Abstract
Classification of porous materials (PM), their properties and characteristics is studied, a comparative analysis is done. An overview of a number of modeling-analytical descriptions of the physical properties of porous bodies is provided. The general issues for producing porous materials by powder metallurgy techniques including various methods of molding and sintering processes are under consideration. The potential applications of porous materials are demonstrated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
In dependence of the chemical composition, the porous materials can be divided into two types: metallic materials and nonmetallic materials. Porous powder materials (PPM), porous fibrous materials of inorganic fibers (PFM), porous mesh materials (PMM), highly porous cellular materials (CM) are produced by powder metallurgy of powdered metals, alloys and refractory compounds.
Porous materials, depending on the type of the pores, can be classified as materials with perforating, closed, and dead-end porosity. The total porosity is the sum of these types of porosity. If the total porosity is less than 7–10 %, all pores are closed, when total porosity is 20–30 % then closed porosity is no greater than 2–3 %. Due to porous structure, the PM can be divided into isotropic and anisotropic ones. Regular alternation of homogeneous structural elements in a space is specific property of isotropic structures, which is typical for materials consisted of spherical particles of the same size as well as for meshes of a regular structure. Anisotropic pore structures have some distribution in numbers, sizes, etc., in one or more directions.
1 Comparative Analysis of the Properties of the Porous Body
Properties of PM are dependent on the chemical composition and shape of the feedstock, processing methods and modes of obtaining the material, its porosity, and other factors [1–3].
The pore sizes depend mainly on the size of the particles (fibers, wires, etc.) of the source materials and porosity. Comparative assessment of different kinds of materials is convenient to carry out in the coordinates: relative pore size—porosity (Fig. 4.1). The highest average pore sizes are typical for cellular materials and for the materials made of the wires and fibers. Maximum porosity of PMM from grid fabrics is limited by the porosity of grids and minimal degree of their compression required to produce the material. The potential of PM can be estimated for practical use basing on the ranges of porosity. From Fig. 4.1 follows that it is possible to use materials of powder particles and fiber as the materials of the filter elements of fine purification, which are characterized by a small pore size, while grid materials—for the more coarse filter. It is known that materials with the porosity of 0.6–0.7 and more are necessary to use as sound-absorbing materials and therefore fiber fabrics, mesh knitted and cellular materials are among them. In the cases where the permeable materials of low porosity with high hydraulic resistance are required, the powder materials consisted of |nonspherical particles and woven meshes are applied.
The ranges of porosity of permeable materials [3]
An important property of porous materials is their permeability. Minimum values of the coefficient of hydraulic resistance in all modes of filtration have been observed in the case of porous materials made of smooth fibers, wires, spheres. The increase of hydraulic resistance of materials compared to similar materials made from smooth particles and fibers is observed with increasing roughness of the precursor particles and fibers, as well as with increasing the tortuosity of porous channels.
Filtration properties of PM are mainly determined by pore size distributions, and mechanisms of filtration of liquid or gas through the pores. Porous materials made of rough particles of nonspherical shapes have the best retention properties during filtration of liquids and gases. Uneven distribution of porosity on the surface of PM significantly reduces quality of the filtration. Permeable materials produced of meshes as well as materials of the fibers have the best strength properties.
Cost of PM is composed of material costs up to 40–60 %. A fraction of overheads increases under conditions of pilot production.
Comparative analysis of the properties of PM shows that several options can be recommended for a specific application. The final choice of material should be carried out taking into account the additional requirements arising from the conditions of PM exploration. The most effective cases of application of materials are those that use multiple properties of PM.
The properties of all materials, including porous ones, can be classified into two groups. Properties that do not depend on the structure, in particular, the coefficients of thermal expansion, magnetic susceptibility and specific heat capacity, which are the same for the porous and nonporous states of materials. Other properties are structurally sensitive. Here we can distinguish properties determined by porosity characteristics of the object: magnitude of porosity, pore sizes, pore shapes and distributions, and tortuosity. These properties include hydraulic and capillary, filter characteristics, etc. Studies of bodies, formed from ultrafine and nanoparticles, have shown the existence of dependence of their properties, including physical ones, on particle size and, as a consequence, on the pore size. These effects occur when the average crystal grain size not greater than 100 nm, and most clearly seen when their size is less than 10 nm.
Very important and wide area of durable and successful application of small particles in the PM production is catalysis of chemical reactions. Catalyzed reactions are typically run at lower temperature than uncatalyzed reactions and are more selective. The number of atoms in an isolated metal particle is small, so the distance between the energy levels ~δ ~E F /N (E F —Fermi energy, N—number of atoms in the particle) is comparable with the thermal energy kT. In the limit when δ > kT, the levels are discrete and the particle loses metallic properties.
