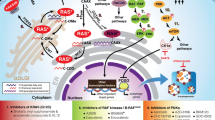
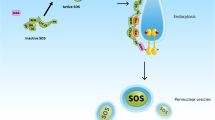
Abstract
Ras research has experienced a considerable boost in recent years, not least prompted by the Ras initiative launched by the NCI in 2013 (https://www.cancer.gov/research/key-initiatives/ras), accompanied and conditioned by a strongly reinvigorated determination within the Ras community to develop therapeutics attacking directly the Ras oncoproteins. As a member of the small G-protein superfamily, function and transforming activity of Ras all revolve about its GDP/GTP loading status. For one thing, the extent of GTP loading will determine the proportion of active Ras in the cell, with implications for intensity and quality of downstream signaling. But also the rate of nucleotide exchange, i.e., the Ras-GDP/GTP cycling rate, can have a major impact on Ras function, as illustrated perhaps most impressively by newly discovered fast-cycling oncogenic mutants of the Ras-related GTPase Rac1. Thus, while the last years have witnessed memorable new findings and technical developments in the Ras field, leading to an improved insight into many aspects of Ras biology, they have not jolted at the basics, but rather deepened our view of the fundamental regulatory principles of Ras activity control. In this brief review, we revisit the role and mechanisms of Ras nucleotide loading and its implications for cancer in the light of recent findings.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Vetter IR, Wittinghofer A (2001) The guanine nucleotide-binding switch in three dimensions. Science 294:1299–1304
Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420:629–635
Nobes CD, Hall A (1995) Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53–62
Novick P, Zerial M (1997) The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol 9:496–504
Pereira-Leal JB, Seabra MC (2001) Evolution of the Rab family of small GTP-binding proteins. J Mol Biol 313:889–901
Weis K (2003) Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 112:441–451
Li HY, Cao K, Zheng Y (2003) Ran in the spindle checkpoint: a new function for a versatile GTPase. Trends Cell Biol 13:553–557
Nie Z, Hirsch DS, Randazzo PA (2003) Arf and its many interactors. Curr Opin Cell Biol 15:396–404
Pasqualato S, Renault L, Cherfils J (2002) Arf, Arl, Arp and Sar proteins: a family of GTP-binding proteins with a structural device for 'front-back' communication. EMBO Rep 3:1035–1041
Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410:37–40
Matallanas D et al (2006) Distinct utilization of effectors and biological outcomes resulting from site-specific Ras activation: Ras functions in lipid rafts and Golgi complex are dispensable for proliferation and transformation. Mol Cell Biol 26:100–116
Ellis CA, Clark G (2000) The importance of being K-Ras. Cell Signal 12:425–434
Rajalingam K, Schreck R, Rapp UR, Albert S (2007) Ras oncogenes and their downstream targets. Biochim Biophys Acta 1773:1177–1195
Bos JL, Rehmann H, Wittinghofer A (2007) GEFs and GAPs: critical elements in the control of small G proteins. Cell 129:865–877
Wittinghofer A, Pai EF (1991) The structure of Ras protein: a model for a universal molecular switch. Trends Biochem Sci 16:382–387
Ahearn IM, Haigis K, Bar-Sagi D, Philips MR (2012) Regulating the regulator: post-translational modification of RAS. Nat Rev Mol Cell Biol 13:39–51
Karnoub AE, Weinberg RA (2008) Ras oncogenes: split personalities. Nat Rev 9:517–531
Vigil D, Cherfils J, Rossman KL, Der CJ (2010) Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer 10:842–857
Downward J (2006) Cancer biology: signatures guide drug choice. Nature 439:274–275
Schubbert S, Shannon K, Bollag G (2007) Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 7:295–308.
