Abstract
There is an unmet clinical need for repairing osteochondral (OC) lesions. Tissue engineering and regenerative medicine (TERM) strategies advance with the possibility of regenerating different tissues by means of using cells, scaffolds and growth factors, alone or together. The use of bioreactor systems for developing mature tissues in vitro is also appealing. This book chapter aims to overview the main aspects related to structure and functions of articular cartilage, subchondral bone, and bone. The components of the tissue engineering and the most relevant reports on their use for treating OC lesions are concisely covered. Several treatment strategies are available; however, the gold standard does not exist. The biofunctional knowledge of these tissues has been uncovered by the development of advanced characterization techniques including medical imaging allowing visualization from sub-cellular to macro level. These techniques have been helping scientists not only to understand how these tissues function but also to develop multiscale TERM strategies. Thus, this hot topic is also briefly discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Clinical practice is often challenged by the need for treating lesions of several musculoskeletal tissues, in which osteochondral (OC) tissues have an important share. Figure 2.1a shows an X-ray image of a human OC tissue obtained from a patient diagnosed with knee osteoarthritis (OA); Fig. 2.1b and c show, respectively, the 2D and 3D reconstruction of the images that were obtained by the micro-computed tomography (\(\upmu \)-CT) analysis. OC lesions affect both the articular cartilage and the underlying bone. Figure 2.1d presents an OC lesion in the human knee. Clinical need for repairing OC lesions is not yet fully satisfied. It is difficult to repair the OC tissue by surgical means alone. Lesions in OC tissues may lead to arthritis if not treated correctly in the early stages [1]. However, usually OA develops without a known cause [2]. OA is the most common disease affecting the joints, and it is seen usually in the knee, hips, hands and spine [3]. The majority of the population over 65 years old have evidence of OA [4].
a An X-ray image of a freeze-dried human OC tissue (OA knee) acquired with a high-resolution micro-computed tomography equipment, Skyscan 1072 scanner, (SkyScan, Kontich, Belgium). b 2D and c 3D reconstructions of the images using CT Analyser and CTvox, image-processing softwares from SkyScan. d An OC lesion in the human knee
OA is characterized by the imbalance of anabolic and catabolic activities of cartilage cells [5]. It is a progressive degenerative disease with gradual loss of articular cartilage [6] leading to joint deformation and pain [7]. Many tissues can be damaged during OA: articular cartilage, subchondral bone (SB), ligaments, capsule and synovium, and muscles [5, 7]. Degeneration of articular cartilage and remodelling of SB are usually seen in OA [7].
Insufficient self-repair capacity of articular cartilage is well known. The complexity and difficulty of treating articular cartilage lesions are largely agreed [8]. A wide variety of surgical methods has been considered [2, 8–12]. However, none of these applied methods has superiority proven by a controlled clinical study [13]. Articular cartilage tissue cannot function as required or it can function only for a short period of time if it does not have the special biological and mechanical properties that it normally has [9]. Cartilage cells have limited proliferation capacity and cannot migrate within the matrix. Therefore, strategies for the treatment of cartilage lesions have been developed in which a new cell population are brought to the lesion site [13]. One such strategy is enhancing the cartilage healing by migration of stem cells from the bone marrow to the injury site. Some examples of methods based on this strategy are drilling of SB [14], joint debridement [15] and spongialization [16]. It has been reported that none of these methods is actually helping cartilage to regenerate the original tissue since the new formed tissue is a fibrous tissue. It has been attempted to treat OC lesions with auto- and allografts as well [10, 11]. The eventual fibrous tissue formation caused biomechanical drawbacks, such as instability and reduced mechanical strength and congruency of articular surfaces [12]. In addition, low availability of material and donor site morbidity are also important limitations [17].
The goal of tissue engineering and regenerative medicine (TERM) is to regenerate tissues by preferably using patient’s own cells, biodegradable biomaterials, and relevant growth factors, alone or in a combination to increase the effectiveness. Conceptually, in a tissue regeneration process, the cells can be obtained by means of biopsy from the patient, grown in vitro and seeded into a porous scaffold, followed by the cultivation of the scaffold-cell construct for some time in vitro in a cell culture medium. This construct is implanted into the defect, and after the implantation, eventually, cells synthesize their extracellular matrix (ECM) and the scaffold degrades gradually. However, the whole process is challenging. The challenge is bigger when regeneration of more than one type of tissue is required. For example, in the case of OC lesions, both articular cartilage and SB need to be treated.
2 Articular Cartilage
Articular cartilage plays important roles in the body. It covers the articulating ends of the bones inside the synovial joints to form a low-friction gliding surface. The cartilage reduces the peak stresses on the SB and also serves as a shock absorber [8]. Articular cartilage is an avascular, aneural and alymphatic tissue with a generally anaerobic metabolism [18]. There are two main components in the cartilage: cells called chondrocytes and the ECM which surrounds the cells [7]. Figure 2.2a presents a histological image of an articular cartilage. The chondrocytes and the ECM are interdependent. The chondrocytes are responsible for the synthesis and the breakdown of the ECM. In return, the ECM surrounds the cells and protects them from mechanical impacts while transmitting signals to the chondrocytes upon loading of the cartilage [19].
a A 5-mm-thick histological sections of fresh healthy human articular cartilage after staining with haematoxylin-eosin showing the chondrocytes in the ECM. b A 5-mm-thick histological sections of newly formed bone within a HAp scaffold after staining with haematoxylin-eosin showing the ECM, osteocytes, osteoblasts and bone lining cells
Chondrocytes take up only 1–10 % of the cartilage volume [12]. They synthesize many enzymes, cytokines and growth factors that affect the anabolic and catabolic activities [3, 6]. Mechanical loading affects the functions of chondrocytes [20]. Some chondrocytes have short cilia that reach into the matrix, which may have a function in detecting changes in the matrix. Even though individual chondrocytes have active metabolism, the articular cartilage still has slow metabolic activity due to low cellularity [19].
Histological properties and health of cartilage and its mechanical properties are interrelated. For example, the Young’s modulus of human cartilage decreases with increasing degrees of degeneration [21]. The ECM provides the tissue’s mechanical and biochemical properties and affects cellular function by cell-matrix interactions [22]. The ECM has two phases: a fluid phase and a solid organic matrix that is mainly composed of collagens, non-collagenous proteins, and proteoglycans (PGs). The fluid phase is mainly composed of water, and some of the water is free and can move in and out of the tissue [19]. Water constitutes 65–85 % of the weight of the cartilage. Water is important for processes such as the transport of nutrients and wastes into/from the tissue, and the lubrication of the gap between articulating surfaces. Water content of the cartilage can be affected by pathological conditions, for instance in osteoarthritic cartilage the water content is above the normal condition [8]. The increase in the water content causes cartilage to become softer and more permeable [23].
Table 2.1 presents indicative range of values of the mechanical properties of articular cartilage and bone from [24]. The values vary depending on many conditions including loading direction, the anatomical location, age and health of donors [24]. The biomechanical properties of the tissues relate to the structure of and interactions in the collagen network. Collagens are proteins that interact with cells and affect cell functioning such as adhesion, growth and differentiation [22]. Collagens constitute 10–20 % of the weight of the cartilage. Collagen type II is the major component of cartilage that gives tensile strength, and it is the predominant collagen type representing 90–95 % of the collagen content. Other collagen types present in the articular cartilage are VI, IX, X and XI [8]. Collagen type II is a sign for differentiated chondrocytes, while collagen type I, which is not found in articular cartilage, is a sign for fibrocartilage as a result of chondrocyte dedifferentiation to fibroblast [18]. PGs take up 10–20 % of the cartilage weight and give compressive strength [8]. PGs consist of a protein core to which glycosaminoglycans (GAGs) are attached [18]. PGs can help matrix stabilization by binding to other macromolecules and also may affect the cell function by binding to growth factors [19].