With decreasing particle size the surface contribution to the free energy is increased. To reduce the total energy of the system such deformation of the crystal, which will decrease the surface energy, can be more profitable. Surface energy has a minimum for the close-packed structures, so face-centered cubic (FCC) or hexagonal close-packed (HCP) structure are most preferred for nanocrystalline particles, that is observed experimentally. Electron diffraction study of niobium, tantalum, molybdenum and tungsten nanocrystals of 5–10 nm showed that they have the FCC or HCP structure, whereas in a normal state, these metals have a body-centered cubic (BCC) lattice.
Dependence of surface energy on the particle size determines relationship between the melting point and the size of the nanoparticle. Experimental lowering the melting temperature of small particles was observed in many studies. Maximum reduction of the melting temperature of the clusters of Sn, Ga and Hg was 152, 106, and 95 K, respectively.
Among the properties of porous objects most research attention are paid to the properties of the conductivity, in particular, electrical and thermal conductivity, as well as certain mechanical characteristics, mainly tensile strength and modulus of elasticity. In the descriptions of the influence of porosity on the conductivity of the body, assumptions of different authors vary considerably, however, lead to approximately identical results, which are in satisfactory agreement with the experimental data that generally correlates with the degree of sensitivity of this property to the macro-structure of the porous material. A linear dependence on porosity is valid for the electrical conductivity of the porous material (with ε < 0.667):
M. Balshin, using his representation of the contact section of the porous body, showed that the electrical conductivity of the body with connected pores has the form:
while for the body with isolated pores we have:
With the amount of porosity of more than 70 … 80 % very significant differences in the conduction of bodies with matrix and random structure can be observed.
Accounting for formation of random clusters and network of physically continuous phase in the statistical mixture of quasi-spherical particles are proposed by V. Skorokhod as early as 1959 by introducing also randomly oriented infinite cylinders along with the spherical particles, as components of the continuous phase, into a model of the porous body. The following dependence of conductivity on the porosity for such an ideal model of the body is obtained:
where ε S and ε C —the proportion of spherical and cylindrical phases of matter, respectively, and ɛ s = 1 − ɛ(1 + logɛ), ɛ c = ɛ log ɛ. Equation (4.4) is valid for any porous body with perfect inter-particle contacts: powder, fiber or with a mixed structure in the whole range of porosity values.
Significant dependence of structure-sensitive properties of the powder body on quality of interparticle contacts is reflected in the ambiguity of relation of such properties with porosity of the material.
The most common dependence of tensile strength on the porosity is the power-law dependence based on the concept of the contact section of porous bodies which was introduced by M. Balshin:
wherein σ в —tensile strength of nonporous material; m = 2 … 10. Minimum values of the exponent m, corresponding to the maximum value of the contact section and strength are 2 and 3, respectively, for porous, fiber bodies, and powder compacts.
B. Pines, A. Syrenko, and N. Sukhinin suggested the dependence of strength on the porosity, which takes into account the weakening of inter-particle contacts by pores:
where S is the weakening coefficient.
V. Troshchenko, assuming spherical shape for pores, the independence of their size and shape on the porosity as well as changes of porosity being proportional to the initial value during loading, obtained the following relationship:
where A—coefficient of the porosity variation (tension—A > 1, compression—A < 1), B—coefficient of inhomogeneity of the pore distribution on sections of a prototype. It should be noted that the relation (4.7) satisfactorily approximates the experimental data to the porosities in the range of 45 … 50 %. For large values of porosity, the relation (4.7) loses its meaning, because the spherical shape, adopted in the model, does not correspond to the actually observed shape in highly porous objects.
Mention should be made concerning dependence of strength on the porosity, obtained by other authors also, although their practical significance is significantly limited by the complexity of decoding various coefficients. These dependencies are the following expressions:
F. Knudsen’s:
where L—size of the grain; к, a, b—constants;
D. Harvey’s:
where \(k = \frac{{\sqrt {3{\raise0.7ex\hbox{$\varepsilon $} \!\mathord{\left/ {\vphantom {\varepsilon \pi }}\right.\kern-0pt} \!\lower0.7ex\hbox{$\pi $}}} }}{2}\);
D. Hasselman’s:
where A—parameter;
A. Vail’s:
where A—parameter;
R. Herman’s:
where ε = 10 … 40 %; m ~ 4 … 7; D = 4 … 60 μm—diameter of spherical particles of the initial powder.