Vanhaesebroeck B, Stephens L, Hawkins P (2012) PI3K signalling: the path to discovery and understanding. Nat Rev 13:195–203
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501
Castellano E, Downward J (2011) RAS interaction with PI3K: more than just another effector pathway. Genes Cancer 2:261–274
Bos JL (1989) Ras oncogenes in human cancer: a review. Cancer Res 49:4682–4689
Castellano E, Santos E (2011) Functional specificity of ras isoforms: so similar but so different. Genes Cancer 2:216–231
Bar-Sagi D, Feramisco JR (1985) Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell 42:841–848
Noda M et al (1985) Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature 318:73–75
Bandaru P et al (2017) Deconstruction of the Ras switching cycle through saturation mutagenesis. eLife 6:e27810
Biankin AV et al (2012) Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491:399–405
Tran E et al (2016) T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med 375:2255–2262
Vaughn CP, Zobell SD, Furtado LV, Baker CL, Samowitz WS (2011) Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer 50:307–312
Prior IA, Lewis PD, Mattos C (2012) A comprehensive survey of Ras mutations in cancer. Cancer Res 72:2457–2467
Jebar AH et al (2005) FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene 24:5218–5225
Willumsen BM, Christensen A, Hubbert NL, Papageorge AG, Lowy DR (1984) The p21 ras C-terminus is required for transformation and membrane association. Nature 310:583–586
Willumsen BM, Norris K, Papageorge AG, Hubbert NL, Lowy DR (1984) Harvey murine sarcoma virus p21 ras protein: biological and biochemical significance of the cysteine nearest the carboxy terminus. EMBO J 3:2581–2585
Whyte DB et al (1997) K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem 272:14459–14464
Chandra A et al (2011) The GDI-like solubilizing factor PDEdelta sustains the spatial organization and signalling of Ras family proteins. Nat Cell Biol 14:148–158
Zimmermann G et al (2013) Small molecule inhibition of the KRAS-PDEdelta interaction impairs oncogenic KRAS signalling. Nature 497:638–642
Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM (2013) K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503:548–551
Lenzen C, Cool RH, Prinz H, Kuhlmann J, Wittinghofer A (1998) Kinetic analysis by fluorescence of the interaction between Ras and the catalytic domain of the guanine nucleotide exchange factor Cdc25Mm. Biochemistry 37:7420–7430
Trahey M, Mccormick F (1987) A cytoplasmic protein stimulates normal N-Ras P21 Gtpase, but does not affect oncogenic mutants. Science 238:542–545
Ebinu JO et al (1998) RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science 280:1082–1086
Lorenzo PS, Beheshti M, Pettit GR, Stone JC, Blumberg PM (2000) The guanine nucleotide exchange factor RasGRP is a high -affinity target for diacylglycerol and phorbol esters. Mol Pharmacol 57:840–846
Teixeira C, Stang SL, Zheng Y, Beswick NS, Stone JC (2003) Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood 102:1414–1420
Reuther GW et al (2002) RasGRP4 is a novel Ras activator isolated from acute myeloid leukemia. J Biol Chem 277:30508–30514
Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A (2005) A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol 25:4426–4441
Zheng Y et al (2005) Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and RAS signaling systems in B cells. Blood 105(9):3648–3654
Grabarek Z (2006) Structural basis for diversity of the EF-hand calcium-binding proteins. J Mol Biol 359:509–525
Gifford JL, Walsh MP, Vogel HJ (2007) Structures and metal-ion-binding properties of the Ca2+−binding helix-loop-helix EF-hand motifs. Biochem J 405:199–221
Tazmini G et al (2009) Membrane localization of RasGRP1 is controlled by an EF-hand, and by the GEF domain. Biochim Biophys Acta 1793:447–461
