Abstract
From the beginning, the basic idea underlying the development of arterial aneurysm treatment with endoluminal approach was to replace the surgical suture by a different element of fixation of the fabric graft to the arterial wall. Since the introduction of this approach in the early 1990s, the technology has progressed rapidly. The endovascular treatment era started with “home-made” devices, as they were named, and has now improved to third-generation devices that are being manufactured by several medical companies. The evolution of catheter-based methods stemming from percutaneous transluminal balloon angioplasty has made these techniques a viable first-choice option also in the treatment of stenotic disease of the thoracic and abdominal aorta. Based on new devices, improved techniques, and operator skills, a sizable percentage of patients are candidate for treatment with minimally invasive procedures. Improvements in the safety and effectiveness of the endovascular treatment of aneurysmal and stenotic disease of the aorta represent an achievement of which also physicians are responsible and may be justifiably proud. In the present chapter, we will describe the endovascular treatment of aneurysms located in the abdominal and thoracic aorta. Moreover, endovascular treatment of coarctation of the aorta and abdominal aorta stenosis will also be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
From the beginning, the basic idea underlying the development of arterial aneurysm treatment with endoluminal approach was to replace the surgical suture by a different element of fixation of the fabric graft to the arterial wall. Since the introduction of this approach in the early 1990s, the technology has progressed rapidly. The endovascular treatment era started with “home-made” devices, as they were named, and has now improved to third-generation devices that are being manufactured by several medical companies.
The evolution of catheter-based methods stemming from percutaneous transluminal balloon angioplasty (PTA) has made these techniques a viable first-choice option also in the treatment of stenotic disease of the thoracic and abdominal aorta. Based on new devices, improved techniques, and operator skills, a sizable percentage of patients are candidate for treatment with minimally invasive procedures. Improvements in the safety and effectiveness of the endovascular treatment of aneurysmal and stenotic disease of the aorta represent an achievement of which also physicians are responsible and may be justifiably proud.
In the present chapter, we will describe the endovascular treatment of aneurysms located in the abdominal and thoracic aorta. Moreover, endovascular treatment of coarctation of the aorta and abdominal aorta stenosis will also be discussed.
Abdominal Aortic Aneurysm
Indications for Intervention
The main reason to treat asymptomatic patients is to prevent the risk of abdominal aortic aneurysm (AAA) rupture, which brings a high mortality rate. Because mortality in elective AAA repair is dramatically lower than that associated with rupture, the emphasis must be on early detection and elective repair. The risk of rupture is strongly related to AAA size: aneurysms between 5.5 and 5.9 cm in diameter have an annual risk of rupture of 9.4 %, and those between 6 and 6.9 cm have an annual risk of rupture of 10.2 %. The speed of aneurysm growth and the eccentric or saccular shape (which have considerably increased wall stress) are other factors that can influence the rupture risk. ACC/AHA Guidelines for peripheral arterial disease suggest intervention for infrarenal aortic aneurysms larger than 5.5 cm and rapidly expanding aneurysms with lower diameter.
Endovascular Abdominal Aortic Aneurysm Repair
The History
On September 7, 1990, Dr. Juan C. Parodi and his team at the Instituto Cardiovascular de Buenos Aires in Argentina performed the first endovascular AAA repair (EVAR) [1]. The aneurysm was excluded endoluminally with a Dacron graft that was anchored at the proximal infrarenal neck with a stainless steel balloon-expandable stent. The system was assembled by affixing with sutures the fabric tube to an undeployed stent mounted on a large-diameter angioplasty balloon. The system was then sheathed inside a large-bore catheter that served as the delivery system. The access to the aorta for delivery and deployment was obtained through the surgically exposed common femoral artery. This system was the first generation of endografts, the home-made ones, and obtained exclusion and depressurization of the large aneurysm in a patient who had been deemed an unsuitable candidate for standard surgical repair. Two years later, Parodi and Claudio Schonolz performed the first endovascular AAA repair in the United States that ushered in a new era for the interventional vascular practice [2]. Not surprisingly, but unbeknownst to them at the time, they were not alone in these endeavors. The Ukrainian surgeon Nicholas Volodos performed the first endovascular repair of a traumatic thoracic aortic aneurysm (TAA) as early as 1986 [3]. These initial experiences were the driving force and stimulus for the truly explosive growth in interest, creativity, and investment that followed. Indeed, subsequent years have seen a tremendous surge in both the number of endovascular AAA repairs performed worldwide, technological improvements in endograft design, and application of the technique to other vascular beds.
EVAR Devices
Zenith Flex Endovascular Graft (Cook Inc., Bloomington, IN)
The Zenith Flex endograft for AAA with the introduction system H&L-B One-Shot is a modular bifurcated stent composed of Z-shaped self-expandable stainless steel exoskeleton, covered with woven polyester (Fig. 34.1). The main body of the endograft includes the aortic portion and is introduced through the H&L-B One-Shot system. It is provided with a long iliac extremity (ipsilateral) and another short iliac extremity (contralateral), both of which work as a link for the branch extensions. A bare-metal stent is attached to the top of the main body, cephalad to the fabric graft. Multiple steel barbs are welded in a staggered configuration to the bare-metal stent to provide additional aortic fixation.
Talent Abdominal Stent Graft (MedtronicVascular, Santa Rosa, CA)
The device is a self-expanding modular system composed of serpentine-shaped nitinol stents integrated into a woven polyester matrix (Fig. 34.2). The stents are discontinuous and are spaced along a full-length nitinol spine. The latter wire provides columnar strength to a graft that is otherwise flexible enough to accommodate aortoiliac angulations. The spine also prevents twisting and longitudinal infolding of the endograft during deployment. The proximal aortic fixation end has a 1.5-cm uncovered nitinol frame that allows for transrenal fixation of the device. These characteristics may allow increased potential for treating patients with challenging aortic neck configurations [4, 5]. Since its original introduction, the endograft has been modified to include the use of a thinner, low-profile graft fabric to create the Talent Low-Porosity System (LPS). Later, the Talent endograft nitinol springs were chemically treated to enhance durability, and the position of the iliac longitudinal connecting bar was moved from lateral to medial to improve conformability and decrease risk of kinking and thrombosis, resulting in the latest-generation Talent eLPS device. The Talent Abdominal Stent endograft has a 22F delivery system with a hydrophilic coating which does not require a delivery sheath. A 24F delivery system is required for 30 to 36-mm devices and allows treatment of up to 32-mm neck diameters with a neck length of 10 mm and <60° of angulation.
Endurant Stent Graft (MedtronicVascular, Santa Rosa, CA)
This device features a polyester graft material externally supported by an electropolished nitinol stent structure attached to the graft by sutures (Fig. 34.3). An M-shaped proximal stent with anchoring hooks is designed to fix the endograft above the renal arteries. The hydrophilic, low-profile delivery system for the main body measures between 18F and 20F, while for the iliac extensions, the sizes are 14F and 16F. A tip-capture mechanism allows separate control-release deployment of the suprarenal stent. The Endurant endograft is loaded into a low-profile delivery system, with an outer diameter ranging from 18F to 20F for the main body and from 14F to 16F for the extenders. The delivery system features a tip-capture release mechanism to ensure accurate control of suprarenal stent deployment.
AneuRx Abdominal Stent Graft (MedtronicVascular, Santa Rosa, CA)
This is a modular endograft system that utilizes an exoskeleton of 1-cm self-expanding elements (Fig. 34.4). The self-expanding, thin-wall polyester graft material, supported by diamond-shaped elements, supplies high radial force for reliable and secure sealing without barbs, hooks, or balloons. To date, the AneuRx Abdominal Stent Graft has undergone eight modifications since the original clinical trial. The first-generation device consisted of a stiff-body design with a pre-reduced porosity graft material and a bullet delivery system. In 1998, the endograft flexibility was increased by changing the body of the endograft from a single 5-cm nitinol stent to a series of 1-cm diamond-shaped rings. Furthermore, the graft material was changed to a reduced porosity material. In 2002, the delivery system was changed to the Xpedient Delivery System with a tapered nose cone, allowing the device to be placed without a sheath. In 2004, the graft material was again changed to the Resilient graft material, which was associated with the greatest amount of sac shrinkage as compared to other contemporary graft materials [6]. In 2005, the Xcelerant Delivery System was added, allowing for easier deployment of the endograft. In 2006, the AneuRx AAAdvantage Stent Graft was offered, which added an extended aortic body of 4 cm, contoured stent rings, longer, larger, and flared iliac limbs to decrease the number of components required for repair and enhanced radiopaque markers. Finally, in 2008 a hydrophilic coating was added to the delivery system. Currently, the AneuRx AAAdvantage Stent Graft has a 21F delivery system and may be used to treat up to 26-mm aortic neck diameters that are 15 mm in length with <45° of angulation.
Excluder Aortic Endograft (W.L. Gore & Associates, Flagstaff, AZ)
This device is a modular system covered with expanded polytetrafluoroethylene (ePTFE) and supported by a nitinol stent frame (Fig. 34.5). The endograft material is bonded to the nitinol frame and thus has no suture holes in the material. The microstructure in the original device permitted selective permeability of serous fluid in a subset of patients that could be associated with endotension [7, 8], but with the addition of a non-permeable, very thin, and highly durable fluoropolymer layer, this drawback was overcome [9, 10]. The endograft consists of a bifurcated main body with a single docking limb and assorted iliac limbs. Iliac and proximal extenders are also available. The main proximal portion of the main body has a scalloped end that is entirely covered with paired nitinol anchors for fixation set at 45°. There is no mechanism for suprarenal fixation. The proximal edge is identifiable by three gold markers designed to be placed immediately below the most inferior renal artery. The main body of the device is contained in an ePTFE jacket that is stitched with a single strand of ePTFE string. This is the mechanism for deployment: the string is pulled and the stitches are released, causing endograft deployment (proximal to distal). Rotational orientation is facilitated by different length markers on the ipsilateral and contralateral endograft sides. In the new C3 Delivery System, deployment is now a three-step maneuver, including the option of re-constraining and repositioning the device. If the position is believed to be too high or too low with regard to the renal arteries, the device can be easily adjusted to reach the ideal final location. Similarly, reorientation of the contralateral gate is possible, which makes cannulation easier and less time consuming. Currently, introducer sheaths are available in 30-cm length as 12F, 18F, and 20F sheaths. Main trunks with ipsilateral leg are available in 23-mm, 26-mm, and 28.5-mm diameters with 12-mm and 14.5-mm iliac diameters. The endoprosthetic length ranges from 12 to 18 cm in 2-cm increments. The recommended aortic neck diameter ranges from 19 to 28 mm. The ipsilateral iliac treatment diameter ranges from 10 to 13.5 mm. All these devices can be delivered via the 18F sheath except for the 31-mm endograft, which requires the 20F sheath. The contralateral endograft ranges from 12 to 20 mm in 2-mm increments and is available in 9.5-cm, 10-cm, 11.5-cm, 12-cm, 13.5-cm, and 14-cm length increments. The larger diameter contralateral leg endograft (16, 18, and 20 mm) can also be used as iliac extenders. The Excluder device contains flared iliac extenders to attach to aneurysmal iliac vessels. Dedicated iliac extenders range from 10 to 14.5 mm and are available in 7-cm length increments. The recommended iliac treatment diameters range from 8 to 18.5 mm. All may be delivered via the 12F sheath except for the 16-mm to 20-mm endograft, which requires an 18F sheath. Aortic extenders range 23, 26, and 28.5 mm and are available in 3.3-cm length increments. Their recommended aortic neck diameter is 19 to 26 mm in the United States, and a range of 19 to 29 mm has been used outside the United States.
Anaconda AAA Stent Graft System (Vascutek, Scotland, UK)
The system is tri-modulare and is composed of a bifurcated body to which the ipsilateral and contralateral branch may engage (Fig. 34.6). A wide range of bodies and bifurcated iliac branches allows to treat patients with an aortic neck from 17.5 to 31 mm and iliac arteries from 8.5 to 21 mm. The tri-modulare system allows adapting the stent to the size and characteristic anatomy of each patient. The flexibility of the body is allowed by the fact that distally to the two rings of nitinol for the proximal anchorage, the prosthesis is supported by two other very flexible rings, which, while supporting the bifurcated body, will not impair flexibility. The end of the contralateral side has a thin support of nitinol and radiopaque markers to ensure an open and visible lumen during the procedure of cannulation. The iliac branches, straight and/or flared, are equipped with support rings and radiopaque markers for the correct positioning inside the main body. The rings of support are varied but independent of one another so as to ensure an excellent flexibility to the iliac branches and prevent kinking. The iliac branches can be used to treat isolated aneurysmal iliac arteries. The flexibility characterizes also the introduction and release system that allows placing the stent in vessels anatomically very tortuous. The proximal part of the aortic bifurcated body has two rings of nitinol supported by four pairs of hooks that ensure safe anchorage of the device.
AFX Endovascular AAA System (Endologix, Irvine, CA)
This Endovascular AAA System integrates anatomical fixation with an advanced delivery system and graft material technology (Fig. 34.7).
Incraft Stent Graft System (Cordis Corporation, Florida, USA)
This new system was designed so that a few sizes will fit most vessels with respect to both diameters and length (Fig. 34.8). The modular components can be tailored during the implant procedure, with “in situ length adjustment” of the limb prostheses. This permits up to 2–3 cm of variability that can be achieved by varying the modular overlap length on each side reducing the risk of inadvertent coverage of the hypogastric orifice and, at the same time, allowing the maximum length of iliac fixation for migration prevention. The endograft design provides appropriate radial force over a relatively large range of diameters, so that four sizes can be used for aortic diameters ranging from 17 to 31 mm. Similarly, five different iliac limb diameters will accommodate iliac arteries ranging from 7 to 22 mm in size. Each iliac limb is supplied in four distinct lengths ranging from 8 to 14 cm, allowing a total aortoiliac coverage length between 13 and 21 cm. Therefore, a combination of 23 different modular components allows the operator to customize the device to the wide variety of AAA anatomy, with a significant reduction in the complexity of device selection, which is so critical in emergency settings. The Incraft device has a flared bare transrenal stent with 8–10 laser-cut barbs (size depending) located on the most cranial part of the stent for suprarenal fixation. The transrenal stent, the short segmented sealing infrarenal endoskeleton stent and the non-tilting deployment mechanism maximize the proximal seal, especially in the presence of a challenging aortic neck. The endograft is constructed of a seamless, woven polyester material supported by a series of laser-cut nitinol stents throughout its entire length. Such a design was chosen for kinking prevention, which has been associated with limb thrombosis. The Incraft stents are attached to the graft with a unique suture scheme to minimize micromotion between the stent and graft component, a mechanism believed to have a negative impact on endograft integrity with prior devices. Finally, one of the most innovative features of the device is the ultra-low profile: the integrated delivery system is 13F with a 14F (4.7 mm) outer diameter. The “catheter-like” flexibility and the elimination of sheath exchange minimize delivery problems, particularly in patients with small, diseased, and otherwise challenging access anatomy.