Phenomenological approach of V. Skorokhod, based on the concept of rms stresses and strains in the material of the porous body, deserves special attention when accounting for the strength of the structure factor. V. Skorokhod proposed the following expression for the dependence of tensile strength on the porosity of the ductile-hardening material with taking into consideration the reduction of the cross-section during deformation process:
where the fracture porosity ε cr is connected with the initial porosity ε (before a test) using the expression of true strain by relative necking of nonporous material ψ 0:
Some dependencies for the yield strength look like the same as for the tensile strength. As an example, the binomial power dependence proposed by M. Balshin in the form of Eq. (4.3), which also describes the relative yield strength of the material in compression as a function of porosity, may be noted. There are known the following basic expressions suggested by M. Nakamura, it is based on the analysis of the elastic stress distribution around the pores (A and B—coefficients):
N. A. Fleck and R. A. Smith:
Shalak:
where b—factor expressing the intensity of the influence of porosity on the yield stress.
Porous materials made of the compacted metal powders and sintered are usually anisotropic objects due to the anisotropy of the size and shape of the initial dispersed particles. Rather simple estimate of the expected anisotropy of properties can be carried out according to the normal elastic modulus. Under the assumption of independence of the integrated elastic rigidity of a plastically deformable porous body on strain and under absence of formation of the new contacting surfaces, the elastic modulus of pressurized body has the following expressions:
in a direction perpendicular to the pressing direction,
in a direction parallel to the pressing direction,
where ε 0 and ε—initial and current porosity.
The most prominent feature in this case is to reduce the modulus of elasticity in the direction of compressing with decreasing porosity of the material.
Further development of theoretical approaches to the description of the elastic properties of porous bodies is using the method of “self-consistency” to the statistical mixture of quasi-spherical elements, with the formation of random clusters and networks of physically continuous phase, which was applied by V. Skorokhod. Corresponding equation, describing the dependence of the shear modulus of the porosity in the whole range of change, has the form:
The above equation is valid for any type of structure—powder, fiber and mixed one.
2 Processes of Powder Metallurgy for Porous Materials
The process of creating the porous material by powder metallurgy method typically includes three basic steps: preparation of the dispersed particles; imparting a predetermined shape to a conglomerate of particles—molding, providing a predetermined set of physical and mechanical properties resulting from high temperature processing—sintering.
2.1 Methods for Forming Porous Materials
Pressing in closed molds are widely used for making articles of simple shape (disk, cone, hub, etc.). Distinguish between unilateral and bilateral pressing. A unilateral compression is used to mold products with a ratio of height to diameter not greater than 1. In all other cases, a bilateral compression is applied. The disadvantages of the method of compression in closed molds should include the limited shapes and sizes of manufactured products, as well as uneven distribution of porosity in the compacts as a result of frictional forces arising between the powder and the walls of the mold, which leads to uneven distribution of permeability and pore sizes in finished products. Advantages of the method are high-dimensional accuracy and higher performance. Increased dispersion of powders, especially of nanoparticles is accompanied by a marked decrease in their compressibility.
Isostatic pressing is a method of compressing the powder in the elastic shell under hydrostatic compression. A variation of this process is hydrostatic and gasostatic pressing and also pressing in thick-walled flexible shells placed into the steel mold.
The method of the hydrostatic compression is based on transmission of the pressure by fluid in the high pressure vessel to metal powder enclosed in an elastic shell. Pressing pressure is usually not more than 15–20 MPa. This method is mainly used to manufacture pipes and sleeves with bottomed. Magnitude of the hydrostatic pressure and pressing power to produce uniform density briquettes is smaller during the hydrostatic pressing than when pressing in a closed mold. This is due to the lack of pressure losses for external friction, due to that under the uniform compression of the powder its slipping relative to the shell does not occur. The disadvantages of hydrostat pressing include difficulty of obtaining products with exact geometric dimensions, the relatively high cost of manufactured products, low productivity. The advantages of the method include the possibility of obtaining products of large size and complicated shape with a uniform distribution of porosity.
By pressing in the resilient thick-walled shells, an elastic matrix inserted into a steel mold plays the role of a pressure-transmitting medium. The shell material should provide a uniform and thorough transfer of pressure on a compressible powder, and have the Poisson coefficient of 0.5. Rubber, a resin, a wax, paraffin, polyurethane, etc., are used as the shell materials. The compression scheme in the elastic shell is shown in Fig. 4.2. Disadvantages of this method of formation are the difficulty of obtaining accurate product shape and size, and the advantage is the ability to manufacture complex shapes.
Rolling of the powders is a continuous molding of pre-forms from powders by rolls. The essence of the method consists in feeding the powder from the hopper into a nip between two rotating rolls toward one another. Powder is entrained in the gap by friction between it and the surface of the rotating rollers and pressed into a strip that is strong enough to carry in a sintering furnace. The process is realized in rolling mills in dissimilar ways, different with arrangement of the axes plane of rolls (vertical, horizontal and inclined rolling) and powder feeding (gravity, forced). The main advantage of the rolling is the possibility of PPM manufacturing in thin ribbons and strips of width up to 550 and of thickness up to 5 mm with the porosity of more than 20 %. The disadvantages include the simplicity of the form and low strength of PPM.
