Abstract
Imaging modalities such as CT and magnetic resonance imaging (MRI) are powerful tools to detect and assess focal injury such as hemorrhagic lesions and edema and brain swelling in severe injury. However, acute and chronic injury at a cellular level is sometimes difficult to discern from normal features by anatomical imaging. Magnetic resonance spectroscopy (MRS) offers a unique noninvasive approach to assess injury at microscopic levels by quantifying cellular metabolites. The findings obtained with MRS in concussion and more severe head trauma are heterogeneous, reflecting the different time after injury, degree of injury and different physiologic and pathologic response of the brain to injury in individuals. The most important findings are that elevated lactate (and lipids) in apparently normal tissue observed 2–5 days after injury are indicators of severe global hypoxic injury and poor outcome. Also, N-acetylaspartate (NAA), a marker for “healthy” neurons and axons, is generally reduced in traumatic brain injury signaling neuronal and axonal loss/damage. The extent of NAA reduction after injury is an objective and quantitative surrogate marker for the severity of injury and is useful for outcome prediction. In the cases of mild traumatic brain injury, choline (Cho) has been shown to be reflective of diffuse axonal injury and alterations in neurotransmitters such as glutamate (Glu) and gamma amino butyric acid (GABA) have also been shown.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Traumatic Brain Injury
- Head Injury
- Magnetic Resonance Spectroscopy
- Severe Traumatic Brain Injury
- Mild Traumatic Brain Injury
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Traumatic brain injury (TBI) results when forces acting upon the head interrupt normal brain function. This type of injury is especially prevalent in children, resulting in over 475,000 emergency department visits each year [1]. In its most severe form, head trauma accounts for 80% of the traumas that lead to death in children, including falls, motor vehicle accidents, and abuse, making it one of the leading causes of death. In addition, “mild” traumatic brain injury (mTBI), otherwise known as concussions, are also a great concern, especially in children ages 0–4 years where the rates of head injury are highest compared to all ages, including adults. Sports-related head injuries are also becoming of increasing concern, especially in older children where 18% of head injuries were sports-related and where the long term effects are unknown.
Magnetic resonance spectroscopy (MRS) offers a unique tool for ascertaining the physiological changes that occur after a traumatic brain injury. What is the metabolic profile of the brain after traumatic injury? Studying head trauma with MRS, as with any tool, is inherently challenging. The type and severity of injury and the time after injury when an MRS study is performed will vary unavoidably. It is therefore expected that the pattern of MR spectroscopy of head trauma is heterogeneous reflecting the different location, degree, and stage of injury and different physiologic and pathologic response of the brain to injury in individuals. There is no single characteristic “finger print” for trauma. As studies emerge, it appears that the brain biochemistry is reflective of this spectrum of traumatic brain injury. As such, this chapter will focus on the metabolic changes observed in the acute and chronic stages of severe traumatic brain injury as well as the acute and chronic stages of mild traumatic brain injury.
Severe Traumatic Brain Injury
Traumatic Brain Injury Is (Almost) Always Associated with a Decrease of NAA in White Matter and Gray Matter
Reduced NAA (or NAA/Cr ratio) after traumatic injury due to diffuse axonal injury and neuronal loss has been consistently reported [2]. The qualifier, “almost”, should not be interpreted as an indicator that there is traumatic head injury without any neuronal/axonal loss. Slightly varying normal levels of NAA in individual subjects, inaccuracies of the MR method, and the absence of a pre-injury baseline scans make it difficult to detect small decreases of NAA in less severe injury [3]. Also, if MRS is carried out soon (within 24 h) after initial injury, a loss of NAA may not yet be established [4].
Is recovery of NAA a marker for healthy neurons and axons? If yes, what is the interpretation? Clinical as well as animal studies are indeed strongly suggestive for a recovery of NAA in both white and gray matter [5–7]. In patients with moderate to severe traumatic brain injury, a continuing decrease of NAA consistent with continuing neuronal loss (or metabolic dysfunction) was observed for gray and white matter at 1.5 and 3 months after injury in longitudinal studies. However, in both brain regions an increase of NAA, albeit to levels that were still less than normal, was observed at the 6-months follow-up study [5].
Assuming that there is no neurogenesis this observation is likely due to one or a combination of the following mechanisms:
-
(a)
Surviving neurons can sprout healthy fibers into the spaces once occupied by damaged axons. This increases the partial volume of healthy axons and will thus increase the measured NAA concentration. This mechanism would predict a more substantial recovery in fibrous white matter than in gray.