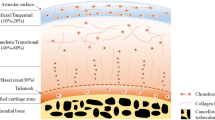
Illustration of the structure of an OC tissue showing the arrangement of the cells and collagen fibres within the articular cartilage. Articular cartilage has four layers: the superficial layer, the intermediate layer, the deep layer, and the calcified layer. The superficial layer is the thinnest layer with flattened chondrocytes and collagen fibres that are parallel to the surface. The intermediate layer represents the thickest layer of the cartilage with spherical chondrocytes and thicker collagen fibrils, which are randomly aligned. The deep layer has spherical chondrocytes that are aligned in columns and collagen fibrils are parallel to each other and perpendicular to the articulating surface. The calcified layer is a thin layer with hypertrophic cells and separated from the deep layer by the tidemark. Subchondral bone is located below the cartilage while the cement line forms the interface between the calcified layer and the subchondral bone
Articular cartilage is a four-layered structure: (i) the superficial layer, (ii) the intermediate or transitional layer, (iii) the deep layer, and (iv) the calcified layer that is separated from the deep layer with the 3D border called tidemark. These layers differ in cellularity, cell morphology, concentration of PGs, collagen fibril content and orientation, water content, and thickness [8]. Figure 2.3 illustrates an OC tissue with distinct layers of articular cartilage and subchondral bone. Articular cartilage is a highly organized tissue and possesses a particular cellular and molecular structure [18]. Thus, it is obvious that the properties of the cartilage matrix cannot be achieved just by combining the components of the ECM in right concentrations. Chondrocytes are in charge of the synthesis, organization and maintenance of the ECM in the articular cartilage. Therefore, in response to a damage in the cartilage matrix, the local chondrocytes detect the changes occurring within the matrix and determine the new needs of the matrix. Subsequently, the chondrocytes will give the needed response, synthesizing the required components in right amounts, and assemble and organize them in the matrix [7, 19].
3 Bone and Subchondral Bone
Bone is composed of organic and inorganic components. Minerals, principally hydroxyapatite (HAp), comprise 50–70 % of the bone, collagen type I rich organic matrix 20–40 %, water 5–10 %, and lipids less than 3 % [25]. HAp contributes to the rigidity and load-bearing strength, while the organic matrix provides flexibility and elasticity of the tissue. Four cell types are present in bone: osteoblasts, osteoclasts, osteocytes and bone lining cells. Osteoblasts are the mature bone-forming cells found on the bone surface, while osteocytes are embedded in the lacunae encircled with mineralized matrix (Fig. 2.2b). They support the structure and metabolism of the bone [25]. Osteoclasts are the cells that resorb bone. Bone-lining cells are present in the non-remodelling surface of the bone. They play an important role in mineral homeostasis and can be stimulated to proliferate and differentiate into osteogenic cells [26].
Bones in the body undergo growth, modelling and remodelling throughout life to preserve the strength and mineral balance and adapt to new biomechanical conditions. Mature bones in the body can be classified structurally into cortical bone and trabecular bone. Cortical bone or compact bone covers the outer surface of the bones and has a dense structure with a porosity less than 5 % [25]. Trabecular bone is also known as cancellous or spongy bone, and it is composed of trabeculae that are porous interconnected irregular arrays of lamellar bone plates and rods [24]. Trabecular bone is present near the ends of long bones, inside the small bones as well as between the surfaces of flat bones. Trabecular bone and cortical bone show anisotropic mechanical behaviour due to their structure [27]. Trabecular bone is not as dense as the cortical bone. The metabolic activity of trabecular bone is higher than that of cortical bone [25].
SB lesions are often related with damage in the articular cartilage. SB affects the articular cartilage both biomechanically and biochemically [28]. It absorbs the mechanical stress and maintains the shape of the joint [29]. It forms a transitional layer between the articular cartilage on the surface and the interior bone volume [28]. The calcified layers of the articular cartilage and the SB make contact at a thin interface called the cement line [30]. SB is composed of the SB plate and subarticular spongiosa, which represent cortical and trabecular bone respectively. However, usually the term SB is referred to both regions, regardless of their mechanical and physiological differences [31]. The SB plate is the bony lamella or cortical endplate found under the calcified layer of the articular cartilage, while the subarticular spongiosa refers to the trabeculae under the SB plate [32].
The subchondral region shows large variations in anatomy, for example, in the contour of the tidemark and cement line, and in composition and thickness [33]. SB adapts to the applied stress by altering its density and strength [29]. Long-term mechanical load distribution within a joint surface affects the density, thickness, vascularity and biochemical composition of the SB plate [32]. In patients with OA, the stiffness, the density and the mineral content of SB are lower as compared to normal tissue [34].
The components of tissue engineering and their role in achieving tissue regeneration. Cells are the basic unit of tissues, they proliferate and generate the tissue matrix; scaffolds are engineered from biomaterials and host the cells provisionally during regeneration process; growth factors and physical stimulation affect the cell function and tissue formation, while bioreactors can mimic the in vivo conditions by supplying controlled environment and conditions for cell culturing
4 Tissue Engineering and Regenerative Medicine Strategies
4.1 The Components: Cells, Scaffolds, Growth Factors, and Bioreactors
The different components of tissue engineering and their role in achieving tissue regeneration are illustrated in Fig. 2.4. Cells are the basic unit of tissues that generate the tissue matrix, while scaffolds are engineered from biomaterials and host the cells during the regeneration process. Growth factors and physical stimulation contribute to the tissue regeneration with their influence on the cell function, while bioreactors can mimic the in vivo conditions for cell culturing. Cells can be categorized as mature differentiated and stem cells. Mature differentiated cells perform highly specialized functions in a specific tissue. Stem cells are in an undifferentiated state having the ability to self-renew and differentiate into specialized cell types to create new tissues. Stem cells can be classified by the tissue type they are isolated from: embryonic [35] and adult stem cells [36]. Embryonic stem cells are isolated during the embryonic stages of development and thus, have a greater natural potential, whereas adult stem cells are relatively more specialized and partially differentiated. Stem cells can also be classified by their potential based on their natural tendency: totipotent, pluripotent, multipotent and unipotent. Totipotent and pluripotent stem cells have an embryonic origin, and totipotent stem cells possess the greatest potential since they have the ability to differentiate into all cell types in the body. Multipotent and unipotent stem cells are adult stem cells. Multipotent stem cells have the potential of forming a limited subset of cells [37].
Employing autologous cells, i.e. patient’s own cells, avoids the risks of immunological responses like rejections. Therefore, it has been considered the gold standard for tissue engineering [38]. There are challenges with the use of autologous differentiated cells, for example with chondrocytes, such as donor site morbidity, low availability of material [17] and dedifferentiation [39]. Chondrocytes in their native tissue are characterised by the macromolecules they synthesize such as collagen type II and aggrecans. When chondrocytes are cultured in a monolayer, they can dedifferentiate to fibroblast-like cells, synthesizing more collagen type I and less collagen type II and aggrecans. For OC tissue regeneration, the mature cells of bone and cartilage can be harvested and grown in vitro. For the cartilage part, Brittberg et al. [40] described a cell-based therapy called autologous chondrocyte implantation (ACI). Patient’s own chondrocytes isolated from a small biopsy of a low-weight bearing site of the cartilage were cultured in vitro for 2–3 weeks to obtain a high enough number of cells. Then, the defect surface is covered with a periosteum patch and the cell suspension injected into the area of the lesion. It has been reported that this method had some clinically good results, at 2 years follow-up. However, the predictability and reproducibility of the outcomes of the approach were not high. Moreover, at least two operations are required, one to obtain, and the other to implant cells, risking donor site morbidity and lack of structural support for the cells [41].
