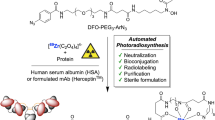
Abstract
Immunoglobulin-based positron emission tomography (ImmunoPET) is making increasingly significant contributions to the nuclear imaging toolbox. The exquisite specificity of antibodies combined with the high-resolution imaging of PET enables clinicians and researchers to localize diseases, especially cancer, with a high degree of spatial certainty. This review focuses on the radiopharmaceutical preparation necessary to obtain those images—the work behind the scenes, which occurs even before the patient or animal is injected with the radioimmunoconjugate. The focus of this methods review will be the chelation of four radioisotopes to their most common and clinically relevant chelators.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Schroeder HW Jr, Cavacini L (2010) Structure and function of immunoglobulins. J Allergy Clin Immunol 125(2 Suppl 2):S41–S52. https://doi.org/10.1016/j.jaci.2009.09.046
Ljungars A, Martensson L, Mattsson J, Kovacek M, Sundberg A, Tornberg UC, Jansson B, Persson N, Emruli VK, Ek S, Jerkeman M, Hansson M, Juliusson G, Ohlin M, Frendeus B, Teige I, Mattsson M (2018) A platform for phenotypic discovery of therapeutic antibodies and targets applied on Chronic Lymphocytic Leukemia. NPJ Precis Oncol 2:18. https://doi.org/10.1038/s41698-018-0061-2
Sundaresan G, Yazaki PJ, Shively JE, Finn RD, Larson SM, Raubitschek AA, Williams LE, Chatziioannou AF, Gambhir SS, Wu AM (2003) 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J Nucl Med 44(12):1962–1969
Lepin EJ, Leyton JV, Zhou Y, Olafsen T, Salazar FB, McCabe KE, Hahm S, Marks JD, Reiter RE, Wu AM (2010) An affinity matured minibody for PET imaging of prostate stem cell antigen (PSCA)-expressing tumors. Eur J Nucl Med Mol Imaging 37(8):1529–1538. https://doi.org/10.1007/s00259-010-1433-1
Pandit-Taskar N, Postow MA, Hellmann MD, Harding JJ, Barker CA, O’Donoghue JA, Ziolkowska M, Ruan S, Lyashchenko SK, Tsai F, Farwell M, Mitchell TC, Korn R, Le W, Lewis JS, Weber WA, Behera D, Wilson I, Gordon M, Wu AM, Wolchok JD (2020) First-in-humans imaging with (89)Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J Nucl Med 61(4):512–519. https://doi.org/10.2967/jnumed.119.229781
Farwell M, Gamache R, Pandit-Taskar N, Postow M, Gordon M, Wilson I, Mascioni A, Wu A, Le W, Weiss A, Korn R (2020) 294 CD8 PET imaging of tumor infiltrating T cells in advanced solid tumors: a phase I first-in-human study of 89Zr-IAB22M2C, a radiolabeled anti-CD8 minibody. J Immunother Cancer 8(Suppl 3):A179–A180. https://doi.org/10.1136/jitc-2020-SITC2020.0294
Wei W, Rosenkrans ZT, Liu J, Huang G, Luo QY, Cai W (2020) ImmunoPET: concept, design, and applications. Chem Rev 120(8):3787–3851. https://doi.org/10.1021/acs.chemrev.9b00738
Holland JP, Sheh Y, Lewis JS (2009) Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol 36(7):729–739. https://doi.org/10.1016/j.nucmedbio.2009.05.007
Anderson CJ, Ferdani R (2009) Copper-64 radiopharmaceuticals for PET imaging of cancer: advances in preclinical and clinical research. Cancer Biother Radiopharm 24(4):379–393. https://doi.org/10.1089/cbr.2009.0674
