Abstract
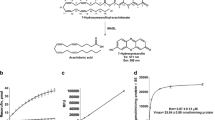
Monoacylglycerol lipase (MGL/MAGL/MGLL) is a serine hydrolase involved in the biological deactivation of the endocannabinoid 2-arachidonoyl-sn-glycerol (2-AG). 2-AG is the most abundant endogenous lipid agonists for cannabinoid receptors in the brain and elsewhere in the body. In the central nervous system (CNS), MGL is localized to presynaptic nerve terminals of both excitatory and inhibitory synapses, where it controls the regulatory actions of 2-AG on synaptic transmission and plasticity. In this chapter, we describe an in vitro method to assess MGL activity by liquid chromatography/mass spectrometry (LC/MS)-based quantitation of its reaction product. The method may be used to determine basal or altered MGL activity in cells or tissues after pharmacological, genetic, or biological interventions. In addition, the assay can be used for MGL inhibitor screening using purified recombinant enzyme or MGL-overexpressing cells.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Dinh TP, Carpenter D, Leslie FM et al (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA 99:10819–10824
Karlsson M, Contreras JA, Hellman U et al (1997) cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem 272:27218–27223
Blankman JL, Simon GM, Cravatt BF (2007) A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol 14:1347–1356
Marrs WR, Blankman JL, Horne EA et al (2010) The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci 13:951–957
Labar G, Bauvois C, Borel F et al (2010) Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. Chembiochem 11:218–227
Bertrand T, Augé F, Houtmann J et al (2010) Structural basis for human monoglyceride lipase inhibition. J Mol Biol 396:663–673
Gulyas AI, Cravatt BF, Bracey MH et al (2004) Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 20:441–458
Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884
Chanda PK, Gao Y, Mark L et al (2010) Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol 78:996–1003
Schlosburg JE, Blankman JL, Long JZ et al (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13:1113–1119
Jung KM, Clapper JR, Fu J et al (2012) 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab 15:299–310
Dinh TP, Kathuria S, Piomelli D (2004) RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol 66:1260–1264
King AR, Duranti A, Tontini A et al (2007) URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol 14:1357–1365
Fredrikson G, Tornqvist H, Belfrage P (1986) Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim Biophys Acta 876:288–293
Astarita G, Piomelli D (2009) Lipidomic analysis of endocannabinoid metabolism in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 877:2755–2767
Piomelli D, Astarita G, Rapaka R (2007) A neuroscientist’s guide to lipidomics. Nat Rev Neurosci 8:743–754
Kayacelebi AA, Schauerte C, Kling K et al (2017) Cross-validated stable-isotope dilution GC-MS and LC-MS/MS assays for monoacylglycerol lipase (MAGL) activity by measuring arachidonic acid released from the endocannabinoid 2-arachidonoyl glycerol. J Chromatogr B Analyt Technol Biomed Life Sci 1047:151–159
Muccioli GG, Labar G, Lambert DM et al (2008) CAY10499, a novel monoglyceride lipase inhibitor evidenced by an expeditious MGL assay. Chembiochem 9:2704–2710
Del Carlo S, Manera C, Chicca A et al (2015) Development of an HPLC/UV assay for the evaluation of inhibitors of human recombinant monoacylglycerol lipase. J Pharm Biomed Anal 108:113–121
Granchi C, Rizzolio F, Caligiuri I et al (2018) Rational development of MAGL inhibitors. Methods Mol Biol 1824:335–346
Miceli M, Casati S, Ottria R et al (2019) Set-up and validation of a high throughput screening method for human monoacylglycerol lipase (MAGL) based on a new red fluorescent probe. Molecules 24:2241
King AR, Dotsey EY, Lodola A et al (2009) Discovery of potent and reversible monoacylglycerol lipase inhibitors. Chem Biol 16:1045–1052
Acknowledgments
This work was supported by the National Institutes on Drug Abuse (NIDA) center grant P50DA044118-01 (to D.P.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Jung, KM., Piomelli, D. (2023). Assay of Monoacylglycerol Lipase Activity. In: Maccarrone, M. (eds) Endocannabinoid Signaling. Methods in Molecular Biology, vol 2576. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2728-0_24
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2728-0_24
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2727-3
Online ISBN: 978-1-0716-2728-0
eBook Packages: Springer Protocols