The challenges experienced with differentiated cells can be overcome by means of employing stem cells. For example, mesenchymal stem cells (MSCs) [42] (marrow stromal cells) are multipotent stem cells and have high capacity to differentiate into multiple mesenchymal tissue types including bone and cartilage under certain manipulation and culturing conditions. MSCs are often isolated from bone marrow but can be also obtained from muscle, adipose or synovial tissues.
Scaffolds have important functions in the TERM strategies. A scaffold, which is typically a 3D porous structure from biomaterials, carries the cells to the target site and provides a provisional residence for the cells where the cells attach, proliferate, function, and eventually regenerate the tissue. A scaffold should function as more than a simple mechanical structure; it should interact with the cells and the environmental factors by mimicking the ECM of the target tissue as much as possible, so that the process of tissue regeneration is promoted. A scaffold must be biocompatible and biodegradable. The rate of biodegradation should match that of tissue regeneration. The main characteristics of an ideal biodegradable 3D scaffold for tissue engineering applications [10, 43–46] can be summarized as: (i) it should present an adequate structural architecture and surface properties to allow cell adhesion and growth, (ii) it should be highly porous to create a large surface for cell-scaffold interactions and to allow cell migration, (iii) the pores should have an appropriate size, and be interconnected for cell ingrowth, (iv) it must be biocompatible (neither the scaffold nor its degradation products should cause acute inflammation or toxicity), (v) it should provide the needed temporary mechanical support to the site, (vi) the degradation rate of the scaffold should be matched with the regeneration rate of the tissue, and (vii) it should have the appropriate size and shape. Scaffolds can be considered typically as 3D porous structures into where cells can be seeded, or as hydrogels in which the cells can be encapsulated. The scaffolds can be bioceramic-based, biopolymer-based or based on their composites. Decellularized ECMs [47] are also used as a scaffold in tissue engineering. In general, bioceramics are mechanically strong and show excellent bone integration. Commonly used bioceramics [48, 49] include HAp, calcium phosphates and bioactive glass. There is an extremely wide range of polymeric biomaterials and processing techniques to produce scaffolds with various forms [46, 50, 51]. Polymer-based scaffolds can be produced from: (i) various protein-based polymers such as collagen [52], gelatine [53], fibrin [54] or silk fibroin [55]; or (ii) various carbohydrate-based polymers as, for example, alginate [56], agarose [57], hyaluronan [58], chitosan [59], starch [60] or gellan gum [61]; or (iii) synthetic polymers such as polylactic acid [62], polyglycolic acid [63] and polycaprolactone [64].
Polymers can also be processed into hydrogels [65], which are 3D hydrophilic polymeric networks that have large capacity of absorbing and retaining water. Using hydrogels as a scaffold material is a promising strategy. Cells can be encapsulated in hydrogels [66], which resemble the ECM of tissues and provide homogeneous and efficient cell seeding. They can be prepared in injectable formulations [67]. Injectability allows minimally invasive procedures, which is especially important for defects with an irregular shape. In the case that the hydrogel is photopolymerizable, the cells can be homogenised within the polymer before applying the polymerization process. Using a photopolymerizable hydrogel can be advantageous in TERM strategies [68] such as: (i) scaffold production and cell seeding can become a one-step process, (ii) a homogeneous distribution of cells with high cell viability can be achieved, and (iii) the treatment can be minimally invasive by injecting the system and initiating the polymerization in situ after the injection. Photopolymerizable hydrogels [69] are hydrogels that polymerize in the presence of a photo-initiator upon UV light exposure. Photo-polymerization can be defined as a chain polymerization process initiated by light, where the molecular weight increases and the pre-existing macromolecules are cross-linked. Light in the UV-visible spectral range is absorbed by photo-initiators, and converted into chemical energy as free radicals and reactive cations that initiate polymerization [70].
Gellan gum is a bacterial polysaccharide [71] and was suggested originally by our group [72] as a candidate biomaterial for cartilage tissue engineering. Even though it has been used as drug delivery devices and also in the food industry, it is considered as a new biomaterial for tissue engineering applications [73]. Gellan gum can form thermoreversible gels. Gelation of gellan gum is temperature-dependent, and presence of cations induces the gelling process. The mechanical properties of gellan gum can be tuned by changing the degree of acetylation. One advantage of gellan gum is that it can be processed into hydrogels without using harsh reagents. Gellan gum can be dissolved in water at high temperatures and can form gel upon a decrease in temperature. Another advantageous feature of gellan gum is that it can be also prepared into an injectable system [61]. Silva-Correia et al. [74] developed photo-cross-linkable methacrylated gellan gum hydrogels for tissue engineering applications by the reaction of gellan gum solution with glycidyl methacrylate.
Scaffolds can be manufactured with various methods. Figure 2.5 presents some examples of scaffolds that were processed with different methods into different structures. Figure 2.5a–d present, respectively, a macroporous HAp scaffold produced by sintering [75], a starch-polycaprolactone mesh produced by fibre bonding [76], a methacrylated gellan gum hydrogel obtained by ionic cross-linking [74], and a chitosan sponge obtained by freeze-drying [59].
Rapid prototyping (RP) is an interesting group of non-conventional scaffold manufacturing techniques. With RP, a physical construct can be created layer-by-layer using a computer-aided design data [77–79]. One of the RP techniques is 3D plotting [80], which is a melt-dissolution deposition based technique. In 3D plotting, liquids or hydrogels can be dispensed into a liquid medium through a nozzle that moves on the horizontal plane to build a layer, then the next layers will be created on top of the previous layer by the movement of the nozzle on the vertical plane [77]. For the first time, Landers et al. [81] processed hydrogels into a scaffold with defined pore-size and shape with this technique. RP techniques bring several advantages. It is possible to produce scaffolds with customized structural design based on the computer-aided design data and this will make it possible to produce patient-specific scaffolds [78]. The advantage of customized design is that it gives the opportunity to produce scaffolds with desired porosity and pore size. It is also possible to change the plotting parameters to control the architecture and mechanical properties [82]. These parameters include nozzle size, speed of nozzle arm, speed of extrusion, and distance between the strands [81]. Furthermore, when a computerized manufacturing technique is used, minimum manpower is required and thus, higher throughput manufacturing is possible [78]. Another advantage is the possibility of including the cells and growth factors into the biomaterial before the scaffold is manufactured [81].
Growth factors represent a large number of polypeptides that have a specific effect on the activities of cells by transmitting signals [83, 84]. They can bind to the specific receptors found on the surface or inside the target cells. The effect could be inhibition or stimulation of differentiation, proliferation, adhesion, migration and gene expression of the cells thereby affecting degradation of the tissue and the ECM synthesis of cells. They can also influence secretion and activation of other growth factors. Effects of growth factors depend on the concentration. New generation strategies incorporate also growth factors into the tissue engineering constructs to promote the regeneration. Frequently employed growth factors for cartilage or bone tissue engineering include bone morphogenetic proteins (BMPs), insulin-like growth factors (IGFs), transforming growth factor-\(\upbeta \) (TGF-\(\upbeta \)), fibroblastic growth factors (FGFs), and platelet-derived growth factor (PDGF). For example, insulin-like growth factor-1 is the main anabolic growth factor for cartilage; it has effects on stimulation of synthesis and inhibits the breakdown of PGs and cartilage homeostasis. Also, it may improve the tissue integration and decrease the synovial inflammation in vivo [85]. Fortier et al. [86] showed in a horse model that IGF-1 introduced to chondrocyte-fibrin grafts may facilitate the repair process of the full thickness defects.