Selection Criteria for EVAR
General accepted guidelines for EVAR include at least 10–15 mm of non-aneurysmal proximal neck, proximal neck diameter less than 30 mm, adequate access vessels (femoral/iliac arteries) at least 7 mm in diameter, minimal vessel calcification and thrombus, and a neck–body aneurysm angle ≤60°. The 2005 Excluder instructions for use recommend the following selection criteria: adequate iliac/femoral access, infrarenal aortic neck diameter ranging from 19 to 26 mm, minimum aortic neck length of 15 mm, proximal aortic neck angulation ≤60°, iliac artery treatment diameter ranging from 8 to 18.5 mm, and iliac distal vessel seal zone of at least 10 mm. Device delivery may be hindered by excessive tortuosity, calcification, or occlusive disease of femoral or iliac arteries. Additional considerations relate to the adequacy of collateral flow for occluded branches, such as an indispensable inferior mesenteric artery or accessory renal artery. The most common obstacle for EVAR is inadequate proximal anatomy. Self-expanding stents may be used more frequently to treat AAA with neck dilatation compared with balloon-expandable stents. An analysis comparing Excluder and the balloon-expandable Lifepath found this factor to be correlated with a higher rate of device migration seen in the Excluder device in this series [11]. A review from the EUROSTAR database analyzed 1,152 patients treated with EVAR having severe neck angulation (>60°) with Excluder, Talent, or Zenith endografts [12]. All three had acceptable outcomes in patients with severe neck angulation, with the Excluder and Zenith having increased risk for short-term type I endoleak and device migration and the Talent device having increased long-term risk of type I endoleak and secondary intervention. Favorable anatomic criteria allow use of any of the current commercially available endografts for EVAR. However, it has become apparent that certain features of proximal neck diameter, length, and angulation, or iliac anatomy characteristics favor some devices over another. The low-profile delivery is one such example. Also, the scalloped proximal edge makes precision delivery possible for tight proximal anatomy. A unique characteristic of the Excluder is its rapid deployment mechanism. This rapid deployment of a self-expanding device with proximal fixation in the setting of tortuosity and path rotation may lead to unexpected shortening and a more distal position than expected in inexperienced hands. Potential consequences of unexpected distal migration could include inadvertent hypogastric artery occlusion. Whittaker et al. found that this event occurred in 11 % of limbs studied with a shortening of graft path 1 cm or more [13]. Moreover, they noted that graft path shortening was affected more by anatomic factors than by Excluder device factors. Graft rotation was the most difficult pattern to predict preoperatively. Most patients will be assessed with three-dimensional reconstruction of multi-detector CT angiography (MDCTA). This has replaced previous modalities such as “road-map” angiogram or marker catheter as the best method for pre-intervention planning. Features such as tortuosity and rotation require special consideration in preoperative planning. Whittaker et al. reported that three-dimensional computer-aided measurement, planning, and simulation software was very accurate for preoperative planning with the Excluder device and more accurate than using marker catheter angiography [14]. Availability of several commercial devices with different properties with regard to proximal fixation, deployment accuracy, endograft flexibility, and size of introduction system enables to tailor the device selection according to each patient’s AAA anatomy. Zenith endografts were mainly used for short proximal necks in view of the suprarenal fixation of the bare stent with hooks and barbs. In addition, in view of the versatility of the Zenith Tri-Fab system in which the length of both limbs can be chosen after insertion of the main body, the endograft may be appropriate also in aneurysms where accurate length measurement proves difficult (e.g., angulation or short common iliac arteries). This feature is shared also by the Incraft Stent Graft System. The Talent endografts were initially used for proximal necks with a large diameter, but this advantage was lost when the Zenith equally featured larger proximal diameter sizes. Excluder endografts were preferred in patients with narrow and angulated iliac arteries because of flexible and thinner wall limbs. In July 2004, the Excluder underwent an important modification with the introduction of a lower porosity graft material. The Talent prosthesis was recently replaced by Medtronic with the Endurant. This new prosthesis, always with suprarenal attachment, has greater flexibility and conformability in the aortic vessel and requires thinner, dedicated introducers.
EVAR Procedure
The deployment technique for a two-piece modular device involves passage through one femoral artery of a sheath containing a body and the ipsilateral iliac limb. The superior end of the body is positioned just below the lowermost renal artery. The contralateral iliac limb is inserted through the opposite femoral artery and is overlapped with a short stump in the body of the device. The end result is a percutaneous Y-graft, with some important differences as compared to surgical grafts: the attachment sites in the infrarenal aortic neck, iliac arteries, and within the endograft are not sutured, and the aortic side branches are not ligated. Since the attachment sites are not hand sutured, they rely on the radial force of the stents to provide a hemostatic seal. Fixation at the attachment sites is also important to prevent endograft migration. Some manufacturers achieve this by adding hooks or barbs on the infrarenal stent. Some devices incorporate a bare suprarenal stent with or without hooks. This suprarenal stent may extend as high as the superior mesenteric artery.
Access-Related Issues
EVAR requires adequate vessel size in order to place the device and to exclude the aneurysm. Indeed, small external iliac arteries, vessel tortuosity, and heavily calcified vessels with aortoiliac occlusive disease make EVAR more difficult. For each of the currently available devices, the external iliac and common iliac arteries need a minimum diameter of 7 mm for the main device. Certain anatomical conditions will make access more difficult, but certain technical maneuvers can overcome these conditions.
Femoral Artery Access
Standard femoral artery access is achieved via a transverse or longitudinal incision. Use of small transverse incisions just below the inguinal ligament may be beneficial during EVAR. With this exposure, dissection just below the inguinal ligament gains access to the common femoral artery, typically in a relatively soft area of the artery. Furthermore, the femoral bifurcation is avoided, making control of the femoral artery easier and avoiding dissection of the profunda and superficial femoral arteries. When using this approach even in patients with heavily calcified vessels, it is usually possible to find a soft spot on the anterior wall of the femoral artery just distal to the inguinal ligament that can be used to puncture the artery. If the vessel is still heavily calcified, the inguinal ligament can be divided to allow access to the very distal external iliac artery. Once the femoral artery is dissected out, proximal and distal control of the vessel is obtained with vessel loops. Wire access is then obtained using the standard Seldinger technique. There is growing experience with a truly percutaneous approach using a technique, often referred to as the “pre-close technique.” With this technique, sutures are percutaneously placed before the insertion of the sheath and are used to close the femoral entry site at the end of the procedure. Using ultrasound guidance to confirm access to the common femoral artery, the artery is accessed and a 7F sheath is placed. A femoral arteriography is performed to confirm the sheath is within the common femoral artery. The 7F sheath is removed, and either a single 10F Prostar (Abbott Vascular, Santa Clara, CA) or two 6F ProGlides (Abbott Vascular, Santa Clara, CA), the second 45°–90° to the first, can be advanced over the guidewire with the sutures deployed within the vessel wall. Once the sutures are deployed, guidewire access is regained through the rapid-exchange port of the device. The suture device is then removed, and either an 11F or 16F sheath can be placed for hemostasis. This technique can be used with any of the currently available devices. When first learning this technique, it is best to avoid small, calcified vessels, as well as extremely tortuous vessels. A fully percutaneous procedure presents some advantages: less bleeding, reduced risk of femoral nerve injury, faster recovery, and, in most cases, earlier ambulation.
Aortic Angiogram
After femoral access is achieved, a marker pigtail angiography catheter is inserted into the sheath and advanced over a J-tipped guidewire up to the suprarenal aorta under fluoroscopic control. The guidewire is removed, and an aortogram is obtained with a pressure injector and recorded on the fluoroscopy screen with road-mapping technology. Aortic angiography with the marker catheter, which has radiopaque markers at 1-cm intervals, allows accurate measurement of the distance between the point of proposed placement of the proximal attachment system (below the lowest renal artery) and that of the distal attachment system (above the ipsilateral hypogastric artery). This serves to confirm preoperative MDCTA measurements and to assure that the appropriate endograft length had been selected.
Endograft Positioning and Deployment
Once guidewire access is obtained, before placing any large sheath or device, it is best to have a stiffer guidewire in place for the sheath and device to track. Stiff guidewire that are available to straighten out the vessels include the Amplatz Super Stiff (Cook Medical, Bloomington, IN), the Meier Wire (Boston Scientific Corporation, Natick, MA), and the Lunderquist (Cook Medical, Bloomington, IN). In vessels with extreme tortuosity and calcification, even advancing a stiff guidewire through a catheter can be difficult, with the stiff guidewire not advancing and the catheter coming back. This can sometimes be overcome with the use of a “buddy guidewire.” Initially, if a stiff guidewire does not pass through a catheter, a stiff Glidewire (Terumo Interventional Systems, Somerset, NJ) can be passed though the catheter, which will usually track easily. Leaving the stiff Glidewire within the catheter, a second guidewire – typically a Glidewire – can be placed within the sheath and into the descending thoracic aorta. A catheter is then advanced over the Glidewire. With the stiff Glidewire and catheter already in place, a stiffer guidewire, such as an Amplatz Super Stiff or a Lunderquist guidewire, can be advanced through the second catheter to straighten out the external iliac artery for placement of the device. Gradually increasing the size of small vessels bypassing dilators can be an adjunctive maneuver besides using a stiff guidewire for device placement. In some patients with severely diseased external iliac arteries or iliofemoral vessels of inadequate size, a conduit end-to-end sutured to the common iliac artery can be used to obtain adequate access for placement of the endograft. A 10-mm Dacron graft allows for passage of all devices. This can be extremely beneficial in preventing damage and/or rupture of the external iliac artery. Once the endograft sheath is introduced over the stiff guidewire and advanced under fluoroscopic control up the iliac segment and well into the aneurysm, the patients receive 5,000 IU intravenous heparin. With the guidewire left in place, the angiography catheter is removed, and the endovascular deployment assembly (trunk-ipsilateral component) is prepared for insertion into the sheath. The assembly is loaded over the guidewire and advanced under fluoroscopic control up the introducer sheath to the aortic position. The introducer sheath, initially advanced into the aorta, is backed into the external iliac artery, which permits the endovascular prosthesis portion of the assembly to be positioned within the aneurysm. The proximal attachment system is positioned just below the renal arteries, the distal attachment system is positioned in the common iliac artery, and the trunk-ipsilateral component of the endograft is deployed. In order to maximize and improve proximal fixation, there are some maneuvers that can be used. The first of these is magnification views and appropriate angulation of the fluoroscopy unit. Typically, adjustment of the image intensifier in the caudal direction will open up the infrarenal neck and show its true length. Typically, the infrarenal aortic neck begins to angle anteriorly following the course of the lumbar spine. Adjusting the image intensifier 10º to 20º is usually sufficient to open up the proximal neck. After guidewire cannulation of the contralateral “gate,” the contralateral leg of the endograft is then inserted in the opposite common femoral artery and advanced until overlap is obtained on the short contralateral leg of the aortic endograft placed in the abdominal aorta. An elastomeric balloon is then used to secure the endograft to the proximal aortic neck. This step is usually performed during cardiac pacing at high frequency to reduce blood pressure and to avoid that the displacing forces of the high aortic flow may push the inflated balloon causing downward migration of the endograft from its position. New balloons, such as the Tri-Lobe (Fig. 34.9), allow blood flow through the device itself and do not require temporary pacing. A pigtail angiography catheter is then reinserted over the guidewire into the suprarenal aorta, and a completion angiogram is obtained. Technical success is achieved when there has been successful access to the arterial system using a remote site, successful deployment of the endograft with secure proximal and distal fixation, absence of either a type I or type III endoleak (see complications), and a patent endograft without significant twist, kinks, or obstruction by intraoperative measurements [13–15]. The sheath and guidewire are removed, and the access site is sutured or hemostasis is obtained with the pre-close technique.
Vascular Anatomy Issues
Proximal Aortic Neck
It has been demonstrated that placing the endograft as close to the renal arteries as possible at the original implantation significantly decreases the risk of migration. There are certainly some conditions that are going to increase the risk of migration or proximal type I endoleaks. These risks include a short angled neck as well as a reverse funnel neck. Currently, all devices are approved for use with a proximal neck length of 15 mm except the Talent abdominal endograft, which is approved for use with a 10-mm neck. The technique and precision of device implantation, along with patient selection are significant factors in predisposing patients to subsequent adverse events in the future, especially migration. Migration has been reported with all devices with an incidence between 2.3 and 9.5 % in clinical trials with a follow-up of 1–4 years [16–19]. When evaluating device-specific outcomes, Ouriel et al. found no significant difference among various devices regarding the risk of migration, which ranged from 0 % with the Talent and Ancure to 8.2 % with the Zenith [18]. In a more recent study, Abbruzzese et al. evaluated 177 Zenith, 111 Excluder, and 277 AneuRx endografts [19]. In the study, 39.3 % of devices were placed outside of at least one of the instruction for use parameters. Mean follow-up was 30 ± 21 months and was significantly shorter for the Zenith (20 months) compared to the Excluder (35 months) and AneuRx (31 months), respectively. Overall actuarial 5-year freedom from aneurysm-related death, reintervention, and graft-related event rates were similar among the three devices.
Iliac Artery Aneurysms
In patients with aortoiliac aneurysms, there are three options available to the implanting physician. For ectatic iliac vessels, use of flared limbs has simplified the repair of aneurysms with large common iliac arteries. Flared limbs as large as 24 mm allow vessels up to 20–22 mm to be safely treated without the need of coil embolization of the internal iliac artery. This is possible with both the AneuRx AAAdvantage and Talent abdominal endografts. However, when using these flared limbs, it is still important to try to achieve between 20 and 25 mm of seal to prevent a retrograde type I endoleak. With aneurysmal common iliac arteries, it is usually safer to occlude the hypogastric artery by coil embolization or vascular plug deployment and bring the endograft into the external iliac artery (Fig. 34.10).
Embolization of the hypogastric artery before EVAR. (a) Deployment of an Amplatzer vascular plug (arrow) in the right hypogastric artery. (b) MDCTA (multiplanar reconstruction) showing final result after EVAR. Note the deployed Amplatzer vascular plug (arrow) with complete occlusion of the right hypogastric artery and the endograft leg that was brought into the external iliac artery
Complications Following EVAR
Migration
As the attachment sites are not sutured, there may be movements at the aortic or iliac attachments or at points of connection within modular endografts. Device migration is defined as any report of postprocedure device movement. These migrations include reports of migrations of the trunk-ipsilateral, contralateral leg, and extender components. It is well known that preprocedure planning, which includes appropriate patient anatomical measurements and device selection, will minimize migration events. Even when placing the device as close to the renal arteries as possible during initial deployment, there is continued risk of migration, especially if there is a short aortic neck, significant neck disease, including thrombus and calcification, neck angulation, and adverse neck contour, such as a reverse funnel neck [18–21]. However, it is important to remember that endograft relies on three points of fixation, including the proximal aortic neck and the right and left common iliac arteries. In vivo animal studies have shown that by maximizing the distal iliac fixation, the amount of force required to displace the endograft is increased by 67 %. This has been demonstrated with both infrarenal and suprarenal devices with no significant improvement when hooks were present [22–25].
Endoleaks
The term endoleak was first used in 1996 and defines the persistent perfusion of the aneurysm sac after EVAR due to incomplete sealing. The presence of an endoleak implies failure to exclude the aneurysm or a vessel segment. A leak can appear during the first 30 days after implantation. This type of leak is called “primary endoleak.” “Secondary endoleak” is one that occurs after 30 days. Persistent blood flow within the aneurysm sac eventually may result in aneurysm rupture despite endovascular repair. Review of EUROSTAR data suggested that the presence of an endoleak can predict rupture, since 69 % of patients whose aneurysm ruptured following EVAR had a preexisting endoleak [26]. The incidence of endoleak varies from 10 to 50 %, and its treatment depends on the type, site, and size of the endoleak.
Endoleak Classification
The anatomic classification of endoleaks is based upon the source of the inflow into the aneurysm sac, regardless of the number and type of other vessels involved in the outflow. The initial definition differentiated between four types of endoleak, but it was expanded in 1999 to include endotension (type V) (Table 34.1).