-
(b)
Neurons that reestablish their connections start to increase communication with each other. A tight coupling between cerebral glucose metabolism and (glutamate) neurotransmitter flux in humans has been proposed by Magistretti [8]. Although this theory is not universally accepted, it would explain a higher rate of glycolysis and TCA-cycle activity as neurons resume their communication with neighboring neurons. Since NAA reduction has been associated with mitochondrial dysfunction in experimental head trauma [9], it can at least be speculated that mitochondrial NAA synthesis may increase with a normalization of neuronal activity.
-
(c)
After injury, consolidation of remaining intact tissue and atrophy is well documented by neuropathological studies and by cortical atrophy on MR images. Thus the relative number of healthy neurons and axons per tissue volume will actually increase and a more prominent NAA peak will be observed.
-
(d)
NAA can increase or decrease in response to hyper-osmolar or hypo-osmolar states.
Total Choline Is Elevated
Most of the choline in human brain is stored in large, water insoluble molecules and rendered MR invisible under normal circumstances. Elevated total choline would be expected in head trauma because of at least three mechanisms:
-
(a)
In acute injury, choline-containing metabolites may be released to the MR-visible pool as a result of shear injury and damage to cell membranes and myelin. It is known that free choline accumulates rapidly in necrotic tissue [10, 11]. This mechanism therefore offers an explanation for elevated choline in acute and sub-acute injury. In the spectrum of acute and severe injury (Fig. 7.1), tCho was 35% above normal. Free choline can be taken up by the cells and recycled to form phosphatidylcholine (PtdCho).
Fig. 7.1 The MRS and T2-weighted MRI of a child (17 months old at time of event) 4 days after traumatic brain injury (left) and 2½ years after injury (right). The MRI report at baseline mentions an increase of signal intensity in the parietal lobes at the vertex bilaterally. The sulci were effaced in that region as well. MRS changes were dramatic. NAA was reduced to 35% of normal and lactate, not detectable in normal brain, was strikingly prominent. Cr and mI were also reduced to 75% of normal whereas tCho was elevated (135% of normal). Glutamine was threefold higher than normal. There is only a small increase in the lipid signal at this early stage after injury. MRS is consistent with severe hypoxic injury subsequent to low/disrupted perfusion and poor outcome was predicted in the MRS report. Follow-up MRI shows general volume loss. The ROI selected for MRS is similar to the anatomy chosen for the initial study. Due to the massive atrophy little brain tissue is included and the spectrum shows lactate (possible from CSF) and only traces of creatine and choline. No trace of NAA is detected indicating that any tissue within the ROI does not contain viable neurons or axons. Clinical outcome was poor (nonverbal, seizures, dystonia, spasticity, profound cognitive loss). Spectra were acquired using a PRESS sequence with TE = 35 ms. at Children’s Hospital Los Angeles
-
(b)
Increased synthesis of cell membranes during repair might result in higher levels of total choline. For example, both the newborn (fast growing) brain and many tumors with a high rate of cell duplication have elevated total choline indicating up-regulation of membrane precursor production. Of the choline containing compounds, it is now phosphocholine (PC) which is expected to be above normal. Unfortunately the MR signal of free choline and PC cannot be separated with proton MRS in vivo. Proton-decoupled phosphorous MRS would be required for this task, which is available only at very few sites.
-
(c)
In patients with more chronic injury, an alternative explanation could be diffuse glial proliferation which is known to be associated with increased tCho but also with elevated mI and Cr [12–14]. Indeed, Ross et al. [15] observed in some subjects elevated mI, tCho, and Cr persistently in white matter even 18 months after injury. In that study mI, tCho, and Cr were essentially normal in gray matter. This would be consistent with glial proliferation predominantly in the white matter. The authors of that study also offered as an alternative explanation for the generally elevated metabolite concentration: the possibility that the white matter is in a hyper-osmolar state.
Indeed, elevated tCho or tCho/Cr ratios were reported by a number of investigators [5, 15–19]. In longitudinal patient studies, tCho was significantly increased at 1.5 months for both gray and white matter and remained elevated 3 and 6 months after injury, with a trend (not significant) to lower tCho concentrations [5].
Magnetic Resonance Spectroscopy of Acute Severe Brain Injury with Disruption or Significantly Impaired Perfusion Resembles That of Hypoxic Brain Injury and Lactate Is a Predictor for Poor Outcome
In acute and severe injury, increased lactate and lipids also accompanies the reduction of NAA in gray and white matter ROI’s are markers of neuronal/axonal loss and cell death. Lactate is the product of anaerobic glycolysis and increases when subsequent oxidation of lactate in the TCA-cycle is impaired (for example by lack of oxygen or mitochondrial disorders). As a result, the MRS pattern of lactate resembles that of hypoxic injury due to global anoxia (Fig. 7.1). Several groups found that lactate found in acute injury may be useful to predict outcome [15, 17, 20–22].
