Abstract
Mitral valve congenital anomalies may involve one or more components of the valve and may result in stenosis, insufficiency, or a combination of both. Classification of these anomalies is challenged by the different anatomic conditions that produce the aberrations causing hemodynamic compromise. A segmental approach is useful from an anatomic point of view. Stenotic lesions can be characterized as supravalvar (encircling rings), annular hypoplasia, valvar restrictions, and subvalvar conditions. Congenital regurgitant lesions, which are less common, can be characterized as annular dilatation, valvar deficiencies, chordal abnormalities, and papillary muscle anomalies. Advanced diagnostic imaging modalities and improved surgical techniques have resulted in reproducible and excellent clinical outcomes; most patients can now be treated with valve repair techniques instead of valve replacement options.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Mitral valve congenital anomalies may involve one or more components of the valve and may result in stenosis, insufficiency, or a combination of both. Classification of these anomalies is challenged by the different anatomic conditions that produce the aberrations causing hemodynamic compromise. A segmental approach is useful from an anatomic point of view. Stenotic lesions can be characterized as supravalvar (encircling rings), annular hypoplasia, valvar restrictions, and subvalvar conditions. Congenital regurgitant lesions, which are less common, can be characterized as annular dilatation, valvar deficiencies, chordal abnormalities, and papillary muscle anomalies. Advanced diagnostic imaging modalities and improved surgical techniques have resulted in reproducible and excellent clinical outcomes; most patients can now be treated with valve repair techniques instead of valve replacement options.
22.1 Normal Mitral Valve Anatomy, Relationships, and Classification
The normal mitral valve is a complex anatomic structure comprised of an annulus, leaflets, chordae tendineae, and papillary muscles. Mitral valve function relies on the integrity of its components and the contractility of the left ventricle. Figure 22.1 is a cross-sectional representation of the heart that shows the mitral valve and its anatomic relationships. A fibrous annulus divides the left atrium from the left ventricle. The leaflets are attached to the annulus; important adjacent structures are noted. The mitral valve annulus projects along the left atrioventricular (AV) sulcus; it is fixed medially to the central cardiac skeleton at the aortic root. The mitral valve is a bileaflet structure and is further defined by two well-delineated commissures in the anterolateral and posteromedial positions. The posterior (mural) leaflet is longer and narrower than the anterior leaflet and is generally divided into three scallops. The anterior (septal) leaflet is larger and wider than the posterior leaflet and is shaped like a trapezoid. Each leaflet has a more proximal clear zone and a thicker basal zone at the region of attachment to the annulus. The chordae tendineae arise from two papillary muscles that are attached to the anterolateral and posteromedial ventricular wall. The posterior descending coronary artery supplies the posterior muscle; the circumflex coronary artery supplies the anterolateral papillary muscle. Each of the papillary muscles supports chordae tendineae from both leaflets. The papillary muscle anatomy varies considerably between individuals. Some are broad-based, others are fingerlike, and still others are broad-based and have fingerlike characteristics.