Type I
These endoleaks are caused by failure of the circumferential seal at the fixation points of either the proximal (type IA) or distal (type IB) end of the endograft. Type IC endoleak is due to non-occluded iliac artery in patients with aorto-mono-iliac grafts and fem–fem bypass. With type I endoleaks, the aneurysm is perfused directly from the aorta or iliac arteries (inflows). These leaks usually communicate through a channel (sometimes multiple channels) with the aneurysm sac. There are several outflow vessels, mainly lumbar arteries and inferior mesenteric artery that communicate with the channel and or the sac. The pressure within a type I leak is systemic. The tension on the aortic wall remains high. Causes of primary type I endoleaks include inappropriate anatomy, such as a significantly angulated neck, severe calcification/plaque at the proximal or distal landing zones, a noncircular landing zone, endograft malpositioning and under-dilation, and endograft type. Secondary type I endoleaks can be due to aneurysm remodeling, resulting in endograft migration, progressive dilatation of the proximal neck, design and dimensions of endograft, or unfavorable infrarenal necks, including the conically shaped neck and neck shorter than 15 mm. Treatment is mandatory because the aneurysm sac is at systemic pressure. Untreated type I endoleaks are at high risk of aneurysm rupture. Indeed, a rupture rate of 3.37 % has been reported [27]. Treatment requires satisfactory circumferential apposition between the endoluminal surface of the vessel and the external aspect of the endograft. Gentle expansion of the device with a compliant “molding” balloon will seal the majority of primary type I leaks. Occasionally, despite prolonged molding, the leak will persist. A giant Palmaz stent may then be used to prevent recoil and improve stent apposition to the proximal neck. If this procedure fails and the leak is significant, a limited laparotomy will enable an external band to be placed around the proximal neck, without opening the aneurysm sac. Open conversion is occasionally required and was more frequent in the past for very large endoleaks due to the migration of home-made devices. If the endoleak is due to endograft malposition or moderate distal migration, the endograft is extended to increase the length of neck contact or the distal seal in the case of a leaking limb. Hence, a proximal aortic cuff or a graft extension limb may be required, and these are generally oversized by 10–20 % to prevent further endoleaks.
Type II
This type of endoleaks corresponds to the retrograde filling of the aneurysm, mainly from lumbar arteries and/or inferior mesenteric artery but also, in rare situations, from sacral, gonadal, accessory renal artery arteries, or iliac artery branches (Fig. 34.11). The leak always communicates with another “outflow” vessel. These are the commonest endoleaks, affecting up to 43 % of cases, and may be associated with aneurysm expansion and rupture. However, this risk is much less than with the type I and III endoleaks (0.5 % vs. 3.4 %) [26, 28]. Of note, they are associated with a significant rate (up to 40 %) of spontaneous closure. The current consensus is that a type II endoleak in the setting of a shrinking aneurysm can generally be followed without the need of immediate intervention. Treatment is required only for endoleaks that persist for more than a year in an aneurysm of increasing size. Intervention is usually by percutaneous embolization, either by a transarterial or a translumbar route. Percutaneous CT-guided or ultrasound-guided thrombin injection in appropriate patients can be a useful treatment option and should be considered when the more conventional methods are difficult [29, 30]. In some instances, coil or plug embolization of feeding vessels before endograft deployment is a viable option for type II endoleak prevention [31] (Fig. 34.12).
Follow-up angiography after EVAR in a patient with type II endoleak. (a) Early-phase subtraction angiography showing patency and correct position of the endograft. (b) Selective angiography of the feeding collateral vessel (arrow) originating from the external left iliac artery. (c–d) Late-phase subtraction angiography showing the endoleak (arrows)
Type III
These endoleaks are caused by structural failure of the endograft due to separation of its modular components (type IIIA) or a tear in the graft material (type IIIB) (Fig. 34.13). As in the case of type I endoleaks, type III endoleaks represent direct perfusion of the aneurysm sac at systemic pressure and have a relatively high rupture rate. Primary type IIIA endoleaks are readily apparent during the initial procedure, but it can be difficult to differentiate them from type I endoleaks. They are usually caused by inadequate overlapping of the junctional sections of a modular endograft. Rarely, the cause may be defects in the graft material. Primary type IIIA leaks almost always resolve after balloon molding of the junction(s). If there is insufficient overlap of the components, an additional component may be required. If the endoleak is from the main body of the endograft or at the flow divider, a second endograft should be deployed within the first. Use of an aorto-mono-iliac device and a surgical fem–fem crossover helps to reduce the total amount of graft material. If no suitable graft is available, open surgical conversion must be considered. Delayed type IIIA endoleaks were seen commonly in the first-generation devices due to a short overlap between the main body and the limb [32]. They are generally due to sac remodeling, causing the graft components to separate, or to mechanical stress causing stent and fabric failure. They are very dangerous, since there is acute repressurization of the sac, and should be treated aggressively as primary type I leaks, accepting that there is a significant open conversion rate [26].
Type IV
Type IV endoleaks are caused by porosity of the endograft fabric during the primary procedure and are seen at the time of device implantation, as a faint blush on the postimplantation angiogram, when patients are fully anticoagulated. They were reported almost exclusively with the first-generation devices and seal spontaneously. Rupture has not been reported.
Type V
This type of endoleak is caused by continued expansion of the aneurysm sac in the absence of an endoleak visible on conventional imaging. The term “endotension” has also been coined for this phenomenon, which is thought to reflect the continued pressurization of the aneurysm sac. It is important to exclude the presence of a subtle endoleak by further investigation such as contrast-enhanced ultrasound. If found, this needs to be treated as described previously. In some cases, the expansion may be due to ultrafiltration through first-generation ePTFE endografts, resulting in the formation of a hygroma (Fig. 34.14), or to tiny endoleaks through suture holes in the endograft material. Sac expansion due to ultrafiltration of serous material can be left untreated even if rupture occurs. However, the inside of the endograft can be relined with a second device [33], and open surgical conversion remains a viable option (Fig. 34.15).
Operating room picture during open surgical conversion (Same patient of Fig. 34.14). Note the proteinaceous material deposited inside the aneurysm sac indicative of hygroma formation
Endoleak Diagnosis
Contrast MDCTA is considered the imaging technique of choice for endoleak detection. Indeed, MDCTA is reported to be superior to aortography for the demonstration of small leaks. However, selective angiography is superior to MDCTA for the detection of inflow and outflow vessels (Fig. 34.11b). Deceitful MDCTA images, including calcifications, contrast within the folds of unsupported portions of the endograft, and residual contrast inside the aneurysm sac from the initial procedure may erroneously suggest the presence of endoleaks when noninvasive angiographic follow-up is obtained within 1–3 days after implantation. Of note, “pseudo-endoleaks” are seen in up to 57 % of patients.
Color Doppler ultrasound (CDUS) is a noninvasive and cost-effective imaging modality that can be used for endoleak assessment. It is highly dependent on the operator and has limitations in obese patients and in those with excessive bowel gas. Patients should be evaluated after 5–6 h fasting, in the supine and lateral position. The aorta is evaluated both transversally and longitudinally. A leak is suspected when a reproducible color and Doppler signal inside the aneurysm is visualized. Variable success is reported for the detection and localization of the source of endoleaks with ultrasound, depending on technical factors, imaging protocol, and image quality. Overall, the reported sensitivity for endoleak detection ranges between 12 and 100 %, with specificities ranging between 74 and 99 % [34]. Although these noninvasive techniques are reliable in detecting an endoleak, the characterization and type of endoleak, as well as endovascular treatment planning can still be difficult.
Structural Failure and Graft Distortion
Metals subjected to aortic pulsation pressures may fracture. The iliac limbs of the device may also become distorted and angulated within the residual space of the aneurysm.
Non-endoleak-Related Complications
These adverse events during or after EVAR can be categorized as complications owing to surgical exposure of the access arteries or the percutaneous approach, ischemic complications owing to intentional or inadvertent clot embolization or covering of an aortic side branch, stenosis or occlusion of an endograft limb, infection of the endograft or aneurysm sac, and contrast-induced nephropathy.
Local Wound Complications in the Groin
Local wound complications following surgical exposure of the access arteries include groin hematoma, infection, or lymphocele. Their reported incidence is 1–10 % [35]. In some cases, CDUS or MDCTA evaluation may be needed to evaluate the extent of the lesion. Clinical surveillance with or without medical treatment or surgical repair are mostly enough for definitive treatment.
Access Artery Injury
Arterial thrombosis, dissection, or pseudoaneurysm formation can occur in up to 3 % of EVAR procedures. Besides correct surgical and interventional skill, a thorough preoperative CDUS or MDCTA evaluation of the common femoral and iliac arteries with special attention to access vessel diameter, tortuosity, and degree of calcification is mandatory. The introduction of a stiff and large catheter system in the presence of small and heavily calcified iliofemoral arteries can induce vessel wall dissection or even perforation. In case of a postprocedural groin pseudoaneurysm, CDUS-guided thrombin injection is not always successful as the pseudoaneurysm neck can be too large, making surgical repair the only definitive treatment option.
Ischemic Complications
Ischemic complications may occur immediately after EVAR. They can be due to thrombus formation and embolization into aortic side branches and include colonic, renal, and pelvic ischemia. Another cause of ischemia is endograft misplacement and partial or complete covering of an aortic or iliac side branch, resulting in renal or pelvic ischemia.
Colonic Ischemia
Bowel ischemia occurs in 1–3 % of cases after open aortic aneurysm repair, and the incidence seems to be in the same range for EVAR [36, 37]. Postoperative bowel ischemia after aortic aneurysm repair still remains a serious complication with a mortality rate of 50 % within 1 month. However, the pathophysiological mechanisms of colonic ischemia most probably differ in open versus endovascular repair. Whereas interruption of the inferior mesenteric or iliac arteries has been suggested to be the cause of bowel ischemia in open procedures, the same mechanism does not seem to be important for EVAR. Zhang et al. suggested that thrombotic deposits and atheroma in the suprarenal aorta may play a role in major bowel ischemia in EVAR [38]. Thrombus and atheromatous material may be dislodged while the proximal part of the endograft is being positioned, deployed, and balloon dilated just below the renal arteries. These maneuvers can induce upstream flushing of mural clot or atherosclerotic debris into the superior mesenteric artery. They may also migrate into the renal, inferior mesenteric, and internal iliac and lower-limb circulation resulting in segmental, skipped, or patchy ischemia of the embolized areas. This embolization mechanism may also explain why bowel ischemia after EVAR mostly presents as multifocal patchy ischemia. This type of bowel ischemia is not seen after open repair, most probably because suprarenal aortic and ostial inferior mesenteric arterial clamping during surgery makes distal embolization unlikely. These observations underline the importance of a careful preprocedural evaluation of the proximal aneurysmal neck and the need to identify thrombus and atheroma that may make patients poor candidates for EVAR.
Spinal Cord Ischemia
Spinal cord ischemia after EVAR is very rare. Indeed, in the EUROSTAR database, an incidence of 0.21 % in 2,862 patients has been reported [39]. The mechanism is not completely understood, but atheromatous embolization and interruption of collateral circulation from lumbar and internal iliac arteries together with a variable anatomy of the artery of Adamkiewicz seem to be the most important contributing factors. The treatment is the same as for paraplegia after thoracoabdominal EVAR or open repair and consists in cerebrospinal fluid drainage and, if indicated, recanalization of occluded collateral arteries like the internal iliac artery [40–44].
Renal Artery Occlusion
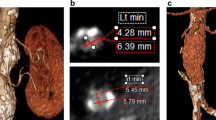
Inaccurate endograft placement with partial or total coverage of one or both renal arteries occurs in less than 5 % of cases [45] and may be due to lack of high-quality imaging technology for guiding the EVAR procedure and limited experience of the endovascular team. In their early learning curve with EVAR, Kalliafas et al. described renal artery occlusion in 5 of 204 patients, resulting in renal failure with chronic hemodialysis need in two of them [46]. If misplacement is detected during EVAR, a bailout attempt can be made, using a pull-down maneuver with an inflated angioplasty balloon or by tugging caudally on a guidewire placed across the endograft bifurcation and exteriorized from both femoral arteries. Using this technique, Görich et al. could move the stent graft from 5 to 27 mm more distally [47]. It is noteworthy that these authors did not use this bailout technique on endografts with barbs at the proximal part of the suprarenal stent. Finally, in case of partial coverage of one renal artery, stent placement in the renal artery with slight protrusion of the stent over the proximal part of the endograft into the aorta can also be used to manage this complication [48]. Rarely, renal ischemia may also occur when the top of the endograft main body needs to be placed close to the main renal artery in the presence of an accessory renal artery originating from the infrarenal neck (Fig. 34.16).
Volume-rendering MDCTA (a) and invasive angiography (b) showing a left accessory renal artery (arrows). (c) The endograft main body was deployed close to the main renal artery occluding the accessory renal artery (arrow), and (d) multiplanar reconstruction MDCTA performed 3 days after EVAR showed infarct of the lower renal pole (arrow)
Early and Late Limb Occlusion After EVAR
Endograft limb thrombosis is a known complication of EVAR, especially in unsupported endografts. Indeed, it can occur in as many as 40 % of cases treated with this type of devices [49, 50]. The underlying mechanism is most frequently kinking of the unsupported endograft limb. Second-generation supported endografts perform significantly better with regard to limb thrombosis and have shown a significantly lower rate of limb occlusion, ranging between 0 and 5 % [51]. Most of the limb thrombotic events occur within the first 2 months after EVAR, and the underlying causes are endograft kinking and extension of the small-diameter endograft into the external iliac artery [52, 53]. Recently, it has been demonstrated that limb occlusion may also occur 4–5 years after EVAR [54]. The mechanism of late limb occlusion can be migration and dislocation of an endograft component causing major hemodynamic turbulence and, eventually, limb or entire endograft thrombosis. Treatment of limb occlusion includes various surgical and endovascular revascularization techniques. The best treatment option depends on the patient’s general status as well as on specific endograft and excluded aneurysm changes.
In contrast to limb thrombosis, incidentally found mural thrombotic deposits are much more frequent in both first-generation endografts (20 %) and second-generation supported endografts (17–33 %) [55]. A circumferential layer of thrombotic material has been observed more in the Zenith endografts than in the Excluder endografts, but they are clinically silent and are not associated with potential endograft thrombosis or distal embolization (Fig. 34.17). Additionally, there is no difference in survival among patients presenting with mural deposits in their endograft compared with patients without this thrombotic layer. Based on these observations, additional treatment in the form of relining the endograft with another endograft or administering any type of anticoagulant therapy cannot be recommended.
Infection
The incidence of endograft infection is 0.5–1 %. If untreated, infection can result in generalized sepsis and death [56]. There are multiple causes of this complication. Endograft contamination during EVAR seems to be the source of early infection. Secondary infection from a remote source is another pathophysiological mechanism. van den Berg et al. reported an endograft infection following septic complication of a kidney stone 1 year after EVAR [57]. Another case of endograft infection after EVAR has been reported in a patient who underwent an appendectomy for appendicitis and peri-appendicular abscess formation 1 month prior to EVAR. Another cause of infection is an aortoenteric fistula. Multiple mechanisms of aortoenteric fistula creation have been described and include endograft migration, erosion of the aorta and duodenum by embolization coils, fabric rupture, inflammatory nature of the aneurysm, and bacterial aortitis with chronic duodenal erosion [58–61]. Diagnosis of endograft infection is based on clinical and radiological findings. Leukocytosis, fever, and back pain are typical clinical signs, while MDCTA may show fluid collection around the rim of the aneurysmal sac. Air bubbles may also be seen within the aneurysm sac [62, 63]. Puncture of the collection or sac for microbiological analysis often gives the definitive diagnosis. After intravenous administration of antibiotics, treatment is always resection of the endograft and aneurysm sac, followed by extra-anatomic bypass or in situ venous bypass.
Contrast-Induced Nephropathy and Acute Kidney Injury
Although patients receiving EVAR are spared the ischemic insult of aortic cross-clamping and have less perioperative hemorrhage [64, 65], the potential nephrotoxicity of large contrast volume must be considered. Correct positioning and deployment of the endograft strictly needs high-quality fluoroscopy and digital subtraction angiography imaging using injection of a contrast agent. In addition, repeated administration of contrast during periprocedural evaluation and follow-up surveillance with MDCTA represents an additional risk for progressive renal dysfunction [66, 67]. Although an average contrast volume of 50–100 mL is commonly needed for an EVAR procedure, higher amounts are not infrequently used as a result of multiple angiographies that may be needed, particularly in complex anatomy for correct endograft positioning and for assessment and treatment of type I endoleak at the proximal or distal fixation sites after endograft implantation. Contrast-induced nephropathy, resulting in acute renal failure, occurs in 6.7 % of cases according to a nationwide survey by Wald et al. [68]. To avoid contrast-induced renal complications, carbon dioxide (CO2) can be used as an alternative contrast agent [69, 70].