Sadeghi M, Aboudzadeh M, Zali A, Zeinali B (2009) (86)Y production via (86)Sr(p,n) for PET imaging at a cyclotron. Appl Radiat Isot 67(7–8):1392–1396. https://doi.org/10.1016/j.apradiso.2009.02.038
Halime Z, Frindel M, Camus N, Orain PY, Lacombe M, Bernardeau K, Cherel M, Gestin JF, Faivre-Chauvet A, Tripier R (2015) New synthesis of phenyl-isothiocyanate C-functionalised cyclams. Bioconjugation and (64)Cu phenotypic PET imaging studies of multiple myeloma with the te2a derivative. Org Biomol Chem 13(46):11302–11314. https://doi.org/10.1039/c5ob01618e
Ferdani R, Stigers DJ, Fiamengo AL, Wei L, Li BT, Golen JA, Rheingold AL, Weisman GR, Wong EH, Anderson CJ (2012) Synthesis, Cu(II) complexation, 64Cu-labeling and biological evaluation of cross-bridged cyclam chelators with phosphonate pendant arms. Dalton Trans 41(7):1938–1950. https://doi.org/10.1039/c1dt11743b
Zeglis BM, Lewis JS (2015) The bioconjugation and radiosynthesis of 89Zr-DFO-labeled antibodies. J Vis Exp 96. https://doi.org/10.3791/52521
Sharma SK, Sevak KK, Monette S, Carlin SD, Knight JC, Wuest FR, Sala E, Zeglis BM, Lewis JS (2016) Preclinical 89Zr Immuno-PET of high-grade serous ovarian cancer and lymph node metastasis. J Nucl Med 57(5):771–776. https://doi.org/10.2967/jnumed.115.167072
Kristensen LK, Christensen C, Jensen MM, Agnew BJ, Schjoth-Frydendahl C, Kjaer A, Nielsen CH (2019) Site-specifically labeled (89)Zr-DFO-trastuzumab improves immuno-reactivity and tumor uptake for immuno-PET in a subcutaneous HER2-positive xenograft mouse model. Theranostics 9(15):4409–4420. https://doi.org/10.7150/thno.32883
Vugts DJ, Klaver C, Sewing C, Poot AJ, Adamzek K, Huegli S, Mari C, Visser GWM, Valverde IE, Gasser G, Mindt TL, van Dongen G (2017) Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for (89)Zr-immuno-PET. Eur J Nucl Med Mol Imaging 44(2):286–295. https://doi.org/10.1007/s00259-016-3499-x
Natarajan A, Patel CB, Habte F, Gambhir SS (2018) Dosimetry prediction for clinical translation of (64)Cu-pembrolizumab ImmunoPET targeting human PD-1 expression. Sci Rep 8(1):633. https://doi.org/10.1038/s41598-017-19123-x
Natarajan A, Mayer AT, Reeves RE, Nagamine CM, Gambhir SS (2017) Development of novel ImmunoPET tracers to image human PD-1 checkpoint expression on tumor-infiltrating lymphocytes in a humanized mouse model. Mol Imaging Biol 19(6):903–914. https://doi.org/10.1007/s11307-017-1060-3
Cai Z, Anderson CJ (2014) Chelators for copper radionuclides in positron emission tomography radiopharmaceuticals. J. Label. Compd. Radiopharm 57(4):224–230. https://doi.org/10.1002/jlcr.3165
Woo SK, Jang SJ, Seo MJ, Park JH, Kim BS, Kim EJ, Lee YJ, Lee TS, An GI, Song IH, Seo Y, Kim KI, Kang JH (2019) Development of (64)Cu-NOTA-Trastuzumab for HER2 targeting: a radiopharmaceutical with improved pharmacokinetics for human studies. J Nucl Med 60(1):26–33. https://doi.org/10.2967/jnumed.118.210294
Bailly C, Gouard S, Lacombe M, Remaud-Le Saec P, Chalopin B, Bourgeois M, Chouin N, Tripier R, Halime Z, Haddad F, Faivre-Chauvet A, Kraeber-Bodere F, Cherel M, Bodet-Milin C (2018) Comparison of Immuno-PET of CD138 and PET imaging with (64)CuCl2 and (18)F-FDG in a preclinical syngeneic model of multiple myeloma. Oncotarget 9(10):9061–9072. https://doi.org/10.18632/oncotarget.23886