Extruded pressing is a molding of performs from the mixture of powder with a plasticizer by forcing it through the opening. Bakelite alcoholic solutions, a starch paste, a wax, polystyrene solutions, etc., are used as the plasticizers. Its amount in the mixture is usually 6–10 % (by weight). Porous powder materials in the form of rods or tubes of great length (1 m) with uniform distribution of porosity in length are obtained by extruded pressing. The porosity of produced materials is 60–70 %. The disadvantage is the need to introduce suitable plasticizers into a powder, that complicates the subsequent sintering process and contaminate the sintered body with undesirable impurities. This method can produce PM without the use of plasticizers, but with addition of the pore-forming agents.
Vibratory compaction is a compression of the powders in the molds with vibration. The method is based on the drastic reduction of frictional forces between the particles of powder as well as between the powder and the walls of the mold. The static pressure applied simultaneously to the vibration is typically 0.5–5 MPa for various metal powders. The absence of such pressure can lead not to compaction, and to loosen the powder. Compaction of powders under the action of vibration occurs in a few seconds. Vibratory compaction mode is characterized with frequency that varies from 5 to 100 Hz and the amplitude of 5–30 microns. A high frequency of vibration, an amplitude increase, and application of a greater load to the powder, which should increase with decreasing surface roughness and accuracy of particle shape, must be chosen with decreasing particle size. Application of vibration reduces compaction pressure tenfold. This significantly facilitates the fabrication of complex shapes. Appropriate to apply the graphite matrixes, which could be subsequently used to sinter the product.
Free filling of powder into a form is the easiest way to forming PPM without applying pressure. The form under backfilling is subjected to vibration for the best filling with powder, elimination of the “arch effect” and production of the PPM with a uniform porosity. The main requirement for the material of the form is the lack of powder sintering to the form at the sintering temperature. Steel or graphite molds are used for powders of nonferrous metals, and ceramic—for iron. To prevent sintering of the powder particles to the walls of the mold, it is necessary to coat those walls by a suitable suspension (e.g., alumina with water) and subsequent drying. Dimensions of a billet, molded by free backfill of powder into the form, are not limited; they are determined by the size of the working space of sintering furnaces.
Slip casting is a molding of preforms by pouring the slurry, which is a concentrated homogenous suspension of powder in a liquid, into a porous form followed by drying. Typically, the slurry fills the plaster mold, which is the negative of the porous preform of a desired configuration. After filling a large portion of the liquid absorbed by the gypsum form; solid particles dry out and adhere firmly to one another. The mold is then opened and the casting is recovered dried up, and subjected to final drying and sintering. Fine powders (1–2 microns, at most 10 microns) are used to produce the slip, because when using a coarse powder a stable and uniform suspension can not be obtained.
Water is used as the coupling fluid for nonoxidizing metal powders and alcohols—for oxidizing metal powders. To avoid the formation of coagulant in a solution, the additions of hydrochloric and acetic acids, alkalis, etc., are used. The amount of solids introduced into the slurry is 40–70 % by volume. Slip casting process can be intensified by vacuuming a form, applying overpressure to the slip or simply heating the slurry. Slip casting is used for manufacturing articles of complex shape and large sizes, which are difficult to obtain by conventional powder metallurgy techniques.
Table 4.1 shows the advantages, disadvantages, and recommended fields of the application of molding techniques that have found the greatest use in the manufacture of PPM.
In the manufacture of porous fiber materials (PFM), the following methods of molding are used: various types of compression, rolling, hot extrusion, and sintering under pressure. Pre-fabricated fiber felts are made using several embodiments of felting: liquid, air, gravity, under the action of electric or magnetic fields, vibration.
When the wet felting is applied, slurry of fibers in a viscous fluid (e.g., glycerol) is prepared, and it is poured into a porous mold. A shape corresponding to the configuration of the finished product is subjected to a vacuum, whereby when the liquid is sucked off, the fibers are deposited on the bottom and walls of the mold, forming a billet in the form of felt with entangled fibers. During the deposition, density is self-regulating. Permeability decreases in a high-density layer, and the slurry supply slows, so the deposition of fibers is inhibited and in areas with a lower density (more permeable) the fibers are deposited until the density of the felt is aligned.
When the air felting is applied a porous material is obtained by precipitating the fibers from the air stream. However, consistency and uniformity of felt a little bit worse than in the wet felting.