Fig. 22.1
There are essentially two classification systems for congenital mitral lesions, as described by Carpentier and by Metruka and Lamberti. These classifications characterize the different lesion sets by leaflet motion, deformed annulus, cleft leaflets, agenesis, commissural fusion, chordal shortening or elongation, and subvalvar apparatus anatomy. Some of the more common lesions are described below, with traditional methods for treatment.
22.2 Mitral Stenosis Lesions
22.2.1 Supravalvar and Intravalvar Mitral Rings
Supravalvar mitral stenosis is a restrictive, fibrous membrane that is attached and continuous with the mitral valve annulus. This thick, fibrous plate of tissue can resemble a membrane in its central portion. It is usually attached at the level of the annulus or just above it. The membrane generally has a single, central opening that may be eccentric in position. The size of the opening defines the grade of obstruction and correlates well with the severity of symptoms. The underlying mitral valve may be functionally normal, but more often the valve is small, and it may be anatomically abnormal in some patients. Supravalvar mitral stenosis should not be confused with cor triatriatum, in which a fibrous membrane divides the atrium into two chambers. The atrial appendage originates downstream from the membrane in cor triatriatum, whereas the left atrial appendage is upstream from a supravalvar ring.
Figure 22.2 shows a lateral cutaway view of supravalvar mitral stenosis. The plane between the supravalvar ring and the mitral orifice has been exaggerated to highlight the anatomic relationship. The operation to repair this anomaly is performed using aortobicaval cardiopulmonary bypass with aortic cross clamping and cardioplegic arrest. Generally, these patients have long-standing mitral stenosis and have a large left atrium, making the approach through the interatrial groove preferable. The key to proper exposure is to employ a large left atrial incision with traction annular sutures to bring the annulus into the operative field for the membrane excision (Fig. 22.3a). A Beaver blade (Fig. 22.3b) is employed to find the dissection plane between the fibrous ring and the annulus, using endarterectomy techniques. The dissection plane can be developed much like removal of a subvalvar aortic ridge. Careful and deliberate dissection will effectively remove the fibrous ring without injury to the underlying mitral annulus. With complete excision of the ring, a much larger mitral orifice can be anticipated (Fig. 22.3c). The mitral valve can then be tested for competence, using a bulb syringe filled with cold saline, and the left ventricular vent can be reinserted. The atrium is carefully closed, the air maneuvers can be performed, and the cross clamp can be removed. After rewarming and separation from cardiopulmonary bypass, transesophageal echocardiography can be used to interrogate the repair. Occasionally, the mitral valve ring will be situated within the body of the mitral valve, as shown in Figure 22.4. Under these circumstances, the valve is approached and exposed in the same manner as for supravalvar stenosis. The dissection plane is developed using endarterectomy techniques, and the fibrous ring is carefully dissected from the surrounding valvar tissue, with special care not to injure the underlying valve. If unwanted mitral leaflet perforation complicates the excision, a piece of CorMatrix® (CorMatrix Cardiovascular; Roswell, Georgia, USA) can be used to repair the leaflet at the point of perforation. Ordinarily, the orifice of the mitral valve can be enlarged significantly. When the subvalvar papillary muscle structure is also stenotic, carefully calculated papillary muscle incisions between the chordae can be accomplished to allow further mobility of the leaflets to maximize inflow to the body of the left ventricle (Fig. 22.4).

Fig. 22.2

Fig. 22.3

Fig. 22.4
22.2.2 Double-Orifice Mitral Valve
Double-orifice mitral valve is a curious anatomic abnormality of the mitral valve in which there are two reasonably formed inlets into the left ventricle. Figure 22.5a shows a left atrial superior view of a double-orifice mitral valve. Figure 22.5b shows the same valve in a coronal view. Typically, the smaller of the two orifices is in the right lateral position and the larger orifice is in the left medial position. The degree of stenosis is variable, and effective orifice-enlarging operations have not been reported. The most important tenet when approaching this valve is not to cut the isthmus between the orifices. Cutting the isthmus will result in severe mitral regurgitation that currently cannot be repaired owing to a lack of subtended supporting chordae tendineae in the middle of the valve. The best approach to a stenotic double-orifice valve is to look for and treat any secondary causes of stenosis such as papillary muscle obstructive lesions.

Fig. 22.5
Sometimes a cleft will be present in the anterior leaflet of the larger orifice. Practical wisdom will determine whether the cleft should be closed, depending on the amount of resultant stenosis if the cleft is closed or the amount of regurgitation if the cleft is not repaired.
22.2.3 Mitral Valve Hypoplasia and Arcade (Hammock) Mitral Valve
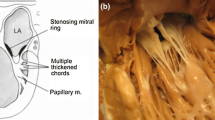
When mitral valve stenosis and annular hypoplasia is associated with an inadequate left ventricle, the patient is generally treated along the single-ventricle pathway en route to Fontan physiology. A hypoplastic valve and annulus can occasionally be associated with a functionally adequate left ventricle, especially if a ventricular septal defect is present. The hypoplastic valve may appear quite similar to a normal mitral valve, having two leaflets with corresponding chordae tendineae that appear appropriate. The leaflets are generally functional, with nonfused commissures. Alternatively, the leaflets may be thickened and the commissures may be fused. These valves can be associated with thickened, short, and inadequate chordae tendineae with poorly developed interchordal spaces. If the chordae are shortened, the valve may have a funnel-like appearance. Most often, these characteristics match up very well with what most anatomists call an arcade mitral valve. The anterior and posterior group of papillary muscles joins together and fuses with the entire edge of the leaflet, without the presence of well-formed chordae. In some patients, only one papillary muscle may participate in the arcade. The arcade mitral configuration can produce mitral stenosis, insufficiency, or a mixed lesion. It may also be associated with a parachute valve. In general, therapy is aimed at incising the elongated and shortened papillary muscle that is responsible for the central obstructing orifice and making fenestrations in the existing valve leaflets. Figure 22.6a shows a cutaway AV view and Figure 22.6b, c show atrial views of a stenotic parachute valve; the anatomic characteristics of parachute valves are similar to those of an arcade/hammock mitral valve. (see Sect. 22.2.4) Once the appropriate incisions and fenestrations are performed, the valve orifice can be measured with staged and measured dilators. The predicted adequacy of the valvuloplasty can be evaluated by comparison to standard measurements of normal mitral valves for somatic size. The mitral valve competence can be assessed with the use of a saline-filled bulb syringe.