Lieberman et al. [84] and Linkhart et al. [87] reviewed the use of growth factors for bone repair. Fortier et al. [88] reviewed the role of growth factors for cartilage tissue regeneration, and their effects on MSCs. Each growth factor affects the cell and the tissue in a specific way; however, the cumulative effects of growth factors are still poorly understood, and should be further investigated since their adequate combination is a must for appropriate tissue regeneration.
Bioreactors are devices that can control the culture media conditions such as the temperature, pH, oxygen ratio, osmolality and nutrients, and thereby can facilitate more advanced tissue regeneration in vitro. They can also promote uniform cell seeding, and facilitate the mass transfer between the culture and the cells. Moreover, with bioreactors it can be possible to have interstitial fluid flow, or mechanical stimulation such as pressure and compression [89]. Various bioreactors have been developed for tissue engineering purposes. For example: (i) spinner flasks [90] are simple bioreactors for cell seeding onto scaffolds where turbulent dynamic flow of medium is generated by a magnetic stirrer; (ii) rotating wall vessels [91] are made of horizontally rotating cylinders filled with culture medium, and provide low-turbulence laminar dynamic flow as well as adequate oxygenation; (iii) flow perfusion bioreactors [92] have a system that can continuously provide direct flow of culture medium through the porous structure of the scaffold and in this way enhance the mass transfer to the interior of the scaffold; and (iv) bioreactors that provide mechanical loading have been developed since it is known that cartilage and bone tissues are affected by mechanical loading. For example, Pei et al. [93] used a bioreactor that can provide a mechanically active environment with efficiently mixed media, low velocity laminar flow and shear stress. They showed that this kind of bioreactor can enhance the structural, functional and molecular properties of in vitro-generated cartilage compared to the use of Petri dishes [93].
4.2 Strategies for Tissue Regeneration
One of the two routes [10] that can be followed for tissue regeneration is implanting the cells and scaffolds into the lesion site for enhancing the regeneration process in vivo. The other route is implanting the entirely in vitro developed construct into the lesion site. When an appropriate bioreactor is used, cell metabolism and ECM production could be controlled better in vitro than in vivo. Mostly, the first route is preferred. It brings also the opportunity of non-destructive characterization of the tissue prior to implantation. The main drawbacks of the second route are mostly associated with tissue integration and mechanical fixation. Also, providing correct mechanical loading is a challenge in vitro. In the first route, the tissue will adapt and integrate better since it is formed in situ under physiological conditions as the result of mechanical loading. However, on the long term, control of cellular activities is more difficult.
Scaffolds may be used with or without cells, but cells are usually incorporated into scaffolds. Various approaches can be used to design scaffolds for OC tissue engineering [17]. These include using different scaffolds for bone and cartilage; using a scaffold only for bone but not for cartilage; or using a single scaffold for both bone and cartilage. Scaffolds can be homogenous or heterogeneous and can consist of a single layer or more layers. In the study of Schaefer et al. [94], different scaffolds were used for bone and cartilage parts. They developed in vitro engineered structures composed of a cell-seeded scaffold and a SB support to be press-fitted into large defects in OC tissue located in the rabbit knee joint. Allogeneic rabbit chondrocytes were dynamically seeded onto a non-woven polyglycolic acid scaffold. SB support was an osteoconductive sponge made of bovine collagen type. As controls, the defects were either treated with cell-free scaffolds or kept empty. Their results showed that the treatments done with composites were structurally superior to the ones done with cell-free scaffolds or kept empty. Composites withstood the physiological loads and showed remodelling into OC tissue with preserved cartilage at the articulating surface and subchondral regeneration. However, the integration of the composites with the host cartilage was not good, whereas a good integration was achieved with the host bone.
In the study of Kandel et al. [95] a scaffold was used only for the bone part. They developed biphasic constructs using cartilaginous tissues grown and fixed on top of porous calcium polyphosphate substrates. Isolated chondrocytes were seeded on top of the substrate and were grown with autologous serum for 8 weeks to generate the cartilaginous tissues. These in vitro-formed constructs were subsequently implanted into OC defects in sheep and maintained up to 9 months. The results supported the suitability of the strategy to treat OC defects. The constructs withstood in vivo loading up to 9 months with good integration to native cartilage and bone ingrowth into the substrate. In another study, Oliveira et al. [59] developed HAp/chitosan bilayered scaffolds through a combination of sintering and freeze-drying methods for OC tissue engineering. Preliminary in vitro tests showed that goat marrow stromal cells grew and differentiated into osteoblasts and chondrocytes, respectively in HAp and chitosan layers. The physicochemical properties and biological performance of the scaffolds revealed their great potential to be employed in the regenerative strategies for treating OC lesions.
As mentioned previously, hydrogels are a group of scaffolding materials for tissue engineering. The conventional hydrogel-based regenerative strategies for cartilage involve hydrogels with cells to achieve a homogeneous tissue rather than the zonal structure of the cartilage [96]. An approach for regeneration of cartilage in a zonal manner is using chondrocytes that are isolated from different zones of the cartilage. Following this interesting strategy, Kim et al. [97] showed an experimental model where chondrocytes were isolated selectively from different layers of bovine articular cartilage, and multi-layered photo-polymerizable polyethylene glycol diacrylate hydrogel scaffold-cell constructs were developed. That study showed that chondrocytes of different layers present differences in gene expression and proliferation kinetics. Cells survived and stayed in the layer in which they were encapsulated. Histological studies of the layers showed similar results as native articular cartilage. However, several improvements are in great need for further clinical application. For example, gradual transition between the layers, an additional layer in the construct for the regeneration of the calcified layer of the cartilage and using a biodegradable hydrogel.
Another approach could be using hydrogels with different properties for each layer. Ng et al. [98] developed chondrocyte-seeded bilayered agarose constructs to mimic the layer-dependent inhomogeneity of cartilage. As a combination of approaches mentioned above, in a later study, Ng et al. [99] introduced chondrocytes that are isolated from different layers of the bovine calf knee cartilage into the bilayered cartilage construct. In the study, layers with different mechanical properties were obtained for 2 or 3 % agarose hydrogels seeded with chondrocytes from different layers to mimic both the cellular and mechanical inhomogeneity of the native tissue. Although the approach followed in the aforementioned studies is for cartilage regeneration only, it can be also applied in strategies aiming to regenerate OC tissue.
Harley et al. [100] manufactured heterogeneous scaffolds from collagen-glycosaminoglycan and nano-calcium phosphate to mimic the composition, structure and mechanical behaviour of articular cartilage and SB as well as gradual transition between them. Layered scaffolds were mimicking the normal OC tissue with the inter-diffused regions having differential pore microstructure, mechanical properties, and chemical composition, and a gradual transition interface. Also, Jiang et al. [101] developed a multi-phased scaffold from agarose hydrogel, and microspheres of poly(lactic-co-glycolic acid) and bioactive glass. Region-specific co-culture of chondrocytes and osteoblasts were introduced into the scaffolds in a controlled manner. Within the scaffolds, three phases were designed: (i) a hydrogel-chondrocyte phase to mimic the cartilage region, (ii) a hydrogel-polymer-bioactive glass-chondrocyte phase as an interface layer to mimic calcified cartilage, (iii) and a polymer-bioactive glass-osteoblast phase to mimic bone. The in vitro results showed the formation of three distinct yet continuous layers of cartilage, calcified cartilage and bone-like matrices.