A method of forming a porous material in the production of metal fibers from a hanging drop of melt by a heat-receiver, which is rotating, is called the pendant drop melt extraction (PDME). When using the PDME the lower end of a vertical rod is melted to form a hanging drop of the melt. Vertex of the working edge of the rotating cooled heat-receiver is in contact with the drop, which is tuned in the form of an isosceles triangle. The melt is cooled in the zone of contact at a speed up to 106 K/s, which leads to the formation of a microcrystalline or amorphous structure. Due to the rotation of the heat-receiver, the solidified material is taken out of the melt in the form of fibers, and is discharged from the top of the working edge with the speed of about 30 m/s under the influence of centrifugal forces. A fiber with equivalent diameter of 30–80 microns falls on the receiving surface, which moves relative to the place of falling of the fiber. As a result, a porous material from fibers of steel and nickel-based alloys, copper, etc., having the porosity up to 98 % (Fig. 4.3), is formed.
Molding due to vibration produces felt with oriented location of fibers. The intensity of this method of felting depends on the mode of vibro-processing and on sizes of fibers. For example, formation of cylindrical and sleeve billets most effectively occurs at frequencies of 50–100 Hz, the amplitude of 40–50 microns and a peak pulse shape fluctuations; fibers of 5–7 mm in length fit almost two times faster than the same fibers with length of 10–12 mm.
Application of the rolling enables to form continuous sheet of a single- or a multilayer material of fibers with controlled porosity. The thickness of sheets is from several tens of micrometers to hundreds of millimeters. The degree of compression of the order of 75 % does not cause a noticeable closing of the pores at the surface of products. During compaction of metal fibers, a contact, as well as reversible elastic and irreversible plastic bending deformation occurs. After removing the compaction pressure, instantaneous purely elastic deformation should be realized, then further prolonged (tens of hours) relaxation of residual stresses with decreasing rate, and the total strain reaches tens of percent, is followed. Large deformation effects related to the design of the fiber body, close to the structure of the spring. Relatively small elastic deformations of the individual fibers, overlapping, eventually give greater total deformation. The maximum strain in the compacts of the fibers is observed in the direction of application of pressing force, i.e., along the height and 4.5 times superior to that of the width. In carrying out of the rolling, the suspension of metal fibers in the viscous liquid is fed onto the grid coated filter paper. Grid is moved continuously with the help of rollers and passes over chambers for suction of liquids. The molded felt is then passed through the nip rolls and introduced into a sintering furnace.
When forming PFM by extrusion, a significant difference in the processes of structure formation compared with extrusion powders was found. Samples of the extruded fibers have three deformation zones. The first (peripheral) zone differs due to defectiveness and has a thickness of 0.5 mm. The second (middle) zone is widest whose fibers are oriented along the extrusion axis. In the third (central) zone the fibers are arranged randomly. With increasing draw ratio, the middle zone is expanding at the expense of the peripheral and central zones. Preliminary impregnation of the felt with wax, resin or a mixture of salts (44.5 % KNO3 and 55.5 % NaNO3) at the temperature of 190 °C allows using the method of extrusion in the manufacture of porous products in the form of tubes and cylinders of different sizes.
Porous mesh materials (PMM) are produced on the basis of knitted and woven nets. Production of crosslinked materials is to prepare nets (washing, degreasing), cutting, a package stacking, pressing and sintering the package. The form of a grid is the criterion for selecting the type of manufacturing operations and their modes.
Highly porous cellular metallic materials (CM) with a porosity of 80–98 % are formed by several groups of ways:
-
1.
Casting methods,
-
2.
Methods of powder metallurgy,
-
3.
Metal deposition from the gaseous state,
-
4.
Electroplating methods.
Detailed consideration of the ways is given in the review [4].
Methods of foaming molten metal (usually aluminum and its alloys, zinc, etc.) are realized in two basic ways: a gas is introduced into the molten metal from an external source, or gas bubbles are formed in the melt by dissociation of the particles of chemical compounds. In the first case, for deceleration of the floating rate of gas bubbles in order to increase its viscosity, from 10 to 20 % (by volume) of SiC, Al2O3, or MgO particles of 10–20 microns are previously introduced into the melt. The advantages of this technique are high-performance and low-density (ε = 80–98 %). In the second case, the molten metal is pre-injected by calcium of 1.5–3.0 %, which forms dispersed particles of CaO, CaAl2O4 that increases the melt viscosity. After reaching a predetermined value of viscosity, from 0.5 to 1.6 % of ZrH2 or TiH2 particles are introduced into the melt. As a result of the decomposition of hydrides, hydrogen is generated, and foams the melt. The method allows to achieve high uniformity of the pore size distribution in the material.
