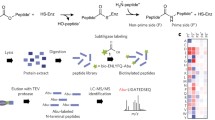
Abstract
The physiological relevance of site-specific precursor processing for the biogenesis of peptide hormones and growth factors can be demonstrated in genetic complementation experiments, in which a gain of function is observed for the cleavable wild-type precursor, but not for a non-cleavable precursor mutant. Similarly, cleavable and non-cleavable synthetic peptides can be used in bioassays to test whether processing is required for bioactivity. In genetic complementation experiments, site-directed mutagenesis has to be used to mask a processing site against proteolysis. Peptide-based bioassays have the distinctive advantage that peptides can be protected against proteolytic cleavage by backbone modifications, i.e., without changing the amino acid sequence. Peptide backbone modifications have been employed to increase the metabolic stability of peptide drugs, and in basic research, to investigate whether processing at a certain site is required for precursor maturation and formation of the bioactive peptide. For this approach, it is important to show that modification of the peptide backbone has the desired effect and does indeed protect the respective peptide bond against proteolysis. This can be accomplished with the MALDI-TOF mass spectrometry-based assay we describe here.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Schaller A, Stintzi A, Rivas S, Serrano I, Chichkova NV, Vartapetian AB, Martínez D, Guiamét JJ, Sueldo DJ, van der Hoorn RAL, Ramírez V, Vera P (2018) From structure to function—a family portrait of plant subtilases. New Phytol 218(3):901–915. https://doi.org/10.1111/nph.14582
Stührwohldt N, Schaller A (2019) Regulation of plant peptide hormones and growth factors by post-translational modification. Plant Biol 21(S1):49–63. https://doi.org/10.1111/plb.12881
Schaller A (2004) A cut above the rest: the regulatory function of plant proteases. Planta 220(2):183–197. https://doi.org/10.1007/s00425-004-1407-2
Van Der Hoorn RAL (2008) Plant proteases: from phenotypes to molecular mechanisms. Annu Rev Plant Biol 59:191–223. https://doi.org/10.1146/annurev.arplant.59.032607.092835
Overall CM, Blobel CP (2007) In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol 8(3):245–257. https://doi.org/10.1038/nrm2120
Schardon K, Hohl M, Graff L, Schulze W, Pfannstiel J, Stintzi A, Schaller A (2016) Precursor processing for plant peptide hormone maturation by subtilisin-like serine proteinases. Science 354(6319):1594–1597. https://doi.org/10.1126/science.aai8550
Ghorbani S, Hoogewijs K, Pečenková T, Fernandez A, Inzé A, Eeckhout D, Kawa D, De Jaeger G, Beeckman T, Madder A, Van Breusegem F, Hilson P (2016) The SBT6.1 subtilase processes the GOLVEN1 peptide controlling cell elongation. J Exp Bot 67(16):4877–4887. https://doi.org/10.1093/jxb/erw241
Stührwohldt N, Ehinger A, Thellmann K, Schaller A (2020) Processing and formation of bioactive CLE40 peptide are controlled by posttranslational proline hydroxylation. Plant Physiol 184(3):1573–1584. https://doi.org/10.1104/pp.20.00528
Reichardt S, Piepho H-P, Stintzi A, Schaller A (2020) Peptide signaling for drought-induced tomato flower drop. Science 367(6485):1482–1485. https://doi.org/10.1126/science.aaz5641
Grauer A, König B (2009) Peptidomimetics—a versatile route to biologically active compounds. Eur J Org Chem 30:5099–5111. https://doi.org/10.1002/ejoc.200900599
Vagner J, Qu H, Hruby VJ (2008) Peptidomimetics, a synthetic tool of drug discovery. Curr Opin Chem Biol 12(3):292–296. https://doi.org/10.1016/j.cbpa.2008.03.009
Syrén P-O (2018) Enzymatic hydrolysis of tertiary amide bonds by anti nucleophilic attack and protonation. J Org Chem 83(21):13543–13548. https://doi.org/10.1021/acs.joc.8b02053
Syrén P-O, Hult K (2011) Amidases have a hydrogen bond that facilitates nitrogen inversion, but esterases have not. ChemCatChem 3(5):853–860. https://doi.org/10.1002/cctc.201000448
Bizzozero SA, Dutler H (1981) Stereochemical aspects of peptide hydrolysis catalyzed by serine proteases of the chymotrypsin type. Bioorg Chem 10(1):46–62. https://doi.org/10.1016/0045-2068(81)90042-0
Liu B, Schofield CJ, Wilmouth RC (2006) Structural analyses on intermediates in serine protease catalysis. J Biol Chem 281(33):24024–24035. https://doi.org/10.1074/jbc.M600495200
Schaller A (1998) Action of proteolysis-resistant systemin analogues in wound signalling. Phytochemistry 47(4):605–612. https://doi.org/10.1016/S0031-9422(97)00523-2
Beloshistov RE, Dreizler K, Galiullina RA, Tuzhikov AI, Serebryakova MV, Reichardt S, Shaw J, Taliansky ME, Pfannstiel J, Chichkova NV, Stintzi A, Schaller A, Vartapetian AB (2018) Phytaspase-mediated precursor processing and maturation of the wound hormone systemin. New Phytol 218(3):1167–1178. https://doi.org/10.1111/nph.14568
Reichardt S, Repper D, Tuzhikov AI, Galiullina RA, Planas-Marques M, Chichkova NV, Vartapetian AB, Stintzi A, Schaller A (2018) The tomato subtilase family includes several cell death-related proteinases with caspase specificity. Sci Rep 8(1):10531. https://doi.org/10.1038/s41598-018-28769-0
Rappsilber J, Ishihama Y, Mann M (2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem 75(3):663–670. https://doi.org/10.1021/ac026117i
Acknowledgments
Our work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB1101 project D06) to Andreas Schaller. We also thank Bianca Pflüger for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Wang, X., Pfannstiel, J., Stintzi, A., Schaller, A. (2022). Peptide Backbone Modifications for the Assessment of Cleavage Site Relevance in Precursors of Signaling Peptides. In: Klemenčič, M., Stael, S., Huesgen, P.F. (eds) Plant Proteases and Plant Cell Death. Methods in Molecular Biology, vol 2447. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2079-3_7
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2079-3_7
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2078-6
Online ISBN: 978-1-0716-2079-3
eBook Packages: Springer Protocols