An alternative to the direct transplantation of cells as applied in the studies above, is recruiting endogenous cells. A proof of this concept was demonstrated by Lee et al. [102] who showed that the entire articulating surface of a joint can be regenerated in vivo by using endogenous cells. They manufactured anatomically correct composite scaffolds from poly-\(\varepsilon \)-caprolactone/HAp using a computer-aided design. The scaffolds were infused with TGF-\(\upbeta \)3-containing or TGF-\(\upbeta \)3-free collagen hydrogel, and transplanted acellularly into the rabbit condyle. The results showed that inclusion of TGF-\(\upbeta \)3 into the scaffolds leads to roughly 130 % more cells in the regenerated cartilage than in the absence of TGF-\(\upbeta \)3. After 4 months, TGF-\(\upbeta \)3-infused scaffolds were fully covered with hyaline cartilage in the articular surface with similar mechanical properties as of the native cartilage, whereas TGF-\(\upbeta \)3-free scaffolds showed inferior results such as only isolated cartilage formation with relatively lower density and thickness [102]. This approach may be useful for the cartilage part of an OC regeneration strategy. In another study, Huang et al. [103] produced calcium phosphate/poly(L-lactic acid) composite scaffolds that were incorporated with basic fibroblast growth factor. The scaffolds were implanted into OC defects of rabbits without in vitro cell seeding. It was reported that the defects were filled with regenerated tissue [103]. The surface was covered with a layer of cartilage tissue with a good integration in the surrounding native tissue. In addition, high levels of collagen type II and aggrecan were reported. With respect to SB regeneration, a continuous layer of trabecular bone was formed below the cartilage [103].
As an alternative to the scaffolding materials used in the studies mentioned above, decellularized tissues can also be used as scaffolds in tissue engineering applications. Yang et al. [104] developed porous decellularized scaffolds from bovine articular cartilage for cartilage regeneration. The scaffolds maintained the collagen and glycosaminoglycan components of cartilage. Rabbit bone marrow MSCs were seeded into the decellularized scaffolds. After implantation of constructs into the knee cartilage defects of rabbits, better histological scores were achieved as compared to control groups. Small intestinal submucosa (SIS) has also been studied experimentally for tissue engineering applications. To repair articular cartilage defects, Peel et al. [105] seeded chondrocytes onto porcine SIS to generate cartilaginous tissue. Full-thickness articular cartilage defects were treated with these constructs. Based on that work, it was suggested that the SIS–cartilaginous tissue constructs might be useful for joint resurfacing. The results of a study with SIS performed by Suckow et al. [106] also suggested that SIS may be a promising biomaterial for bone repair.
5 Multiscale Tissue Engineering and Regenerative Medicine Strategies
The current trend in TERM is towards multiscale strategies in which different fields of expertise such as tissue engineering, information technology, and medical imaging collaborate. This part briefly overviews these multiscale approaches. The Laboratory for Multiscale Regenerative Technologies (http://lmrt.mit.edu/) directed by Sangeeta Bhatia, is researching micro- and nanotechnology applications for tissue repair and regeneration. They study how micro-environmental signals influence fate and function of liver cells and use this knowledge to develop robust models of animal and human liver for in vitro [107] and in vivo studies. They use microelectronic circuits [108] to study the role of cell–cell interactions in liver constructs, and to use novel extracellular matrix microarrays [109] to study the role of matrix combinations in liver functions. Moreover, their studies include hydrogels, stem cells, and bioreactors [110] to provide gradients of soluble stimuli. The systematic approach they developed is oriented towards hepatic tissue engineering. However, it can be taken as a reference point to employ new tools leading to a multiscale approach in TERM for other tissues.
Step-wise strategy for the patient-specific treatment of an OC defect. The data obtained from medical imaging, such as magnetic resonance imaging (MRI) or computed tomography (CT) is used in the design of a scaffold that is specific for the patient. Autologous cells are isolated and expanded in vitro and encapsulated into the photopolymerizable polymer solution where also growth factors are introduced. Then, the construct (cell/scaffold) is manufactured with a rapid prototyping technique under UV light to obtain the anatomical correct shape and size. The cellular scaffold is dynamically conditioned in vitro with a bioreactor. The tissue engineered OC construct is then implanted into the OC defect of the patient
In the studies of Moroni et al. [111] and Ballyns et al. [112] anatomically shaped scaffolds are produced by using the data obtained from medical imaging. In those studies, the meniscus was chosen as a possible application, but the approach might be used for other tissues as well. Moroni et al. [111] combined the computed tomography (CT) and magnetic resonance imaging (MRI) data of menisci with a RP method to manufacture 3D fibre-deposited scaffolds. The scaffolds were in anatomical shape, and the manufacturing technique allowed for tailoring scaffold architecture and mechanical properties to mimic the native meniscus. Ballyns et al. [112] showed the possibility of developing engineered tissues using tissue injection moulding technique combined with computer-aided design based on the anatomic shapes obtained via medical imaging modalities such as CT and MRI. Depending on the size of the lesion, hydrogels could be injected with a minimally invasive operation or could be pre-formed into anatomical shape. A step-wise strategy for the treatment of a patient with OC defect is illustrated in Fig. 2.6, where the data obtained from the medical images is used in the design of scaffolds having anatomical correct shape and size. Cells are cultured in vitro and encapsulated into the photopolymerizable polymer solution while also growth factors are added into the mixture. With a RP technique, the scaffold is produced under UV light. The cellular scaffold is dynamically conditioned in vitro with a bioreactor. The tissue engineered OC construct is then implanted into the OC defect of the patient.
In another study by Schek et al. [113], a biphasic poly-L-lactic acid/HAp composite scaffolds for OC tissue engineering were developed by means of a RP method with image-based design, which resulted in scaffolds with a matched articular shape and load bearing features. The polymeric phase was seeded with chondrocytes whereas fibroblasts transduced with an adenovirus expressing BMP-7 was introduced into the HAp part. The subcutaneous implantation of the constructs into mice demonstrated the potential of this strategy for OC tissue regeneration.
Lima et al. [114] used finite element modelling to study the biophysical stimuli occurring within the structure under dynamic deformational loading. They developed bilayered OC constructs composed of a cell-seeded agarose gel on the top and a bone part at the bottom where in the middle an interface region of gel/bone was formed. The results showed that relatively more inhomogeneous mechanical signals, such as strain, fluid flow or fluid pressure, occurred within the gel region of the OC composite, compared to the structures only made of gel. That study showed that the cells in the gel may sense these radially and axially varied signals, and it may be beneficial to achieve an inhomogeneity in engineered OC constructs.
6 Final Remarks
TERM is a truly multidisciplinary field with the ultimate goal of regenerating damaged/diseased tissues. Several strategies are available for cartilage and bone tissue regeneration. However, the gold standard does not exist. Many factors affect the outcome of the strategies: the type and manufacturing technique of biomaterials, source, type and culturing conditions of cells, employment of growth factors and bioreactors, and their cumulative effect when used in combination. It is also noteworthy that the depth and size of the lesion, and the patient’s age and condition affect the repair response. As herein discussed, many options exist for each of the components of tissue engineering. Each strategy has its own advantages and disadvantages. Nevertheless, the new generation strategies of TERM show the trend to be patient-specific and to be built on a multiscale approach, specially benefiting from basic science, engineering, and medical imaging and modelling.
References
Sanders, R. K., & Crim, J. R. (2001). Osteochondral injuries. Seminars in Ultrasound Ct and Mri, 22(4), 352–370.
Buckwalter, J. A., & Mankin, H. J. (1997). Articular cartilage. Part II. Degeneration and osteoarthrosis, repair, regeneration, and transplantation. Journal of Bone and Joint Surgery-American Volume, 79A(4), 612–632.
Westacott, C. I., & Sharif, M. (1996). Cytokines in osteoarthritis: Mediators or markers of joint destruction? Seminars in Arthritis and Rheumatism, 25(4), 254–272.
Goldring, M. B., & Goldring, S. R. (2007). Osteoarthritis. Journal of Cellular Physiology, 213(3), 626–634.
