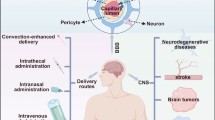
Abstract
Multiple sclerosis (MS) is a neurodegenerative autoimmune disorder of the central nervous system (CNS) infecting 2.5 million people worldwide. It is the most common nontraumatic neurological impairment in young adults. The blood–brain barrier rupture for multiple sclerosis pathogenesis has two effects: first, during the onset of the immunological attack, and second, for the CNS self-sustained “inside–out” demyelination and neurodegeneration processes. In addition to genetic variations, environmental and lifestyle variables can also significantly increase the risk of developing MS. Dimethyl fumarate (DMF) and sphingosine-1-phosphate (S1P) receptor modulators that may pass the blood–brain barrier and have positive direct effects in the CNS with quite diverse mechanisms of action raise the possibility that a combination therapy could be successful in treating MS. Lipid nanocarriers are recognized as one of the best drug delivery techniques to the brain for effective brain delivery. Numerous scientific studies have shown that lipid nanoparticles can enhance the lipid solubility, oral bioavailability, and brain availability of the drugs. Nanolipidic carriers for DMF delivery could be derived through vitamin D, tocopherol acetate, stearic acid, quercetin, cell-mimicking platelet-based, and chitosan–alginate core–shell–corona-shaped nanoparticles. Clinical and laboratory diagnosis of MS can be performed mainly through magnetic resonance imaging. The advancements in nanotechnology have enabled the clinicians to cross the blood–brain barrier and to target the brain and central nervous system of the patient with multiple sclerosis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Torkildsen O, Myhr KM, Bø L (2016) Disease-modifying treatments for multiple sclerosis - a review of approved medications. Eur J Neurol 23 Suppl 1:18–27. https://doi.org/10.1111/ENE.12883
Lassmann H, Brück W, Lucchinetti C, Rodriguez M (1997) Remyelination in multiple sclerosis. Mult Scler 3:133–136. https://doi.org/10.1177/135245859700300213
Kumar P, Sharma G, Kumar R et al (2016) Promises of a biocompatible nanocarrier in improved brain delivery of quercetin: biochemical, pharmacokinetic and biodistribution evidences. Int J Pharm 515:307–314. https://doi.org/10.1016/j.ijpharm.2016.10.024
Ortiz GG, Pacheco-Moisés FP, Macías-Islas MÁ et al (2014) Role of the blood-brain barrier in multiple sclerosis. Arch Med Res 45:687–697. https://doi.org/10.1016/j.arcmed.2014.11.013
Nicholas JA, Lee Boster A, Imitola J et al (2014) Design of oral agents for the management of multiple sclerosis: benefit and risk assessment for dimethyl fumarate. Drug Des Devel Ther 8:897–908. https://doi.org/10.2147/DDDT.S50962
Meissner M, Valesky EM, Kippenberger S, Kaufmann R (2012) Dimethyl fumarate - only an anti-psoriatic medication? J Dtsch Dermatol Ges 10:793–801. https://doi.org/10.1111/J.1610-0387.2012.07996.X
Deshmukh P, Unni S, Krishnappa G, Padmanabhan B (2017) The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys Rev 9:41–56. https://doi.org/10.1007/S12551-016-0244-4
Kumar P, Sharma G, Kumar R et al (2017) Enhanced brain delivery of dimethyl fumarate employing tocopherol-acetate-based nanolipidic carriers: evidence from pharmacokinetic, biodistribution, and cellular uptake studies. ACS Chem Neurosci 8:860–865. https://doi.org/10.1021/ACSCHEMNEURO.6B00428
Kumar P, Sharma G, Kumar R et al (2017) Stearic acid based, systematically designed oral lipid nanoparticles for enhanced brain delivery of dimethyl fumarate. Nanomedicine 12:2607–2621. https://doi.org/10.2217/nnm-2017-0082
Franco-Pons N, Torrente M, Colomina MT, Vilella E (2007) Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol Lett 169:205–213. https://doi.org/10.1016/j.toxlet.2007.01.010
Brain targeting drug delivery systems for the management of brain disorders: molecular targets and nanotechnological strategies - Google Search