PM can be obtained by the casting method without foaming of the metal. According to that process, the porous preform of polymer with interconnected porosity, e.g., polyurethane is filled with a liquid solution of sufficiently refractory material, for example a mixture of mullite, phenolic resin, and calcium carbonate. The polymer is removed then by heat treatment, and the melt is poured into the open interconnecting voids which replicate the original polymer foam structure. After removal of the ceramic filler material (e.g., by pressurized water), metal structure which accurately replicates the original polymer structure is obtained. Difficulties in this process include the achievement of complete filling of the structure of the polymer with metal and the filler material removal without damaging the structure. The method is used to produce porous products from aluminum alloys, copper, and magnesium.
Metal powders can be use to duplicate highly porous structure of reticulate-cellular polymer such as polyurethane foam. Polymer with open through porosity is used for that. Polymer billets are cut into the samples of specified sizes, and are impregnated with prepared suspension of a metal powder. The average particle diameter of the powder used is determined by the fact that the powder must form the slurry layers at the reticulate-cellular porous polymer surface containing minimally 3–5 rows of metal particles along its thickness. The average particle diameter should be no greater than 5 microns to produce high quality preforms. Impregnation is carried out in immersion baths and by deformation of billets. After impregnation, the excess of slurry is removed and its distribution throughout the volume of the preform is aligned by the compression operation. The degree of compression is adjusted so as to provide a desired preform density. The preform is a composite material, since the polymer surface is uniformly coated with the dried slurry. With increasing density of preforms their structure changes somewhat and films of suspension, spanning part of the pores between the cells, appear. Removal of the polymer from the workpieces is carried out at the temperature of 200–600 °C and the heating rate is not more than 100 deg/h. A content of no more than 1–1.5 % of the starting polymer remains in blanks at the temperature of 550 °C. Spatial structure of the workpiece is not violated.
Metal ions from the electrolyte are deposited onto the open cells polymeric foam, which is later removed when using methods of electroplating. Application of electrochemical methods requires the electrical conductivity of the polymer. This is achieved by lowering the polymer in a liquid conductive solution, based on graphite. The polymer can be removed by heat treatment after electrodeposition. This method provides a porous preform made of nickel, copper or nichrome. Porous nichrome is prepared making layered coating of nickel and chromium. The material is then annealed at high temperatures. As a result of diffusion, the alloy is formed.
Technology of nickel carbonyl production can be applied when using methods of CVD. The porous preform of the polymer can be maintained at the temperature of nickel carbonyl decomposition (~120 °C). The resulting nickel is deposited onto the polymer, forming a metallic coating. After cooling, the polymer can be removed by thermal or chemical treatment.
3 Sintering of Porous Materials
Sintering is a thermally activated process, spontaneous or initiated by the external influence of the transition of contacting solids or porous medium in a thermodynamically equilibrium state by reducing the free surface.
Conventionally, shrinking process (compaction) of the powder body in an isothermal sintering can be subdivided into three sequential steps.
The early stage. The density of the powder body is small and the rate of densification is determined by processes occurring in the contact areas, the structure and geometry of which play a significant role. The rate of displacement relative to each other and the volume deformation of the particles, leading to shrinkage of the porous structure, are high.
The intermediate stage. The density of the powder body is large enough and reduction of the volume of each of the pores, which in the aggregate constitute a single ensemble, can occur almost independently. Porous matrix of the particles behaves like a viscous medium and its densification is evenly throughout the volume (with a uniform distribution of pores).
The late stage. Powder body contains some isolated pores that are healed (overgrown) by the diffusion dissolution in the matrix with outlet of vacancies on the external (overall) surface of the sintered preform.
There is no sharp and distinct boundaries between these three stages, the first of which is typical for nonisothermal sintering, and densification of powder body at an intermediate stage can be determined by the processes, which are characteristic for the early and (or) late stage.
One of the characteristic features of the shrinkage of the heated powder body is slowing its rate under isothermal exposure. With an increase in the sintering temperature the rate of density enlarge also increases, but this rate decreases more intense with an increase in the sintering temperature. General behavior of the analytic curves of isothermal volumetric shrinkage V/ΔV can be expressed as power functions in the following form:
where V and ΔV—the current pore volume and its change in the reporting time τ of isothermal exposure, respectively; K—constant. Equation (4.21) is valid in a relatively small interval of time, usually no more than a few hours.
V. Ivensen showed that the speed ratio of the pore volume reduction during isothermal sintering of any metal powders at the two arbitrarily moments of time τ 1 and τ 2, for selected isothermal sintering, is equal to:
where V 1 and V 2, are the total pore volumes at time τ 1 and τ 2, respectively. This regularity under constant value of the exponent n (which characterizes the intensity of braking shrinkage) is maintained throughout the sintering process, including the very rapid reduction of the pore volume at the beginning of the sintering and the slow reduction—at the end. The ratio of (4.22) was proved valid for isothermal sintering duration >50 h, as well as for sintering powders of different metals with any methods for their preparation.