Nesic, D., Whiteside, R., Brittberg, M., Wendt, D., Martin, I., & Mainil-Varlet, P. (2006). Cartilage tissue engineering for degenerative joint disease. Advanced Drug Delivery Reviews, 58(2), 300–322.
Goldring, M. B. (2000). The role of the chondrocyte in osteoarthritis. Arthritis and Rheumatism, 43(9), 1916–1926.
Buckwalter, J. A., Mankin, H. J., & Grodzinsky, A. J. (2005). Articular cartilage and osteoarthritis. Instructional Course Lectures, 54, 465–480.
Bhosale, A. M., & Richardson, J. B. (2008). Articular cartilage: Structure, injuries and review of management. British Medical Bulletin, 87(1), 77–95.
Buckwalter, J. A., & Mankin, H. J. (1998). Articular cartilage repair and transplantation. Arthritis and Rheumatism, 41(8), 1331–1342.
Hunziker, E. B. (2002). Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthritis and Cartilage, 10(6), 432–463.
Vaquero, J., & Forriol, F. (2012). Knee chondral injuries: Clinical treatment strategies and experimental models. Injury-International Journal of the Care of the Injured, 43(6), 694–705.
Wirth, C. J., & Rudert, M. (1996). Techniques of cartilage growth enhancement: A review of the literature. Arthroscopy, 12(3), 300–308.
Aigner, T., & Stove, J. (2003). Collagens—major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Advanced Drug Delivery Reviews, 55(12), 1569–1593.
Pridie, K. H., & Gordon, G. (1959). A method of resurfacing osteoarthritic knee joints. Journal of Bone and Joint Surgery-British, 41(3), 618–619.
Sprague, N. F. (1981). Arthroscopic debriment for degenerative knee-joint disease. Clinical Orthopaedics and Related Research, 160, 118–123.
Ficat, R. P., Ficat, C., Gedeon, P., & Toussaint, J. B. (1979). Spongialization—new treatment for diseased patellae. Clinical Orthopaedics and Related Research, 144, 74–83.
Martin, I., Miot, S., Barbero, A., Jakob, M., & Wendt, D. (2007). Osteochondral tissue engineering. Journal of Biomechanics, 40(4), 750–765.
Heath, C. A., & Magari, S. R. (1996). Mini-review: Mechanical factors affecting cartilage regeneration in vitro. Biotechnology and Bioengineering, 50(4), 430–437.
Buckwalter, J. A., & Mankin, H. J. (1997). Articular cartilage. Part I: Tissue design and chondrocyte-matrix interactions. Journal of Bone and Joint Surgery-American Volume, 79A(4), 600–611.
Grodzinsky, A. J., Levenston, M. E., Jin, M., & Frank, E. H. (2000). Cartilage tissue remodeling in response to mechanical forces. Annual Review of Biomedical Engineering, 2, 691–713.
Kleemann, R. U., Krocker, D., Cedraro, A., Tuischer, J., & Duda, G. N. (2005). Altered cartilage mechanics and histology in knee osteoarthritis: Relation to clinical assessment (ICRS Grade). Osteoarthritis and Cartilage, 13(11), 958–963.
Gelse, K., Pöschl, E., & Aigner, T. (2003). Collagens—Structure, function, and biosynthesis. Advanced Drug Delivery Reviews, 55(12), 1531–1546.
Armstrong, C. G., & Mow, V. C. (1982). Variations in the intrinsic mechanical properties of human articular-cartilage with age, degeneration and water-content. Journal of Bone and Joint Surgery-American, 64(1), 88–94.
Black, J., & Hastings, G. W. (Eds.). (1998). Handbook of biomaterials properties. London: Chapman and Hall.
Clarke, B. (2008). Normal bone anatomy and physiology. Clinical Journal of the American Society of Nephrology, 3, S131–S139.
Miller, S. C., Saintgeorges, L., Bowman, B. M., & Jee, W. S. S. (1989). Bone lining cells—structure and function. Scanning Microscopy, 3(3), 953–961.
Yaszemski, M. J., Payne, R. G., Hayes, W. C., Langer, R., & Mikos, A. G. (1996). Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials, 17(2), 175–185.
Suri, S., & Walsh, D. A. (2012). Osteochondral alterations in osteoarthritis. Bone, 51(2), 204–211.
Kawcak, C. E., McIlwraith, C. W., Norrdin, R. W., Park, R. D., & James, S. P. (2001). The role of subchondral bone in joint disease: A review. Equine Veterinary Journal, 33(2), 120–126.
Lyons, T. J., McClure, S. F., Stoddart, R. W., & McClure, J. (2006). The normal human chondro-osseous junctional region: Evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. Bmc Musculoskeletal Disorders, 7, 52.
Burr, D. B. (2004). Anatomy and physiology of the mineralized tissues: Role in the pathogenesis of osteoarthrosis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society, 12 Suppl A, S20–S30.
Madry, H., van Dijk, C. N., & Mueller-Gerbl, M. (2010). The basic science of the subchondral bone. Knee Surgery Sports Traumatology Arthroscopy, 18(4), 419–433.
Clark, J. M., & Huber, J. D. (1990). The structure of the human subchondral plate. Journal of Bone and Joint Surgery-British, 72(5), 866–873.
Li, B. H., & Aspden, R. M. (1997). Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Annals of the Rheumatic Diseases, 56(4), 247–254.
Rippon, H. J., & Bishop, A. E. (2004). Embryonic stem cells. Cell Proliferation, 37(1), 23–34.
Young, H. E., & Black, A. C. (2004). Adult stem cells. Anatomical Record Part a-Discoveries in Molecular Cellular and Evolutionary Biology, 276A(1), 75–102.
Alison, M. R., Poulsom, R., Forbes, S., & Wright, N. A. (2002). An introduction to stem cells. Journal of Pathology, 197(4), 419–423.
Corona, B. T., Ward, C. L., Harrison, B. S., & Christ, G. J. (2010). Regenerative medicine: Basic concepts, current status, and future applications. Journal of Investigative Medicine, 58(7), 849–858.
Darling, E. M., & Athanasiou, K. A. (2005). Rapid phenotypic changes in passaged articular chondrocyte subpopulations. Journal of Orthopaedic Research, 23(2), 425–432.
Brittberg, M., Lindahl, A., Nilsson, A., Ohlsson, C., Isaksson, O., & Peterson, L. (1994). Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New England Journal of Medicine, 331(14), 889–895.
Bedi, A., Feeley, B. T., & Williams, R. J, I. I. I. (2010). Management of articular cartilage defects of the knee. Journal of Bone and Joint Surgery-American, 92A(4), 994–1009.
Caplan, A. I. (1991). Mesenchymal stem-cells. Journal of Orthopaedic Research, 9(5), 641–650.
Freyman, T. M., Yannas, I. V., & Gibson, L. J. (2001). Cellular materials as porous scaffolds for tissue engineering. Progress in Materials Science, 46(3–4), 273–282.
Kinner, B., Capito, R. M., & Spector, M. (2005). Regeneration of articular cartilage. In: Yannas, I. V. (ed.). Regenerative medicine II: Clinical and preclinical applications, (Vol. 94). Advances in Biochemical Engineering-Biotechnology, (pp. 91–123). New York: Springer.
Noeth, U., Rackwitz, L., Steinert, A. F., & Tuan, R. S. (2010). Cell delivery therapeutics for musculoskeletal regeneration. Advanced Drug Delivery Reviews, 62(7–8), 765–783.
Puppi, D., Chiellini, F., Piras, A. M., & Chiellini, E. (2010). Polymeric materials for bone and cartilage repair. Progress in Polymer Science, 35(4), 403–440.
Gilbert, T. W., Sellaro, T. L., & Badylak, S. F. (2006). Decellularization of tissues and organs. Biomaterials, 27(19), 3675–3683.