Dighriri IM, Aldalbahi AA, Albeladi F et al (2023) An overview of the history, pathophysiology, and pharmacological interventions of multiple sclerosis. Cureus 15. https://doi.org/10.7759/CUREUS.33242
Wong AD, Ye M, Levy AF et al (2013) The blood-brain barrier: an engineering perspective. Front Neuroeng 6:7. https://doi.org/10.3389/FNENG.2013.00007/ABSTRACT
Spencer JI, Bell JS, DeLuca GC (2018) Vascular pathology in multiple sclerosis: reframing pathogenesis around the blood-brain barrier. J Neurol Neurosurg Psychiatry 89:42–52. https://doi.org/10.1136/JNNP-2017-316011
Charabati M, Wheeler MA, Weiner HL, Quintana FJ (2023) Multiple sclerosis: neuroimmune crosstalk and therapeutic targeting. Cell 186:1309–1327. https://doi.org/10.1016/J.CELL.2023.03.008
Merjane J, Chung R, Patani R, Lisowski L (2023) Molecular mechanisms of amyotrophic lateral sclerosis as broad therapeutic targets for gene therapy applications utilizing adeno-associated viral vectors. Med Res Rev. https://doi.org/10.1002/MED.21937
de Sèze J, Maillart E, Gueguen A et al (2023) Anti-CD20 therapies in multiple sclerosis: from pathology to the clinic. Front Immunol 14. https://doi.org/10.3389/FIMMU.2023.1004795
Graham EL (2023) Neuroimmunological disorders: the gender effect. Neurol Clin 41. https://doi.org/10.1016/J.NCL.2022.10.004
Lassmann H, Van Horssen J, Mahad D (2012) Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol 8:647–656. https://doi.org/10.1038/nrneurol.2012.168
Zéphir H (2018) Progress in understanding the pathophysiology of multiple sclerosis. Rev Neurol (Paris) 174:358–363. https://doi.org/10.1016/j.neurol.2018.03.006
Balasa R, Barcutean L, Mosora O, Manu D (2021) Reviewing the significance of blood–brain barrier disruption in multiple sclerosis pathology and treatment. Int J Mol Sci 22:8370. https://doi.org/10.3390/IJMS22168370
Goldman MD, Ward M, Goldman MD (2022) Epidemiology and pathophysiology of multiple sclerosis. Contin Lifelong Learn Neurol 28:988–1005. https://doi.org/10.1212/CON.0000000000001136
Ward M, Goldman MD (2022) Epidemiology and pathophysiology of multiple sclerosis. Continuum (Minneap Minn) 28:988–1005. 1 - Google Search
Maier S, Barcutean L, Andone S et al (2023) Recent progress in the identification of early transition biomarkers from relapsing-remitting to progressive multiple sclerosis. Int J Mol Sci 24. https://doi.org/10.3390/IJMS24054375
Freeman L, Longbrake EE, Coyle PK et al (2022) High-efficacy therapies for treatment-Naïve individuals with relapsing-remitting multiple sclerosis. CNS Drugs 36:1285–1299. https://doi.org/10.1007/S40263-022-00965-7
Ransohoff RM (2023) Multiple sclerosis: role of meningeal lymphoid aggregates in progression independent of relapse activity. Trends Immunol 44. https://doi.org/10.1016/J.IT.2023.02.002
Gilli F, Ceccarelli A (2023) Magnetic resonance imaging approaches for studying mouse models of multiple sclerosis: a mini review. J Neurosci Res. https://doi.org/10.1002/JNR.25193
Leray E, Moreau T, Fromont A, Edan G (2016) Epidemiology of multiple sclerosis. Rev Neurol (Paris) 172:3–13. https://doi.org/10.1016/J.NEUROL.2015.10.006
Pilotto S, Zoledziewska M, Fenu G et al (2023) Disease-modifying therapy for multiple sclerosis: implications for gut microbiota. Mult Scler Relat Disord 73. https://doi.org/10.1016/J.MSARD.2023.104671
El Ouaamari Y, Van den Bos J, Willekens B et al (2023) Neurotrophic factors as regenerative therapy for neurodegenerative diseases: current status, challenges and future perspectives. Int J Mol Sci 24. https://doi.org/10.3390/IJMS24043866
Furman MJ, Meuth SG, Albrecht P et al (2023) B cell targeted therapies in inflammatory autoimmune disease of the central nervous system. Front Immunol 14. https://doi.org/10.3389/FIMMU.2023.1129906
Spuch C, Navarro C (2012) Transport mechanisms at the blood-cerebrospinal-fluid barrier: role of megalin (LRP2). Recent Pat Endocr Metab Immune Drug Discov 4:190–205. https://doi.org/10.2174/1872214811004030190
