Abstract
Survival of bacteria on biotic and abiotic surfaces is an important part in understanding food contamination. Temperature and relative humidity (RH) play important roles in influencing bacterial survival on surfaces. Surface type and inoculum diluent also appear to influence bacterial survival. This study examines how RH, temperature, and inoculum diluent affected the survival of Enterobacter aerogenes on stainless steel, polyvinyl chloride, and ceramic tile. While surface type had little effect on survival, temperature showed a clear effect. E. aerogenes survived better at 7 °C at 15% and 50% RH on all surfaces. Inoculum diluent composition influenced survival and allowed apparent growth under some high RH conditions. Understanding the impact that methods for the inoculation of surfaces have on bacterial survival will enable a better understanding of inconsistent research findings for the survival of bacteria on surfaces.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Key words
1 Introduction
Cross-contamination of foods by pathogens present on contaminated surfaces can lead to foodborne illness, so understanding the survival of microorganisms on food contact surfaces is an important part of managing cross-contamination risk. The Centers for Disease Control and Prevention (CDC) estimates there are greater than nine million episodes of foodborne illness, including 56,000 hospitalizations, and 1300 deaths caused by known agents each year in the United States [1]. The CDC publishes reports that summarize data on surveillance for foodborne disease outbreaks in the United States. This report summarizes more than 30 contributing factors that may contribute to foodborne disease each year. Cross-contamination from surfaces (not including ill workers) is commonly a “top ten” contributing factor each year [2, 3]. The survival of pathogenic organisms on surfaces is an important driver of cross-contamination . Many pathogenic organisms including Escherichia coli and Salmonella enterica can survive for long periods of time on nonbiological surfaces [4,5,6,7,8]. Many factors influence the ability for these organisms to survive on surfaces, including temperature [4, 9,10,11,12], relative humidity (RH) [10, 13, 14], surface type [4, 9, 15], and microbial matrix [7, 16,17,18], while other factors can influence the transfer of and surviving bacteria from surfaces to food [16, 19].
Stainless steel, ceramic tile, and polyvinyl chloride (PVC) are surfaces commonly found in homes, restaurants, and food processing facilities. Surface free energy, hydrophobicity, and porosity have all been shown to effect bacteria attachment and biofilm formation, which can be important factors in bacterial survival on surfaces [20,21,22,23,24,25,26,27]. Stainless steel has been shown to promote biofilm formation and allow microorganisms to survive longer vs. other metals, which can in turn promote cross-contamination in food processing facilities [8, 28, 29]. The survival of pathogenic organisms on ceramic tile has been shown to be a potential cause of foodborne disease outbreaks [30]. PVC is a thermoplastic that is widely used in a variety of ways in food facilities and produce packinghouses, including as a food contact surface [31]. Understanding survival of bacteria on these surfaces can help to create a better understanding of when cross-contamination can occur to help manage the risk foodborne disease [32].
The suspending diluent (commonly a buffer) used to inoculate a microbial suspension onto a food or surface in a laboratory experiment is generally assumed to have a minimal effect on the experimental results. Typical suspending matrices (e.g., dilute peptone or phosphate-buffered saline) reduce osmotic stress to the suspended cells. The concentration of peptone in a buffer can affect microbial survival on surfaces [16, 33]. Studies have evaluated the ability of different peptone buffers to recover and subsequently culture bacteria like E. coli and S. enterica from foods [33,34,35]. S. enterica survival on surfaces has been shown to be greater when suspended in Tryptic Soy Broth (TSB) rather than when suspended in phosphate buffered saline (PBS), likely due to TSB’s nutrient content [15].
2 Materials
2.1 Preparation of Surfaces
Stainless steel (0.018″ thickness, 16 gauge), polyvinyl chloride (1/8″ thickness), and ceramic tile purchased online or locally and were cut to 5 cm × 5 cm tiles for use in this study. Many other surfaces such as wood, cardboard, and rubber have also been used in other survival studies, and preparation of these surfaces are typically similar [9, 36, 37]. Reused tiles may need to be wiped with a clean paper towel to remove any visible dust or dirt. Tiles were then wrapped in aluminum foil to keep the surfaces separated and prevent possible contamination if the surfaces needed to be moved or handled. Foil can be cut into squares about twice the size of the surfaces in order to wrap the foil completely around the tiles. Tiles wrapped in foil were placed into autoclavable containers and then autoclaved for 15 min at 250 °C. Tiles were removed from the autoclave and allowed to cool. The foil should prevent most moisture that condenses on the surface from the autoclave, but some additional drying may be necessary before inoculation . Tiles were sprayed with 70% ethanol and allowed to dry to minimizes any cross-contamination after sterilization. The tiles can be either left in the sterilized tin foil or put into an open sterile petri dish, so that they could be flipped over, and both sides could be sprayed with ethanol. A nitrile glove or sterile tongs should be used when handling surfaces to prevent contamination from bacteria on the hands.