Hench, L. L. (1991). Bioceramics—from concept to clinic. Journal of the American Ceramic Society, 74(7), 1487–1510.
Best, S. M., Porter, A. E., Thian, E. S., & Huang, J. (2008). Bioceramics: Past, present and for the future. Journal of the European Ceramic Society, 28(7), 1319–1327.
Mano, J. F., Silva, G. A., Azevedo, H. S., Malafaya, P. B., Sousa, R. A., Silva, S. S., et al. (2007). Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. Journal of the Royal Society Interface, 4(17), 999–1030.
Hutmacher, D. W. (2000). Scaffolds in tissue engineering bone and cartilage. Biomaterials, 21(24), 2529–2543.
Yan, L.-P., Wang, Y.-J., Ren, L., Wu, G., Caridade, S. G., Fan, J.-B., et al. (2010). Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. Journal of Biomedical Materials Research Part A, 95A(2), 465–475.
Hoshikawa, A., Nakayama, Y., Matsuda, T., Oda, H., Nakamura, K., & Mabuchi, K. (2006). Encapsulation of chondrocytes in photopolymerizable styrenated gelatin for cartilage tissue engineering. Tissue Engineering, 12(8), 2333–2341.
Eyrich, D., Brandl, F., Appel, B., Wiese, H., Maier, G., Wenzel, M., et al. (2007). Long-term stable fibrin gels for cartilage engineering. Biomaterials, 28(1), 55–65.
Yan, L. P., Oliveira, J. M., Oliveira, A. L., & Reis, R. L. (2012). Development of a bilayered scaffold based on silk fibroin and silk fibroin/nano-calcium phosphate for osteochondral regeneration. Journal of Tissue Engineering and Regenerative Medicine, 6, 24–24.
Popa, E. G., Gomes, M. E., & Reis, R. L. (2011). Cell delivery systems using alginate-carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules, 12(11), 3952–3961.
Pelaez, D., Huang, C.-Y. C., & Cheung, H. S. (2009). Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells and Development, 18(1), 93–102.
Stillaert, F. B., Di Bartolo, C., Hunt, J. A., Rhodes, N. P., Tognana, E., Monstrey, S., et al. (2008). Human clinical experience with adipose precursor cells seeded on hyaluronic acid-based spongy scaffolds. Biomaterials, 29(29), 3953–3959.
Oliveira, J. M., Rodrigues, M. T., Silva, S. S., Malafaya, P. B., Gomes, M. E., Viegas, C. A., et al. (2006). Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials, 27(36), 6123–6137.
Rodrigues, A. I., Gomes, M. E., Leonor, I. B., & Reis, R. L. (2012). Bioactive starch-based scaffolds and human adipose stem cells are a good combination for bone tissue engineering. Acta Biomaterialia, 8(10), 3765–3776.
Oliveira, J. T., Santos, T. C., Martins, L., Picciochi, R., Marques, A. P., Castro, A. G., et al. (2010). Gellan gum injectable hydrogels for cartilage tissue engineering applications: In vitro studies and preliminary in vivo evaluation. Tissue Engineering Part A, 16(1), 343–353.
Antunes, J. C., Oliveira, J. M., Reis, R. L., Soria, J. M., Gomez-Ribelles, J. L., & Mano, J. F. (2010). Novel poly(L-lactic acid)/hyaluronic acid macroporous hybrid scaffolds: Characterization and assessment of cytotoxicity. Journal of Biomedical Materials Research Part A, 94A(3), 856–869.
Xue, D., Zheng, Q., Zong, C., Li, Q., Li, H., Qian, S., et al. (2010). Osteochondral repair using porous poly(lactide-co-glycolide)/nano-hydroxyapatite hybrid scaffolds with undifferentiated mesenchymal stem cells in a rat model. Journal of Biomedical Materials Research Part A, 94A(1), 259–270.
Erisken, C., Kalyon, D. M., Wang, H., Oernek-Ballanco, C., & Xu, J. (2011). Osteochondral tissue formation through adipose-derived stromal cell differentiation on biomimetic polycaprolactone nanofibrous scaffolds with graded insulin and beta-glycerophosphate concentrations. Tissue Engineering Part A, 17(9–10), 1239–1252.
Drury, J. L., & Mooney, D. J. (2003). Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials, 24(24), 4337–4351.
Uludag, H., De Vos, P., & Tresco, P. A. (2000). Technology of mammalian cell encapsulation. Advanced Drug Delivery Reviews, 42(1–2), 29–64.
Temenoff, J. S., & Mikos, A. G. (2000). Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials, 21(23), 2405–2412.
Chan, B. P., & Leong, K. W. (2008). Scaffolding in tissue engineering: General approaches and tissue-specific considerations. European Spine Journal: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society, 17(Suppl 4), 467–479.
Nguyen, K. T., & West, J. L. (2002). Photopolymerizable hydrogels for tissue engineering applications. Biomaterials, 23(22), 4307–4314.
Kaur, M., & Srivastava, A. K. (2002). Photopolymerization: A review. Journal of Macromolecular Science-Polymer Reviews, C42(4), 481–512.
Jansson, P. E., Lindberg, B., & Sandford, P. A. (1983). Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas-Elodea. Carbohydrate Research, 124(1), 135–139.
Oliveira, J. T., Martins, L., Picciochi, R., Malafaya, I. B., Sousa, R. A., Neves, N. M., et al. (2009). Gellan gum: A new biomaterial for cartilage tissue engineering applications. Journal of Biomedical Materials Research Part A, 93A(3), 852–863.
Smith, A. M., Shelton, R. M., Perrie, Y., & Harris, J. J. (2007). An initial evaluation of gellan gum as a material for tissue engineering applications. Journal of Biomaterials Applications, 22(3), 241–254.
Silva-Correia, J., Oliveira, J. M., Caridade, S. G., Oliveira, J. T., Sousa, R. A., Mano, J. F., et al. (2011). Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. Journal of Tissue Engineering and Regenerative Medicine, 5(6), E97–E107.
Oliveira, J. M., Silva, S. S., Malafaya, P. B., Rodrigues, M. T., Kotobuki, N., Hirose, M., et al. (2009). Macroporous hydroxyapatite scaffolds for bone tissue engineering applications: Physicochemical characterization and assessment of rat bone marrow stromal cell viability. Journal of Biomedical Materials Research Part A, 91A(1), 175–186.
Oliveira, J. M., Sousa, R. A., Kotobuki, N., Tadokoro, M., Hirose, M., Mano, J. F., et al. (2009). The osteogenic differentiation of rat bone marrow stromal cells cultured with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles. Biomaterials, 30(5), 804–813.
Landers, R., Pfister, A., Hubner, U., John, H., Schmelzeisen, R., & Mulhaupt, R. (2002). Fabrication of soft tissue engineering scaffolds by means of rapid prototyping techniques. Journal of Materials Science, 37(15), 3107–3116.
Leong, K. F., Cheah, C. M., & Chua, C. K. (2003). Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials, 24(13), 2363–2378.
Yeong, W. Y., Chua, C. K., Leong, K. F., & Chandrasekaran, M. (2004). Rapid prototyping in tissue engineering: Challenges and potential. Trends in Biotechnology, 22(12), 643–652.
Landers, R., & Mulhaupt, R. (2000). Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromolecular Materials and Engineering, 282(9), 17–21.
Landers, R., Hubner, U., Schmelzeisen, R., & Mulhaupt, R. (2002). Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials, 23(23), 4437–4447.
Son, J., & Kim, G. (2009). Three-dimensional plotter technology for fabricating polymeric scaffolds with micro-grooved surfaces. Journal of Biomaterials Science-Polymer Edition, 20(14), 2089–2101.