Zeng D, Guo Y, White AG, Cai Z, Modi J, Ferdani R, Anderson CJ (2014) Comparison of conjugation strategies of cross-bridged macrocyclic chelators with cetuximab for copper-64 radiolabeling and PET imaging of EGFR in colorectal tumor-bearing mice. Mol Pharm 11(11):3980–3987. https://doi.org/10.1021/mp500004m
Dearling JL, Paterson BM, Akurathi V, Betanzos-Lara S, Treves ST, Voss SD, White JM, Huston JS, Smith SV, Donnelly PS, Packard AB (2015) The ionic charge of copper-64 complexes conjugated to an engineered antibody affects biodistribution. Bioconjug Chem 26(4):707–717. https://doi.org/10.1021/acs.bioconjchem.5b00049
Lovqvist A, Humm JL, Sheikh A, Finn RD, Koziorowski J, Ruan S, Pentlow KS, Jungbluth A, Welt S, Lee FT, Brechbiel MW, Larson SM (2001) PET imaging of (86)Y-labeled anti-Lewis Y monoclonal antibodies in a nude mouse model: comparison between (86)Y and (111)In radiolabels. J Nucl Med 42(8):1281–1287
Nikula TK, Curcio MJ, Brechbiel MW, Gansow OA, Finn RD, Scheinberg DA (1995) A rapid, single vessel method for preparation of clinical grade ligand conjugated monoclonal antibodies. Nucl Med Biol 22(3):387–390. https://doi.org/10.1016/0969-8051(94)00126-5
Tijink BM, Perk LR, Budde M, Stigter-van Walsum M, Visser GW, Kloet RW, Dinkelborg LM, Leemans CR, Neri D, van Dongen GA (2009) (124)I-L19-SIP for immuno-PET imaging of tumour vasculature and guidance of (131)I-L19-SIP radioimmunotherapy. Eur J Nucl Med Mol Imaging 36(8):1235–1244. https://doi.org/10.1007/s00259-009-1096-y
Kumar K, Ghosh A (2021) Radiochemistry, production processes, labeling methods, and ImmunoPET imaging pharmaceuticals of Iodine-124. Molecules 26(2). https://doi.org/10.3390/molecules26020414
Aerts HJWL, Dubois L, Perk L, Vermaelen P, van Dongen GAMS, Wouters BG, Lambin P (2009) Disparity between in vivo EGFR expression and (89)Zr-labeled cetuximab uptake assessed with PET. J Nucl Med 50(1):123–131. https://doi.org/10.2967/jnumed.108.054312
Bailly C, Gouard S, Guerard F, Chalopin B, Carlier T, Faivre-Chauvet A, Remaud-Le Saec P, Bourgeois M, Chouin N, Rbah-Vidal L, Tripier R, Haddad F, Kraeber-Bodere F, Bodet-Milin C, Cherel M (2019) What is the best radionuclide for Immuno-PET of multiple myeloma? A comparison study between (89)Zr- and (64)Cu-labeled anti-CD138 in a preclinical syngeneic model. Int J Mol Sci 20(10). https://doi.org/10.3390/ijms20102564
Acknowledgments
We gratefully acknowledge the Radiochemistry and Molecular Imaging Probes Core (RMIP Core) at MSK, which is supported by NIH grant P30 CA08748. This work was also supported by NIH NCI R35 CA232130 (JSL).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Arroyo, A., Lyashchenko, S.K., Lewis, J.S. (2024). Methods for the Production of Radiolabeled Bioagents for ImmunoPET. In: Witney, T.H., Shuhendler, A.J. (eds) Positron Emission Tomography. Methods in Molecular Biology, vol 2729. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3499-8_8
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3499-8_8
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3498-1
Online ISBN: 978-1-0716-3499-8
eBook Packages: Springer Protocols