In addition to contrast agents, other factors may be responsible for acute kidney injury after EVAR. Manipulation and positioning of the endograft within the aneurysm together with balloon inflation at the proximal fixation site may result in thromboembolism of the renal arteries and renal infarction. Moreover, the type of endograft used for proximal fixation in AAA may have an influence on renal function. Indeed, suprarenal fixation of the endograft is still creating concerns in regards to long-term effects. Interference with renal artery flow, narrowing of the renal ostium, renal infarction, and biological response of the aorta may be the result of continued injury from the suprarenal fixation stent and may play a role in renal function deterioration over time [71, 72].
Post-EVAR Follow-Up
The most common method of evaluating changes in aneurysm sac size is serial MDCTA and measuring the widest diameter for comparison (Fig. 34.14). Ultrasound screening is useful to evaluate sac diameter and to look for evidence of endoleaks, but it may not be sensitive enough to adequately assess early changes. Also, lateral plain X-ray has been used to evaluate graft migration by correlating graft markers with bony landmarks. The most sensitive method for detecting sac changes appears to be three-dimensional volumetric analysis by MDCTA [8]. Serial MDCTA has the benefit of enhanced information compared with ultrasound or plain radiograph. However, the financial burden, cumulative radiation exposure, and potential renal impairment from repeated contrast agent loads are a concern. It has been suggested that if an aneurysm is stable or reduced in size at 12-month MDCTA, it could safely be followed with clinical and ultrasound evaluation. This recommendation is based, in part, on the improved performance of the low-permeability endograft that markedly reduced the rates of endotension and sac expansion at 1-year follow-up. Although this improved short-term performance is encouraging, late expansion is still a concern and the importance of long-term serial evaluation should be emphasized. Another screening approach is to obtain a non-contrast CT image at follow-up. This would provide good information about device migration or deformation, aneurysm sac size, and stent fracture without exposing the patients to contrast agent administration. Screening with magnetic resonance angiography (MRA) is another option and is suitable for the Talent, Excluder, and Quantum low-permeability endografts but not for Zenith or Lifepath endografts due to artifact susceptibility [73].
Thoracic Aortic Aneurysm
Although, in general, cardiac surgeons manage thoracic segments of the aorta and vascular surgeons take care of abdominal aneurysms, the diagnosis and medical care of most aortic pathologies is frequently the responsibility of cardiologists. In light of this fact and considering the emergence of endovascular interventions for this disorder, it is important for cardiologists to gain confidence in managing at least the most frequent presentations of thoracic aortic aneurysms (TAA). Accordingly, it is also important for cardiologists to know the clinical and endovascular management of patients with TAA.
Indications for Intervention
Asymptomatic TAA are initially managed medically. Symptomatic and expanding aneurysms, or those more than 55 mm in diameter in the ascending aorta or more than 60 mm regardless of site or symptoms, are managed surgically. A novel predictor of TAA rupture, the aortic size index, could help predict rupture, dissection, or death. This index uses body surface area information (i.e., mm aortic diameter per m2 body surface area), enabling improved selection for surgical repair on a case-by-case basis and stratification of patients according to risk. A value of 2.75 cm/m2 or less represents low risk of rupture (approximately 4 % per year), 2.75–4.24 cm/m2 represents moderate risk (approximately 8 % per year), and more than 4.25 cm/m2 represents high risk (approximately 20 % per year), further underlining the importance of relative aortic size for predicting complications [74].
Endovascular Repair of Thoracic Aortic Aneurysm
For aneurysmal disease that encompasses the descending thoracic aorta and, in selected cases, the distal aortic arch, the use of endografts for repair of suitable anatomic conditions is emerging as a promising, nonsurgical alternative. Currently, catheter-based endovascular TAA repair (TEVAR) is undergoing clinical investigation [75–81]. As expected, outcomes have improved with growing technical expertise, use of commercially manufactured endografts, and the availability of appropriate patient selection criteria. Overall, technically successful device deployment is currently achieved in 85–100 % of cases. Data from 457 patients treated with endografts (113 emergency and 344 elective cases) and enrolled in the Talent Thoracic Retrospective Registry showed that among the 422 patients who survived the interventional procedure, mortality during follow-up was 8.5 % [80]. Of the 36 patients who died, 11 died from the aortic disease. Persistent endoleaks were reported in 64 cases, of which 44 cases were primary (9.6 %) and 21 occurred during follow-up (4.9 %). Kaplan-Meier overall survival estimates were 90.9 % at 1 year, 85.4 % at 3 years, and 77.5 % at 5 years [80]. At the same time intervals, the rates of freedom from a second procedure (either open conversion or endovascular) were 92.5 %, 81.3 %, and 70.0 %, respectively. Endovascular treatment for isolated TAA is considered a procedure with low early morbidity and mortality, even in patients treated on an emergency basis or with an arch aneurysm requiring hybrid intervention with surgical de-branching and subsequent endograft placement [80]. Follow-up data suggest substantial durability of such a hybrid procedure with high freedom from procedure-related death and secondary interventions [80]. Notably, patients included in registries had a high burden of comorbidities, and many would, therefore, not have been candidates for open surgery [81]. With only mid-term follow-up data after TEVAR available, any direct comparison with long-term outcome after successful surgical repair is not yet justified. However, the low rate of adverse events and neurological complications could eventually favor endovascular repair.
Hybrid Procedures for Aortic Arch Pathology
The aortic arch morphology is challenging due to angulation and proximity of supra-aortic branches that need to be preserved. Traditionally, the safest open arch reconstruction technique includes hypothermic cardiac arrest, extracorporal circulation, and selective cerebral perfusion. However, open procedures for any arch pathology carry a high risk for in-hospital mortality (2–9 %) and neurological complications (4–13 %) [82–84]. Therefore, surgery is often reserved for low-risk patients. As an alternative strategy, hybrid arch procedures may provide a viable solution in higher-risk patients combining first-stage de-branching bypasses with second-stage endovascular exclusion of the affected arch. Hybrid arch procedures are generally performed without hypothermic circulatory arrest or extracorporal circulation and may expand the treatment option to older patients with severe comorbidities or those who need redo surgery and are currently ineligible for open surgical intervention. To treat distal arch aneurysms involving both the left subclavian and the left common carotid artery, these vessels can be translocated upstream to the right common carotid artery approached via a cervical access [85, 86]. For arch aneurysms extending to the innominate artery, the ascending aorta can be used, via sternotomy, as a donor site for de-branching bypasses and serve as a proximal landing zone for the endograft [87, 88].
TEVAR Devices
The Zenith TX2 Thoracic TAA Endovascular Graft (Cook Inc., Bloomington, IN)
This device is a two-piece modular system allowing the physician to customize a graft system to fit each patient’s individual anatomy (Fig. 34.18). Independent, stainless steel z-stent configuration provides graft flexibility. Varied z-stent lengths and diameters promote secure graft/vessel apposition, columnar strength, and graft flexibility. The endograft is covered with a lightweight, strong, and shrink-/stretch-resistant woven polyester (historically used for open surgical TAA and AAA repairs). Four gold markers placed 1 mm from all proximal and distal aspects of the endograft denote its edges to assist in deployment accuracy. Varied proximal component diameters and lengths allow for optimal coverage to fit each patient’s specific anatomical requirements. The proximal end of the proximal component has barbs that protrude through the fabric to inhibit downward migration. Varied distal component diameters and lengths allow for optimal coverage to fit each patient’s specific anatomical requirements and provide maximum overlap with proximal component. The bare stent at the distal end of the distal component contains fixation barbs to reduce the risk of upward migration. Long, tapered hydrophilic dilator tips minimize vessel trauma and provide increased trackability over the wire guide.
The Talent Thoracic Endograft (MedtronicVascular, Santa Rosa, CA)
The Talent thoracic endograft is composed of a Dacron graft material supported by self-expanding nitinol springs. The springs are sewn to the graft material with polyester sutures (Fig. 34.19a). The graft material is a sheet of monofilament polyester with a seam joining the edges to create a cylindrical tube. Opposite the seam is the connecting bar, which attaches the most proximal and distal springs. The connecting bar provides columnar strength to the device and facilitates deployment. During implantation, the connecting bar should be oriented to the outer radius for endograft conformability and kink resistance. All nitinol components are surface treated to enhance long-term fatigue performance. The Talent endograft has many modular sections that can be used to treat a wide variety of thoracic lesions. The endograft has three proximal and distal configurations. The proximal configurations are FreeFlo, Bare Spring (22 mm only), and Open Web, which all have a flared geometry. The FreeFlo design (different from the Bare Spring configuration) has a bare spring and a mini-support spring, which improves sealing for sizes of 24–46 mm. The distal configurations are Bare Spring, Closed Web, and Open Web. The Open Web configuration was designed for use as distal extensions for the Talent endograft system.
The Valiant Endograft (MedtronicVascular, Santa Rosa, CA)
The Valiant thoracic endograft is an evolution of the previously described Talent thoracic endograft (Fig. 34.19b). The Valiant is composed of a woven, monofilament polyester graft sewn to a self-expanding nickel–titanium (nitinol) wire stent. The nitinol scaffolding is composed of a series of 5 peaked springs stacked in a tubular configuration and sewn with braided polyester non-absorbable sutures onto the outside of the polyester graft material. The proximal and distal stents differ from the “body” springs in that they have 8 peaks and range in height from 15 to 17 mm. The endograft does not have a connecting bar between springs in an attempt to increase conformability of the device. The spring spacing is designed to allow adjacent peaks to contact each other when loaded into the Xcelerant delivery system providing the necessary column strength for deployment. The proximal and distal ends of the endograft come in both open (FreeFlo) and closed (Closed Web) configurations. Seven radiopaque platinum–iridium markers are sewn to the endograft fabric. Four proximal 8-shaped markers and two distal 0-shaped markers indicate the proximal and distal extremities of the stent material. A single 8-shaped “mid-marker” indicates the minimum amount of overlap required when deploying multiple segments. The Closed Web configuration is not designed for use as a primary section or proximal extension. A segment with a FreeFlo configuration should not be deployed within another segment because of the risk of material rupture. The Valiant is currently available in diameters from 24 to 46 mm. Junctions between sections require oversizing to achieve a seal. Multiple sections may be required to achieve disease coverage and fixation. These should be sized to give appropriate oversizing, using tapered segments if necessary, and sufficient length, taking the requirement for adequate overlap into account. The Xcelerant delivery system consists of a single-use, disposable catheter with an integrated handle to provide the user with controlled deployment of the Valiant endograft. The delivery system has a 22–25F outer diameter and is designed to track over a 0.035″ guidewire. The delivery part of the system is composed of an inner member with a flexible tapered tip, a middle member incorporating the stent-stop, and an outer cover. A stainless steel braid stiffens the outer cover. The outer cover restrains the endograft during tracking and releases the endograft upon retraction. The middle member helps tracking through tortuous anatomy and has a flexible stent-stop to maintain the endograft position during deployment. Rotating or releasing and retracting the integrated handle deploy the endograft by retraction of the outer sheath cover. Should the handle jam, it can be disassembled to allow direct retraction of the outer sheath while the inner and middle members are held firmly.
Bolton Medical Relay Thoracic Endograft (Bolton Medical Inc, International Parkway Sunrise, FL)
This device, together with its “Transport” delivery system, has been specifically designed for the thoracic aorta and has an indication to treat every region, particularly the aortic arch (Fig. 34.20). The Relay endograft is made of a Dacron-woven graft and of a proprietary electropolished ultrasmooth nitinol. The device is available in two configurations featuring a proximal bare stent (Relay Plus) or a proximal crown stent (Relay NBS Plus). The device is characterized by a dedicated radial force distribution: the radial force expressed by each stent is different, according to its role (high for proximal and distal sealing stents, intermediate for middle stents, and low for the proximal bare stent). A “spiral support strut,” “double S” shaped in three dimensions, is also available with the aim of absorbing the forces expressed by the heartbeat and the blood flow forces onto the aorta, giving columnar strength and support. The “Transport” delivery system is a unique two-stage introducer system: while the primary sheath gives support in the navigation of the iliacs, the flexible secondary inner sheath allows to take the device through every tortuous anatomy. A proximal capture system allows a precise positioning and repositioning of the endograft prior to deployment. Diameters of the Relay endograft vary from 22 to 46 mm, and lengths from 100 to 250 mm, for straight and tapered configurations. A custom-made program is also available.
The TAG (W.L. Gore & Associates, Flagstaff, AZ)
The TAG is a flexible nitinol-supported PTFE device available in diameters of 26–40 mm and in 10-cm, 15-cm, and 20-cm lengths (Fig. 34.21). The exoskeleton is bonded to the graft material without sutures and is constrained by an ePTFE sleeve. The device profile depends on the size of endograft and requires a 20–24F sheath. Deployment is very rapid and occurs with the release of the constraining sleeve. The TAG expansion is designed to start from the middle of the endograft and progress toward its end. This is designed to avoid the displacing forces of the high arterial flow when aortic endografts are partially deployed in a standard deployment mode. The device is then additionally expanded with a specially designed trilobed balloon that allows flow to continue during inflation, thus avoiding the need for rapid pacing (Fig. 34.9). If more than one device is used, the most proximal device should be deployed first, and then the second must follow distal to this with at least 2 cm of overlap. The original device contained two longitudinal spines for columnar support. The device has been redesigned recently: the longitudinal spine was removed, and the ePTFE material was reinforced.
TEVAR Indications
Thoracic Aortic Aneurysm
In approximately 60 % of cases, TAA involves the ascending aorta, in 40 % the descending aorta, and in 10 % the arch, while 10 % of patients show a thoracoabdominal extension. Etiology, natural history, and treatment of TAA differ for each of these segments [89]. The suitability of a given patient for endovascular repair is based on both clinical and anatomical considerations. To date, endograft placement is a first-choice option when TAA is distal to the aortic arch and when an abdominal aorta location is present. As with AAA, the endograft bridges the aneurysm sac to exclude it from high-pressure aortic blood flow, thereby promoting sac thrombosis and aorta remodeling. Successful TAA exclusion similarly requires normal segments of native aorta at both lesion ends of at least 12–25 mm to ensure adequate deployment and contact between the endograft and the aortic wall with a tight circumferential seal. Devices are oversized by 10–20 % in diameter to provide sufficient radial force for fixation. The preferred and most common site of vascular access is the common femoral artery (41–58 %). Less frequently, access to the iliac artery (9–44 %) via an extraperitoneal approach is required [90, 91]. Proximal attachment of the endograft may require intentional coverage of the left subclavian artery ostium [92]. With this technique, it is important to identify patients with anomalous arch vessels in order to allow the requisite revascularization surgery before endograft deployment [93]. Since the first human case of TEVAR was performed in 1986 [94], a primary technical success rate of 80–90 % has been reported [95–101]. Major procedure-related neurological complications, including stroke and spinal cord ischemia, were seen in up to 8 % of patients. However, compared with open surgical repair, endovascular treatment does appear to halve perioperative mortality with similar late survival and almost identical rates of reinterventions and ischemic spinal cord complications [102, 103].