2.2 Preparation of Culture Media
Bacteria culture and recovery media may be prepared several days in advance and stored at refrigerated temperatures . TSB is a general growth media for mesophilic bacteria , it can be made by combining 25 g of media per 1 L of distilled water. Media should then be heated on a hot plate until boiling and then allowed to boil for 10 min and autoclaved for 15 min at 250 °C. Once removed and sealed, it can be stored at room temperature for up to 1 month and potentially longer at refrigeration temperature . In this study, the microorganism used for testing was resistant to nalidixic acid, which was added in the correct concentration to all media prior to use. The addition of antimicrobials can allow for the increase of shelf life of the media, prevent contamination of the culture media, and prevent recovery of any accidental surface contaminants.
2.3 Preparation of Bacterial Strains
A high cell concentration of overnight broth culture was prepared to use for inoculation of the surfaces. Our lab has previously used E. aerogenes strain B199A, a nonpathogenic microorganism [38] that has shown attachment characteristics similar to S. enterica on chicken skin [39], which was used for all experiments (Vivolac Cultures, Indianapolis, Ind). This strain is resistant to nalidixic acid and control experiments showed that no nalidixic acid-resistant E. aerogenes were found on any surfaces after disinfection. While this study used an organism that was resistant to an antimicrobial, microorganisms that are not resistant to antimicrobials may also be used in surface survival experiments as sterilization of the surfaces should prevent any background microbiota from contaminating the results. Strain selection appears to be important for duration of survival in microorganisms, including on surfaces, and there should be some care in researching the appropriate strains to use before experimentation.
Cultures for our experiments were prepared in a similar manner to that described previously [38, 39]. A frozen stock of E. aerogenes in 80% glycerol solution was streaked onto Tryptic Soy Agar (Difco, BD, Sparks, MD) containing 50 μg/mL of nalidixic acid (Sigma Chemical Co., St. Louis, Mo.), referred to as TSA-na in order to select for a single isolated colony. These plates can be wrapped in parafilm and stored in a refrigerator for use up to 2 weeks. One colony was grown overnight in 10 mL TSB (Difco, BD, Sparks, MD), containing 50 μg/mL of nalidixic acid and incubated at 37 °C for 24 h, consistent with methods used previously for this organism [19, 40]. Inoculum matrices were of the three different types described below. Cells were harvested from the overnight culture in TSA-na by centrifuging at 5000 × g for 10 min and washed twice in either 0.1% peptone water (Difco, BD), 1% phosphate buffered saline solution (Difco, BD), or sterile distilled water. Typically, a final concentration of 108 CFU/mL will be achieved on a nonselective media, such as TSA.
2.4 Preparation of Controlled Environment
Saturated salt solutions can be kept in the bottom of glass desiccators to control for environmental RH with the inoculated surfaces stored above the solutions. A small amount of petroleum jelly can be applied around the lid to ensure tight seal on the glass desiccators . In our study, lithium chloride or potassium carbonate (each 230 g) was slowly mixed into 100 mL of heated water to create saturated salt solutions at 15% and 50% RH , respectively. Potassium sulfate salt (250 g) was mixed into 100 mL of water to create a 100% RH environment. Salt solutions were placed in the bottom of glass desiccators (Thermo Fisher Scientific, Waltham, MA) and given 24 h for the RH to stabilize. A list of additional salts that can be used to achieve a variety of relative humidities was published by Greenspan in 1976 [41]. Data loggers purchased from LASCAR Electronics (Erie, PA) for RH and temperature were used to monitor the environment. Loggers were sensitive to 0.5(±1) °C and 1(±2)% RH . Desiccators were stored on the lab bench to represent room temperature (21 °C), and desiccators were also stored in a walk-in refrigeration unit to achieve a cool (7 °C) storage temperature . Desiccators that were held at room temperature in the lab were found to have very consistent storage temperatures , within ±1 °C, and storage temperature inside of the walk-in refrigerator showed even less variability. RH inside of the desiccators was also fairly consistent, ±3%. There were changes in the RH when the chambers were opened, but the humidity stabilized within 1 h. Incubators that control for both temperature and RH can achieve more precise control; however, these are much more expensive than salt and glass desiccators .
