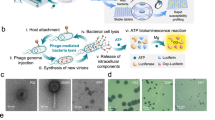
Abstract
It has been over 100 years since bacteriophages (phages) were used as a human therapeutic. Since then, phage production has dramatically evolved. Current phage preparations have fewer adverse effects due to their low bacterial toxin content. As a result, therapeutic phages have become a predominant class of new antimicrobials and are being widely used for compassionate treatment of multidrug-resistant (MDR) infections. We describe herein a protocol for the production and ultrapurification of phages. By this technique, it is possible for a lab experienced with the process to produce >109 plaque-forming units (PFU) per mL of Gram-negative phages that meet FDA endotoxins limits for intravenous infusions in as little as 48 hours. We provide illustrations of the process and tips on how to safely remove bacterial toxins from phage lysates. Although dependent on the phage strain, the approach described can rapidly generate and purify phages for a variety of applications.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Luong T, Salabarria A-C, Roach DR (2020) Phage therapy in the resistance era: where do we stand and where are we going? Clin Ther 9:1659–1680
Hatfull GF, Dedrick RM, Schooley RT (2022) Phage therapy for antibiotic-resistant bacterial infections. Annu Rev Med 1:197–211
Bretaudeau L, Tremblais K, Aubrit F et al (2020) Good manufacturing practice (GMP) compliance for phage therapy medicinal products. Front Microbiol
Murray CJL, Ikuta KS, Sharara F et al (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 10325:629–655
Bruttin A, Brussow H (2005) Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother 7:2874–2878
McCallin S, Alam Sarker S, Barretto C et al (2013) Safety analysis of a Russian phage cocktail: from metagenomic analysis to oral application in healthy human subjects. Virol 2:187–196
Sarker SA, Sultana S, Reuteler G et al (2016) Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine 124–137
Adams MH (1959) Bacteriophages. Interscience Publishers Inc., New York
Fries BC, Varshney AK (2013) Bacterial toxins-staphylococcal enterotoxin B. Microbiol Spectr 2
Pisetsky DS (2012) The origin and properties of extracellular DNA: from PAMP to DAMP. Clin Immunol 1:32–40
Magalhães PO, Lopes AM, Mazzola PG et al (2007) Methods of endotoxin removal from biological preparations: a review. J Pharm Pharm Sci 3:388–404
FDA (2012) Guidance for industry: pyrogen and endotoxins testing: questions and answers. Office of Communications, Division of Drug Information. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-pyrogen-and-endotoxins-testing-questions-and-answers. Accessed 6 Dec 2022
Jault P, Leclerc T, Jennes S et al (2019) Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect Dis 1:35–45
Bourdin G, Schmitt B, Marvin Guy L et al (2014) Amplification and purification of T4-like Escherichia coli phages for phage therapy: from laboratory to pilot scale. Appl Environ Microbiol 4:1469–1476
Yamamoto KR, Alberts BM, Benzinger R et al (1970) Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virol 3:734–744
Zakharova MY, Kozyr AV, Ignatova AN et al (2005) Purification of filamentous bacteriophage for phage display using size-exclusion chromatography. BioTechniques 2:194–198
Hietala V, Horsma-Heikkinen J, Carron A et al (2019) The removal of endo- and enterotoxins from bacteriophage preparations. Front Microbiol
Luong T, Salabarria A-C, Edwards RA et al (2020) Standardized bacteriophage purification for personalized phage therapy. Nat Protoc 9:2867–2890
Schooley RT, Biswas B, Gill JJ et al (2017) Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 10:e00954–e00917
Thurber RV, Haynes M, Breitbart M et al (2009) Laboratory procedures to generate viral metagenomes. Nat Protoc 4:470–483
Carlson K (2005) Working with bacteriophages: common techniques and methodological approaches. Boca Raton, FL
Van Belleghem JD, Merabishvili M, Vergauwen B et al (2017) A comparative study of different strategies for removal of endotoxins from bacteriophage preparations. J Microbiol Methods:153–159
Krueger AP, Ritter RC, Smith SP (1929) The electrical charge of bacteriophage. J Exp Med 6:739–746
Bemberis I, Sakata M, Hirayama C et al (2005) Affinity chromatography removes endotoxins. BioPharm Int:50–57
Bonilla N, Rojas MI, Netto Flores Cruz G et al (2016) Phage on tap–a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ:e2261
Szermer-Olearnik B, Boratyński J (2015) Removal of endotoxins from bacteriophage preparations by extraction with organic solvents. PLoS One 3:e0122672
Wagner PL, Waldor MK (2002) Bacteriophage control of bacterial virulence. Infect Immun 8:3985–3993
Turner D, Adriaenssens EM, Tolstoy I et al (2021) Phage annotation guide: guidelines for assembly and high-quality annotation. PHAGE 4:170–182
Philipson CW, Voegtly LJ, Lueder MR et al (2018) Characterizing phage genomes for therapeutic applications. Viruses 10(4):188
Brüssow H, Canchaya C, Hardt WD (2004) Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev 3:560–602
Steinmetz NF (2010) Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomed J 5:634–641
Dalwadi G, Benson HAE, Chen Y (2005) Comparison of diafiltration and tangential flow filtration for purification of nanoparticle suspensions. Pharm Res 12:2152–2162
Yang H (2013) Establishing acceptable limits of residual DNA. PDA J Pharm Sci Technol 2:155–163
Kienle PC, Pharmacists ASoH-S (2009) Compounding sterile preparations: ASHP’s video guide to chapter workbook
Arndt D, Grant JR, Marcu A et al (2016) PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res W1:W16–W21
Akhter S, Aziz RK, Edwards RA (2012) PhiSpy: a novel algorithm for finding prophages in bacterial genomes that combines similarity- and composition-based strategies. Nucleic Acids Res 16:e126
Song W, Sun H-X, Zhang C et al (2019) Prophage hunter: an integrative hunting tool for active prophages. Nucleic Acids Res W1:W74–W80
Lorenz TC (2012) Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. J Vis Exp 63:e3998
Canchaya C, Proux C, Fournous G et al (2003) Prophage genomics. MMBR 2:238–276
Acknowledgments
We would like to acknowledge Nino Grdzelishvili for her feedback and suggestions. TL is a recipient of the ARCS Foundation Scholarship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Luong, T., Sue, A.D., Roach, D.R. (2024). Rapid Bench to Bedside Therapeutic Bacteriophage Production. In: Azeredo, J., Sillankorva, S. (eds) Bacteriophage Therapy. Methods in Molecular Biology, vol 2734. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3523-0_5
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3523-0_5
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3522-3
Online ISBN: 978-1-0716-3523-0
eBook Packages: Springer Protocols