Penetrating Atherosclerotic Ulcer
The term penetrating atherosclerotic ulcer (PAU) describes a condition in which ulceration of an atherosclerotic plaque penetrates the internal elastic lamina and allows hematoma formation within the media of the aortic wall [104]. PAUs are most frequently located in the descending thoracic aorta, and affected patients tend to be elderly with a history of hypertension, smoking, and other atherosclerotic disease, including preexisting TAA. In a quarter of cases, a PAU may penetrate through the media to form a saccular aortic pseudoaneurysm (Fig. 34.22a) or, less often, it may perforate the adventitia to cause transmural aortic rupture [104, 105]. The entity is associated with a variable extent of localized intramural hematoma but may develop to classic aortic dissection in rare cases [104–106]. Currently, no definite treatment strategy has been established for PAU management. Indeed, patients with a PAU diameter and/or depth of 2 cm, those who are hemodynamically unstable, as well as those with evidence of contained rupture should undergo urgent repair. Continued or recurrent pain, distal embolization, and progressive dilatation are also indications for surgery or endovascular therapy [107]. There is growing optimism that TEVAR may become an alternative to open surgery, since limited aortic disease such as PAU constitutes an ideal condition for this less invasive approach (Fig. 34.22b) [108].
TEVAR Procedure
Based on measurements from transesophageal echocardiography, MDCTA, which is the best technique for unstable patients in an emergency situation, or intravascular ultrasound (IVUS), individually selected endografts should be used. The procedure is best performed in the catheterization laboratory or hybrid operating room using digital angiography. Cerebrospinal fluid drainage is recommended for patients with previous AAA repair and in those in whom coverage of the subclavian artery is planned or requires extensive endograft coverage of the aorta. The femoral artery is the preferred access site. In most cases, it can accommodate a 20–24F endograft system. Using the Seldinger technique to place a sheath, a 260-cm stiff guidewire is placed over a pigtail catheter navigated with a soft guidewire in the true lumen under fluoroscopic guidance. In some centers, transesophageal ultrasound guidance is also used. A 6F sheath is placed in the contralateral femoral artery and a second guidewire is positioned in the ascending aorta. If the endograft will be deployed distal to the subclavian artery, a 6F pigtail catheter will be positioned in the ascending aorta. If the left subclavian artery is going to be excluded with the endograft, this guidewire will serve as a “bailout” access guidewire. In case the endograft is deployed inadvertently over the left carotid artery, a balloon catheter of appropriate size will quickly be advanced over the guidewire and inflated across the proximal end of the device, to allow perfusion to the carotid artery. A 4F sheath and subsequent pigtail catheter are placed in the right brachial artery when the left subclavian artery is to be excluded. The pigtail catheter is positioned in the ascending aorta. When dealing with a very tortuous aorta or an arch with a small radius, the “body floss” maneuver may be helpful in advancing the endograft through the arch. This is accomplished by advancing a 0.035″ × 450-cm hydrophilic guidewire from the right brachial artery to the femoral artery. The device may then be advanced over this guidewire. The pigtail catheter (transfemoral or through right brachial/radial access) is used to perform an aortogram of the area of interest. After the angiogram is performed, the proximal neck is evaluated. The length and the diameter of the proximal and distal neck are therefore measured using the preoperative MDCTA, intraoperative IVUS, as well as angiogram. Based on these measurements, the endograft is chosen, flushed with heparinized solution, and carefully advanced, over the stiff guidewire, into the proximal neck. A repeat angiogram is commonly performed to confirm the device position within aorta. Prior to device deployment, the systolic blood pressure is brought down to 100 mmHg. If the patient has a landing zone proximal to the left subclavian artery, adenosine (35 mg for the first dose, 20 mg for subsequent doses) may be administrated to gain a 4–5 s cardiac arrest. The ventilator is stopped for the deployment in patients under general anesthesia. These two measures improve deployment accuracy. Alternatively, endograft deployment is performed with systolic blood pressure briefly lowered by rapid right ventricular pacing [109]. After deployment, short inflation of a latex balloon is performed to improve endograft apposition to the aortic wall but only if proximal sealing is incomplete. Both Doppler ultrasound and contrast fluoroscopy are instrumental for documenting any circumferential stent malapposition to the proximal or distal landing zone that may lead to endoleak. However, a single aortogram may miss endoleak due to projection overlap. A biplane aortogram is more reliable in excluding any significant intraprocedural endoleak. After the completion angiogram, the introducer sheath is removed leaving the guidewire in the aorta. If there is any concern about iliac artery injury, an iliac artery angiogram is performed. If no iliac lesion is detected and the patient remains hemodynamically stable, the guidewire is removed and the femoral artery is repaired in standard fashion.
Post-TEVAR Complications
Postoperative complications in patients undergoing TEVAR are about the same of those described for EVAR in the previous paragraph. We will limit ourselves here to recall briefly the more specific complications of TEVAR. The most severe complications in the W.L. Gore TAG pivotal clinical trial included stroke (4 %), paraplegia/paraparesis (3 %), peripheral vascular injury (14 %), and death (2 %) [110]. Stroke can occur because the guidewires placed in the aortic arch to direct the endograft into position can dislodge a thrombus or atheromatous debris that can embolize via the cerebral vasculature to the brain. Similarly, thromboembolic material can also lead to limb and mesenteric ischemia. Spinal ischemia may occur when the intercostal blood vessels supplying the spinal cord are covered by the endografts. Paraplegia can be occasionally reversed by elevating blood pressure and draining spinal fluid. Due to their large size, thoracic endograft may damage femoral and iliac arteries as they are advanced into position. The most worrisome concern is complete femoral or iliac avulsion, which can be controlled with balloon occlusion. This is the main reason why guidewires must be left in position until the very end of the case. Endoleaks occur when the aneurysm is not completely isolated from the bloodstream. In the W.L. Gore TAG trial, this complication was found in 6 % and 9 % of patients at 1 and 2 years, respectively [111].
Post-TEVAR Therapy and Follow-Up
The long-term follow-up of patients who underwent successful TEVAR begins with the appreciation of a systemic illness. All patients merit aggressive medical therapy, follow-up visits, and serial imaging. Treatment with effective β-blockade is the cornerstone of medical therapy. By lowering both blood pressure and dp/dt, β-blockers have been shown to retard aortic expansion in Marfan’s syndrome and that associated with chronic AAA. Blood pressure should be titrated to less than 130/80 mmHg in usual patients and to no more than 120/80 mmHg in those with Marfan’s syndrome [112, 113]. Additionally, heart rate should be controlled. Indeed, heart rate <60 bpm significantly decreases secondary adverse events (aortic expansion, recurrent aortic dissection, aortic rupture, and/or need for aortic surgery) compared to conventional control at >60 bpm [114]. Serial imaging of the aorta is an essential component of long-term management after endograft placement (Fig. 34.22b). The choice of imaging modality depends on institutional availability and expertise [112, 113].
Aortic Coarctation and Atherosclerotic Stenosis of the Infrarenal Abdominal Aorta
Coarctation of the aorta (CoA) and atherosclerotic stenosis of the infrarenal abdominal aorta (IAA) will be discussed together because the general and technical principles are similar as well as the devices used for their endovascular treatment.
Aortic Coarctation
CoA is a congenital abnormality that accounts for 5–8 % of the 8/1,000 (4–6/10,000) children born with congenital heart disease. In most cases, CoA is diagnosed in childhood, while in less than 25 % of patients is recognized beyond 10 years. In the adult form, CoA is generally characterized by a narrowing localized in the post-ductal or ligament region, just distal the left subclavian artery origin (Fig. 34.23a). Unlike the infantile CoA, the adult form is typically not associated with a patent arterial duct. Moreover, degenerative aortic wall changes and formation of arterial collateral vessels are usually present (Fig. 34.23b). Coarctation may be an isolated malformation or may occur in association with bicuspid aortic valve and other cardiovascular diseases such as subaortic stenosis, mitral valve abnormalities, ventricular septal defect, and, less frequently, cerebral aneurysms of the Willis circle. In adults, CoA may be recognized because of systemic arterial hypertension and pulse discrepancy between the upper and lower extremities. Patients may complain of exertional headache, leg fatigue, or claudication. Occasionally, the patient may come to medical attention because of a murmur due to a bicuspid aortic valve or ventricular septal defect. Survival in non-operated patients averages 35 years of age, with 75 % mortality by 45 years of age. Systemic arterial hypertension, accelerated coronary artery disease, stroke, aortic dissection, and congestive heart failure are common complications in patients who did not have surgery or underwent surgical correction in later childhood or adult life. Death may be due to congestive heart failure, aortic rupture/dissection, endocarditis/endarteritis, intracerebral hemorrhage, or myocardial infarction [115]. When operated in neonates and small infants, re-coarctation occurs in up to 10 % of cases in most published series. The time of this re-narrowing may vary largely from weeks to years after the first intervention. Patients may also present with aortic wall injury (aneurysm) related to prior CoA repair. Treatment of these aneurysms is surgically challenging.
Atherosclerotic Stenosis of the Infrarenal Abdominal Aorta
Severe, concentric, and diffuse calcific stenosis of the IAA occurs relatively infrequently but presents a challenging management problem [116, 117]. Indeed, focal stenosis of the IAA not extending to the bifurcation or iliac arteries is infrequent and is one of less frequent causes of lower extremity claudication. However, diffuse stenosis of a heavily calcified IAA is an even more uncommon clinical entity and is considered an advanced stage of a severe chronic obliterative atherosclerotic process in the aorta. Patients with IAA stenosis are usually younger and have less extensive atherosclerotic disease than patients who present with iliofemoral or more peripheral disease. These patients have a longer life expectancy than the average patient with claudication. In adult patients, about 90 % of stenosing or occlusive disease of the IAA is caused by atherosclerosis [118]. Open surgery, with either endarterectomy or bypass graft replacement when more extensive disease is present, is an effective treatment modality and traditionally has been the treatment of choice [119, 120]. Both of these treatments have durable results in a high proportion of patients but are highly invasive and carry a significant risk of morbidity and mortality ranging from 9 to 27 % and 1 to 7 %, respectively [121, 122].
Non-invasive Assessment
MRA or MDCTA with three-dimensional reconstruction identifies the precise location and anatomy of CoA and IAA stenosis, as well as collateral vessels (Figs. 34.23 and 34.24). MDCTA is also extremely useful in intervention planning: (1) it helps the physician determine the true diameter of the native aorta that can be underestimated with angiography, and (2) it defines the extent of calcification and (3) the extent of disease (focal or diffuse). Alternatively, IVUS could be used in patients where renal function is suboptimal, but preprocedural MDCTA has the benefit of being able to plan the procedure beforehand. In CoA patients, MRA to search for aneurysms of the intracranial arteries is appropriate and may also be useful to quantify collateral flow.
Endovascular Treatment
Coarctation of the Aorta
Accepted criteria for CoA treatment in adults (native and postoperative) include an anatomically discrete lesion, upper extremity hypertension, and a resting gradient of at least 20 mmHg or 30 mmHg after exercise. However, the best treatment in adults remains the subject of continuous debate [123, 124]. Surgical repair, first performed by Crafoord and Nylin in 1944 [125], was initially the only available option, but in some cases, it was complicated by restenosis, aneurysm formation [126, 127], late hypertension, and premature death, particularly when the repair was performed after the first year of life [128, 129]. Currently, surgical mortality is very low, but surgery is still associated with a small risk of spinal cord damage and consequent irreversible paraplegia [130]. To deal with postoperative complications such as restenosis after surgical repair, balloon dilation of re-CoA was introduced in 1982 [131–133] and demonstrated to be successful in reducing the gradient to <20 mmHg in 85 % of patients, with a restenosis rate of 8 %. Although widely used since then, balloon dilation has excited controversy. Histology and IVUS studies have established that angioplasty increases the internal aortic diameter by tearing the intima and media of the aorta. Although intimal and medial tears may heal, some may progress to aneurysm formation. The incidence of early and late aneurysm formation after balloon angioplasty has been reported to be between 5 and 11.5 % [128, 132, 134–138], a higher rate as compared to their occurrence after surgical repair (3–4 %). The known major complications of angioplasty are sudden death due to ventricular fibrillation, aortic rupture, and aneurysm formation due to intimal tears and the consequent weakening of the aortic wall. Other problems associated with balloon dilation are residual CoA and re-CoA. Similar complications have been encountered in patients with native CoA treated with balloon dilation [134–136, 139]. In the early 1990s, balloon-expandable stents were introduced for CoA treatment in children, and since then, their use increased dramatically [140–145] due to many advantages over balloon angioplasty. In particular, balloon-expandable stents provide support to the vessel wall and apposition of the torn vessel intima to the media and thereby are associated with a lower rate of residual stenosis, restenosis, and late aneurysm formation. Moreover, they may be redilated to accommodate the patient’s somatic growth. Although stenting addresses the concern about angioplasty complications, aortic dissection and aneurysm formation have also been reported after stent implantation in up to 5 % of patients [128, 145, 146]. A meta-analysis of 22 studies performed in adult and adolescent patients with CoA compared interventional to surgical treatment with regard to morbidity, restenosis, requirement for reintervention, and hypertension and found that stenting had the lowest risk of complications, with surgery having a slightly higher risk and angioplasty a significantly higher risk [147]. However, restenosis and reintervention rates were significantly higher following stenting and angioplasty when compared to surgery, and surgical complications were minor (e.g., vasculitis, bleeding), while endovascular complications tended to be more serious. In 1999, a covered stent was used for the first time to treat coexistent CoA and aneurysm of the aorta in a young man [148]. Since then, covered stent demonstrated to significantly improve endovascular treatment of CoA, reducing the risk associated to angioplasty and bare stent implantation [149–154]. Up to now, several different covered stents have been used: (1) the AneuRx (Medtronic, Watford, England) self-expanding stent covered with a stretchable polytetrafluoroethylene membrane [148], (2) the graft Jomed stent (Jomed, Rangendingen, Germany) [149], (3) the self-expanding stent graft (Braile stent, Braile Biomedica, Sao José do Rio Preto, Brazil) [154], and (4) the balloon-expandable Cheatham-Platinum stent covered with ePTFE (NuMed Inc., Hopkinton, NY) (Fig. 34.25) [150, 151, 153, 154]. Covered stents provide increased safety, reducing the risk associated with aorta rupture by “sealing” the dilated area [155, 156], particularly in patients with extreme narrowing, tortuous lesions, genetic aortic wall weakness, and advanced age. Covered stents have been successfully used even in case of stent fracture and for the treatment of late aortic aneurysm after surgical or endovascular CoA repair and for subatretic native CoA [77, 155, 157]. Although the ability to redilate covered stents has been recently demonstrated [155, 156], their use is limited to patients that are nearly fully grown, because currently they can be redilated up to 25 mm only. Moreover, at diameters of up to 22 mm, they can shorten by one-eighth of their initial length. One of the main initial concerns with the use of covered stents was the occlusion of a significant aortic side branch. It is obviously important to avoid covering the main arteries such as the innominate or the left common carotid arteries, but the left subclavian artery can be covered, if needed, because it has been shown that endograft-induced occlusion of the ostial left subclavian artery is tolerated without chronic functional ischemia in the absence of stenotic vertebral and/or carotid arteries and with a documented intact vertebrobasilar system [92]. Furthermore, occlusion of a spinal artery, which would result in the most severe complication, is unlikely after implantation of a covered stent in the thoracic aorta, because the spinal artery originates below the level of the ninth thoracic vertebra in over 90 % of the patients [158, 159]. The risk of subacute stent thrombosis is low due the brisk aortic flow and the large size of the stent. In conclusion, covered stents have become the therapy of choice in Europe and recently in the USA through the FDA Compassionate Use process in patients with complications after CoA repair. Moreover, they provide a safe alternative to surgery for the treatment of the large part of adult patients with native CoA [160, 161].