Lipid signals increase when there is breakdown of cell membrane and release of fatty acids. Lipids are therefore important markers for severe brain injury. Lipids appear to be more prominent in children. Particularly prominent lipid peaks were reported by Haseler et al. [21] in “shaken babies” with poor outcome.
Concentrations of Cerebral Metabolites Are Reduced in Patients with Clinically Diagnosed Syndrome of Inappropriate Antidiuretic Hormone Secretion and Low Serum Sodium
Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) is a frequently observed feature of head injury. Because the metabolites of the 1H spectrum can also function (to variable degrees) as osmolytes, systemic changes can be observed. Absolute quantitation of metabolite concentrations is necessary to depict this condition because a reduction of all metabolites is not apparent when peak ratios are analyzed. The reduction persisted weeks after Na + returned to normal [15].
MRS Can Detect Widespread Injury: In Radiologically Normal Appearing Tissue!
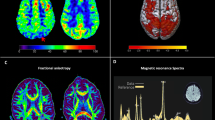
MRS confirms that traumatic brain injury is associated with damage at the microscopic level throughout the brain. This has been—unintentionally—confirmed by all those single-voxel MRS studies of normal appearing tissue where investigators selected different regions of interest (although most investigators pick well established parietal white matter and occipital gray matter locations). Spectral abnormalities were reported in all those studies. Obviously, it is more elegant to employ CSI where widespread metabolic abnormalities apparently affecting radiologically normal appearing tissue is readily detectable in individual subjects (Fig. 7.2). Widespread injury is consistent with the fact that recovery of patients is not well explained by purely focal injury. Rather, it is more likely that the overall behavioral recovery is related to the severity and location of the injury averaged over the whole brain.
Three Tesla magnetic resonance spectroscopic imaging data (STEAM, TE = 20 ms) in traumatic brain injury. The data in the right panels were acquired from a 20-year-old male patient who had suffered a motor-cycle accident (Glasgow Coma Scale = 3) 84 days previously. Although the primary site of injury was frontal, the metabolite images show widespread decreased N-acetyl-aspartate and elevated choline and myo-inositol throughout this radiologically normal-appearing brain slice. Comparison metabolite data from an uninjured 27-year-old male are shown in the left panels. The color map corresponds to metabolite concentrations expressed in institutional units (unpublished data, Hoglund Imaging Center, University of Kansas Medical Center)
Returning to the question of where to measure, the added value of measuring the metabolic profile of apparently normal tissue has been demonstrated by Holshouser et al. [23]. They used susceptibility (T2*) weighted MRI to depict regions of non-hemorrhagic and of hemorrhagic tissue after injury. CSI was then utilized to analyze biochemical changes in hemorrhagic and non-hemorrhagic regions. They found that biochemical changes in apparently normal appearing tissue predict outcome better than alterations in lesions.
The selection of an appropriate region of interest is another problem. As mentioned above, the metabolic fingerprint of tissue depends on tissue type. Accuracy in prescribing a region of interest (and proper documentation for longitudinal studies!) is of great importance in particular for single voxel studies. It is therefore recommended to study brain regions where MRS works and where normal MRS data are readily available for comparison. Two very popular choices are parietal white matter and occipital gray matter which have been studied frequently in head trauma with single voxel MRS [5, 15, 21]. MR spectra of normal gray matter and white matter differ slightly. NAA is present in approximately equal concentration. Creatine is ≈20% higher in gray matter whereas tCho is slightly higher in white matter. Focusing on a small number of well-studied regions may be less of a limitation than one might think. As shown below, alterations of the metabolic state throughout the brain have been observed in traumatic brain injury. When studying head trauma (with single-voxel MRS) one should also avoid acquiring MR spectra from ROIs with obvious focal injury for two reasons: (1) It is most likely, and unsurprising, that metabolism will be abnormal in visibly injured areas and hence MRS does not add very much to MRI. (2) A prerequisite for good quality spectra is a highly homogeneous magnetic field within the region of interest. The presence of blood or blood products, often associated with lesions in TBI, reduces the homogeneity of the local magnetic field due to iron accumulation. Accurate prescription of the region of interest is less a problem for CSI where spectra from a whole slice are being obtained and the position of individual voxels can be adjusted retrospectively via voxel shifting. However, currently CSI is less reproducible than single voxel methods, particularly at the short echo times necessary to evaluate glutamate levels.
