Abstract
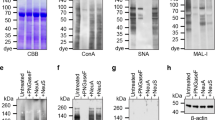
Hemagglutinin (HA) on the surface of influenza viruses binds to sialic acids, mainly N-acetylneuraminic acid (Neu5Ac) or N-glycolylneuraminic acid. Neu5Ac and N-glycolylneuraminic acid lie at the terminal end of sugar chains on the cell surface. Human influenza viruses preferentially bind to sialic acids bound to galactose by the alpha2-6 linkage (Neu5Acα2-6Gal), abundant in the human airway. In contrast, avian influenza viruses preferentially bind to Neu5Acα2-3Gal, abundant in the intestine of ducks. Sambucus nigra lectin (SNA) and Maackia amurensis lectin (MAA) bind to Neu5Acα2-6Gal and Neu5Acα2-3Gal, respectively. These two lectins have therefore been applied to detect sialic acids on the airway epithelium of animals.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Suzuki Y, Ito T, Suzuki T, Holland RE, Chambers TM, Kiso M et al (2000) Sialic acid species as a determinant of the host range of influenza A viruses. J Virol 74:11825–11831
Goldstein IJ, Hayes CE (1978) The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem 35:127–340
Ito T, Suzuki Y, Takada A, Kawamoto A, Otsuki K, Masuda H et al (1997) Differences in sialic acid-galactose linkages in the chicken egg amnion and allantois influence human influenza virus receptor specificity and variant selection. J Virol 71:3357–3362
Ito T, Suzuki Y, Suzuki T, Takada A, Horimoto T, Wells K et al (2000) Recognition of N-glycolylneuraminic acid linked to galactose by the alpha2,3 linkage is associated with intestinal replication of influenza A virus in ducks. J Virol 74:9300–9305
Nicholls JM, Bourne AJ, Chen H, Guan Y, Peiris JS (2007) Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir Res 8:73
Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y (2006) Avian flu: influenza virus receptors in the human airway. Nature 440:435–436
Davis AS, Chertow DS, Moyer JE, Suzich J, Sandouk A, Dorward DW et al (2015) Validation of normal human bronchial epithelial cells as a model for influenza A infections in human distal trachea. J Histochem Cytochem 63:312–328
França M, Stallknecht DE, Howerth EW (2013) Expression and distribution of sialic acid influenza virus receptors in wild birds. Avian Pathol 42:60–71
Van Poucke SG, Nicholls JM, Nauwynck HJ, Van Reeth K (2010) Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J 7:38
Scocco P, Pedini V (2008) Localization of influenza virus sialoreceptors in equine respiratory tract. Histol Histopathol 23:973–978
Lakdawala SS, Jayaraman A, Halpin RA, Lamirande EW, Shih AR, Stockwell TB et al (2015) The soft palate is an important site of adaptation for transmissible influenza viruses. Nature 526:122–125
Matsuoka Y, Suguitan A Jr, Orandle M, Paskel M, Boonnak K, Gardner DJ et al (2014) African green monkeys recapitulate the clinical experience with replication of live attenuated pandemic influenza virus vaccine candidates. J Virol 88:8139–8152
Davis AS, Taubenberger JK, Bray M (2015) The use of nonhuman primates in research on seasonal, pandemic and avian influenza, 1893-2014. Antivir Res 117:75–98
Itoh Y (2016) Translational research on influenza virus infection using a nonhuman primate model. Pathol Int 66:132–141
Nakayama M, Ozaki H, Itoh Y, Soda K, Ishigaki H, Okamatsu M et al (2016) Vaccination against H9N2 avian influenza virus reduces bronchus-associated lymphoid tissue formation in cynomolgus macaques after intranasal virus challenge infection. Pathol Int 66:678–686
Matrosovich MN, Krauss S, Webster RG (2001) H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 281:156–162
Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S et al (2013) Characterization of H7N9 influenza A viruses isolated from humans. Nature 501:551–555
de Wit E, Rasmussen AL, Feldmann F, Bushmaker T, Martellaro C, Haddock E et al (2014) Influenza virus A/Anhui/1/2013 (H7N9) replicates efficiently in the upper and lower respiratory tracts of cynomolgus macaques. mBio 5:e01331–e01314
Shichinohe S, Itoh Y, Nakayama M, Ozaki H, Soda K, Ishigaki H et al (2016) Comparison of pathogenicities of H7 avian influenza viruses via intranasal and conjunctival inoculation in cynomolgus macaques. Virology 493:31–38
Xiong X, Martin SR, Haire LF, Wharton SA, Daniels RS, Bennett MS et al (2013) Receptor binding by an H7N9 influenza virus from humans. Nature 499(7459):496–499. https://doi.org/10.1038/nature12372
Acknowledgments
M.N. is supported by a grant for female researchers from The Naito Foundation and Grants-in-Aid for Scientific Research (20K16217).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Nakayama, M., Itoh, Y. (2022). Lectin Staining to Detect Human and Avian Influenza Virus Receptors in the Airway of Nonhuman Primates. In: Suzuki, Y. (eds) Glycovirology. Methods in Molecular Biology, vol 2556. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2635-1_4
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2635-1_4
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2634-4
Online ISBN: 978-1-0716-2635-1
eBook Packages: Springer Protocols