Fig. 22.6
If any “pearl” can be offered by a surgeon to another regarding this operation, it would be to “establish excellent exposure with retraction sutures in the annulus, measure 10 times, and cut once.” Incision into the papillary muscle must be made accurately, deliberately, and centrally to avoid papillary muscle avulsion and to maintain chordal attachments.
22.2.4 Parachute Mitral Valve
Parachute mitral valve is characterized by total chordal attachment to a single papillary muscle, often causing significant congenital mitral stenosis. In general, the entire leaflet is connected to the posterior papillary muscle, with an absence of the anterior papillary muscle. In rare instances, the entire leaflet is connected to an anterior papillary muscle. Figure 22.6a–c show a severe case of congenital mitral stenosis involving all elements of the valve apparatus, in which the parachute mitral valve is attached to the single papillary muscle arising from the posterior left ventricular wall (Fig. 22.6a). In Figure 22.6b, the dotted lines illustrate the areas of proposed leaflet fenestrations and papillary muscle incision to open the ventricular inlet. The valve is approached in a similar manner as outlined in the previous sections, with the same precautions and the same methods of intraoperative evaluation.
22.3 Mitral Regurgitation Lesions
Most authors and practitioners use the terms “mitral insufficiency” and “mitral regurgitation” interchangeably, but other than reference to a leaking valve, “mitral insufficiency” connotes an absence of valvar tissue, or not enough for a functional valve. “Mitral regurgitation” is more descriptive, as the term refers to a leaking valve without reference to whether there is sufficient tissue. We generally do not refer to a leaking mitral valve as “MI” because that can be confused with myocardial infarction. Most of the time, we refer to a leaking valve as “MR,” which is not likely to be confused with anything else medical. In this atlas, there is no obvious preference for either term; they are used interchangeably, as is done by most practitioners in this field.
Congenital mitral insufficiency can be associated with many of the previously noted abnormalities of mitral valve stenosis. It can also be primarily structural, owing to annular dilatation, chordal elongation, and leaflet anomalies. Mitral insufficiency may also present as a secondary manifestation caused by ventricular dilatation, cardiomyopathy, or aortic insufficiency. In particular, significant mitral regurgitation often occurs in association with an anomalous left coronary artery arising from the pulmonary artery, owing to decreased myocardial oxygen delivery and poor ventricular function. Though it is not a mitral valve, the common AV valve found in a complete AV canal defect is often associated with insufficiency of the left AV orifice. An isolated cleft of the anterior leaflet of the mitral valve may produce important mitral insufficiency. Young patients with Marfan syndrome who present early with mitral insufficiency have abnormal mitral valves with billowing leaflets, elongated chordae, and dilated annuli. Double-orifice mitral valve, discussed above (Fig. 22.5a), also can be associated with a complete AV canal, with a cleft in the larger of the two orifices. This configuration may or may not result in regurgitation. Mitral valve prolapse is characterized by excess valvar tissue involving the posterior leaflet, which projects into the left atrium owing to elongated chordae and annular dilatation.
22.3.1 Repair of Mitral Insufficiency Owing to Annular Dilatation: Carpentier Techniques
Repair of mitral insufficiency owing to annular dilatation has undergone many changes from the introduction by Carpentier of posterior leaflet quadrangular resection, annular reduction, and supporting annular rings. When applied to infants and children, annular reduction techniques can be effective, but annular rings need to be used judiciously, as somatic growth must be considered in the long term. Figure 22.7a–d show the progression of steps that are taken to perform the classic Carpentier posterior leaflet quadrangular resection annular reduction technique with a supportive annular ring. Figure 22.7a shows a mitral valve that is insufficient owing to annular dilatation. The dotted lines depict the valvar incision for the proposed posterior leaflet quadrangular resection. Figure 22.7b shows the quadrangular resection in progress and the completed linear incisions (arrows) that were performed in anticipation of the sliding valvuloplasty. The quadrangular resection involves the middle scallop of the mitral valve posterior leaflet. The resection is carefully performed to remove the middle scallop up to the supporting chordae of the right and left scallops, which will support the valve during systole and prevent insufficiency. Once the sliding incisions are made, a posterior reduction annuloplasty can be performed, as shown in Figure 22.7c. The individual sutures can be reinforced by pledgets, depending on the size of the patient. Once the reduction annuloplasty is performed, the remaining posterior leaflets can be brought into apposition and closed, thereby forming a new posterior leaflet that has annular and chordal support. Figure 22.7d shows the completed repair with a supporting noncircumferential ring. The ring is meant to support the annuloplasty and prevent dehiscence. Not shown in this series of figures is the complete annular ring that encompasses and supports the entire annulus. Also not shown is the incomplete ring that supports the annulus for about 270 degrees from aortic trigone to aortic trigone, the idea being that the aortic/mitral annulus is fixed, thereby requiring only an incomplete ring to support the rest of the mitral annulus for a reduction procedure.