Magnetic Resonance Spectroscopy, a Predictor of Outcome?
Considering that the extent of the decrease of NAA can be seen as a quantitative marker for neuronal loss, questions arise whether MRS can be used to predict outcome and if so, at what (earliest) time after injury can prognostic information be obtained. Significant reduction of NAA, the presence of lipids and elevated lactate are markers of severe (hypoxic) brain injury and MRS as early as 2–5 days after injury (Fig. 7.1) might be a useful tool for triage of patients who remain unconscious several days after injury [15, 17, 20–22]. In less devastating injury, Friedman et al. [18] found that NAA concentrations in occipital gray matter measured 1½ months after injury predicted overall neuropsychological performance measured at 6 months after injury (Fig. 7.3) and correlated with the Glasgow Outcome Score (GOS).
NAA concentrations at 1.5 months after injury versus the composite neuropsychological z-score. A significant correlation was found. Patients with lower NAA concentration have significantly poorer overall cognitive function. (Figure provided by Seth Friedman Ph.D. and reproduced with permission from S.D. Friedman, W.M. Brooks, R.E. Jung, et al. Quantitative proton MRS predicts outcome after traumatic brain injury Neurology 1999;52(7):1384–1391, with permission.)
Other measures of metabolites were not predictive in this study. The prognostic value of MRS in occipital gray matter alone is quite remarkable, considering that other important parts of the brain sensitive to shearing injury were not evaluated in this study. For example, marked decreases of NAA have been reported for sites such as the corpus callosum [24] and frontal lobe [16, 19, 25]. Still, in this cross-sectional study considerable differences of NAA were observed in patients with comparable neuropsychological scores. This cannot be seen as evidence that NAA is an imperfect marker for neuronal loss. As mentioned above, variations of baseline NAA levels (and baseline neurological function) are obviously not accounted for. Therefore the loss of NAA, which is supposedly proportional to the loss of neurons, can only be determined relative to mean NAA in a control group. One wonders whether subjects with high risk for head injury should be studied with prospective MRS to obtain baseline values for NAA.
Another study by Shutter et al. [26] has demonstrated that other metabolites such as glutamate and glutamine and choline have shown predictive value, particularly when incorporating different metabolites from different areas of the brain. For example, GOS at 1 month was best predicted from ratios of NAA/Cr and Glx/Cr from the posterior white matter with an accuracy of 78% in comparison with motor GCS, which had an accuracy of 62%. However, GOS at 6 months was best predicted with NAA/Cho and Cho/Cr from the posterior white matter combined with Glx/Cr from the occipital gray matter, increasing the accuracy up to 94% compared with motor GCS which remained low at 67% at 6 months. It is also important to note, however, that MRS is not a replacement for clinical evaluation as this same study found that the combination of MRS ratios and motor GCS provided the most accurate prediction of outcome (97%) when utilized together. This demonstrates that MRS is highly complementary to existing TBI measures and used in conjunction may provide the greatest diagnostic value to TBI victims.
Mild Traumatic Brain Injury
Mild traumatic brain injury (mTBI) is defined by the Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine as a TBI where the severity of the injury does not exceed the following [27]:
-
Posttraumatic amnesia not greater than 24 h
-
After 30 min, an initial Glasgow Coma Scale (GCS) of 13–15
-
Loss of consciousness of approximately 30 min or less
In addition, persons that have undergone mild TBI will suffer at least one of the following conditions: any period of loss of consciousness; any loss of memory for events immediately before or after the accident; any alteration in mental state at the time of the accident (e.g., feeling dazed, disoriented, or confused); and focal neurological deficit(s) that may or may not be transient.
Mild traumatic brain injury is the most prevalent form of TBI, accounting for approximately 70% of the TBI that occur. Furthermore, 15–30% of mild TBI are so-called malingering TBI where symptoms persist beyond 1 year or more. Even though this type of TBI is called “mild”, the effect on the family and the injured person can be significant, resulting in symptoms such as headache, difficulty thinking, memory problems, attention deficits, mood swings, and frustration.
While a majority of the MR spectroscopy studies have focused on severe traumatic brain injury, recent literature has increasingly focused on this more mild form of brain injury, showing long-term effect sometimes decades after the initial injury. Table 7.1 summarizes some of the major studies of mild TBI.
