, in the future, may help indicate when to initiate treatment and how to assess novel HD therapeutics in clinical trials [37].
A key finding in HD is the pronounced, and selective, loss of GABAergic medium spiny striatal neurons projecting to the substantia nigra and the globus pallidus [38]. MRI demonstrates loss of striatal volume that is evident even before the emergence of HD signs but also changes in white matter (Fig. 5.2a); with time striatal volumes diminish faster than in controls [39, 40]. However, there is also good evidence from neuroimaging studies for early cortical involvement in particular the motor cortex and the occipital lobe [41, 42] indicating that HD is not confined to a single brain region but leads to widespread pathology [38, 43] (Fig. 5.2b). The HTT mutation is present in every tissue. HD predominantly, but not exclusively, affects the brain [44, 45]. This is important because evidence for HD pathology beyond the brain means that peripheral tissues may serve as a source for biomarkers.
Three Tesla structural MRI in manifest HD. (a) The striatum shows decreased gray matter volume (GMV) in manifest HD individuals compared to healthy controls. Results of the 2nd-level ANOVA, p < 0.05, FWE corrected. The figure displays the SPM5 “glass brain” output (bottom, left) together with maps rendered onto the anatomical template implemented in SPM5. (b) More widespread atrophy extending beyond the striatum in manifest HD. Results of the 2nd-level ANOVA, p < 0.001 (uncorrected at the voxel level), p < 0.05 corrected for spatial extent. For illustration purposes, the 2nd-level maps were thresholded at t = 3.4 (corresponding p < 0.001, uncorrected for height) and rendered onto the anatomical template implemented in MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/) (Images courtesy of Dr C Wolf)
As with clinical trials the study of human HD benefits from multi-site efforts following a standard protocol with appropriate quality assessment and quality control. Examples for such efforts are the observational studies COHORT [6], REGISTRY [5], and PREDICT-HD [46], as well as TRACK-HD and TRACK-ON HD [39]. These studies have already contributed enormously to our understanding of human HD.
Diagnosis and Age-at-Onset
The clinical diagnosis of manifest HD can be made with certainty if unequivocal signs of HD are present in an individual with a CAG repeat expansion mutation in the HTT gene. A clinical diagnosis of manifest HD needs to be distinguished from a genetic test result demonstrating a CAG repeat expansion in the HTT gene in someone who is completely well. The result of a genetic test is not a disease; it can be a predictor of a disease in the individual’s future, which is why this is referred to as predictive testing (see above). It is usually very difficult, if not impossible, to precisely determine the age-at-onset of clinical signs of HD. The concept of age-at-onset refers to when a carrier of the mutated HTT gene develops unequivocal signs of HD. The accurate determination of age-at-onset is critical to identify factors that modify age-at-onset and to develop and evaluate therapies that aim to delay it. If a manifest HD patient attends an HD clinic, the clinician estimates age-at-onset retrospectively based on information from the patient, relatives, and carers. Age-at-onset is most commonly defined as the age-at-onset of motor signs; however, in many patients the first sign of HD may be a non-motor sign with motor signs appearing later [47].
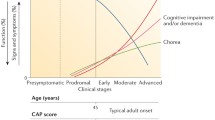
Predicting age-at-onset accurately in the prodromal phase – when the person shows no sign of disease – is also very important with a view to future clinical trials that evaluate the effects of therapeutics that aim to delay age-at-onset. By and large, longer CAG repeat expansions are associated with an earlier onset, so that most, but not all, patients with juvenile HD (see below) have more than 60 CAG repeats; carriers of shorter CAG repeat expansions tend to develop signs of HD later in life (Fig. 5.3). However, the variation of CAG repeat size only explains about 50–60 % of the variability of age-at-onset [10]. This means that in a group of individuals with the same CAG repeat length, the age-at-onset can differ by more than 10 years (Fig. 5.3). A number of algorithms have been devised to help estimate age-at-onset in prodromal HTT expansion mutation carriers (reviewed in [48]). The Langbehn formula [49] uses CAG repeat length and age because of their well-known influences on age-at-onset and calculates the time to a predefined degree of probability of manifesting signs of HD. However, CAG repeat length accounts for only about 50–60 % of the variability, so other factors not modeled in this formula likely influence AAO. For patients very close to onset, i.e., persons at greatest risk, the Langbehn formula may be limited, and it is not helpful comparing prodromal with manifest HD. It may also not be suitable for all CAG repeat lengths and has been established mainly in North American patients. Another formula uses CAG repeat length and parental onset age to estimate AAO. This may accommodate for some other inherited factors as an advantage over the Langbehn formula. However, it was derived from a small sample of affected parent-child pairs and needs to be validated in larger numbers of patients.
In addition to the CAG repeat expansion in the HTT gene, other factors, e.g., genetic or environmental, may influence when signs of HD develop, in which domain the first signs occur and how they evolve over time. At present, many studies of genetic modifiers relate their effect to a general onset of HD. It is possible that there are domain-specific onset modifiers. Such domain-specific modifying effects may be overlooked unless domain-specific onsets are defined.
Clinical Manifestations
Most carriers of the HTT mutation develop clinical signs of HD between the ages of 30 and 50. However, there are very early, so-called juvenile, or even childhood onset, forms of HD [50], as well as late-onset variants with onset later than age 60 [51]. The clinical spectrum of HD comprises progressive motor dysfunction with a mixed movement disorder featuring chorea, dystonia, bradykinesia and rigidity, clumsiness, and gait and balance abnormalities. Patients invariably develop dementia with personality changes and progressive loss of autonomy, while behavioral problems such as depression or irritability are present in many, but not all, patients. In addition, urge incontinence and bowl problems, weight loss, as well as insensitivity to pain may develop; in the absence of an alternative explanation, these clinical features are probably also due to HD. In the following the clinical features of HD will be divided into clinical domains bearing in mind that in practice this may not always be so clear.
Motor Domain
The motor phenotype consists in most patients of chorea and dystonia. Hence HD is considered a predominantly hyperkinetic movement disorder. Chorea as the sole feature of the motor manifestations is, in the author’s experience, the exception rather than the rule. In most patients, the movement disorder is mixed. Chorea is generalized and often involves the face, mouth, tongue, trunk, and arms more than the legs. Patients find it difficult to maintain a posture; motor impersistence affects the eyes, the tongue, or the limbs. Almost always chorea is accompanied by a degree of dystonia; in particular cervical dystonia is common. Parkinsonian features – bradykinesia and rigidity – are also not uncommon and can be the most prominent motor sign in some patients, e.g., those with juvenile onset but also in other patients with a later onset. The type of movements that predominate may reflect inherent differences in other domains, e.g., cognition, with predominantly hypokinetic–rigid patients performing worse on cognitive assessments and being more impaired in day-to-day function [52]. However, cognitive assessments often have a motor component and time limits so that simply being slower may mean that hypokinetic–rigid patients cannot complete tasks with the same speed as predominantly hyperkinetic patients.