3 Methods
3.1 Survival Based on Surface and Temperatures
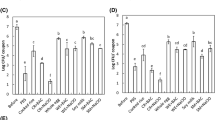
In our study, three surface types (stainless steel, PVC, and ceramic tile) were inoculated with 100 μL containing ~108 CFU/mL in 0.1% peptone of overnight culture after centrifuging and washing. The coupons allowed to dry for approximately 2 h at room temperature and ambient RH for an initial concentration of ~107 CFU per coupon. Coupons can also be placed in a biosafety cabinet which will dry the surfaces quicker because of the air flow. Dried coupons were then placed in previously equilibrated desiccators containing saturated salt solutions at 15, 50, or 100% RH . Desiccators were placed either on the bench top (21 °C) or in a walk-in cooler (7 °C). Tiles were removed from the desiccators at ten time points (from 0 to 21 days). Cell recovery time points may need to be adjusted based on the storage conditions of the coupons, where slower cell decline should have time points taken further out, and conditions that show a more rapid cell decline should take time points in a shorter period of time in order to adequately capture the cell decline. Tiles were removed by using a gloved hand in order to prevent any potential contamination of the surfaces. Some care should be taken to not touch the site of inoculation , in order to prevent premature removal of cells. Each coupon was placed in a sterile 207-mL Whirl-Pak sampling bag (Nasco, Fort Atkinson, WI) and filled with 10 mL of 0.1% peptone water. The rub-shake method was used for 1 min to detach the microorganisms from the surfaces [4]. A clear site of inoculation can typically be seen on the coupons, and it is important to rub well on this spot to properly detach all cells from the surface. Dilutions were plated on TSA-na plates and incubated at 37 °C for 24 h and colonies were counted. Populations were expressed in log CFU per surface.
3.2 Survival Based on Diluent Type
Survival of E. aerogenes in different diluent types was evaluated on only stainless steel. Cultures were washed with either 0.1% peptone, 1% PBS, or sterile distilled water and inoculated onto stainless steel surfaces and placed in a desiccator containing saturated salt solutions at either 15, 50, 100% RH as described above. Different inoculating media and food slurries have also been used to inoculate surfaces in order to test bacteria survival . Slurries of food products have also been used to mimic a potential real-world scenario of contamination of a surface from a food product [18, 42]. Typically, inoculating media that have greater nutrient concentration will allow for greater survival of bacteria [16, 18]. Desiccators were placed on the lab benchtop (21 °C), and tiles were sampled at ten time points (from 0 to 21 days) for peptone and PBS samples and over 10 more frequent time points (from 0 to 168 h) for sterile distilled water samples. Surfaces were placed in sterile Whirl-Pak bags containing 10 mL of the same diluent that was used for inoculation , and the rub-shake method as previously described was used to detach microorganisms from surface then diluted and plated on the TSA-na plates. Colonies were counted and expressed as log CFU per surface.
3.3 Survival at Different Starting Concentrations at a High Humidity
E. aerogenes was inoculated onto stainless steel coupons at starting concentrations of ~2, 4, and 6 log CFU/surface with 0.1% peptone and 1% PBS and ~3, 4, and 5 log CFU/surface using distilled water and placed in desiccators containing saturated potassium sulfate salt solutions to ensure 100% RH . It is important to remember to dilute the initial inoculum in the same matrix that the cells were washed in to prevent possible inconsistencies of results. It is important to note that the concentration put onto the surface will be greater that the concentration recovered after the initial drying time, and the reduction may be >90% under some conditions. We would recommend testing the initial concentration after drying if a more dilute inoculum culture is being used to determine the initial cell concentration on the surfaces. Coupons were removed from the desiccators at ten time points (from 0 to 21 days) for 0.1% peptone and 1% PBS or 10 more frequent time points (from 0 to 7 days). Each coupon was placed in a sterile Whirl-Pak bag with 10 mL of 0.1% peptone water or PBS. The rub-shake method as previously described was again applied for 1 min to detach the bacteria from the surfaces as described above. Dilutions were plated on TSA-na plates and incubated at 37 °C for 24 h and colonies were counted. Populations were expressed in log CFU per surface.
4 Conclusions
Bacteria survival on abiotic surfaces is important in understanding how food can become contaminated due to cross contamination . Cross contamination of foods can potentially lead to illness after consumption of the food. Survival of bacteria can be greatly influenced based on the methodology used to inoculate the surfaces. Important variable includes how the bacteria are grown and the matrix that suspends the inoculated organism. The storage conditions of the surfaces including temperature and RH can also influence survival . These factors should be considered before beginning any experimentation. The methods listed above can help create consistent research practices in order to obtain the best results.