In summary, these mTBI studies find that NAA, a putative neuronal/axonal marker, is decreased in white matter (WM) regions such as the splenium, centrum semiovale, and frontal white matter. Decreases in NAA have been found in other diseases as well [28] and although there is some debate, it is generally accepted in the literature as an indicator of neuronal/axonal health. These studies show that in mTBI, there is definitive axonal/neuronal loss particularly in the WM where fiber tracts are found which correlates well with diffusion tensor imaging studies that have found similar results [29]. Secondary findings such as changes in choline, a marker for cellular proliferation or tissue damage, are inconsistent throughout the studies. It is important to note that some studies have shown changes in creatine, an energy marker but more importantly a commonly used internal reference used for metabolite ratios (i.e., NAA/Cr, Cho/Cr). The assumption is that Cr is generally unchanged in normal brain tissue however if it is affected by mTBI, metabolite ratio measurements would not be accurate as it would be difficult to assess if changes in ratios is due to the metabolite of interest or Cr itself.
Acute Sports-Related Concussion
An estimated 1.8–3.8 million sports-related concussions occur each year in the United States [30]. There is increasing recognition of immediate and long-term neurological problems from these injuries, including headaches, dizziness, behavioral changes, and problems with memory and attention [31]. Some evidence is being accumulated on neurochemical effects of head trauma in general and concussion in particular, including a number of changes in key neurochemical levels in the brain (see below).
Sports-related concussions are a major problem in pediatrics [32]; according to the Center for Disease Control, 10–15 year olds have the highest rate of SRC compared to other age groups [33], and studies have shown that younger athletes require longer recovery times [34]. Despite the magnitude of the clinical problem, there is no information on the neurochemical effects of concussion in this age group, which may be pronounced due to their earlier developmental stage.
Sports-related TBI are generally milder head injuries. To date, there are only three studies that have focused specifically on sports-related TBI: Cimatti et al. [35] found that NAA decreased in two of six adult athletes after head trauma. Henry et al. [36] showed a significant decrease in Glx and NAA in the primary motor cortex and NAA in the prefrontal cortex in 14 concussed compared with non-concussed athletes (ages 20–25 years). Vagnozzi et al. [37] showed in 13 concussed athletes (ages 22–25) a decrease of 18.5% in NAA, with modest recovery at 15 days and full recovery at 30 days. Most importantly, this study also showed that in those with second concussive injuries that took up to 45 days to fully recover, NAA levels did not seem to return to the same level as in those with a single head injury (Fig. 7.4). This has significant implications for the hypothesized cumulative effects of head injury. Unfortunately there are no studies that have been conducted in children and therefore it is unclear whether the same mechanisms are invoked after head injury.
Scatterplot demonstrating the different time course of NAA recovery in athletes having single or double concussive injury, as expressed by the NAA-to-Cr ratio determined by 1 H-MR spectroscopy. The second concussion (occurring between the 10th and the 13th d after the first insult) either provoked a further slight NAA decrease or significantly delayed the process of NAA restoration, which was completed at 45 days instead of at 30 days post-injury. (From Vagnozzi R, Signoretti S, Tavazzi B, et al. Temporal Window of Metabolic Brain Vulnerability to Concussion: A Pilot 1 H-Magnetic Resonance Spectroscopic Study in Concussed Athletes-Part III. Neurosurgery. 2008;62(6):1286–1296, with permission.)
Chronic Sports-Related Head Injury
It is believed that the cumulative head trauma experienced by these athletes is responsible for neurodegenerative changes, which, over time, result in a progressive decline of memory and executive functioning [38]; mood and behavioral disturbances that include depression, apathy, impulsivity, anger, irritability, suicidal behavior [39], and aggressiveness [40, 41]; gait changes that resemble Parkinsonism [40]; and, eventually, progression to dementia [40]. Previously referred to as dementia pugilistica due to its strong association with boxing [42, 43], the chronic effects of head injury has been identified as chronic traumatic encephalopathy (CTE). Autopsy studies by the Boston University Center for the Study of Traumatic Encephalopathy (BUCSTE) and others also reveal a distinct pattern of neuropathological changes in CTE, including: extensive tau-immunoreactive inclusions scattered throughout the cerebral cortex in a patchy, superficial distribution, with focal epicenters at the depths of sulci and around the cerebral vasculature; extensive tau-immunoreactive inclusions in limbic and paralimbic regions as well as brainstem nuclei; generalized atrophy and enlarged ventricles; specific atrophy of the frontal and medial temporal lobes; degenerations of white matter fiber bundles; cavum septum pellucidum, often with fenestrations; thinning of the hypothalamic floor and shrinkage of the mammillary bodies; and a relative absence of beta-amyloid (Aβ) deposits. [40] The BUCSTE has examined over 24 brains of deceased athletes. Neuropathological evidence of CTE has been found in 12 of 12 football players examined to date, three of whom only played in high school and college. CTE was also found in a deceased former professional hockey player, and we have found the initial evidence of the disease in an 18-year-old boy who died after having had multiple sports-related concussions. These initial findings suggest that CTE may be more common than previously known, and may develop without involvement in professional sports. Given the millions of youth, high school, and collegiate athletes participating in contact sports involving head trauma, CTE may represent an important and previously under-recognized public health issue.