Patients become increasingly clumsy because of involuntary movements and declining motor coordination. Household items break more frequently, e.g., crockery, or impaired fine motor skills may affect work performance in particular in jobs that require those skills.
A characteristic feature of HD is the oculomotor disorder. In addition to gaze impersistence – the patient finds it difficult to maintain eye contact, and the eyes seem choreatic – patients may have to induce voluntary eye movements with a blink or head movements. These head movements or eye blinking can be suppressed to a certain degree, but eventually patients may be incapable of initiating saccades without turning their head. This can resemble oculomotor apraxia.
A swallowing disorder develops in most patients with HD. The movement disorder affects muscles responsible for all phases of swallowing. The swallowing disorder often develops from first the incoordination of tongue and pharyngeal muscles so that patients and family report a tendency to swallow too much at a time. The patient may find it difficult to move food in a well-coordinated fashion with the tongue towards the pharynx. Later even swallowing saliva may become difficult with frequent aspiration followed by bouts of coughing. Eventually, the risk for aspiration may increase to a degree that necessitates the placement of a gastric feeding tube.
The assessment of the motor phenotype relies on the motor subscale of the Unified Huntington’s Disease Rating Scale (UHDRS) [53], a categorical, semiquantitative scale susceptible to error and with substantial inter-rater variability [54]. The scale was designed for manifest HD even though it was able to detect small signal changes in preclinical HD [55]. There is considerable interest to develop tools that quantitate the motor assessment objectively including in the preclinical phase of the disease. To this end computer-assisted measurements of a variety of motor acts such as finger tapping [56], grip force, tongue force, or chorea have been developed that are sensitive to change over time [39]. Subtle motor signs may precede the diagnosis of manifest HD by many years [46, 55]. The development of objective quantifiable measures of HD motor signs is important because, in the future, treatments may aim to delay or avoid onset of HD. Thus, monitoring subtle clinical signs that are present in the preclinical phase of HD could be very valuable.
Cognitive Domain
Cognition is invariably affected over the course of HD (for a review see [57]). Some people develop cognitive impairment early on before a diagnosis of motor manifest HD is made [58]. In some it may be the first sign of manifest HD, while others seem less impaired until they are further advanced. Once evident, cognitive skills decline until every HD patient has developed dementia. This very likely reflects ongoing degeneration in the underlying brain structure, in particular fronto-striatal circuits. Executive dysfunction is typical in HD. Patients find it more and more difficult to plan and organize their daily activities and chores and are easily overwhelmed when mental flexibility is required, e.g., in dealing with multiple tasks that they have to attend to at the same time. This can be a complaint at the workplace where it may be noted that the affected individual has slowed down, achieving less and less even when working more hours. The patient may only be able to deal with one task at a time having difficulty to switch attention efficiently. The patient may note these shortcomings himself; as a consequence self-esteem may drop, frustration may rise, and people can become irritable, angry, or depressed. Mental inflexibility can develop into perseveration, and a lack of will and executive dysfunction can develop into apathy (see below).
As the disease progresses, patients become more self-centered and fail to see other people’s viewpoint. The perception of self and others changes, as does the ability to monitor self-appearance and the consequences of actions, e.g., regarding the patient’s social surroundings. Patients progressively neglect themselves. This includes grooming, personal hygiene, and eating but also their relationships with others, in particular their family and carers. This may be related to deficits of emotion recognition abilities [59], in particular concerning negative emotions, as a result of which the relationship with family and carers can suffer a loss of empathy and connectedness when the patient increasingly needs support and, at the same time, changes as a person (for a review see [60]). Not all cognitive abilities decline with the same speed. Language skills, for instance, may be preserved much longer than executive skills. However, as in other forms of dementia, HD patients gradually lose autonomy and become dependent on carers and family.
It is important to distinguish attention deficits with subsequent cognitive impairment from primary cognitive deficits [61]. Inattention and loss of energy and drive can be important features of depression and can lead to reduced cognitive performance. In any HD patient with cognitive impairment, in particular when this evolves rapidly at early disease stages, a mood disorder has to be considered and treated. Psychosis may be another explanation for a (treatable) loss of cognitive skills and can be difficult to diagnose clinically (see below).
Neuropsychiatric Manifestations
The motor signs of HD may be the most noticeable. However, together with the cognitive features, the main neuropsychiatric aspects of HD – depression, anxiety, irritability and aggression, perseveration, apathy, and psychosis –are very often more troublesome. They have a higher negative impact on quality of life for patients, families, and caregivers than the motor manifestations [57, 62]. Consistent with clinical impression, systematic data analysis, using, e.g., principal component analysis, confirms that neuropsychiatric symptoms in HD can be differentiated into those that pertain to affect, irritability/aggression, and apathy [63, 64]. With the exception of apathy, behavioral abnormalities may be common but are not invariably part of HD. This may relate to the episodic nature of many behavioral problems, e.g., depression or psychosis, which are amenable to treatment. HD and depression may be two separate disorders [65]. In this context, it was recently shown that current sub-threshold depressive symptoms in early HD were associated with microstructural changes – without concomitant brain volume loss – in brain regions known to be involved in major depressive disorder (MDD), but not those typically associated with HD pathology [66]. Apathy, in contrast, may reflect degeneration within fronto-striatal-cortical networks as HD advances [39, 67].
Affective Disorders
The most common affective disorder in HD is depression. Anxiety may also be very common, but it can be difficult to say whether anxiety goes beyond the uncertainty and worrying when expecting a terrible disease to strike. Anxiety is very often part of a depressive disorder from which it is therefore difficult to disentangle. Depression can occur at any point in time during the course of HD, with its severity ranging from sub-threshold depressive symptoms to MDD [68]. The estimated prevalence of depression in symptomatic HD varies between 30 and 45 % [46] with an estimated prevalence rate of 16.5 % compared to 5.5 % in the normal population [69]. In HD, depression is highly debilitating and a key determinant of social functioning, life satisfaction, and well-being [62]. The need for complex adjustments coupled with preserved insight into the significance of HD symptoms may be one factor explaining why depressive symptoms seem to be particularly common in early HD [70]. Adverse life circumstances may therefore play an important role in triggering and maintaining depression in individuals with HD. The diagnosis of MDD may be challenging in HD because many of the physical symptoms required to make a diagnosis of MDD according to DSM-IV-TR can also be part of HD even in the absence of the core symptoms of sadness and anhedonia. It is therefore important to use appropriate assessment tools such as the clinician-rated Problem Behaviours Assessment (PBA) [71] and the Hospital Anxiety and Depression Scale [72] self-rating scale. These instruments focus on the core symptoms of depression without any of the physical signs and symptoms that may simply be the result of a degenerative disorder.