References
Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM (2011) Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7
Dewey-Mattia D, Manikonda K, Hall AJ, Wise ME, Crowe SJ (2018) Surveillance for foodborne disease outbreaks—United States, 2009–2015. MMWR Surveill Summ 67:1
Gould LH, Walsh KA, Vieira AR, Herman K, Williams IT, Hall AJ, Cole D (2013) Surveillance for foodborne disease outbreaks—United States, 1998–2008. Morb Mortal Wkly Rep Surveill Summ 62:1–34
Allen RL, Warren BR, Archer DL, Schneider KR, Sargent SA (2005) Survival of salmonella spp. on the surfaces of fresh tomatoes and selected packing line materials. HortTechnology 15:831–836
Edyta M, Nicolas M, Beatrice C-P, Christine ERD, John H (2014) Survival and death kinetics of salmonella strains at low relative humidity, attached to stainless steel surfaces. Int J Food Microbiol 187C:33–40
Kusumaningrum HD, Riboldi G, Hazeleger WC, Beumer RR (2003) Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int J Food Microbiol 85:227–236
Posada-Izquierdo G, Pérez-Rodríguez F, Zurera G (2013) Mathematical quantification of microbial inactivation of Escherichia coli O157: H7 and salmonella spp. on stainless steel surfaces soiled with different vegetable juice substrates. Food Res Int 54:1688–1698
Wilks SA, Michels H, Keevil CW (2005) The survival of Escherichia coli O157 on a range of metal surfaces. Int J Food Microbiol 105:445–454
Helke DM, Wong ACL (1994) Survival and growth characteristics of listeria monocytogenes and salmonella typhimurium on stainless steel and Buna-N rubber. J Food Prot 57:963–968
Hokunan H, Koyama K, Hasegawa M, Kawamura S, Koseki S (2016) Survival kinetics of salmonella enterica and enterohemorrhagic Escherichia coli on a plastic surface at low relative humidity and on low–water activity foods. J Food Prot 79:1680–1692
Kim H, Bang J, Beuchat LR, Ryu J-H (2008) Fate of Enterobacter sakazakii attached to or in biofilms on stainless steel upon exposure to various temperatures or relative humidities. J Food Prot 71:940–945
Williams A, Avery L, Killham K, Jones D (2005) Persistence of Escherichia coli O157 on farm surfaces under different environmental conditions. J Appl Microbiol 98:1075–1083
Bae Y-M, Baek S-Y, Lee S-Y (2012) Resistance of pathogenic bacteria on the surface of stainless steel depending on attachment form and efficacy of chemical sanitizers. Int J Food Microbiol 153:465–473
Zoz F, Iaconelli C, Lang E, Iddir H, Guyot S, Grandvalet C, Gervais P, Beney L (2016) Control of relative air humidity as a potential means to improve hygiene on surfaces: a preliminary approach with listeria monocytogenes. PLoS One 11:e0148418
Cesare AD, Sheldon BW, Smith KS, Jaykus LA (2003) Survival and persistence of campylobacter and salmonella species under various organic loads on food contact surfaces. J Food Prot 66:1587–1594
Dawson P, Han I, Cox M, Black C, Simmons L (2007) Residence time and food contact time effects on transfer of salmonella typhimurium from tile, wood and carpet: testing the five-second rule. J Appl Microbiol 102:945–953
Pérez-Rodríguez F, Posada-Izquierdo GD, Valero A, García-Gimeno RM, Zurera G (2012) Modelling survival kinetics of Staphylococcus aureus and Escherichia coli O157: H7 on stainless steel surfaces soiled with different substrates under static conditions of temperature and relative humidity. Food Microbiol 33(2):197–204
Takahashi H, Kuramoto S, Miya S, Kimura B (2011) Desiccation survival of listeria monocytogenes and other potential foodborne pathogens on stainless steel surfaces is affected by different food soils. Food Control 22:633–637
Miranda RC, Schaffner DW (2016) Longer contact times increase cross-contamination of Enterobacter aerogenes from surfaces to food. In: Applied and Environmental Microbiology. AEM, New York, pp 01838–01816
Donlan RM (2001) Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 33:1387–1392
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890
Mafu AA, Roy D, Goulet J, Magny P (1990) Attachment of listeria monocytogenes to stainless steel, glass, polypropylene, and rubber surfaces after short contact times. J Food Prot 53:742–746
Pringle JH, Fletcher M (1983) Influence of substratum wettability on attachment of freshwater bacteria to solid surfaces. Appl Environ Microbiol 45:811–817