Fig. 22.7
22.3.2 Repair of Mitral Insufficiency Owing to Annular Dilatation: Wooler Technique
Variations of the segmental annuloplasty technique have been described by Wooler and many others. The principles are similar to the Carpentier technique, namely that a reduction annuloplasty procedure will better approximate the mitral leaflets, rendering them competent. The surgical approach is the same as previously noted for other mitral procedures. Figure 22.8a shows forceps identification of the commissure and the adjacent aortic annulus that shares an annulus with the mitral valve. Figure 22.8b shows the placement of pledgeted mattress sutures of nonabsorbable suture material. The sutures are located along the mitral annulus at the commissures. The length and number of sutures determines the degree of annular reduction. Obviously, the purpose of the sutures is to reduce the posterior annulus without disturbing leaflet coaptation. Figure 22.8c shows the completed annular reduction procedure. The orifice can now be calibrated with sized dilators and the valve can be assessed, using a saline-filled bulb syringe to determine competency. The benefit of this technique is that additional sutures can be placed as necessary. Occasionally, the annular plication distorts the leaflet coaptation, requiring re-repair, but this repair can be performed without difficulty, as no incisions were made in the valve apparatus.

Fig. 22.8
22.3.3 Repair of Mitral Insufficiency Owing to Annular Dilatation and Valve Prolapse: The Side-to-Side Valvuloplasty Technique (Alfieri Stitch)
When annular dilatation and valve prolapse are concurrent, another option for valve repair is the side-to-side valvuloplasty Alfieri stitch. A pledgeted mattress suture is place in the middle portion of the anterior mitral leaflet and connected to the middle portion of the posterior leaflet. This mattress suture establishes a bridge between the leaflets and creates a double-orifice mitral valve. The suture prevents leaflet prolapse, which generally is the main cause of the regurgitation (Fig. 22.9). A reduction annuloplasty procedure may not be necessary, making this a very good option for young children who are still undergoing maturation and somatic growth. It is very important to use pledgeted sutures for this procedure, however, as the leaflets tend to be thin, and tearing can result in recurrent regurgitation. Some surgeons use this technique as a primary procedure, but others use it as a “bail out” procedure when all else has failed. Our experience with this technique has been positive; we do not hesitate to use it as a primary intervention when an anticipated long cross clamp time will require expeditious repairs, especially in situations of a redo Fontan operation and multiple valve operations.