The description of George Huntington already emphasized that suicide was a serious risk in HD [1]. Suicidal ideation is probably very common in HD with data suggesting that maybe a third of HD patients entertain suicidal ideas in their lifetime [47, 70, 73]. Low mood is a predictor for suicidal ideas; hence screening for the core symptoms of depression is important. However, many people with HD, in particular in the preclinical phase, consider suicide an option should they lose their autonomy when the disease manifests. Considering the inevitability of a relentlessly progressive loss of abilities once HD symptoms become obvious, this is understandable; suicide as a way out of this conundrum can reassure people with HD that they retain a degree of control over their fate. Once affected by the signs of manifest HD, the increasing loss of insight, energy, and motivation means that very few patients actually go on and kill themselves. In the author’s experience it can be very helpful if patients feel that their physician acknowledges their predicament and does not think that such thoughts are wrong and a sign of illness.
Irritability and Aggression
Relatives and carers of HD patients often report that irritability and outbursts of verbal and physical aggression are the most difficult behavioral challenge. HD patients, particularly when insight is preserved, develop mood swings where they feel extreme anger irresistibly welling up within them leading to an explosive outburst with verbal abuse and aggression towards objects or even towards other people. This is then followed by remorse, sadness, and, sometimes, even ideas of suicide. This resembles rage attacks in the context of impaired impulse control in other circumstances. Often the hostility and aggression are directed towards those closest to the patient. Once insight is lost, it is then mainly the family and carers of patients that report such behavior. The behavior can go on for a long time, often hours or even days, and is out of proportion to the preceding, often only very minor, provocation or inconvenience. A simple reminder of a trivial task can suffice to spark an outburst. People around the patient have to change their behavior, and, when untreated, irritability and aggression can estrange the patient emotionally from those he or she most depends on. As a consequence family and carers may feel that they cannot cope with the situation so that the patient may no longer be able to live at home.
Some degree of irritability is probably quite common in HD [57, 74]. It can be assessed using the clinician-rated PBA, or PBAs, or the Snaith irritability self-rating scale [71, 75]. For management decisions it is important to carefully assess whether other signs and symptoms such as low mood, sleep problems, motor signs, and the patient’s life circumstances can contribute towards irritability. Depression, for instance, can be associated with irritability, and the everyday frustrations of being clumsy or not having anything useful to do can lower self-esteem, impact on mood, and thus contribute to irritability.
Apathy
Apathy denotes a lack of interest, feeling, drive, emotion, or concern. Some patients experience this as very unpleasant and suffer from apathy, e.g., in the context of depression. Others may have lost insight and are not concerned, quite in contrast to their relatives. If pronounced this cluster of symptoms resembles abulia, and patients lack the will to do anything. In HD, apathy develops insidiously so that it may take some time for the symptoms to become noticeable. When it does, however, apathy can be a very frustrating sign of HD for family and carers [57]. The patient has lost his interest in hobbies, spends more time doing very little, and needs a push from those around him. If not reminded by others, the patient may neglect personal hygiene, grooming, and even eating. He may spend a lot of time watching TV and may not even be bothered to change the channel. In contrast to family and carers, the patient is not unduly concerned by his lack of will.
The onset of apathy may predate the onset of unequivocal motor signs of HD and probably reflects degeneration in fronto-striatal-cortical networks [39]. Apathy is thus part of the personality changes that develop with dementia in HD. However, it may also be part of several different underlying psychopathologies including depression and, as a negative syndrome, psychosis [76, 77]. Current concepts of what constitutes apathy, and how to diagnose it, have recently been reappraised [76, 77]. Since apathy is currently not well defined, and its origins may be quite different, it is perhaps not surprising that the association of apathy with the biological load of HD (disease burden, [78]) is not as strong as that of motor or cognitive signs [47].
Perseveration and Obsessive–Compulsive Disorder
Obsessive–compulsive disorder (OCD) is an anxiety disorder. Patients feel they have to do certain things, dwell on a thought, recall an experience, or ruminate on something abstract (ICD-10; http://www.who.int/classifications/icd/en/). Characteristically, at the expense of mounting levels of anxiety, patients need to resist such behavior or thinking, which is qualified as alien to his or her personality. Some of the obsessive behaviors aim to relieve anxiety, worry, fear, or uneasiness in a ritualistic way, e.g., washing in response to fear of contamination. OCD in patients with HD may not be more common than in the general population. However, repetitive behaviors that do not follow on from anxiety-provoking thoughts and are characteristically not perceived as alien or abnormal are very common in HD [57]. The distinction from OCD is important because the underlying pathophysiological concepts and management differ between OCD and perseveration. Perseverative behaviors are most commonly reported by family and carers. The patient, very often when a degree of dementia has already developed, gets stuck on certain ideas or behaviors and is not easily redirected. He prefers certain routines in his day-to-day life and may react angrily if forced to vary from this routine. Simple things such as an appointment with the doctor can provoke discomfort and sleepless nights, and together with a loss of the sense of time, the patient may be restless and urging for the departure for that appointment hours before it would actually be necessary. He may repeatedly ask the same questions or get stuck on a certain topic about which he will go on and on. Without prompting he may return to this topic later in the day, or sometimes even the next day, and will again dwell on this for a long time. While the patient may be unfazed, such perseverative behaviors can cause major distress to family and carers.
Psychosis
Psychosis in HD strikingly resembles schizophrenia with delusions, auditory hallucinations, disordered thinking, social withdrawal, and emotional blunting. The prevalence figures for psychosis in HD range from 3 to 11 % (see [57, 69]). However, the prevalence of psychosis in HD is much lower than for other neuropsychiatric symptoms (see above). Similar to major depressive episodes, the diagnosis of schizophrenia in HD is difficult and, according to the various diagnostic guidelines such as ICD or DSM, not recommended because of the organic basis of HD. Clinically, it can be challenging to distinguish apathy and emotional changes in the context of the degenerative dementia in HD from negative symptoms as a sign of a psychotic episode in particular in the absence of clear evidence of delusions or/and hallucinations. Psychosis can occur at any time in HD including the motor premanifest phase. Hence, prominent negative symptoms and disordered thinking with a rather abrupt onset in an HD patient in whom cognition seemed, until then, fairly intact raise the possibility of a psychosis, in particular if there is no evidence to suggest the presence of a mood disorder. Considering the impact on quality of life, a suspicion of psychosis merits empirical treatment, which can sometimes clarify that distinction.
Juvenile HD
Juvenile HD (JHD) is often defined as HD with an arbitrary age-at-onset before age 20. More importantly, JHD affects individuals who are still developing intellectually, emotionally, and as socially competent independent persons. This may help understand some of the clinical phenomena but also the enormous implications of JHD for the patient and the affected family [50].
Fortunately, JHD is rare. Depending on the epidemiological study, between 1 and 10 % of HD patients have JHD, while childhood HD (onset before age 10) may be even rarer [79]. JHD can manifest before unequivocal signs of HD become apparent in a parent; most commonly, JHD is inherited from an affected father (anticipation, see below).