Pringle JH, Fletcher M (1986) Influence of substratum hydration and adsorbed macromolecules on bacterial attachment to surfaces. Appl Environ Microbiol 51:1321–1325
Sinde E, Carballo J (2000) Attachment of salmonella spp. and listeria monocytogenes to stainless steel, rubber and polytetrafluorethylene: the influence of free energy and the effect of commercial sanitizers. Food Microbiol 17:439–447
Smoot LM, Pierson MD (1998) Effect of environmental stress on the ability of listeria monocytogenes Scott a to attach to food contact surfaces. J Food Prot 61:1293–1298
Teixeira P, Sónia Carina S, Araújo F, Joana A, Rosário O (2007) Bacterial adhesion to food contacting surfaces. In: Communicating current research and educational topics and trends in applied microbiology. FORMATEX
Lagha R, Bellon-Fontaine M-N, Renault M, Briandet R, Herry J-M, Mrabet B, Bakhrouf A, Chehimi MM (2015) Impact of long-term starvation on adhesion to and biofilm formation on stainless steel 316 L and gold surfaces of salmonella enterica serovar typhimurium. Ann Microbiol 65:399–409
Wilks SA, Harold TM, Keevil CW (2006) Survival of listeria monocytogenes Scott a on metal surfaces: implications for cross-contamination. Int J Food Microbiol 111:93–98
Todd EC, Greig JD, Bartleson CA, Michaels BS (2009) Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 6. Transmission and survival of pathogens in the food processing and preparation environment. J Food Prot 72:202–219
Pearson R (1982) PVC as a food packaging material. Food Chem 8:85–96
Gerwen SJCV, Zwietering MH (1998) Growth and inactivation models to be used in quantitative risk assessments. J Food Prot 61:1541–1549
Guerini MN, Arthur TM, Shackelford SD, Koohmaraie M (2006) Evaluation of Escherichia coli O157: H7 growth media for use in test-and-hold procedures for ground beef processing. J Food Prot 69:1007–1011
Baylis C, MacPhee S, Betts R (2000) Comparison of two commercial preparations of buffered peptone water for the recovery and growth of salmonella bacteria from foods. J Appl Microbiol 89:501–510
Margot H, Zwietering MH, Joosten H, O’Mahony E, Stephan R (2015) Evaluation of different buffered peptone water (BPW) based enrichment broths for detection of gram-negative foodborne pathogens from various food matrices. Int J Food Microbiol 214:109–115
Gough NL, Dodd CER (1998) The survival and disinfection of salmonella typhimurium on chopping board surfaces of wood and plastic. Food Control 9:363–368
Li K, Khouryieh H, Jones L, Etienne X, Shen C (2018) Assessing farmers market produce vendors’ handling of containers and evaluation of the survival of salmonella and listeria monocytogenes on plastic, pressed-card, and wood container surfaces at refrigerated and room temperature. Food Control 94:116–122
Chen Y, Jackson KM, Chea FP, Schaffner DW (2001) Quantification and variability analysis of bacterial cross-contamination rates in common food service tasks. J Food Prot 64:72–80
Zhao P, Zhao T, Doyle MP, Rubino JR, Meng J (1998) Development of a model for evaluation of microbial cross-contamination in the kitchen. J Food Prot 61:960–963
Jensen DA, Friedrich LM, Harris LJ, Danyluk MD, Schaffner DW (2013) Quantifying transfer rates of salmonella and Escherichia coli O157: H7 between fresh-cut produce and common kitchen surfaces. J Food Prot 76:1530–1538
Greenspan L (1977) Humidity fixed points of binary saturated aqueous solutions. J Res Natl Bur Stand 81:89–96
Hajime T, Ayumi O, Satoko M, Yukino I, Bon K (2011) Effect of food residues on norovirus survival on stainless steel surfaces. PLoS One 6:e21951
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter
Igo, M.J., Schaffner, D.W. (2021). Survival of Pathogens on Surfaces and the Influence of Inoculating Matrix on Survival Capabilities. In: Magnani, M. (eds) Detection and Enumeration of Bacteria, Yeast, Viruses, and Protozoan in Foods and Freshwater. Methods and Protocols in Food Science . Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1932-2_1
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1932-2_1
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1931-5
Online ISBN: 978-1-0716-1932-2
eBook Packages: Springer Protocols