Kolbe SC, Garcia LM, Yu N et al (2022) Lesion volume in relapsing multiple sclerosis is associated with perivascular space enlargement at the level of the basal ganglia. AJNR Am J Neuroradiol 43:238–244. https://doi.org/10.3174/AJNR.A7398
Ndemazie NB, Inkoom A, Morfaw EF et al (2022) Multi-disciplinary approach for drug and gene delivery systems to the brain. AAPS PharmSciTech 23. https://doi.org/10.1208/s12249-021-02144-1
Hernández-Pedro NY, Espinosa-Ramirez G, De La Cruz VP et al (2013) Initial immunopathogenesis of multiple sclerosis: innate immune response. Clin Dev Immunol 2013. https://doi.org/10.1155/2013/413465
Masserini M (2013) Nanoparticles for brain drug delivery. ISRN Biochem 2013:1–18. https://doi.org/10.1155/2013/238428
Upadhyay RK (2014) Drug delivery systems, CNS protection, and the blood brain barrier. Biomed Res Int 2014. https://doi.org/10.1155/2014/869269
Ortiz GG, Pacheco-Moisés FP, Macías-Islas MÁ et al (2014) Role of the blood–brain barrier in multiple sclerosis. Arch Med Res 45:687–697. https://doi.org/10.1016/J.ARCMED.2014.11.013
Pena SA, Iyengar R, Eshraghi RS et al (2020) Gene therapy for neurological disorders: challenges and recent advancements. J Drug Target 28:111–128. https://doi.org/10.1080/1061186X.2019.1630415
Teixeira MI, Lopes CM, Amaral MH, Costa PC (2023) Surface-modified lipid nanocarriers for crossing the blood-brain barrier (BBB): a current overview of active targeting in brain diseases. Colloids Surf B Biointerfaces 221. https://doi.org/10.1016/J.COLSURFB.2022.112999
Kumar P, Sharma G, Kumar R et al (2017) Vitamin-derived nanolipoidal carriers for brain delivery of dimethyl fumarate: a novel approach with preclinical evidence. ACS Chem Neurosci. acschemneuro.7b00041. https://doi.org/10.1021/acschemneuro.7b00041
Kumar P, Sharma G, Kumar R et al (2017) Enhanced brain delivery of dimethyl fumarate employing tocopherol-acetate-based nanolipidic carriers: evidence from pharmacokinetic, biodistribution, and cellular uptake studies. ACS Chem Neurosci 8:860–865. https://doi.org/10.1021/acschemneuro.6b00428
Kumar P, Sharma G, Kumar R et al (2017) Stearic acid based, systematically designed oral lipid nanoparticles for enhanced brain delivery of dimethyl fumarate. Nanomedicine (Lond) 12:2607–2621. https://doi.org/10.2217/NNM-2017-0082
Kumar P, Sharma G, Gupta V et al (2019) Oral delivery of methylthioadenosine to the brain employing solid lipid nanoparticles: pharmacokinetic, behavioral, and histopathological evidences. AAPS PharmSciTech 20:74. https://doi.org/10.1208/s12249-019-1296-0
Mehdi-alamdarlou S, Ahmadi F, Azadi A et al (2022) A cell-mimicking platelet-based drug delivery system as a potential carrier of dimethyl fumarate for multiple sclerosis. Int J Pharm 625:122084. https://doi.org/10.1016/J.IJPHARM.2022.122084
Ojha S, Kumar B (2018) Preparation and statistical modeling of solid lipid nanoparticles of dimethyl fumarate for better management of multiple sclerosis. Adv Pharm Bull 8:225–233. https://doi.org/10.15171/APB.2018.027
Sinha S, Garg V, Sonali, et al (2021) Chitosan-alginate core-shell-corona shaped nanoparticles of dimethyl fumarate in orodispersible film to improve bioavailability in treatment of multiple sclerosis: preparation, characterization and biodistribution in rats. J Drug Deliv Sci Technol 64:. https://doi.org/10.1016/j.jddst.2021.102645
clinical trials - Google Search
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Subhash, S., Chaurawal, N., Raza, K. (2024). Promises of Lipid-Based Nanocarriers for Delivery of Dimethyl Fumarate to Multiple Sclerosis Brain. In: Ray, S.K. (eds) Neuroprotection. Methods in Molecular Biology, vol 2761. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3662-6_31
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3662-6_31
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3661-9
Online ISBN: 978-1-0716-3662-6
eBook Packages: Springer Protocols