Clinically, as in adult-onset HD, patients have a mixed movement disorder, cognitive impairment, and behavioral abnormalities. However, there are important differences between the phenotype of adult-onset HD and JHD. Instead of chorea, which, if present, appears fairly late, a hypokinetic–rigid syndrome, dystonia, ataxia, and tremor predominate. A considerable number of patients with JHD (about 30 %) have epileptic seizures. The cognitive changes and behavioral abnormalities can be particularly troubling. Depending on the age of the child, this becomes apparent as developmental delay or, in older children, as a loss of cognitive abilities and dementia. Children may show a change of character and personality with aggressive, hostile, and oppositional and antisocial behavior; as in adults with HD, depression and apathy are common. It is sometimes difficult to differentiate signs and symptoms that are directly related to the biology of HD from those that may arise as a consequence of the psychologically and emotionally challenging responses from the environment.
The management and treatment of JHD is even more complex than in adult HD. It always requires close interdisciplinary collaboration of patients and their family and carers, medical doctors, psychologists, teachers, and others around the patient and his family. The interested reader is referred to a very good book on JHD for further reading [50].
Treatment and Management of HD
Standards of Care and Multidisciplinary Clinic
HD management is not limited to the HD patient as the situation affects the whole family. It includes relatives being at risk of having inherited the HD mutation, those knowing they carry the HD gene, carers, and symptomatic family members. This adds to the complexity of HD. Therefore, HD care requires a multidisciplinary approach involving a wide range of services that can support the symptomatic individual in each stage of HD as well as addressing the needs of those around him [80]. A multidisciplinary approach to the family with HD comprises a variety of specialized services, such as neurology, psychiatry, neuropsychology, clinical genetics, physiotherapy, speech and language therapy, dietician, social services, and dentistry. An HD management clinic can serve as the hub where these specialized services come together to benefit the HD family. The role of the HD clinic is to provide information and to establish a management plan in collaboration with partner agencies outside the clinic. These partner agencies include, e.g., acute services and inpatient care, general physicians and primary care services, psychiatric care, psychiatric nursing services, social worker and welfare rights, financial advisors, disability employment advisors, peer support groups, housing support services, day-care services, personal care, occupational therapy services, and drivers’ licensing authorities. For examples of multidisciplinary HD clinics and guidelines, the reader is referred to the websites of the Huntington’s Disease Society of America (www.hdsa.org) or the European Huntington’s Disease Network’s Standards of Care working group (EHDN; www.euro-hd.net).
Management of Clinical Manifestations
The majority of patients attending an HD clinic will have manifest HD. However, it is important to recognize that living with the knowledge of carrying a mutated HTT gene can be very difficult. Even though there are no clinical signs of manifest HD, people with premanifest HD may be in need of support or pharmacological or non-pharmacological treatment. In the absence of causal, disease-modifying treatment options, the approach to treatment will need to be guided by first establishing a hierarchy of problems. This requires taking a careful history from the patient and, importantly, family and caregivers. It is important to identify who has which problem. In particular, behavioral problems, such as apathy or irritability, are sometimes much more troublesome for the family and carers than for the patient who may even deny having any problems at all. Listing the problems in their order of relevance determines in which order the problems need to be addressed. It is very important to explain carefully to the patient and caregivers what the pharmacological and non-pharmacological management options are. This should lead to the definition of the treatment goals and how to measure treatment effects. As a general principle for therapy, one should try and address as many problems from the list with a single intervention. In the course of the evaluation of treatment effects, one has to be mindful of drug interactions when using more than one drug and of trying to differentiate signs of HD from side effects of medication. It is sometimes useful to consider reducing the amount of pharmacotherapy rather than adding yet another drug or increasing the doses since side effects of medication can be mistaken as signs of HD. Finally, it is always important to consider causes other than HD for a particular problem. There is no evidence to suggest that HD patients are protected from other ailments that may thus affect them just as they affect the rest of the population.
Motor Signs and Symptoms
Once it has been established that motor signs and symptoms impair the patient’s day-to-day activities, there are pharmacological and non-pharmacological treatment options (Table 5.1). When considering the treatment options, it is important to be clear about the type of motor symptom – hyperkinetic, hypokinetic–rigid, gait and balance problems, and swallowing – that is to be treated. Symptomatic pharmacological treatment of hyperkinetic motor manifestations most commonly targets the dopaminergic system. Available drugs include dopamine receptor antagonists (“antipsychotics”) that target postsynaptic dopamine receptors and tetrabenazine, a reversible inhibitor of the vesicular monoamine transporter 2 (VMAT-2) that concentrates monoamines such as dopamine within presynaptic vesicles. VMAT-2 inhibition leads to the depletion of presynaptic dopamine. The evidence indicating efficacy of these agents is largely empirical even though small clinical trials suggest antipsychotics are effective, and a placebo-controlled trial demonstrated that tetrabenazine was better than placebo in treating chorea [81, 82]. However, a systematic Cochrane Review of the available data was unable to recommend any drug solely based on the available evidence [83]. It is further important to note that antipsychotics and tetrabenazine can have substantial side effects; adverse affects on cognition, mood, or alertness can outweigh the beneficial effects on chorea and were even recorded in the tetrabenazine trial [82]. It may therefore be premature to derive firm evidence-based guidelines for clinical practice.
In the absence of evidence-based guidelines, recent efforts benefited from large clinical networks in North America and Australia (Huntington Study Group, www.hsg.org) and Europe (European Huntington’s Network, EHDN; www.euro-hd.net) in capturing expert’s experience in the management of HD motor symptoms [84]. This initiative has published an algorithm for the treatment of chorea [84]. This algorithm takes into account that abnormal movements are often accompanied by neuropsychiatric manifestations (see next section). Hence, the choice of treatment depends also on non-motor problems (Table 5.1). In addition, the movement disorder in HD is mixed. While chorea may respond to some degree to treatment, dystonia and hypokinetic–rigid symptoms are more difficult to treat with medication. While it is always worth a trial, hypokinetic–rigid symptoms often do not respond satisfactorily to dopaminergic therapy, at least in the author’s experience. Hypokinetic–rigid symptoms and dystonia can be a side effect of medication, in particular from antipsychotics and tetrabenazine. Dystonia and hypokinetic–rigid symptoms can also contribute to gait and balance disorders, as well as speech and swallowing difficulties. Sometimes, reducing the dose of these medications can have a beneficial effect on these motor symptoms.
Non-pharmacological approaches such as physiotherapy, occupational therapy, and speech and language therapy are very important in the treatment of these motor manifestations. A guideline for physiotherapy published by the EHDN physiotherapy working group can be found on www.euro-hd.net/html/network/groups/physio.
Dysphagia puts patients at risk for aspiration pneumonia. Treatment and prevention of dysphagia remain empirical in the absence of any evidence to support a particular therapeutic strategy [85]. Frequent coughing at meal times suggests the presence of swallowing difficulties. However, it remains unclear if, e.g., video-fluoroscopy assessment is more sensitive to predict aspiration than common clinical sense. The option of placing a gastric feeding tube needs to be discussed with the patient and carers/family when, in the judgment of the physician, the risk of aspiration exists. Patients can benefit greatly from counseling by a dietician whenever dysphagia and/or nutrition (including weight loss) become an issue.