Atherosclerotic Stenosis of the Infrarenal Abdominal Aorta
Grollmann et al., Tegtmeyer et al. and Velasques et al. performed the first PTAs of IAA stenoses in 1980 [162–164]. Since then, several authors [162, 163] have shown that PTA is an effective treatment modality of aortic atherosclerotic occlusive disease associated with a high technical and clinical success rate, few major complications, and encouraging mid- and long-term results.[165–174]. The vast majority of lesions treated were relatively short (<2 cm) with a technical success rate ranging from 95 to 100 % and a patency rate approaching 89 % [169, 174]. By comparison, primary patency rates of 75–90 % and 90–95 % have been reported for bypass grafting and endarterectomy, respectively [121, 122]. During the 1990s, some reports were published indicating that stent placement may be an option in cases where PTA of aortic lesions fails, or is inadequate [171, 175, 176]. Growing clinical experience with aortic stenting has demonstrated that this is a safe and effective treatment [171, 177–179]. Indeed, endovascular treatment with stents achieved immediate technical success rates of 100 % and low complication rates in the majority of published series [164, 175, 180–182]. At present, the durability of primary stenting can be considered acceptable in all the studies and appear to be broadly similar or superior to the results obtained by an open surgical approach. Therefore, primary stenting is recommended as a safe and effective treatment strategy and should be offered to patients as “the treatment of choice” in focal atherosclerotic IAA stenoses.
Implantation Technique
Stent Choice
Only a few stents are available that can be dilated to a diameter ≥20 mm. The Palmaz XL (Cordis, Miami, FL) and the Cheatham-Platinum (CP) stent (NuMed Inc., Hopkinton, NY) are designed for treatment of large vessel lesions. The CP stent is composed of 0.013″ platinum/iridium struts arranged in a “zig” pattern. It allows expansion from 8 to 24 mm, and its variability in length is from 11 to 50 mm. The covered-CP stent is the same bare-metal stent covered with an expandable sleeve of ePTFE. A bare and a covered CP stent pre-mounted on a BIB (balloon in balloon) catheter (NuMed Inc., Hopkinton, NY) became recently available in Europe (Fig. 34.25). The BIB catheter is 110 cm in length, utilizes a 0.035″ guidewire, and has an inner balloon half the diameter of the outer balloon and 1 cm shorter in length. The BIB is designed to prevent stent displacement from the balloon during inflation and to allow adjustment of the balloon/stent position after partial dilation of the stent.
Coarctation of the Aorta
The general and technical principles are the same as those used for performing balloon angioplasty or stenting in other vessels. In pediatric patients, the procedure is usually performed under general anesthesia, while sedation with midazolam and propofol and local anesthesia are preferred in adults. Intravenous heparin (100 UI/kg, maximum of 5,000 UI) is given immediately after femoral access is gained percutaneously with an 8 Fr introducer.
Hemodynamic and Angiographic Assessment
The stenotic segment is crossed with a 6F multipurpose catheter over a floppy hydrophilic guidewire (0.035″ Terumo guidewire). Then, the multipurpose catheter is exchanged for a pigtail catheter over a standard 0.035″ exchange 260-cm guidewire. The pressure gradient is measured between the femoral sheath and the pigtail catheter positioned in the ascending aorta. Anteroposterior, 40° left anterior oblique and lateral angiograms are performed and the following measurements are obtained: (1) diameter and length of the stenotic area, (2) diameter of the descending aorta at the level of the diaphragm, (3) diameter of the aorta at the level of the left subclavian artery, and (4) diameter of the transverse arch (Fig. 34.26a). The maximum balloon diameter is usually chosen based on either the transverse or the distal arch diameter, whichever is greater using catheter magnification or the calibration markers on the catheters. The angioplasty balloon diameter is usually 2.5–3.0 times that of the narrowed segment but should be less than 1.5 times the diameter of the aorta proximal to the CoA. It is clear that for some partially elastic stenoses, it is necessary to employ 1–2-mm larger balloons. This strategy may likely play a role in aneurysm formation, but it is not yet clear whether the use of a smaller balloon and elastic recoil prevention by stent implantation [140] may reduce this risk. Some groups suggest a graduated approach, using repeated procedures to gradually enlarge the stenosis, but evidence that graduated ballooning or stenting carries less risk is still lacking.
Procedural phases of CoA endovascular treatment. (a) Angiography performed with a pigtail cateter advanced in the proximity of the CoA with a transradial approach showing a near-atretic aortic coarctation. (b) A 0.014″ coronary guidewire (arrow) and a 4.0-mm coronary balloon catheter were needed to predilate the lesion. (c) Covered Cheatham-Platinum stent placed across the lesion. (d) Stent deployed with BIB balloon inflation
Stent Delivery
The pigtail catheter is exchanged for a long sheath, usually a Mullins sheath (Cook, Bloomington, IN), ranging from 11F to 14F, over a 0.035″ stiff exchange 260-cm guidewire positioned in the ascending aorta or in the right subclavian artery. The CoA is then predilated with standard peripheral balloons. When a near-atretic CoA is found, predilation with coronary guidewires and balloons may be needed (Fig. 34.26b). The length of the stent is determined by the distance from just beyond the left subclavian artery to about 10–15 mm beyond the CoA site and is always shorter than the balloon length. The stent may be mounted on a BIB catheter. After advancement of the mounted stent to the stenotic site through the long sheath, serial injections of dye through the sheath or a separate catheter confirm the desired position of the stent immediately before implantation. A catheter with radiopaque markers may also be used for this purpose. The fluoroscopic image of the catheter will project over the aortic isthmus and will allow an accurate localization of the narrowing plane, thus confirming that the stent straddles the CoA during balloon inflation (Fig. 34.26c). The balloon is manually inflated up to the pressure recommended by the manufacturer (usually 4–6 atm), and the stent is deployed in the correct position (Fig. 34.26d). Pressure gradient is recorded and angiography is performed after stent placement injecting contrast through the side arm of the long sheath or a pigtail in order to assess the results and to rule out dissection or rupture of the aorta. At the end of the procedure, hemostasis is achieved by manual compression, surgical repair, or percutaneous pre-closure. All patients receive cephalosporin for 24 h and aspirin (≥100 mg) once a day for 6 months after implantation.
Atherosclerotic Stenosis of the Infrarenal Abdominal Aorta
The procedure is usually undertaken under local anesthesia. Initially, bilateral common femoral access is established with a 6F short sheath, followed by systemic anticoagulation with heparin (100 U/kg). Occasionally, transradial access may be used for accurate reference imaging and to pass the initial guidewire in the antegrade direction. This approach may lower the risk of creating a cephalad-extending dissection of the aorta. The IAA-diseased segment is traversed with a 0.035″ hydrophilic guidewire under fluoroscopic guidance, and a pigtail marker angiographic catheter is placed in the aorta for preliminary imaging and to assist in selection of stent length (Fig. 34.27). The hydrophilic guidewire is then exchanged for a 0.035″ stiffer guidewire, and a larger (9–12F) long sheath is advanced retrogradely across the stenosis until its tip is proximal to the lesion. The stenotic segment is dilated with balloon angioplasty with standard technique. The selection for the balloon catheter size is usually 60–80 % of the diameter of the healthy aorta. A stent of an appropriate diameter for the IAA and length for the lesion (as a rule, the selected stent should be longer then diseased segment by 3 mm) is then positioned, sheath is withdrawn, and the stent is deployed after angiographic control via the contralateral approach for exact placement. After stent deployment, a further angiogram is obtained in two planes to determine whether the stented segment requires any additional dilatation (Fig. 34.28). “Kissing-balloon” technique may be used when dealing with a large IAA. Technical success is determined if residual stenosis is less than 30 % at angiography or there is no hemodynamic pressure gradient across the lesion.
Angiography of the distal abdominal aorta showing final result after covered Cheatham-Platinum stent deployment and post-dilation (Same patient seen in Fig. 34.24)
Follow-Up
It is incumbent on all interventionists carrying out endovascular procedures or aortic stenosis treatment to document objective outcomes (e.g., treadmill testing), symptomatic improvement, quality of life, and proof of patency (i.e., duplex ultrasound, MDCTA, MRA, or angiography). Usually, patients undergo MRA or MDCTA (Figs. 34.29 and 34.30) at 3 and 12 months after the procedure. Invasive angiography is usually performed in cases where problems are identified.
References
Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–9.
Parodi JC, Marin ML, Veith FJ. Transfemoral, endovascular stented graft repair of an abdominal aortic aneurysm. Arch Surg. 1995;130:549–52.
Volodos’ NL, Goloborod’ko NK, Mikhailenko VV. Surgical treatment of traumatic rupture of the thoracic aorta. Grudn Khir. 1981;6:76–8.
Criado FJ, Wilson EP, Wellons E, Abul-Khoudoud O, Gnanasekeram H. Early experience with the talent stent-graft system for endoluminal repair of abdominal aortic aneurysms. Tex Heart Inst J. 2000;27:128–35.
Criado FJ, Fairman RM, Becker GJ. Talent LPS AAA stent graft: results of a pivotal clinical trial. J Vasc Surg. 2003;37:709–15.
Broker HS, Foteh KI, Murphy EH, Davis CM, Clagett GP, Modrall JG, Buckley CJ, Arko FR. Device-specific aneurysm sac morphology after endovascular aneurysm repair: evaluation of contemporary graft materials. J Vasc Surg. 2008;47:702–7.
Risberg B, Delle M, Eriksson E, Klingenstierna H, Lonn L. Aneurysm sac hygroma: a cause of endotension. J Endovasc Ther. 2001;8:447–53.
Fillinger M, Excluder Bifurcated Endoprosthesis Clinical Investigators. Three-dimensional analysis of enlarging aneurysms after endovascular abdominal aortic aneurysm repair in the Gore Excluder Pivotal clinical trial. J Vasc Surg. 2006;43:888–95.
Haider SE, Najjar SF, Cho JS, Rhee RY, Eskandari MK, Matsumura JS, Makaroun MS, Morasch MD. Sac behavior after aneurysm treatment with the Gore Excluder low-permeability aortic endoprosthesis: 12-month comparison to the original Excluder device. J Vasc Surg. 2006;44:694–700.
Tanski 3rd W, Fillinger M. Outcomes of original and low-permeability Gore Excluder endoprosthesis for endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2007;45:243–9.
Dalainas I, Nano G, Bianchi P, Ramponi F, Casana R, Malacrida G, Tealdi DG. Aortic neck dilatation and endograft migration are correlated with self-expanding endografts. J Endovasc Ther. 2007;14:318–23.
Hobo R, Kievit J, Leurs LJ, Buth J, EUROSTAR Collaborators. Influence of severe infrarenal aortic neck angulation on complications at the proximal neck following endovascular AAA repair: a EUROSTAR study. J Endovasc Ther. 2007;14:1–11.
Whittaker DR, Dwyer J, Fillinger MF. Prediction of altered endograft path during endovascular abdominal aortic aneurysm repair with the Gore excluder. J Vasc Surg. 2005;41:575–83.
Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, Matsumura JS, May J, Veith FJ, Fillinger MF, Rutherford RB, Kent KC, Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of the Society for Vascular Surgery, American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–60.
Carpenter JP. Midterm results of the multicenter trial of the Powerlink bifurcated system for endovascular aortic aneurysm repair. J Vasc Surg. 2004;40:849–59.
Zarins CK, Bloch DA, Crabtree T, Matsumoto AH, White RA, Fogarty TJ. Stent graft migration after endovascular aneurysm repair: importance of proximal fixation. J Vasc Surg. 2003;38:1264–72.
Ouriel K, Greenberg RK, Clair DG, O’hara PJ, Srivastava SD, Lyden SP, Sarac TP, Sampram E, Butler B. Endovascular repair of abdominal aortic aneurysms: device-specific outcome. J Vasc Surg. 2003;37:991–8.
Abbruzzese TA, Kwolek CJ, Brewster DC, Chung TK, Kang J, Conrad MF, LaMuraglia GM, Cambria RP. Outcomes following endovascular abdominal aortic aneurysm repair (EVAR): an anatomic and device-specific analysis. J Vasc Surg. 2008;48:19–28.
Albertini J, Kalliafas S, Travis S, Yusuf SW, Macierewicz JA, Whitaker SC, Elmarasy NM, Hopkinson BR. Anatomical risk factors for proximal perigraft endoleak and graft migration following endovascular repair of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2000;19:308–12.
Mohan IV, Harris PL, Van Marrewijk CJ, Laheij RJ, How TV. Factors and forces influencing stent-graft migration after endovascular aortic aneurysm repair. J Endovasc Ther. 2002;9:748–55.
Arko FR, Heikkinen M, Lee ES, Bass A, Alsac JM, Zarins CK. Iliac fixation length and resistance to in-vivo stent-graft displacement. J Vasc Surg. 2005;41:664–71.
Murphy EH, Johnson ED, Arko FR. Device-specific resistance to in vivo displacement of stent-grafts implanted with maximum iliac fixation. J Endovasc Ther. 2007;14:585–92.
Heikkinen MA, Alsac JM, Arko FR, Metsänoja R, Zvaigzne A, Zarins CK. The importance of iliac fixation in prevention of stent graft migration. J Vasc Surg. 2006;43:1130–7.
Benharash P, Lee JT, Abilez OJ, Crabtree T, Bloch DA, Zarins CK. Iliac fixation inhibits migration of both suprarenal and infrarenal aortic endografts. J Vasc Surg. 2007;2:250–7.
Enzler MA, van Marrewijk CJ, Buth J, Harris PL. Endovascular therapy of aneurysms of the abdominal aorta: report of 4,291 patients of the Eurostar Register. Vasa. 2002;31:167–72.
van Marrewijk C, Buth J, Harris PL, Norgren L, Nevelsteen A, Wyatt MG. Significance of endoleaks after endovascular repair of abdominal aortic aneurysms: The EUROSTAR experience. J Vasc Surg. 2002;35:461–73.
Zarins CK, White RA, Hodgson KJ, Schwarten D, Fogarty TJ. Endoleak as a predictor of outcome after endovascular aneurysm repair: AneuRx multicenter clinical trial. J Vasc Surg. 2000;32:90–107.
Buth J, Harris PL, van Marrewijk C, Fransen G. The significance and management of different types of endoleaks. Semin Vasc Surg. 2003;16:95–102.
Van den Berg JC, Tutein Nolthenius RP, Casparie JWBM, Moll FL. CT-guided thrombin injection into aneurysm sac in a patient with endoleak after endovascular abdominal aortic aneurysm repair. AJR Am J Roentgenol. 2000;175:1649–51.
Kasthuri RS, Stivaros SM, Gavan D. Percutaneous ultrasound-guided thrombin injection for endoleaks: an alternative. Cardiovasc Intervent Radiol. 2005;28:110–2.
Muthu C, Maani J, Plank LD, Holden A, Hill A. Strategies to reduce the rate of Type II Endoleaks: routine intraoperative embolization of the inferior mesenteric artery and thrombin injection into the aneurysm sac. J Endovasc Ther. 2007;14:661–8.
Fransen GA, Vallabhaneni Sr SR, van Marrewijk CJ, Laheij RJ, Harris PL, Buth J, EUROSTAR. Rupture of infra-renal aortic aneurysm after endovascular repair: a series from EUROSTAR registry. Eur J Vasc Endovasc Surg. 2003;26:487–93.
Goodney PP, Fillinger MF. The effect of endograft relining on sac expansion after endovascular aneurysm repair with the original-permeability Gore Excluder abdominal aortic aneurysm endoprosthesis. J Vasc Surg. 2007;45:686–93.
Bakken AM, Karl A, Illig KA. Long-term follow-up after endovascular aneurysm repair: is ultrasound alone enough? Perspect Vasc Surg Endovasc Ther. 2010;22:145–51.
Liaw JV, Clark M, Gibbs R, Jenkins M, Cheshire N, Hamady M. Update: complications and management of infrarenal EVAR. Eur J Radiol. 2009;71:541–51.
Becquemin JP, Majewski M, Fermani N, Marzelle J, Desgrandes P, Allaire E, Roudot-Thoraval F. Colon ischemia following abdominal aortic aneurysm repair in the era of endovascular abdominal aortic repair. J Vasc Surg. 2008;47:258–63.
Perry RJ, Martin MJ, Eckert MJ, Sohn VY, Steele SR. Colonic ischemia complicating open versus endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2008;48:272–7.