Recently MRS was used to study neurochemical levels in five professional athletes, ages 31–54 years, with histories of concussions and subconcussive trauma. The higher Cho and Glx are statistically significant. In 1D MRS many of the resonances are composite or overlap. Two dimensional (2D) MRS, COrrelated SpectroscopY (COSY), can be employed to separate them in a second frequency [44, 45] (Fig. 7.5). While it is possible to use spectral editing techniques to identify metabolites that overlap in 1D MRS, the advantage of the 2D COSY method is that the second frequency reveals all chemical species within a single scan [46].
Representative spectrum of conventional 1D MRS (top) and 2D COSY MRS (bottom) of a 34-year-old male professional motorcross racer with a long history of repetitive head injury starting at age 9 and through high school and college. Symptoms of CTE include memory loss, anger issues, and confusion. Data acquired at Brigham and Women’s Hospital
In addition to the Cho and Glx changes, the following neurochemicals have been shown to be involved in severe traumatic brain injury, and can be measured with 2D COSY:
-
Aspartate (Asp): An excitatory amino acid released into extracellular space after head injury [47].
-
Possibly threonine (Thr): A structural amino acid that appears to be released when tissue is damaged [48].
-
Lipids (Lip) and macromolecules (mac): Acute brain injury initiates a metabolic cascade that includes activation of phospholipase resulting in the accumulation of lipid and macromolecules [49].
-
Possibly gamma-aminobutyric acid (GABA) and histidine (His): Both are inhibitory neurotransmitters. GABA is initially increased in the brain after head injury and thought to play a neuroprotective role [50]. Histidine has been shown to be excreted for an extended period of time after head injury [46, 51].
It is thought that blows to the skull cause an immediate disruption of neuronal membranes resulting in a massive efflux of potassium into extracellular space that triggers the calcium-dependent release of excitatory amino acids, further stimulating potassium efflux and resulting in a cascade of neurochemical effects on both the acute and long-term level [32]. The ability to measure these metabolites in vivo and noninvasively will provide an important window into the molecular pathophysiology of pediatric sports concussion.
Conclusions
MRS is a unique non-invasive method to study metabolism of tissue in vivo. Proton spectroscopy and in particular the quantitation of NAA, lactate, total choline, and lipids provide unique information about the status of the brain following acute and chronic head trauma.
References
Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20(3):229–38.
Brooks WM, Friedman SD, Gasparovic C. Magnetic resonance spectroscopy in traumatic brain injury. J Head Trauma Rehabil. 2001;16(2):149–64.
Govindaraju V, Gauger GE, Manley GT, Ebel A, Meeker M, Maudsley AA. Volumetric proton spectroscopic imaging of mild traumatic brain injury. AJNR Am J Neuroradiol. 2004;25(5):730–7.
Macmillan CS, Wild JM, Wardlaw JM, Andrews PJ, Marshall I, Easton VJ. Traumatic brain injury and subarachnoid hemorrhage: in vivo occult pathology demonstrated by magnetic resonance spectroscopy may not be “ischaemic”. A primary study and review of the literature. Acta Neurochir (Wien). 2002;144(9):853–62. discussion 862.
Brooks WM, Stidley CA, Petropoulos H, et al. Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J Neurotrauma. 2000;17(8):629–40.
Gasparovic C, Arfai N, Smid N, Feeney DM. Decrease and recovery of N-acetylaspartate/creatine in rat brain remote from focal injury. J Neurotrauma. 2001;18(3):241–6.
Schuhmann MU, Stiller D, Skardelly M, Thomas S, Samii M, Brinker T. Long-time in-vivo metabolic monitoring following experimental brain contusion using proton magnetic resonance spectroscopy. Acta Neurochir Suppl. 2002;81:209–12.
Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283(5401):496–7.