Neuropsychiatric and Cognitive Manifestations
For depression, the treatment and management recommendations are essentially the same as for depression in other contexts [86]. Depending on the severity of the depressive disorder, this can include medication and psychotherapy (Table 5.1). If anxiety and loss of energy predominate, the drug of first choice may be a serotonin reuptake inhibitor (SSRI) such as citalopram or a mixed SSRI/noradrenalin uptake inhibitor (NARI) such as venlafaxine. If insomnia, anxiety, and restlessness are prominent, a sleep-inducing drug such as mirtazapine or agomelatine may be a good first choice. Some patients benefit from a combination of an SSRI, or combined SSRI/NARI, and a sleep-inducing antidepressant. An important distinction is that between insomnia as a biological feature of depression and the fairly common disturbance of the sleep–wake cycle in HD. A sleep-inducing antidepressant can be useful in both situations to improve the quality of sleep. This can have beneficial effects on concentration and thus cognition. It remains to be shown whether improved sleep quality may also contribute to the clearance of unwanted proteins and thus have a biological effect [87].
Apathy can be a particularly troublesome problem in HD since there is no good treatment. Apathy can always, at least in part, occur in the context of depression even if there are no obvious signs of depressed mood. Thus, a trial of an antidepressant, e.g., an SSRI or combined SSRI/NARI like venlafaxine, is warranted (see Table 5.1). If this does not improve the situation, aripiprazole should be tried. In the author’s experience, aripiprazole sometimes improves drive and concentration with beneficial effects on cognition even though this needs to be investigated more systematically. The same is true for bupropion and other pharmacological treatments that are used in other neurodegenerative disorders [88]. It is important to bear in mind that sedation and apathy can be side effects of medication in particular antipsychotics and tetrabenazine. Reducing the dose of these drugs can sometimes have a big effect on apathy. Non-pharmacological interventions are very important even though there is no evidence to advocate any one in particular. These can include scheduled activities, such as physiotherapy and diverse different tasks so that the patient, with the help of a carer who provides support, accepts to adopt a routine.
Irritability and aggression sooner or later cause distress for the patient and his family. Similar to motor treatments and apathy treatments, there is no evidence base that could serve as a treatment guideline. For the same reasons as for chorea, expert opinion has been synthesized into a treatment algorithm for irritability/aggression [89]. Depending on the severity of outbursts, the first choice may be an SSRI if outbursts are verbal but not physical. If depression and insomnia also pose problems, mirtazapine or agomelatine may be good alternatives. Severe aggression with or without physical violent behavior and impulsivity warrant an antipsychotic as first choice (see Table 5.1). The presence of other symptoms also plays a role in the choice of treatment. Concurrent depression, anxiety, or OCD may also respond to an antidepressant, whereas concurrent psychosis requires antipsychotics. In some cases the severity of aggression may require an admission to a psychiatric institution.
Taken together, treatment should be customized to fit the set of problems the patient has. Multiple input including pharmacological treatments and environmental modifications and also psychosocial support and education for caregivers can help them understand and cope with the situation to the benefit of the HD family. Psychological support for patients and relatives is very important, and psychotherapy, in particular in the preclinical phase of HD and in early manifest HD, can be very helpful.
Lifestyle Measures
Environmental enrichment was able to delay the onset, and slow progression, of phenotypical manifestations in HD models [90, 91]. In humans, small effects on age-at-onset were observed in a retrospective analysis of participants’ daily activities. The less active the participants were, the earlier signs and symptoms of HD emerged [92]. Similar to what has been observed in other neurodegenerative disorders such as Parkinson’s disease or Alzheimer’s disease this supports the notion that mental and physical activity has beneficial effects on health [93]. In contrast, the supplementation of essential fatty acids has only had an effect on motor performance in an HD animal model without influencing the degenerative process [94]. In human HD patients, two double-blind placebo-controlled trials also showed no effect of ethyl EPA, an omega-3 fatty acid, over 6 or 12 months [95, 96]. There is data to support the beneficial effects of a Mediterranean diet on cardiovascular disease [97]; however, in the absence of such evidence in HD, no clear recommendations can be given for specific diets in HD.
Outlook for Causal Treatment
HD is a monogenic disease in which the mutant allele causes the disease. Thus, lowering levels of mutant HTT should have a beneficial effect on HD and is thus a very attractive therapeutic option. In conditional model systems of HD, turning off mutant HTT can reverse the phenotype and neuropathology of HD [98, 99]. However, given that in a knockout model abolishing HTT is lethal in the uterus in mice [28], great care has to be taken to ensure that the normal HTT is not interfered with. Targeting the protein is difficult because of its many and largely unknown interactions with other proteins. For this reason inhibiting the expression of mutant HTT at the gene or mRNA level seems particularly promising. To this end, different strategies are being pursued. The first used an approach that was not allele specific. In mouse models, and nonhuman primates, antisense oligonucleotides injected into the brain reduced expression of the mutant and wild-type HTT by 50 %, which was well tolerated [100, 101].
Other approaches are the use of small interfering RNAs or micro RNAs, molecules that contribute to the regulation of gene expression at the RNA level [102]. If targeting the mutant and the normal HTT, there is always the risk that not enough mutant HTT is suppressed, while too little of the normal HTT is left. Even though in nonhuman primates suppression of normal HTT by about 30–50 % seemed safe [103], it is difficult to say how this would be in humans. There is always the possibility that the desired effects may not suffice, while the function of the normal HTT is compromised and causes harm. For this reason, allele-specific treatments would be preferable. Already there is evidence that using, e.g., CAG-directed zinc finger protein (ZFP) repressors [104], or RNAi [105], could do exactly this. With such approaches care has to be taken to ensure that other genes with CAG repeats are not targeted. Apart from the choice of novel therapeutics, two main questions remain before clinical trials can be considered. The first concerns the type of delivery. Delivery of ASO therapeutics given intrathecally would mean the ASOs reach the cortex but not deeper brain areas. The aim would thus be to lower HTT expression in cortical areas. In transgenic animals, such treatment was able to exert a beneficial effect [100]. siRNAs could be administered via a pump implanted into the putamen [106]. ZFPs and miRNAs could be delivered using viral vectors, e.g., adeno-associated viral vectors (AAV) [107]. AAV transduction has the advantage, and maybe the risk, that it only needs to be done once per patient. As AAV viruses integrate into the host genome, presumably a lifetime expression of the HTT suppressing agent is envisioned. Such an approach has been used in ALS, in Parkinson’s disease, and in congenital blindness [108, 109]. In Parkinson’s disease, neurturin AAV delivery was well tolerated, and expression of transgenes was stable several years after the initial transduction [110]. However, depending on the AAV used, an immune response to the transduction of neurons and astrocytes remains a potential risk [111].
The second question concerns the appropriate readouts to use so that the effectiveness of such treatments could be assessed. In models, one can use brain tissue, but for obvious reasons this is not a possibility in humans. Therefore the development and validation of HTT-dependent biological readouts focuses on methods to measure HTT levels, both normal and mutant, or HTT aggregates [112], while at the same time evaluating other potential biomarkers [37].