Zhang WW, Kulaylat MN, Anain PM, Dosluoglu HH, Harris LM, Cherr GS, Dayton MT, Dryjski ML. Embolization as a cause of bowel ischemia after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2004;40:867–72.
Berg P, Kaufmann D, van Marrewijk CJ, Buth J. Spinal cord ischemia after stent-graft treatment for infra-renal abdominal aortic aneurysm. Analysis of the Eurostar database. Eur J Endovasc Surg. 2001;22:342–7.
Fortes DL, Atkins BZ, Chiou AC. Delayed paraplegia following intrarenal abdominal aortic endograft placement: case report and literature review. Vascular. 2004;12:130–5.
Reid JA, Mole DJ, Johnston LC, LEE B. Delayed paraplegia after endovascular repair of abdominal aortic aneurysm. J Vasc Surg. 2003;37:1322–3.
Garcia ND, Tehrani H, Morasch M, Pearce W, Matsumura J. Spinal cord ischemia following endovascular repair of an infrarenal aortic aneurysm. Ann Vasc Surg. 2002;16:509–12.
Rockman CB, Riles TS, Landis R. Lower extremity paraparesis or paraplegia subsequent to endovascular management of abdominal aortic aneurysm. J Vasc Surg. 2001;33:178–80.
Bajwa A, Davis M, Moawad M, Taylor PR. Paraplegia following elective endovascular repair of abdominal aortic aneurysm: reversal with cerebrospinal fluid drainage. Eur J Vasc Endovasc Surg. 2008;35:46–8.
Kalliafas S, Albertini JN, Macierewicz J, Yusuf SW, Whitaker SC, Macsweeney ST, Wenham PW, Hopkinson BR. Incidence and treatment of intraoperative technical problems during endovascular repair of complex abdominal aortic aneurysm. J Endovasc Ther. 2000;7:123–31.
Kalliafas S, Whitaker SC, Hopkinson BR. Use of a bifurcated graft for endovascular repair of a common iliac aneurysm after previous open aortic aneurysm surgery. Eur J Vasc Endovasc Surg. 2000;19:428–9.
Görich J, Krämer S, Rilinger N, Sokiranski R, Sunder-Plassmann L, Pamler R, Kapfer X. Malpositioned or dislocated aortic endoprostheses: repositioning using percutaneous pull-down maneuvers. J Endovasc Ther. 2000;7:123–31.
van Dijk LV, van Sambeek MR, Cademartiri F, Pattynama PM. Partial blockage of the renal artery ostium after stent-graft placement: detection and treatment. J Endovasc Ther. 2003;10:684.
Fairman RM, Baum RA, Carpenter JP, Deaton DH, Makaroun MS, Velazquez OC, Phase II EVT Investigators. Limb interventions in patients undergoing treatment with an unsupported bifurcated aortic endograft system: a review of the Phase II EVT trial. J Vasc Surg. 2002;36:118–26.
Amesur NB, Zajko AB, Orons PD, Makaroun MS. Endovascular treatment of iliac limb stenoses or occlusions in 31 patients treated with the Ancure graft. J Vasc Interv Radiol. 2000;11:421–8.
Maleux G, Koolen M, Heye S, Nevelsteen A. Limb occlusion after endovascular repair of abdominal aortic aneurysm with supported endografts. J Vasc Interv Radiol. 2008;19:1409–12.
Maldonado TS, Rockman CB, Riles E, Douglas D, Adelman MA, Jacobowitz GR, Gagne PJ, Nalbandian MN, Cayne NS, Lamparello PJ, Salzberg SS, Riles TS. Ischemic complications after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2004;40:703–10.
Carroccio A, Faries PL, Morrissey NJ, Teodorescu V, Burks JA, Gravereaux EC, Hollier LH, Marin ML. Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and device-related causes. J Vasc Surg. 2002;36:679–84.
Blom AS, Troutman D, Beeman B, Yarchoan M, Dougherty MJ, Calligaro KD. Duplex ultrasound imaging to detect limb stenosis or kinking of endovascular device after midterm follow-up. J Vasc Surg. 2012;55(6):1577–80.
Maleux G, Koolen M, Heye S, Heremans B, Nevelsteen A. Mural thrombotic deposits in abdominal aortic endografts are common and do not require additional treatment at short-term and midterm follow-up. J Vasc Interv Radiol. 2008;19:1558–62.
Sharif MA, Lee B, Lau LL, Ellis PK, Collins AJ, Blair PH, Soong CV. Prosthetic stent graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2007;46:442–8.
van der Berg HR, Leijdekkers VJ, Vahl A. Aortic stent-graft infection following septic complications of a kidney stone. Cardiovasc Intervent Radiol. 2006;29:443–5.
Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysm: single-center experience and review of the literature. J Endovasc Ther. 2008;15:441–8.
Bergqvist D, Bjorck M, Nyman R. Secondary aortoenteric fistula after endovascular aortic interventions: a systematic literature review. J Vasc Interv Radiol. 2008;19:163–5.
Parra JR, Lee C, Hodgson KJ, Perler B. Endograft infection leading to rupture of aortic aneurysm. J Vasc Surg. 2004;39:676–9.
Baker M, Uflacker R, Robinson JG. Stent graft infection after abdominal aneurysm repair: a case report. J Vasc Surg. 2002;36:180–3.
Mita T, Arita T, Matsunaga N, Furukawa M, Zempo N, Esato K, Matsuzaki M. Complications of endovascular repair for thoracic and abdominal aortic aneurysm: an imaging spectrum. Radiographics. 2000;20:1263–78.
Orton DF, LeVeen RF, Saigh JA, Culp WC, Fidler JL, Lynch TJ, Goertzen TC, McCowan TC. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics. 2000;20:977–93.
Adriansen ME, Bosch JL, Halpern EF, Hunink MGM, Gazelle GS. Elective endovascular versus open surgical repair of abdominal aortic aneurysms: systemic review of short-term results. Radiology. 2002;224:739–47.
Zeebregts CJ, Geelkerken RH, van der Palen J, Huisman AB, De Smit P, Van Det RJ. Outcome of abdominal aneurysm repair in the era of endovascular treatment. Br J Surg. 2004;91:563–8.
Eggebrecht H, Breuckmann F, Martini S, Baumgart D, Herold U, Kienbaum P, Peters J, Jakob H, Erbel R, Mehta RH. Frequency and outcomes of acute renal failure following thoracic aortic stent-graft placement. Am J Cardiol. 2006;98:458–63.
Parmer SS, Fairman RM, Karmacharya J, Carpenter JP, Velazquez OC, Woo EY. A comparison of renal function between open and endovascular aneurysm repair in patients with baseline chronic renal insufficiency. J Vasc Surg. 2006;44:706–11.
Wald R, Waikar SS, Liangos O, Pereira BJ, Chertow GM, Jaber BL. Acute renal failure after endovascular vs open repair of abdominal aortic aneurysm. J Vasc Surg. 2006;43:460–6.
Gahlen J, Hansmann J, Schumacher H, Seelos R, Richter GM, Allenberg JR. Carbon dioxide digital subtraction angiography for endovascular grafting in high-risk patients with infrarenal abdominal aortic aneurysm. J Vasc Surg. 2001;33:646–9.
Chao A, Major K, Kumar SR, Patel K, Trujillo I, Hood DB, Rowe VL, Weaver FA. Carbon dioxide subtraction angiography-assisted endovascular aortic aneurysm repair in azotemic patient. J Vasc Surg. 2007;45:451–8.
Blocker D, Krauss M, Mansmann U, Halawa M, Lange R, Probst T, Raither D. Incidence of renal infarction after endovascular AAA repair: relationship to infrarenal versus suprarenal fixation. J Endovasc Ther. 2003;10:1054–60.
Sun Z, Stevenson G. Transrenal fixation of aortic stent-grafts: short- to midterm effects on renal function. A systematic review. Radiology. 2006;240:65–72.
van der Laan MJ, Bartels LW, Bakker CJ, Viergever MA, Blankensteijn JD. Suitability of 7 aortic stent-graft models for MRI-based surveillance. J Endovasc Ther. 2004;11:366–71.
Davies RR, Gallo A, Coady MA, Tellides G, Botta DM, Burke B, Coe MP, Kopf GS, Elefteriades JA. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169–77.
Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;331:1729–34.
Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, von Kodolitsch Y, Nicolas V, Pierangeli A. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539–45.
Ince H, Petzsch M, Rehders T, Kische S, Körber T, Weber F, Nienaber CA. Percutaneous endovascular repair of aneurysm after previous coarctation surgery. Circulation. 2003;108:2967–70.
Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, Williams D, Cambria RP, Mitchell RS. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg. 2005;41:1–9.
Wheatley 3rd GH, Gurbuz AT, Rodriguez-Lopez JA, Ramaiah VG, Olsen D, Williams J, Diethrich EB. Midterm outcome in 158 consecutive Gore TAG thoracic endoprostheses: single center experience. Ann Thorac Surg. 2006;81:1570–7.
Fattori R, Nienaber CA, Rousseau H, Beregi JP, Heijmen R, Grabenwöger M, Piquet P, Lovato L, Dabbech C, Kische S, Gaxotte V, Schepens M, Ehrlich M, Bartoli JM, Talent Thoracic Retrospective Registry. Results of endovascular repair of the thoracic aorta with the Talent Thoracic stent graft: the Talent Thoracic Retrospective Registry. J Thorac Cardiovasc Surg. 2006;132:332–9.
Demers P, Miller DC, Mitchell RS, Kee ST, Sze D, Razavi MK, Dake MD. Midterm results of endovascular repair of descending thoracic aortic aneurysms with first-generation stent grafts. J Thorac Cardiovasc Surg. 2004;127:664–7.
Kazui T, Washiyama N, Muhammad BA, Terada H, Yamashita K, Takinami M. Improved results of atherosclerotic arch aneurysm operation with a refined technique. J Thorac Cardiovasc Surg. 2001;121:491–9.
Spielvogel D, Halstead JC, Meier M, Kadir I, Lansman SL, Shahani R, Griepp RB. Aortic arch replacement using a trifurcated graft: simple versatile, and safe. Ann Thorac Surg. 2005;80:90–5.
Nakai M, Shimamoto M, Yamazaki F, Fujita S, Aoyama A, Chin T, Nakata T, Yamada T. Long-term results after surgery for aortic arch non-dissection aneurysm. Kyobu Geka. 2002;55:280–4.
Czerny M, Zimpfer D, Fleck T, Hofmann W, Schoder M, Cejna M, Stampfl P, Lammer J, Wolner E, Grabenwoger M. Initial results after combined repair of aortic arch aneurysms by sequential transposition of the supra-aortic branches and consecutive endovascular stent-graft placement. Ann Thorac Surg. 2004;78:1256–60.
Schumacher H, Von Tengg-Kobligk H, Ostovic M, Henninger V, Ockert S, Böckler D, Allenberg JR. Hybrid arch procedures for endoluminal arch replacement in thoracic aneurysms and type B dissection. J Cardiovasc Surg (Torino). 2006;47:509–17.
Saleh HM, Inglese L. Combined surgical and endovascular treatment of aortic arch aneurysms. J Vasc Surg. 2006;44:460–6.
Czerny M, Gottardi R, Zimpfer D, Schoder M, Grabenwoger M, Lammer J, Wolner E, Grimm M. Transposition of the supraaortic branches for extended endovascular arch repair. Eur J Cardiothorac Surg. 2006;29:709–13.
Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–28.
Fann JI, Miller DC. Endovascular treatment of descending thoracic aortic aneurysms and dissections. Surg Clin North Am. 1999;79:551–74.
Dake MD, Miller DC, Mitchell RS, Semba CP, Moore KA, Sakai T. The “first generation” of endovascular stent grafts for patients with aneurysms of the descending thoracic aorta. J Thorac Cardiovasc Surg. 1998;116:689–703.
Rehders TC, Petzsch M, Ince H, Kische S, Korber T, Koschyk DH, Chatterjee T, Weber F, Nienaber CA. Intentional occlusion of the left subclavian artery during stent-graft implantation in the thoracic aorta: risks and relevance. J Endovasc Ther. 2004;11:659–66.
Peterson BG, Eskandari MK, Gleason TG, Morasch MD. Utility of left subclavian artery revascularization in association with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg. 2006;43:433–9.
Volodos’ NL, Shekhanin VE, Karpovich IP, Troian VI, Gur’ev IA. A self-fixing synthetic blood vessel endoprosthesis. Vestn Khir Im I I Grek. 1986;137:123–5.
Eggebrecht H, Nienaber CA, Neuhäuser M, Baumgart D, Kische S, Schmermund A, Herold U, Rehders TC, Jakob HG, Erbel R. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J. 2006;27:489–98.
Leurs LJ, Bell R, Degrieck Y, Thomas S, Hobo R, Lundbom J, EUROSTAR; UK Thoracic Endograft Registry Collaborators. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and United Kingdom Thoracic Endografting registries. J Vasc Surg. 2004;40:670–9.
Cartes-Zumelzu F, Lammer J, Kretschmer G, Hoelzenbein T, Grabenwöger M, Thurnher S. Endovascular repair of thoracic aortic aneurysms. Semin Interv Cardiol. 2000;5:53–7.
Heijmen RH, Deblier IG, Moll FL, Dossche KM, van den Berg JC, Overtoom TT, Ernst SM, Schepens MA. Endovascular stent-grafting for descending thoracic aortic aneurysms. Eur J Cardiothorac Surg. 2002;21:5–9.
Qu L, Raithel D. Two-year single-center experience with thoracic endovascular repair using the EndoFit thoracic stent-graft. J Endovasc Ther. 2008;15:530–8.
Hughes GC, Lee SM, Daneshmand MA, Bhattacharya SD, Williams JB, Tucker Jr SW, McCann RL. Endovascular repair of descending thoracic aneurysms: results with “on-label” application in the post Food and Drug Administration approval era. Ann Thorac Surg. 2010;90:83–9.
Stone DH, Brewster DC, Kwolek CJ, Lamuraglia GM, Conrad MF, Chung TK, Cambria RP. Stent-graft versus open-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg. 2006;44:1188–97.
Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS, Gore TAG Investigators. Endovascular stent-grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;133:369–77.
Coady MA, Rizzo JA, Hammond GL, Pierce JG, Kopf GS, Elefteriades JA. Penetrating ulcers of the thoracic aorta: what is it? How do we recognize it? How do we manage it? J Vasc Surg. 1998;27:1006–15.
Vilacosta I, San Román JA, Aragoncillo P, Ferreirós J, Mendez R, Graupner C, Batlle E, Serrano J, Pinto A, Oyonarte JM. Penetrating atherosclerotic aortic ulcer: documentation by transesophageal echocardiography. J Am Coll Cardiol. 1998;32:83–9.
Hayashi H, Matsuoka Y, Sakamoto I, Sueyoshi E, Okimoto T, Hayashi K, Matsunaga N. Penetrating atherosclerotic ulcer of the aorta: imaging features and disease concept. Radiographics. 2000;20:995–1005.
Tittle SL, Lynch RJ, Cole PE, Singh HS, Rizzo JA, Kopf GS, Elefteriades JA. Midterm follow-up of penetrating ulcer and intramural hematoma of the aorta. J Thorac Cardiovasc Surg. 2002;123:1051–9.
Demers P, Miller DC, Mitchell RS, Kee ST, Chagonjian L, Dake MD. Stent-graft repair of penetrating atherosclerotic ulcers in the descending thoracic aorta: mid-term results. Ann Thorac Surg. 2004;77:81–6.
Dillavou ED, Makaroun MS. Predictors of morbidity and mortality with endovascular and open thoracic aneurysm repair. J Vasc Surg. 2008;48:1114–20.
Nienaber CA, Kische S, Rehders TC, Schneider H, Chatterjee T, Bünger CM, Höppner R, Ince H. Rapid pacing for better placing: comparison of techniques for precise deployment of endografts in the thoracic aorta. J Endovasc Ther. 2007;14:506–12.