Fig. 22.9
22.3.4 Repair of Left-Sided Atrioventricular Valve Regurgitation
Although technically and anatomically not a mitral valve, the left-sided AV valve associated with partial and complete AV canal may require attention either primarily or secondarily after initial repair for left-sided AV valve regurgitation. Figure 22.10 shows the technique for repair of left-sided AV valve regurgitation. As is usually the case, the left-sided AV valve in this drawing is characterized by a cleft in the anterior leaflet, which is shown closed at the zone of apposition using interrupted suture technique. The knots can be tied on the atrial side or the ventricular side of the suture line; no studies have favored one method over the other. Ordinarily, cleft closure should be sufficient to render the valve competent, but if there is annular dilatation, a Wooler annuloplasty can be added, as shown in Figure 22.10. The principles for annular reduction and testing for competence are the same as previously discussed.

Fig. 22.10
22.3.5 Chordal Shortening and Replacement Techniques
Chordal shortening and replacement techniques have undergone many changes since their introduction. The difficulty with these techniques is in ensuring the correct length of the resultant chordal repair and correlating the repair with somatic growth in a growing child so that the need for re-intervention can be delayed or eliminated altogether. The most common indications for application of chordal shortening and replacement techniques are Marfan syndrome, tricuspid valve anomalies associated with a single ventricle, and injured chordal attachments or their congenital absence. Recent strategies have moved away from chordal shortening and replacement techniques in favor of “bicuspidization” of the tricuspid valve (taking advantage of adjacent chords and leaflets with normal configurations) and the side-to-side Alfieri technique. Nevertheless, there are still indications for chordal shortening and replacement techniques, which are presented here for completeness.
Figure 22.11a shows a diagram of redundant chordae that allow prolapse of a segment of the mitral leaflet. Figure 22.11b shows a deliberate and careful incision of the papillary muscle head to produce a trough into which the base of the chordal attachments can be shortened by drawing the segment down into the trough and secured using a pledgeted mattress suture. The choral shortening procedure is reinforced by a second pledgeted mattress suture through both sides of the papillary head (Fig. 22.11c). Figure 22.11d shows the final result. Defining the correct amount of chordal shortening will determine the functional outcome. This maneuver often must be carried out a number of times to achieve an excellent result.

Fig. 22.11
Another method for achieving chordal shortening, replacement, and reinforcement involves using polytetrafluoroethylene (PTFE) artificial chordae. In Figure 22.12a, the length of the redundant, elongated chordae is noted by the solid line “B”. The solid line “A” is noted to indicate the normal length of the adjacent chordae. A mattress suture is placed through the free edge of the leaflet at the site where the chordae are missing or elongated. The mattress suture is then brought down to the head of the papillary muscle, and a pledget is added. Figure 22.12b shows the leaflet being drawn down to the papillary head. This maneuver allows the surgeon to tie the knots over the pledget at the appropriate length (solid line A). Once the knots are tied at the appropriate length, the leaflet can be freed (broken line with pointing hand) so that it can assume a new, adjusted position. Figure 22.12c shows the leaflet elevated to its normal position, where the effect of the artificial chordal attachment on leaflet height is assessed.

Fig. 22.12
Figure Credits
-
Figs. 22.1, 22.8, 22.11, 22.12
-
Reproduced from Mainwaring RD, Lamberti JJ. Congenital anomalies of the mitral valve. In: Mavroudis C, Backer C, editors. Pediatric cardiac surgery. 4th ed. Hoboken: Wiley-Blackwell; 2013. p. 640–58. (Reproduced by permission of John Wiley & Sons, Inc.)
-
Figs. 22.2, 22.4–22.7
-
Reproduced from Zias EA, Mavroudis C, Backer CL, Kohr LM, Gotteiner NL, Rocchini AP. Surgical repair of the congenitally malformed mitral valve in infants and children. Ann Thorac Surg. 1998;66:1551–9. (Reproduced with permission from Elsevier.)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag London
About this chapter
Cite this chapter
Mavroudis, C. (2015). Mitral Valve Repairs. In: Mavroudis, C., Backer, C. (eds) Atlas of Pediatric Cardiac Surgery. Springer, London. https://doi.org/10.1007/978-1-4471-5319-1_22
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5319-1_22
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5318-4
Online ISBN: 978-1-4471-5319-1
eBook Packages: MedicineMedicine (R0)