Signoretti S, Marmarou A, Tavazzi B, Lazzarino G, Beaumont A, Vagnozzi R. N-Acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. J Neurotrauma. 2001;18(10):977–91.
Miller BL. A review of chemical issues in 1 H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991;4(2):47–52.
Jope RS, Jenden DJ. Choline and phospholipid metabolism and the synthesis of acetylcholine in rat brain. J Neurosci Res. 1979;4(1):69–82.
Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15(3–5):289–98.
Badar-Goffer RS, Ben-Yoseph O, Bachelard HS, Morris PG. Neuronal-glial metabolism under depolarizing conditions. A 13C-n.m.r. study. Biochem J. 1992;282(Pt 1):225–30.
Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med. 1999; 41(2):276–84.
Ross BD, Ernst T, Kreis R, et al. 1 H MRS in acute traumatic brain injury. J Magn Reson Imaging. 1998;8(4):829–40.
Garnett MR, Blamire AM, Corkill RG, Cadoux-Hudson TA, Rajagopalan B, Styles P. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain. 2000;123(Pt 10):2046–54.
Holshouser BA, Ashwal S, Shu S, Hinshaw Jr DB. Proton MR spectroscopy in children with acute brain injury: comparison of short and long echo time acquisitions. J Magn Reson Imaging. 2000;11(1):9–19.
Friedman SD, Brooks WM, Jung RE, et al. Quantitative proton MRS predicts outcome after traumatic brain injury. Neurology. 1999;52(7):1384–91.
Ricci R, Barbarella G, Musi P, Boldrini P, Trevisan C, Basaglia N. Localised proton MR spectroscopy of brain metabolism changes in vegetative patients. Neuroradiology. 1997;39(5):313–9.
Holshouser BA, Ashwal S, Luh GY, et al. Proton MR spectroscopy after acute central nervous system injury: outcome prediction in neonates, infants, and children. Radiology. 1997;202(2):487–96.
Haseler LJ, Arcinue E, Danielsen ER, Bluml S, Ross BD. Evidence from proton magnetic resonance spectroscopy for a metabolic cascade of neuronal damage in shaken baby syndrome. Pediatrics. 1997;99(1):4–14.
Condon B, Oluoch-Olunya D, Hadley D, Teasdale G, Wagstaff A. Early 1 H magnetic resonance spectroscopy of acute head injury: four cases. J Neurotrauma. 1998;15(8):563–71.
Holshouser BA, Tong KA, Ashwal S. Proton MR spectroscopic imaging depicts diffuse axonal injury in children with traumatic brain injury. AJNR Am J Neuroradiol. 2005;26(5):1276–85.
Cecil KM, Hills EC, Sandel ME, et al. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J Neurosurg. 1998;88(5):795–801.
Choe BY, Suh TS, Choi KH, Shinn KS, Park CK, Kang JK. Neuronal dysfunction in patients with closed head injury evaluated by in vivo 1 H magnetic resonance spectroscopy. Invest Radiol. 1995;30(8):502–6.
Shutter L, Tong KA, Holshouser BA. Proton MRS in acute traumatic brain injury: role for glutamate/glutamine and choline for outcome prediction. J Neurotrauma. 2004;21(12):1693–705.
Kay T, Harrington DE, et al. Definition of mild traumatic brain injury. J Head Trauma Rehabilitat. 1993;8(3):86–7.
Lin A, Ross BD, Harris K, Wong W. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx. 2005;2(2):197–214.
Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(10):2508–19.
Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–8.
Iverson GL, Gaetz M, Lovell MR, Collins MW. Cumulative effects of concussion in amateur athletes. Brain Inj. 2004;18(5):433–43.
Meehan 3rd WP, Bachur RG. Sport-related concussion. Pediatrics. 2009;123(1):114–23.
CDC. Nonfatal traumatic brain injuries from sports and recreation activities–United States, 2001–2005. MMWR. 2007;56(29):733–7.
Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546–53.
Cimatti M. Assessment of metabolic cerebral damage using proton magnetic resonance spectroscopy in mild traumatic brain injury. J Neurosurg Sci. 2006;50(4):83–8.
Henry LC, Tremblay SB, Boulanger Y, Ellemberg D, Lassonde M. Neurometabolic changes in the acute phase following sports concussions correlate with symptom severity. J Neurotrauma. 2009;27:65–76.
Vagnozzi R, Signoretti S, Tavazzi B, et al. Temporal window of metabolic brain vulnerability to concussion: A pilot 1 H-magnetic resonance spectroscopic study in concussed athletes-Part III. Neurosurgery. 2008;62(6):1286–96.