Conclusions
Twenty years after the discovery of the causal CAG repeat expansion mutation in the HTT gene, Huntington’s disease remains an incurable devastating disorder. However, great progress has been made in understanding the pathophysiology of HD. This has led to the development of the first gene therapy approaches with promising results in HD model systems. This raises hopes that HD, a monogenetic fully penetrant autosomal dominant disorder, may be a model for novel therapeutics in neurodegenerative disorders. In addition, thanks to the efforts of the HD community – families, clinicians, health professionals, and researchers – standards of care are improving patients’ and families’ quality of life. HD networks (HSG, EHDN, RLAH), and their observational studies, have further laid the groundwork for conducting clinical trials of high quality on a global stage. This includes collaboration with clinical trial sponsors in designing and conducting clinical trials, preparing and training investigators, and developing the right assessment tools [113]. The time seems right for the clinical trials of the future that hopefully will change our treatment options to relieve the plight of all those affected by HD.
References
Huntington G. On chorea. Med Surg Rep. 1872;26:320–1.
Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature. 1983;306:234–8.
The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–83.
Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98.
Orth M, Handley OJ, Schwenke C, Dunnett S, Wild EJ, Tabrizi SJ, et al. Observing Huntington’s disease: the European Huntington’s Disease Network’s REGISTRY. J Neurol Neurosurg Psychiatry. 2011;82:1409–12.
Dorsey ER, Beck CA, Darwin K, Nichols P, Brocht AF, Biglan KM, et al. Natural history of Huntington disease. JAMA Neurol. 2013;70(12):1520–30.
Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov Disord. 2012;27:1083–91.
Hayden MR, MacGregor JM, Beighton PH. The prevalence of Huntington’s chorea in South Africa. S Afr Med J. 1980;58:193–6.
Wright HH, Still CN, Abramson RK. Huntington’s disease in black kindreds in South Carolina. Arch Neurol. 1981;38:412–4.
Wexler NS, Lorimer J, Porter J, Gomez F, Moskowitz C, Shackell E, et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc Natl Acad Sci U S A. 2004;101:3498–503.
Harper P. Huntington’s disease: genetic and molecular studies. In: Bates G, Harper P, Jones L, editors. Huntington’s disease: genetic and molecular studies. 3rd ed. Oxford: Oxford University Press; 2002. p. 113–58.
Gonitel R, Squitieri F. Molecular mechanisms in juvenile Huntington’s disease. In: Quarrell OWJ, Brewer H, Squitieri F, Barker RA, Nance MA, Landwehrmeyer GB, editors. Juvenile Huntington’s disease. Oxford: Oxford University Press; 2009. p. 79–100.
Warner JP, Barron LH, Brock DJ. A new polymerase chain reaction (PCR) assay for the trinucleotide repeat that is unstable and expanded on Huntington’s disease chromosomes. Mol Cell Probes. 1993;7:235–9.
Riess O, Noerremoelle A, Soerensen SA, Epplen JT. Improved PCR conditions for the stretch of (CAG)n repeats causing Huntington’s disease. Hum Mol Genet. 1993;2:637.
Losekoot M, van Belzen MJ, Seneca S, Bauer P, Stenhouse SA, Barton DE. EMQN/CMGS best practice guidelines for the molecular genetic testing of Huntington disease. Eur J Hum Genet. 2013;21:480–6.
Quarrell OW, Handley O, O’Donovan K, Dumoulin C, Ramos-Arroyo M, Biunno I, et al. Discrepancies in reporting the CAG repeat lengths for Huntington’s disease. Eur J Hum Genet. 2012;20:20–6.
MacLeod R, Tibben A, Frontali M, Evers-Kiebooms G, Jones A, Martinez-Descales A, et al. Recommendations for the predictive genetic test in Huntington’s disease. Clin Genet. 2013;83:221–31.
Tibben A. Genetic counselling and presymptomatic testing. In: Bates G, Harper P, Jones L, editors. Huntington’s disease. 3rd ed. Oxford: Oxford University Press; 2002. p. 198–250.
Codori AM, Slavney PR, Rosenblatt A, Brandt J. Prevalence of major depression one year after predictive testing for Huntington’s disease. Genet Test. 2004;8:114–9.
de Die-Smulders CE, de Wert GM, Liebaers I, Tibben A, Evers-Kiebooms G. Reproductive options for prospective parents in families with Huntington’s disease: clinical, psychological and ethical reflections. Hum Reprod Update. 2013;19:304–15.
Van Rij MC, De Rademaeker M, Moutou C, Dreesen JC, De Rycke M, Liebaers I, et al. Preimplantation genetic diagnosis (PGD) for Huntington’s disease: the experience of three European centres. Eur J Hum Genet. 2012;20:368–75.
Andrade MA, Bork P. HEAT repeats in the Huntington’s disease protein. Nat Genet. 1995;11:115–6.
Li W, Serpell LC, Carter WJ, Rubinsztein DC, Huntington JA. Expression and characterization of full-length human huntingtin, an elongated HEAT repeat protein. J Biol Chem. 2006;281:15916–22.
Li SH, Li XJ. Huntingtin-protein interactions and the pathogenesis of Huntington’s disease. Trends Genet. 2004;20:146–54.
Shirasaki DI, Greiner ER, Al-Ramahi I, Gray M, Boontheung P, Geschwind DH, et al. Network organization of the huntingtin proteomic interactome in mammalian brain. Neuron. 2012;75:41–57.
La Spada AR, Taylor JP. Repeat expansion disease: progress and puzzles in disease pathogenesis. Nat Rev Genet. 2010;11:247–58.
Cattaneo E, Zuccato C, Tartari M. Normal huntingtin function: an alternative approach to Huntington’s disease. Nat Rev Neurosci. 2005;6:919–30.
Zeitlin S, Liu JP, Chapman DL, Papaioannou VE, Efstratiadis A. Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington’s disease gene homologue. Nat Genet. 1995;11:155–63.
Dragatsis I, Levine MS, Zeitlin S. Inactivation of Hdh in the brain and testis results in progressive neurodegeneration and sterility in mice. Nat Genet. 2000;26:300–6.
Lo Sardo V, Zuccato C, Gaudenzi G, Vitali B, Ramos C, Tartari M, et al. An evolutionary recent neuroepithelial cell adhesion function of huntingtin implicates ADAM10-Ncadherin. Nat Neurosci. 2012;15:713–21.
Olshina MA, Angley LM, Ramdzan YM, Tang J, Bailey MF, Hill AF, et al. Tracking mutant huntingtin aggregation kinetics in cells reveals three major populations that include an invariant oligomer pool. J Biol Chem. 2010;285:21807–16.
Sathasivam K, Neueder A, Gipson TA, Landles C, Benjamin AC, Bondulich MK, et al. Aberrant splicing of HTT generates the pathogenic exon 1 protein in Huntington disease. Proc Natl Acad Sci U S A. 2013;110:2366–70.
Busch A, Engemann S, Lurz R, Okazawa H, Lehrach H, Wanker EE. Mutant huntingtin promotes the fibrillogenesis of wild-type huntingtin: a potential mechanism for loss of huntingtin function in Huntington’s disease. J Biol Chem. 2003;278:41452–61.
Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–6.
Krainc D. Clearance of mutant proteins as a therapeutic target in neurodegenerative diseases. Arch Neurol. 2010;67:388–92.
Jeong H, Then F, Melia Jr TJ, Mazzulli JR, Cui L, Savas JN, et al. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell. 2009;137:60–72.
Weir DW, Sturrock A, Leavitt BR. Development of biomarkers for Huntington’s disease. Lancet Neurol. 2011;10:573–90.
Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–84.
Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos RA, et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12:637–49.
Paulsen JS, Magnotta VA, Mikos AE, Paulson HL, Penziner E, Andreasen NC, et al. Brain structure in preclinical Huntington’s disease. Biol Psychiatry. 2006;59:57–63.
Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, et al. Cerebral cortex and the clinical expression of Huntington’s disease: complexity and heterogeneity. Brain. 2008;131:1057–68.
Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, et al. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8:791–801.
Thu DC, Oorschot DE, Tippett LJ, Nana AL, Hogg VM, Synek BJ, et al. Cell loss in the motor and cingulate cortex correlates with symptomatology in Huntington’s disease. Brain. 2010;133:1094–110.
Sassone J, Colciago C, Cislaghi G, Silani V, Ciammola A. Huntington’s disease: the current state of research with peripheral tissues. Exp Neurol. 2009;219:385–97.
van der Burg JM, Bjorkqvist M, Brundin P. Beyond the brain: widespread pathology in Huntington’s disease. Lancet Neurol. 2009;8:765–74.
Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–80.
Orth M, Handley OJ, Schwenke C, Dunnett SB, Craufurd D, Ho AK, et al. Observing Huntington’s disease: the European Huntington’s Disease Network’s REGISTRY. PLoS Curr. 2010;2:RRN1184.
Langbehn DR, Hayden MR, Paulsen JS. CAG-repeat length and the age of onset in Huntington disease (HD): a review and validation study of statistical approaches. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:397–408.
Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65:267–77.
Quarrell OWJ, Brewer HM, Squitieri F, Barker RA, Nance MA, Landwehrmeyer GB. Juvenile Huntington’s disease. Oxford: Oxford University Press; 2009.
Kremer B. Clinical neurology of Huntington’s disease. In: Bates G, Harper P, Jones L, editors. Huntington’s disease. 3rd ed. Oxford: Oxford University Press; 2002. p. 28–61.
Hart EP, Marinus J, Burgunder JM, Bentivoglio AR, Craufurd D, Reilmann R, et al. Better global and cognitive functioning in choreatic versus hypokinetic-rigid Huntington’s disease. Mov Disord. 2013;28:1142–5.
Huntington Study Group. Unified Huntington’s disease rating scale: reliability and consistency. Mov Disord. 1996;11:136–42.
Hogarth P, Kayson E, Kieburtz K, Marder K, Oakes D, Rosas D, et al. Interrater agreement in the assessment of motor manifestations of Huntington’s disease. Mov Disord. 2005;20:293–7.
Biglan KM, Ross CA, Langbehn DR, Aylward EH, Stout JC, Queller S, et al. Motor abnormalities in premanifest persons with Huntington’s disease: the PREDICT-HD study. Mov Disord. 2009;24:1763–72.
Bechtel N, Scahill RI, Rosas HD, Acharya T, van den Bogaard SJ, Jauffret C, et al. Tapping linked to function and structure in premanifest and symptomatic Huntington disease. Neurology. 2010;75:2150–60.
Craufurd D, Snowden J. Neuropsychological and neuropsychiatric aspects of Huntington’s disease. In: Bates G, Harper P, Jones L, editors. Huntington’s disease. 3rd ed. Oxford: Oxford University Press; 2002. p. 62–94.
Paulsen JS, Smith MM, Long JD. Cognitive decline in prodromal Huntington disease: implications for clinical trials. J Neurol Neurosurg Psychiatry. 2013;84:1233–9.
Sprengelmeyer R, Young AW, Calder AJ, Karnat A, Lange H, Homberg V, et al. Loss of disgust. Perception of faces and emotions in Huntington’s disease. Brain. 1996;119(Pt 5):1647–65.
Henley SM, Novak MJ, Frost C, King J, Tabrizi SJ, Warren JD. Emotion recognition in Huntington’s disease: a systematic review. Neurosci Biobehav Rev. 2012;36:237–53.
Wolf RC, Gron G, Sambataro F, Vasic N, Wolf ND, Thomann PA, et al. Brain activation and functional connectivity in premanifest Huntington’s disease during states of intrinsic and phasic alertness. Hum Brain Mapp. 2012;33:2161–73.
Ho AK, Gilbert AS, Mason SL, Goodman AO, Barker RA. Health-related quality of life in Huntington’s disease: Which factors matter most? Mov Disord. 2009;24:574–8.
Kingma EM, van Duijn E, Timman R, van der Mast RC, Roos RA. Behavioural problems in Huntington’s disease using the Problem Behaviours Assessment. Gen Hosp Psychiatry. 2008;30:155–61.
Rickards H, De Souza J, van Walsem M, van Duijn E, Simpson SA, Squitieri F, et al. Factor analysis of behavioural symptoms in Huntington’s disease. J Neurol Neurosurg Psychiatry. 2011;82:411–2.
Folstein S, Abbott MH, Chase GA, Jensen BA, Folstein MF. The association of affective disorder with Huntington’s disease in a case series and in families. Psychol Med. 1983;13:537–42.
Sprengelmeyer R, Orth M, Müller HP, Wolf RC, Grön G, Depping MS, et al. The neuroanatomy of subthreshold depressive symptoms in Huntington’s disease: a combined diffusion tensor imaging (DTI) and voxel-based morphometry (VBM) study. Psychol Med. 2013;7:1–12.
Thompson JC, Snowden JS, Craufurd D, Neary D. Behavior in Huntington’s disease: dissociating cognition-based and mood-based changes. J Neuropsychiatry Clin Neurosci. 2002;14:37–43.
Epping EA, Paulsen JS. Depression in the early stages of Huntington disease. Neurodegener Dis Manag. 2011;1:407–14.
van Duijn E, Kingma EM, Timman R, Zitman FG, Tibben A, Roos RA, et al. Cross-sectional study on prevalences of psychiatric disorders in mutation carriers of Huntington’s disease compared with mutation-negative first-degree relatives. J Clin Psychiatry. 2008;69:1804–10.
Paulsen JS, Hoth KF, Nehl C, Stierman L. Critical periods of suicide risk in Huntington’s disease. Am J Psychiatry. 2005;162:725–31.
Craufurd D, Thompson JC, Snowden JS. Behavioral changes in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:219–26.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Hubers AA, van Duijn E, Roos RA, Craufurd D, Rickards H, Bernhard Landwehrmeyer G, et al. Suicidal ideation in a European Huntington’s disease population. J Affect Disord. 2013;151:248–58.