Cho JS, Haider S, Makaroun MS. Endovascular therapy of thoracic aneurysms: gore TAG trial results. Semin Vasc Surg. 2006;19:18–24.
Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903.
Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U, Taylor J, Zollikofer C, Klein WW, Mulder B, Providencia LA, Task Force on Aortic Dissection, European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642–81.
Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, Eggebrecht H, Elefteriades JA, Erbel R, Gleason TG, Lytle BW, Mitchell RS, Nienaber CA, Roselli EE, Safi HJ, Shemin RJ, Sicard GA, Sundt 3rd TM, Szeto WY, Wheatley 3rd GH, Society of Thoracic Surgeons Endovascular Surgery Task Force. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85:S1–41.
Kodama K, Nishigami K, Sakamoto T, Sawamura T, Hirayama T, Misumi H, Nakao K. Tight heart rate control reduces secondary adverse events in patients with type B acute aortic dissection. Circulation. 2008;118 Suppl 1:S167–70.
Warnes CA, Williams RG, Bashore TM, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults with Congenital Heart Disease); American Society of Echocardiography; Heart Rhythm Society; International Society for Adult Congenital Heart Disease; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–263.
Ali AT, Modrall JG, Lopez J, Brawley JG, Welborn MB, Clagett GP, Valentine RJ, Jackson MR. Emerging role of endovascular grafts in complex aortoiliac occlusive disease. J Vasc Surg. 2003;38:486–91.
Poncyljusz W, Falkowski A, Garncarek J, Karasek M, England S, Zawierucha D. Primary stenting in the treatment of focal atherosclerotic abdominal aortic stenoses. Clin Radiol. 2006;61:691–5.
Ramzi Cotran S, Robbins SL, Kumar V. Robbins pathologic basis of disease. 4th ed. Philadelphia: Saunders; 1989.
DeLaurentis DA, Friedman P, Wolferth Jr CC. Atherosclerosis and the hypoplastic aortoiliac system. Surgery. 1978;83:27–37.
Constantino MJ, Smith RB, Perdue GD. Segmental aortic occlusion. An unusual lesion found in menopausal women. Arch Surg. 1979;114:317–8.
Nevelsteen A, Wouters I, Suy R. Aorto-femoral Dacron reconstruction for aortoiliac occlusive disease: a 25 years survey. Eur J Surg. 1991;5:179–86.
Ar N, Ah-See AK, Engeset J. Aortoiliac endarterectomy: an 11 year review. Br J Surg. 1990;77:190–3.
Rao PS. Should balloon angioplasty be used instead of surgery for native aortic coarctation? Br Heart J. 1995;74:578–9.
Fawzy ME, Sivanandam V, Galal O, Dunn B, Patel A, Rifai A, von Sinner W, Al Halees Z, Khan B. One- to ten-year follow-up results of balloon angioplasty of native coarctation of the aorta in adolescents and adults. J Am Coll Cardiol. 1997;30:1542–6.
Crafoord C, Nylin G. Congenital coarctation of the aorta and its surgical treatment. J Thorac Surg. 1945;14:347–61.
Bromberg BI, Beekman RH, Rocchini AP, et al. Aortic aneurysm after patch aortoplasty repair of coarctation: a prospective analysis of prevalence, screening tests and risks. J Am Coll Cardiol. 1989;14:734–41.
Parks WJ, Ngo TD, Plauth WH, et al. Incidence of aneurysm formation after Dacron patch aortoplasty repair for coarctation of the aorta: long term results and assessment utilizing magnetic resonance angiography with three dimensional surface rendering. J Am Coll Cardiol. 1995;26:266–71.
Ovaert C, Benson LN, Nykanen D, Freedom RM. Transcatheter treatment of coarctation of the aorta: a review. Pediatr Cardiol. 1998;19:27–44.
Cohen M, Fuster V, Steele PM, Driscoll D, McGoon DC. Coarctation of the aorta. Long term follow-up and prediction of outcome after surgical correction. Circulation. 1989;80:840–5.
Gibbs JL. Interventional catheterisation. Opening up I: the ventricular outflow tracts and great arteries. Heart. 2000;83:111–5.
Singer MI, Rowen M, Dorsey TJ. Transluminal aortic balloon angioplasty for coarctation of the aorta in the newborn. Am Heart J. 1982;103:131–2.
Hijazi ZM, Fahey JT, Kleinman CS, Hellenbrand WE. Balloon angioplasty for recurrent coarctation of the aorta: immediate and long term results. Circulation. 1991;84:1150–6.
Maheshwari S, Bruckheimer E, Fahey JT, Hellenbrand WE. Balloon angioplasty of postsurgical recoarctation in infants: the risk of restenosis and long term follow-up. J Am Coll Cardiol. 2000;35:209–13.
Fletcher SE, Nihill MR, Grifka RG, O’Laughlin MP, Mullins CE. Balloon angioplasty of native coarctation of the aorta: midterm follow-up and prognostic factors. J Am Coll Cardiol. 1995;25:730–4.
Rao PS, Galal O, Smith PA, Wilson AD. Five to nine year follow-up results of balloon angioplasty of native coarctation in infants and children. J Am Coll Cardiol. 1996;27:462–70.
Mendelsohn AM, Lloyd TR, Crowley DC, Sandhu SK, Kocis KC, Beekman 3rd RH. Late follow-up of balloon angioplasty in children with a native coarctation of the aorta. Am J Cardiol. 1994;74:696–700.
Marshall A, Perry SB, Keane JF, Lock JE. Early results and medium-term follow-up of stent implantation for mild residual or recurrent aortic coarctation. Am Heart J. 2000;139:1054–60.
McCrindle BW, Jones TK, Morrow WR, Hagler DJ, Lloyd TR, Nouri S, Latson LA. Acute results of balloon angioplasty of native coarctation versus recurrent aortic obstruction are equivalent. J Am Coll Cardiol. 1996;28:1810–7.
Tynan M, Finley JP, Fontes V, Hess J, Kan J. Balloon angioplasty for the treatment of native coarctation: results of the valvuloplasty and angioplasty of congenital anomalies registry. Am J Cardiol. 1990;65:790–2.
Ebeid MR, Prieto LR, Latson LA. Use of balloon expandable stents for coarctation of the aorta: initial results and intermediate term follow-up. J Am Coll Cardiol. 1997;30:1847–52.
Cheatham JP. Stenting of coarctation of the aorta. Catheter Cardiovasc Interv. 2001;54:112–25.
Magee AG, Brzezinska-Rajszys G, Qureshi SA, Rosenthal E, Zubrzycka M, Ksiazyk J, Tynan M. Stent implantation for aortic coarctation and recoarctation. Heart. 1999;82:600–6.
Thanopoulos BD, Hadjinikolaou L, Konstadopoulou GN, Tsaousis GS, Triposkiadis F, Spirou P. Stent treatment for coarctation of the aorta: intermediate term follow-up and technical considerations. Heart. 2000;84:65–70.
Hamdan MA, Maheshwari S, Fahey JT, Hellenbrand WE. Endovascular stents for coarctation of the aorta: initial results and intermediate term follow-up. J Am Coll Cardiol. 2001;38:1518–23.
De Lezo JS, Pan M, Romero M. Immediate and follow-up findings after stent treatment for severe coarctation of aorta. Am J Cardiol. 1999;83:400–6.
Varma C, Benson LN, Butany J, McLaughlin PR. Aortic dissection after stent dilatation for coarctation of the aorta: a case report and literature review. Catheter Cardiovasc Interv. 2003;59:528–35.
Carr JA. The results of catheter-based therapy compared with surgical repair of adult aortic coarctation. J Am Coll Cardiol. 2006;47:1101–7.
Gunn J, Cleveland T, Gaines P. Covered stent to treat coexistent coarctation and aneurysm of the aorta in a young man. Heart. 1999;82:351.
DeGiovanni JV. Covered stents in the treatment of aortic coarctation. J Interv Cardiol. 2001;14:187–90.
Qureshi SA, Zubrzycka M, Brzezinska-Rajszys G, Kosciesza A, Ksiazyk J. Use of covered Cheatham-platinum stents in aortic coarctation and recoarctation. Cardiol Young. 2004;14:50–4.
Ewert P, Abdul-Khalid H, Peters B, Nagdyman N, Schubert S, Lange PE. Transcatheter therapy of long extreme subatretic aortic coarctations with covered stents. Catheter Cardiovasc Interv. 2004;63:236–9.
Forbes T, Matisoff D, Dysart J, Aggarwal S. Treatment of coexisting coarctation and aneurysm of the aorta with covered stent in a pediatric patient. Pediatr Cardiol. 2003;24:289–91.
Sadiq M, Malick NH, Qureshi SA. Simultaneous treatment of native coarctation of the aorta combined with patent ductus arteriosus using a covered stent. Catheter Cardiovasc Interv. 2003;59:387–90.
Pedra CA, Fontes VF, Esteves CA, Arrieta SR, Braga SL, Justino H, Kambara AM, Moreira SM, Sousa JE. Use of covered stents in the management of coarctation of the aorta. Pediatr Cardiol. 2005;26:431–9.
Butera G, Gaio G, Carminati M. Redilation of e-PTFE covered CP stents. Catheter Cardiovasc Interv. 2008;72:273–7.
Butera G, Dua J, Chessa M, Carminati M. Covered Cheatham-Platinum stents for serial dilatation of severe native aortic coarctation. Catheter Cardiovasc Interv. 2010;75:472. author reply 473.
Butera G, Piazza L, Chessa M, Abella R, Bussadori C, Negura D, Carminati M. Covered stents in patients with congenital heart defects. Catheter Cardiovasc Interv. 2006;67:466–72.
Wada T, Yao H, Miyamoto T, Mukai S, Yamamura M. Prevention and detection of spinal cord injury during thoracic and thoracoabdominal aortic repairs. Ann Thorac Surg. 2001;72:80–4.
Connolly JE. Prevention of spinal cord complications in aortic surgery. Am J Surg. 1998;176:92–101.
Forbes TJ, Moore P, Pedra CA, et al. Intermediate follow-up following intravascular stenting for treatment of coarctation of the aorta. Catheter Cardiovasc Interv. 2007;70:569–77.
Ewert P, Schubert S, Peters B, Abdul-Khaliq H, Nagdyman N, Lange PE. The CP stent-short, long, covered-for the treatment of aortic coarctation, stenosis of pulmonary arteries and caval veins, and Fontan anastomosis in children and adults: an evaluation of 60 stents in 53 patients. Heart. 2005;91:948–53.
Velasquez G, Castaneda-Zuniga W, Formanek A, Zollikofer C, Barreto A, Nicoloff D, Amplatz K, Sullivan A. Nonsurgical aortoplasty in Leriche syndrome. Radiology. 1990;134:359–60.
Grollman Jr JH, Del Vicario M, Mittal AK. Percutaneous transluminal abdominal aortic angioplasty. AJR Am J Roentgenol. 1980;134:1053–4.
Tegtmeyer CJ, Wellons HA, Thompson RN. Balloon dilation of the abdominal aorta. JAMA. 1980;244:2636–7.
Heeney D, Bookstein J, Daniels E, Warmath M, Horn J, Rowley W. Transluminal angioplasty of the abdominal aorta: report of 6 cases in women. Radiology. 1983;148:81–3.
Tegtmeyer CJ, Kellum CD, Kron IL, Mentzer Jr RM. Percutaneous transluminal angioplasty in the region of the aortic bifurcation. The two-balloon technique with results and long-term follow-up study. Radiology. 1985;157:661–5.
Charlebois N, Saint-Georges G, Hudon G. Percutaneous transluminal angioplasty of the lower abdominal aorta. AJR Am J Roentgenol. 1986;146:369–71.
Morag B, Rubinstein Z, Kessler A, Schneiderman J, Levinkopf M, Bass A. Percutaneous transluminal angioplasty of the distal abdominal aorta and its bifurcation. Cardiovasc Intervent Radiol. 1987;10:129–33.
Yakes WF, Kumpe DA, Brown SB, Parker SH, Lattes RG, Cook PS, Haas DK, Gibson MD, Hopper KD, Reed MD. Percutaneous transluminal aortic angioplasty: techniques and results. Radiology. 1989;172:965–70.
Gross-Fengels W, Steinbrich W, Pichlmaier H, Erasmi H. Percutaneous transluminal angioplasty of the infrarenal abdominal aorta. Radiologe. 1990;30:235–41.
el Ashmaoui A, Do DD, Triller J, Stirnemann P, Mahler F. Angioplasty of the terminal aorta: follow-up of 20 patients treated by PTA or PTA with stents. Eur J Radiol. 1991;13:113–7.
Hallisey MJ, Meranze SG, Parker BC, Rholl KS, Miller WJ, Katzen BT, van Breda A. Percutaneous transluminal angioplasty of the abdominal aorta. J Vasc Interv Radiol. 1994;5:679–87.
Steinmetz OK, McPhail NV, Hajjar GE, Barber GG, Cole CW. Endarterectomy versus angioplasty in the treatment of localized stenosis of the abdominal aorta. Can J Surg. 1994;37:385–90.
Hedeman Joosten PP, Ho GH, Breuking Jr FA, Overtoom TT, Moll FL. Percutaneous transluminal angioplasty of the infrarenal aorta: initial outcome and long-term clinical and angiographic results. Eur J Vasc Endovasc Surg. 1996;12:201–6.
Westcott MA, Bonn J. Comparison of conventional angioplasty with the Palmaz stent in the treatment of abdominal aortic stenoses from the STAR registry. SCVIR transluminal angioplasty and revascularization. J Vasc Interv Radiol. 1998;9:225–31.
Therasse E, Cote G, Soulez G. Infrarenal aortic stenting with the Palmaz stent: midterm results. J Vasc Interv Radiol. 1989;9:169–70.
Long AL, Gaux JC, Raynaud AC, Faintuch JM, Pagny JY, Lacombe P, Fiessinger JN, Relland JY, Beyssen BM. Infrarenal aortic stents: initial clinical experience and angiographic follow-up. Cardiovasc Intervent Radiol. 1993;16:203–8.
Wolf YG, Schatz RA, Knowles HJ, Saeed M, Bernstein EF, Dilley RB. Initial experience with the Palmaz stent for aortoiliac stenoses. Ann Vasc Surg. 1993;7:254–61.
Yilmaz S, Sindel T, Yeğin A, Erdoğan A, Lüleci E. Primary stenting of focal atherosclerotic infrarenal aortic stenoses: long-term results in 13 patients and a literature review. Cardiovasc Intervent Radiol. 2004;27:121–8.
Audet P, Therasse E, Oliva VL, Soulez G, Côté G, Wistaff R, Nguyen PV, Blair JF, Bui BT, Cusson JR. Infrarenal aortic stenosis: long-term clinical and hemodynamic results of percutaneous transluminal angioplasty. Radiology. 1998;209:357–63.
Ravimandalam K, Rao VR, Kumar S, Gupta AK, Joseph S, Unni M, Rao AS. Obstruction of the infrarenal portion of the abdominal aorta: results of treatment with balloon angioplasty. AJR Am J Roentgenol. 1991;156:1257–60.
McPherson SJ, Laing AD, Thomson KR, Mitchell PJ, Milne P, Atkinson N, Cox G. Treatment of infrarenal aortic stenosis by stent placement: a 6-year experience. Australas Radiol. 1999;43(2):185–91.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Teruzzi, G., Bartorelli, A.L. (2014). Endovascular Management of Aortic Aneurysmal and Stenotic Disease. In: Thompson, C. (eds) Textbook of Cardiovascular Intervention. Springer, London. https://doi.org/10.1007/978-1-4471-4528-8_34
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4528-8_34
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4527-1
Online ISBN: 978-1-4471-4528-8
eBook Packages: MedicineMedicine (R0)