Belanger HG, Spiegel E, Vanderploeg RD. Neuropsychological performance following a history of multiple self-reported concussions: A meta-analysis. J Int Neuropsychol Soc. 2009;11:1–6.
Omalu BI, Bailes J, Hammers JL, Fitzsimmons RP. Chronic traumatic encephalopathy, suicides and parasuicides in professional American athletes: The role of the forensic pathologist. Am J Forensic Med Pathol. 2010;31:130–2.
McKee A, Cantu R, Nowinski C, et al. Chronic traumatic encephalopathy in athletes. J Neuropath and Exp Neurol. 2009;68:709–35.
Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903–9.
Martland HS. PUNCH DRUNK. J Am Med Assoc. 1928;91(15):1103–7.
Millspaugh J. Dementia Pugilistica. US Naval Medical Bulletin. 1937;35(3):297–303.
Ramadan S, Mountford CE. Two-dimensional magnetic resonance spectroscopy on biopsy and in vivo. In: Webb GA, editor. Annual reports on NMR spectroscopy, vol. 65. Burlington: Academic; 2009. p. 161–99.
Thomas MA, Hattori N, Umeda M, Sawada T, Naruse S. Evaluation of two-dimensional L-COSY and JPRESS using a 3 T MRI scanner: from phantoms to human brain in vivo. NMR Biomed. 2003;16(5):245–51.
Lin A, Ramadan S, Stanwell P, et al. In vivo L-COSY MR distinguishes glutamate from glutamine and shows neuropathic pain to cause a buildup of glutamine in the brain. Proc Int Soc Magn Reson Med. 2010;18:381.
Gopinath SP, Valadka AB, Goodman JC, Robertson CS. Extracellular glutamate and aspartate in head injured patients. Acta Neurochir. 2000;76:437–8.
Bullock R, Zauner A, Woodward JJ, et al. Factors affecting excitatory amino acid release following severe human head injury. J Neurosurg. 1998;89(4):507–18.
Shohami E, Shapira Y, Yadid G, Reisfeld N, Yedgar S. Brain phospholipase A2 is activated after experimental closed head injury in the rat. J Neurochem. 1989;53(5):1541–6.
Nilsson P, Hillered L, Ponten U, Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J Cereb Blood Flow Metab. 1990;10(5):631–7.
Deutschman CS, Konstantinides FN, Raup S, Thienprasit P, Cerra FB. Physiological and metabolic response to isolated closed-head injury. Part 1: Basal metabolic state: correlations of metabolic and physiological parameters with fasting and stressed controls. J Neurosurg. 1986;64(1):89–98.
Cecil KM, Hills EC, Sandel ME, et al. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J Neurosurg. 1998;88(5):795–801.
Garnett MR, Blamire AM, Rajagopalan B, Styles P, Cadoux-Hudson TAD. Evidence for cellular damage in normal-appearing white matter correlates with injury severity in patients following traumatic brain injury: a magnetic resonance spectroscopy study. Brain. 2000;123(7):1403–9.
Govindaraju V, Gauger GE, Manley GT, Ebel A, Meeker M, Maudsley AA. Volumetric proton spectroscopic imaging of mild traumatic brain injury. Am J Neuroradiol. 2004;25(5):730–7.
Kirov I, Fleysher L, Babb JS, Silver JM, Grossman RI, Gonen O. Characterizing ‘mild’ in traumatic brain injury with proton MR spectroscopy in the thalamus: Initial findings. Vol 21: Informa Healthcare; 2007:1147–1154.
Cohen BA, Inglese M, Rusinek H, Babb JS, Grossman RI, Gonen O. Proton MR spectroscopy and MRI-volumetry in mild traumatic brain injury. Am J Neuroradiol. 2007;28(5):907–13.
Nakabayashi M, Suzaki S, Tomita H. Neural injury and recovery near cortical contusions: a clinical magnetic resonance spectroscopy study. J Neurosurg. 2007;106(3):370–7.
Gasparovic C, Yeo R, Mannell M, et al. Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: a 1H Magnetic resonance spectroscopy study. J Neurotrauma. 2009;26:1635–43.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Lin, A.P., Blüml, S. (2013). Traumatic Brain Injury and Concussion. In: Blüml, S., Panigrahy, A. (eds) MR Spectroscopy of Pediatric Brain Disorders. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-5864-8_7
Download citation
DOI: https://doi.org/10.1007/978-1-4419-5864-8_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-5863-1
Online ISBN: 978-1-4419-5864-8
eBook Packages: MedicineMedicine (R0)