Reedeker N, Bouwens JA, Giltay EJ, Le Mair SE, Roos RA, van der Mast RC, et al. Irritability in Huntington’s disease. Psychiatry Res. 2012;200:813–8.
Snaith RP, Constantopoulos AA, Jardine MY, McGuffin P. A clinical scale for the self-assessment of irritability. Br J Psychiatry. 1978;132:164–71.
Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry. 2008;79:1088–92.
Robert P, Onyike CU, Leentjens AF, Dujardin K, Aalten P, Starkstein S, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24:98–104.
Penney Jr JB, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington’s disease. Ann Neurol. 1997;41:689–92.
Quarrell O, O’Donovan KL, Bandmann O, Strong M. The prevalence of Juvenile Huntington’s disease: a review of the literature and meta-analysis. PLoS Curr. 2012;4:e4f8606b742ef3.
Nance MA, Westphal B. Comprehensive care in Huntington’s disease. In: Bates G, Harper P, Jones L, editors. Huntington’s disease. 3rd ed. Oxford: Oxford University Press; 2002. p. 475–500.
Venuto CS, McGarry A, Ma Q, Kieburtz K. Pharmacologic approaches to the treatment of Huntington’s disease. Mov Disord. 2012;27:31–41.
Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2006;66:366–72.
Mestre T, Ferreira J, Coelho MM, Rosa M, Sampaio C. Therapeutic interventions for symptomatic treatment in Huntington’s disease. Cochrane Database Syst Rev. 2009;(3):CD006456.
Burgunder JM, Guttman M, Perlman S, Goodman N, van Kammen DP, Goodman L. An international survey-based algorithm for the pharmacologic treatment of chorea in Huntington’s disease. PLoS Curr. 2011;3, RRN1260.
Heemskerk AW, Roos RA. Dysphagia in Huntington’s disease: a review. Dysphagia. 2011;26:62–6.
Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381:1672–82.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7.
Krishnamoorthy A, Craufurd D. Treatment of apathy in Huntington’s disease and other movement disorders. Curr Treat Options Neurol. 2011;13:508–19.
Groves M, van Duijn E, Anderson K, Craufurd D, Edmondson MC, Goodman N, et al. An international survey-based algorithm for the pharmacologic treatment of irritability in Huntington’s disease. PLoS Curr. 2011;3, RRN1259.
van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington’s in mice. Nature. 2000;404:721–2.
Hockly E, Cordery PM, Woodman B, Mahal A, van Dellen A, Blakemore C, et al. Environmental enrichment slows disease progression in R6/2 Huntington’s disease mice. Ann Neurol. 2002;51:235–42.
Trembath MK, Horton ZA, Tippett L, Hogg V, Collins VR, Churchyard A, et al. A retrospective study of the impact of lifestyle on age at onset of Huntington disease. Mov Disord. 2010;25:1444–50.
Spires TL, Hannan AJ. Nature, nurture and neurology: gene-environment interactions in neurodegenerative disease. FEBS Anniversary Prize Lecture delivered on 27 June 2004 at the 29th FEBS Congress in Warsaw. FEBS J. 2005;272:2347–61.
Van Raamsdonk JM, Pearson J, Rogers DA, Lu G, Barakauskas VE, Barr AM, et al. Ethyl-EPA treatment improves motor dysfunction, but not neurodegeneration in the YAC128 mouse model of Huntington disease. Exp Neurol. 2005;196:266–72.
Huntington Study Group TEND-HD Investigators. Randomized controlled trial of ethyl-eicosapentaenoic acid in Huntington disease: the TREND-HD study. Arch Neurol. 2008;65:1582–9.
Puri BK, Leavitt BR, Hayden MR, Ross CA, Rosenblatt A, Greenamyre JT, et al. Ethyl-EPA in Huntington disease: a double-blind, randomized, placebo-controlled trial. Neurology. 2005;65:286–92.
Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90.
Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101:57–66.
Martin-Aparicio E, Yamamoto A, Hernandez F, Hen R, Avila J, Lucas JJ. Proteasomal-dependent aggregate reversal and absence of cell death in a conditional mouse model of Huntington’s disease. J Neurosci. 2001;21:8772–81.
Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron. 2012;74:1031–44.
Boudreau RL, McBride JL, Martins I, Shen S, Xing Y, Carter BJ, et al. Nonallele-specific silencing of mutant and wild-type huntingtin demonstrates therapeutic efficacy in Huntington’s disease mice. Mol Ther. 2009;17:1053–63.
Davidson BL, Monteys AM. Singles engage the RNA interference pathway. Cell. 2012;150:873–5.
Grondin R, Kaytor MD, Ai Y, Nelson PT, Thakker DR, Heisel J, et al. Six-month partial suppression of Huntingtin is well tolerated in the adult rhesus striatum. Brain. 2012;135:1197–209.
Garriga-Canut M, Agustin-Pavon C, Herrmann F, Sanchez A, Dierssen M, Fillat C, et al. Synthetic zinc finger repressors reduce mutant huntingtin expression in the brain of R6/2 mice. Proc Natl Acad Sci U S A. 2012;109:E3136–45.
Yu D, Pendergraff H, Liu J, Kordasiewicz HB, Cleveland DW, Swayze EE, et al. Single-stranded RNAs use RNAi to potently and allele-selectively inhibit mutant huntingtin expression. Cell. 2012;150:895–908.
Boudreau RL, Rodriguez-Lebron E, Davidson BL. RNAi medicine for the brain: progresses and challenges. Hum Mol Genet. 2011;20:R21–7.
Grieger JC, Samulski RJ. Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol. 2012;507:229–54.
Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, et al. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum Gene Ther. 2012;23:377–81.
Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4:120ra15.
Bartus RT, Baumann TL, Brown L, Kruegel BR, Ostrove JM, Herzog CD. Advancing neurotrophic factors as treatments for age-related neurodegenerative diseases: developing and demonstrating “clinical proof-of-concept” for AAV-neurturin (CERE-120) in Parkinson’s disease. Neurobiol Aging. 2013;34:35–61.
Ciesielska A, Hadaczek P, Mittermeyer G, Zhou S, Wright JF, Bankiewicz KS, et al. Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses. Mol Ther. 2013;21:158–66.
Baldo B, Paganetti P, Grueninger S, Marcellin D, Kaltenbach LS, Lo DC, et al. TR-FRET-based duplex immunoassay reveals an inverse correlation of soluble and aggregated mutant huntingtin in Huntington’s disease. Chem Biol. 2012;19:264–75.
HORIZON Investigators of the Huntington Study Group and European Huntington’s Disease Network. A randomized, double-blind, placebo-controlled study of latrepirdine in patients with mild to moderate Huntington disease. JAMA Neurol. 2013;70:25–33.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Orth, M. (2014). Huntington’s Disease: Clinical Phenotypes and Therapeutics. In: Micheli, F., LeWitt, P. (eds) Chorea. Springer, London. https://doi.org/10.1007/978-1-4471-6455-5_5
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6455-5_5
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6454-8
Online ISBN: 978-1-4471-6455-5
eBook Packages: MedicineMedicine (R0)