Abstract
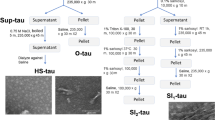
Tau protein in Alzheimer’s disease (AD) and tauopathies becomes insoluble due to hyperphosphorylation, conformational alterations, and aggregation. To analyze insoluble tau and pathological tau species, this study employs a methodology that utilizes wild-type and transgenic tau mice (P310S Tau) tissue extraction using 1% Sarkosyl or N-Lauroylsarcosine sodium salt and the radio immunoprecipitation assay (RIPA) buffer. However, the commonly used methods to study the insoluble tau fraction using detergents like Sarkosyl and RIPA require a large amount of homogenate, which can pose challenges when dealing with small tissue samples. Additionally, the study employs immunohistochemistry to visualize and quantify the pathological tau species in the brain tissue of transgenic mice, aiming to identify and analyze pathological tau species such as hyperphosphorylated tau to further our understanding of tauopathies such as Alzheimer’s disease.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Barbier P, Zejneli O, Martinho M, Lasorsa A, Belle V, Smet-Nocca C et al (2019) Role of tau as a microtubule-associated protein: structural and functional aspects. Front Aging Neurosci 11:204
Wolfe MS (2012) The role of tau in neurodegenerative diseases and its potential as a therapeutic target. Scientifica (Cairo) 2012:796024
Huang Y, Li X, Luo G, Wang J, Li R, Zhou C, Wan T, Yang F (2022) Pyroptosis as a candidate therapeutic target for Alzheimer’s disease. Front Aging Neurosci 14:996646
Rajmohan R, Reddy PH (2017) Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J Alzheimers Dis 57:975–999
Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K et al (2002) Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci 22(21):9340–9351
Selvarasu K, Singh AK, Iyaswamy A, Gopalkrishnashetty Sreenivasmurthy S, Krishnamoorthi S, Bera AK, Huang JD, Durairajan SSK (2022) Reduction of kinesin I heavy chain decreases tau hyperphosphorylation, aggregation, and memory impairment in Alzheimer’s disease and tauopathy models. Front Mol Biosci 9:1050768
Iyaswamy A, Venkatasubramanian L, Singh N, Jagannathan R, Krishnamoorthy E (2021) Qingyangshen mitigates amyloid-β and tau aggregate defects involving PPARΑ-TFEB activation in transgenic mice of Alzheimer’s disease. Phytomedicine 91:153648
Myeku N, Clelland CL, Emrani S, Kukushkin NV, Yu WH, Goldberg AL, Duff KE (2015) Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat Med 22(1):46–53
Durairajan SS, Liu LF, Lu J, Tang SC, Viswanath A, Shanmugam MK et al (2012) Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol Aging 33(12):2903–2919
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Singh, A.K., Selvarasu, K., Durairajan, S.S.K. (2024). Isolation and Detection of Pathological Tau Species in a Tauopathy Mouse Model. In: Ray, S.K. (eds) Neuroprotection. Methods in Molecular Biology, vol 2761. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3662-6_23
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3662-6_23
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3661-9
Online ISBN: 978-1-0716-3662-6
eBook Packages: Springer Protocols