The dependence of shrinkage on the sintering duration, described by the Eq. (4.22), is explained by the fact that the supply of the free energy per unit mass of powder is determined not only by its dispersivity, but also by the surface state and the degree of defectiveness of the particles to a considerable extent. Powders with a high surface area and high density of defects are compacted with the highest rate, as having a large supply of free energy, in the process of isothermal exposure at sintering. Attenuation of the shrinkage is due to occurring decrease in free energy of the powder body during isothermal exposure.
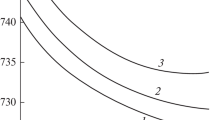
An essential feature of the shrinkage of compacts is that in the case of further temperature increase after prolonged isothermal sintering shrinkage, when shrinkage is almost ceased, the shrinkage rate increases again. Figure 4.4 shows the effect of stepwise heating on shrinkage of silver powder compacts at three temperatures, and each new rise in temperature leads to the intensification of the shrinking process, proceeding at different stages about the same.
V. Ivensen proposed the dependence, taking into account the change of the pore volume in the sintering and the concentration of defects:
where ΔE = E b − E a , E b , E a —the activation energies of flow processes of the crystalline solid and the elimination of defects, respectively; V 0, N 0—the pore volume and concentration of defects, respectively, at the beginning of isothermal sintering; τ0—time. Equation (4.23) allows to describe simultaneously both the timing and the temperature dependence of shrinkage.
Sintering of fine powders has a number of features. With their sintering, the densification processes go so intense that a significant portion of shrinkage occurs already during heating to the sintering temperature. The densification rate sharply is attenuated with isothermal exposure. Initial densification rate for highly active superfine powders during sintering can be six to eight orders of magnitude larger than predicted by the theory of diffusion of vacancies for diffusion- viscous flow, even taking into account the role of grain boundary diffusion. The observed effects can be explained by the following reasons.
Systems of nanopowders are subjected to increased capillary pressure, since the radius of curvature of the quasi-spherical nanoparticle is equal to half of its linear dimension. Laplace’s pressure on the particle surface at r = 10 nm and surface tension s ~1.5–2 kJ/m2 is 150 … 200 MPa.
Size effect has two aspects in relation to diffusion. First, the reduction of grain size increases the concentration of the boundaries along which the diffusion coefficients significantly larger than those in the bulk. In addition, it is found experimentally that self-diffusion coefficients, in nanocrystalline materials at relatively low temperatures, are higher by many orders of magnitude than expected ones which are extrapolated from high temperatures for grain -boundary diffusion. Thus, diffusion processes take place in nanostructured systems with much higher speeds than in conventional systems. A difference between diffusion parameters gradually disappears when the temperature rises due to relaxation of the nonequilibrium structure.
A key feature of sintering nanocrystalline PM is a competition between two major processes: shrinkage and enlargement of the microstructure. Both processes are controlled by the same mechanisms of mass transfer: surface and grain-boundary diffusion. Parameters of the thermal activation of these processes are similar to each other and, as a consequence, shrinkage and evolution of the microstructure occur simultaneously.
During sintering of nanoparticles their size remains approximately constant over a wide range of porosity and their intensive growth begins at the porosity below 10 % and depends on the porosity type as well as the pore size distribution and the pore volume. During the sintering, it is necessary to control the pore size distribution while maintaining the channel open porosity. The grain size distribution is controlled at the final stage. During sintering of nanopowders, the special tendency to agglomerate and isolation of local shrinkage resulting in inhibition of densification, especially observed in the later stages of sintering, should also be considered. A good example of a fully localized sintering, not accompanied with densification of pressing in a whole, is sintering of tin oxide nanopowder. Compacts from SnO2 powder with the particle size of 100 nm, having the porosity of 45 %, were sintered in air for 1 h at 500–1100 °C. Total shrinkage of briquettes was absent during sintering. The pore size distribution was measured and specific surface area was determined by mercury porosimetry for the porous samples. As a result of high-temperature sintering of active nanopowder, the pore size was increased fourfold, indicating the intensive zonal isolation process which takes place in the absence of overall shrinkage. The only way to combat the negative impact of localization shrinkage is to ensure maximum uniformity of particle size distribution of the powders and to use special (e.g., slurry) molding methods, excluding the formation of large pores while still forming.
Sintering the porous fibrous material is carried out under sintering conditions of powders of similar composition. Enlargement of sizes of fiber pellets during sintering occur most strongly in the direction of the previous extrusion (along a height). In the plane perpendicular to the direction of the forming force in the felting and pressing, a sample size is increased much smaller. Growth of samples is enhanced with increasing porosity along the height and width.