Babensee, J. E., McIntire, L. V., & Mikos, A. G. (2000). Growth factor delivery for tissue engineering. Pharmaceutical Research, 17(5), 497–504.
Lieberman, J. R., Daluiski, A., & Einhorn, T. A. (2002). The role of growth factors in the repair of bone—Biology and clinical applications. Journal of Bone and Joint Surgery-American, 84A(6), 1032–1044.
Schmidt, M. B., Chen, E. H., & Lynch, S. E. (2006). A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis and Cartilage, 14(5), 403–412.
Fortier, L. A., Mohammed, H. O., Lust, G., & Nixon, A. J. (2002). Insulin-like growth factor-I enhances cell-based repair of articular cartilage. Journal of Bone and Joint Surgery-British, 84B(2), 276–288.
Linkhart, T. A., Mohan, S., & Baylink, D. J. (1996). Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone, 19(1), S1–S12.
Fortier, L. A., Barker, J. U., Strauss, E. J., McCarrel, T. M., & Cole, B. J. (2011). The role of growth factors in cartilage repair. Clinical Orthopaedics and Related Research, 469(10), 2706–2715.
Vunjak-Novakovic, G., Meinel, L., Altman, G., & Kaplan, D. (2005). Bioreactor cultivation of osteochondral grafts. Orthodontics and Craniofacial Research, 8(3), 209–218.
Vunjak-Novakovic, G., Obradovic, B., Martin, I., Bursac, P. M., Langer, R., & Freed, L. E. (1998). Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnology Progress, 14(2), 193–202.
Schwarz, R. P., Goodwin, T. J., & Wolf, D. A. (1992). Cell culture for three-dimensional modeling in rotating-wall vessels: An application of simulated microgravity. Journal of Tissue Culture Methods: Tissue Culture Association Manual of Cell, Tissue, and Organ Culture Procedures, 14(2), 51–57.
Bancroft, G. N., Sikavitsas, V. I., & Mikos, A. G. (2003). Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Engineering, 9(3), 549–554.
Pei, M., Solchaga, L. A., Seidel, J., Zeng, L., Vunjak-Novakovic, G., Caplan, A. I., et al. (2002). Bioreactors mediate the effectiveness of tissue engineering scaffolds. Faseb Journal, 16(10), 1691–1711.
Schaefer, D., Martin, I., Jundt, G., Seidel, J., Heberer, M., Grodzinsky, A., et al. (2002). Tissue-engineered composites for the repair of large osteochondral defects. Arthritis and Rheumatism, 46(9), 2524–2534.
Kandel, R. A., Grynpas, M., Pilliar, R., Lee, J., Wang, J., Waldman, S., et al. (2006). Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a Sheep model. Biomaterials, 27(22), 4120–4131.
Klein, T. J., Rizzi, S. C., Reichert, J. C., Georgi, N., Malda, J., Schuurman, W., et al. (2009). Strategies for zonal cartilage repair using hydrogels. Macromolecular Bioscience, 9(11), 1049–1058.
Kim, T. K., Sharma, B., Williams, C. G., Ruffner, M. A., Malik, A., McFarland, E. G., et al. (2003). Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis and Cartilage, 11(9), 653–664.
Ng, K. W., Wang, C. C. B., Mauck, R. L., Kelly, T. A. N., Chahine, N. O., Costa, K. D., et al. (2005). A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. Journal of Orthopaedic Research, 23(1), 134–141.
Ng, K. W., Ateshian, G. A., & Hung, C. T. (2009). Zonal chondrocytes seeded in a layered agarose hydrogel create engineered cartilage with depth-dependent cellular and mechanical inhomogeneity. Tissue Engineering Part A, 15(9), 2315–2324.
Harley, B. A., Lynn, A. K., Wissner-Gross, Z., Bonfield, W., Yannas, I. V., & Gibson, L. J. (2010). Design of a multiphase osteochondral scaffold III: Fabrication of layered scaffolds with continuous interfaces. Journal of Biomedical Materials Research Part A, 92A(3), 1078–1093.
Jiang, J., Tang, A., Ateshian, G. A., Guo, X. E., Hung, C. T., & Lu, H. H. (2010). Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Annals of Biomedical Engineering, 38(6), 2183–2196.
Lee, C. H., Cook, J. L., Mendelson, A., Moioli, E. K., Yao, H., & Mao, J. J. (2010). Regeneration of the articular surface of the rabbit synovial joint by cell homing: A proof of concept study. Lancet, 376(9739), 440–448.
Huang, X., Yang, D., Yan, W., Shi, Z., Feng, J., Gao, Y., et al. (2007). Osteochondral repair using the combination of fibroblast growth factor and amorphous calcium phosphate/poly(L-lactic acid) hybrid materials. Biomaterials, 28(20), 3091–3100.
Yang, Z., Shi, Y., Wei, X., He, J., Yang, S., Dickson, G., et al. (2010). Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Engineering Part C-Methods, 16(5), 865–876.
Peel, S. A. F., Chen, H., Renlund, R., Badylak, S. F., & Kandel, R. A. (1998). Formation of a SIS-cartilage composite graft in vitro and its use in the repair of articular cartilage defects. Tissue Engineering, 4(2), 143–155.
Suckow, M. A., Voytik-Harbin, S. L., Terril, L. A., & Badylak, S. F. (1999). Enhanced bone regeneration using porcine small intestinal submucosa. Journal of Investigative Surgery, 12(5), 277–287.
Khetani, S. R., & Bhatia, S. N. (2008). Microscale culture of human liver cells for drug development. Nature Biotechnology, 26(1), 120–126.
Bhatia, S. N., Balis, U. J., Yarmush, M. L., & Toner, M. (1999). Effect of cell-cell interactions in preservation of cellular phenotype: Cocultivation of hepatocytes and nonparenchymal cells. Faseb Journal, 13(14), 1883–1900.
Flaim, C. J., Chien, S., & Bhatia, S. N. (2005). An extracellular matrix microarray for probing cellular differentiation. Nature Methods, 2(2), 119–125.
Allen, J. W., & Bhatia, S. N. (2003). Formation of steady-state oxygen gradients in vitro—application to liver zonation. Biotechnology and Bioengineering, 82(3), 253–262.
Moroni, L., Lambers, F. M., Wilson, W., van Donkelaar, C. C., de Wijn, J. R., Huiskesb, R., et al. (2007). Finite element analysis of meniscal anatomical 3D scaffolds: Implications for tissue engineering. The Open Biomedical Engineering Journal, 1, 23–34.
Ballyns, J. J., Gleghorn, J. P., Niebrzydowski, V., Rawlinson, J. J., Potter, H. G., Maher, S. A., et al. (2008). Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Engineering Part A, 14(7), 1195–1202.
Schek, R. M., Taboas, J. M., Segvich, S. J., Hollister, S. J., & Krebsbach, P. H. (2004). Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Engineering, 10(9–10), 1376–1385.
Lima, E. G., Mauck, R. L., Han, S. H., Park, S., Ng, K. W., Ateshian, G. A., et al. (2004). Functional tissue engineering of chondral and osteochondral constructs. Biorheology, 41(3–4), 577–590.
Acknowledgments
The authors thank the financial support of the MultiScaleHuman project (Contract number: MRTN-CT-2011-289897) in the Marie Curie Actions—Initial Training Networks.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Cengiz, I.F., Oliveira, J.M., Reis, R.L. (2014). Tissue Engineering and Regenerative Medicine Strategies for the Treatment of Osteochondral Lesions. In: Magnenat-Thalmann, N., Ratib, O., Choi, H. (eds) 3D Multiscale Physiological Human. Springer, London. https://doi.org/10.1007/978-1-4471-6275-9_2
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6275-9_2
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6274-2
Online ISBN: 978-1-4471-6275-9
eBook Packages: Computer ScienceComputer Science (R0)