Progressive increase in the volume of samples with increasing porosity is a phenomenon inherent only for materials of a fiber structure, in which changes of the number and strength of contacts between the individual fibers under the influence of residual stresses, generated during the pressing, occur. Relaxation of residual stresses in the fiber compacts ends in the early stages of sintering (at heating to the temperature of exposure). Although further shrinkage will occur, a cumulative effect of manifestation of reversible and irreversible deformations in the form of a “growth” of samples is fixed more often at this stage.
4 Application of Porous Materials
Porous materials are widely used for the purification of liquids and gases, environmental protection, and human health protection. PM are used for purification of potable, waste and process water, alkali, acid and salt solutions, fuels and lubricants, food products (milk, wine, juices, vegetable oil), varnishes and paints, melts of polymers and metals, liquefied and compressed gases. They are used for deferrization of water by a method based on the oxidation of iron ions, followed by filtration of oxidized water during electro-filtration decontamination of drinking water and food liquids, disinfection of sewage water, desalination of water in an electric field using porous electrodes. Filtration is also proposed to use in an electric field through the highly porous cellular materials for purification of the air from fumes having the particle size less than 1 micron.
One of the promising areas of application of porous materials, determined by their high capillary and filtering properties, is cleaning and drying of compressed gases from mechanical impurities and moisture.
They are used for separation processes in the chemical, petrochemical and biological production.
Important properties of the filter are the degree of purification, high permeability, good reconditioning, high service life, and good corrosion resistance.
Materials with a high interconnected porosity could be used as a heat exchanger between a gas and a liquid or solid. Another application of the PM—is the heat pipes.
The efficiency of catalysis is critically dependent on the high magnitude of surface contact between the catalyst and the liquids or gases which are involved in the reaction. Therefore, the catalyst is a highly porous structure or, if not, is applied to a porous ceramic material. Porous metals may substitute ceramics, even if they cannot compete with it in the specific surface area, because they have high thermal conductivity and ductility. New area of catalysis at the nanoparticles is photocatalysis using semiconductor particles and nanostructured semiconductor films, which is promising, for example, for photochemical purification of wastewater from a variety of organic pollutants through their photocatalytic oxidation and mineralization.
One of the oldest applications of porous powder metal materials is self-lubricating materials in which the oil is stocked in the pores between the particles and is released in the operation process during the heating, replacing the oil used in this manner. Furthermore, the porous materials can reduce unwanted liquid flow in partially filled containers.
The most common sound-absorbing materials are porous materials. Losses of sound energy in the PM occur due to viscous friction of air in the narrow pores of the material, as well as sequential compression and rarefaction of air in contact with the sound wave, which is accompanied by the air heating, and due to the reflection of sound waves from the gas, disposed in the pores of the material back to the source. To reduce noise, dual mufflers are used at both low and high frequencies. Porous materials may also restrain sudden pressure changes occurring in the compressors, or pneumatic devices.
Saturation of liquids with gas by passing it through a porous elements—an effective way to intensify the processes in chemical technology, biological treatment of wastewater, fine cleaning and disinfection of drinking water and process water, as well as food, pharmaceutical and microbiological industry, municipal and industrial water treatment systems.
Porous metals may be used to stop spread of flame in the fuel gases.
Porous fiber material from alloys of Fe–Cr–Al is used in the neutralization of exhaust gases of diesel automotive engines. Highly porous aluminum can ideally perform three functions: absorption of energy in emergency situations, sound isolation, and heat extraction.
Porous titanium is used for the manufacture of prosthetic or dental implants because of their biocompatibility with living tissues.
References
Kostornov AG (2002) Material science of dispersible and porous metals and alloys. Naukova Dumka, Kiev (in Russian)
Belov SV (ed) (1987) Porous permeable materials Handbook, Metallurgiya, Moscow (in Russian)
Tumanovich MV et al (2010) Porous powder materials and items on their base for protection of human health and environment: production, properties and applications. Byeloruss, Navuka, Minsk (in Russian)
Liu PS, Liang КM (2001) J Mat Sci 36:5059
Banhart J (2001) Prog Mater Sci 46:559
Colombo P, Degischer HP (2010) J Mater Sci Technol 26(10):1145
Luyten J, Mullens S, Thijs I (2010) Kona Powder Particle J 28:131
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Serov, M.M. (2014). Powder Metallurgy. In: Structural Properties of Porous Materials and Powders Used in Different Fields of Science and Technology. Engineering Materials and Processes. Springer, London. https://doi.org/10.1007/978-1-4471-6377-0_4
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6377-0_4
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6376-3
Online ISBN: 978-1-4471-6377-0
eBook Packages: EngineeringEngineering (R0)