Zahedi B et al (2011) Phosphoinositide 3-kinase regulates plasma membrane targeting of the Ras-specific exchange factor RasGRP1. J Biol Chem 286:12712–12723
Iwig JS et al (2013) Structural analysis of autoinhibition in the Ras-specific exchange factor RasGRP1. eLife 2:e00813
Daley SR et al (2013) Rasgrp1 mutation increases naive T-cell CD44 expression and drives mTOR-dependent accumulation of Helios+ T cells and autoantibodies. eLife 2:e01020
Myers DR, Norlin E, Vercoulen Y, Roose JP (2019) Active tonic mTORC1 signals shape baseline translation in naive T cells. Cell Rep 27:1858–1874 e1856
Vercoulen Y et al (2017) A Histidine pH sensor regulates activation of the Ras-specific guanine nucleotide exchange factor RasGRP1. eLife 6:e29002
Ksionda O, Limnander A, Roose JP (2013) RasGRP Ras guanine nucleotide exchange factors in cancer. Front Biol (Beijing) 8:508–532
Stone JC (2011) Regulation and function of the RasGRP family of Ras activators in blood cells. Genes Cancer 2:320–334
Baltanas FC et al (2013) Functional redundancy of Sos1 and Sos2 for lymphopoiesis and organismal homeostasis and survival. Mol Cell Biol 33:4562–4578
Sondermann H, Soisson SM, Bar-Sagi D, Kuriyan J (2003) Tandem histone folds in the structure of the N-terminal segment of the ras activator Son of Sevenless. Structure 11:1583–1593
Chen RH, Corbalan-Garcia S, Bar-Sagi D (1997) The role of the PH domain in the signal-dependent membrane targeting of Sos. EMBO J 16:1351–1359
Soisson SM, Nimnual AS, Uy M, Bar-Sagi D, Kuriyan J (1998) Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein. Cell 95:259–268
Nimnual AS, Yatsula BA, Bar-Sagi D (1998) Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science 279:560–563
Li N et al (1993) Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature 363:85–88
Buday L, Downward J (1993) Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell 73:611–620
Buday L, Egan SE, Rodriguez Viciana P, Cantrell DA, Downward J (1994) A complex of Grb2 adaptor protein, Sos exchange factor, and a 36-kDa membrane-bound tyrosine phosphoprotein is implicated in ras activation in T cells. J Biol Chem 269:9019–9023
Aronheim A et al (1994) Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell 78:949–961
Christensen SM et al (2016) One-way membrane trafficking of SOS in receptor-triggered Ras activation. Nat Struct Mol Biol 23:838–846
Margarit SM et al (2003) Structural evidence for feedback activation by Ras.GTP of the Ras-specific nucleotide exchange factor SOS. Cell 112:685–695
Gureasko J et al (2010) Role of the histone domain in the autoinhibition and activation of the Ras activator Son of Sevenless. Proc Natl Acad Sci U S A 107:3430–3435
Gureasko J et al (2008) Membrane-dependent signal integration by the Ras activator Son of sevenless. Nat Struct Mol Biol 15:452–461
Sondermann H et al (2004) Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell 119:393–405
Das J et al (2009) Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell 136:337–351
Kubiseski TJ, Chook YM, Parris WE, Rozakis-Adcock M, Pawson T (1997) High affinity binding of the pleckstrin homology domain of mSos1 to phosphatidylinositol (4,5)-bisphosphate. J Biol Chem 272:1799–1804
Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D (2007) Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol 9:706–712
Yadav KK, Bar-Sagi D (2010) Allosteric gating of Son of sevenless activity by the histone domain. Proc Natl Acad Sci U S A 107:3436–3440
Jun JE, Rubio I, Roose JP (2013) Regulation of Ras exchange factors and cellular localization of Ras activation by lipid messengers in T cells. Front Immunol 4:239
Lemmon MA, Ferguson KM (2000) Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J 350(Pt 1):1–18
Parry DA, Fraser RD, Squire JM (2008) Fifty years of coiled-coils and alpha-helical bundles: a close relationship between sequence and structure. J Struct Biol 163:258–269
Bahler M, Rhoads A (2002) Calmodulin signaling via the IQ motif. FEBS Lett 513:107–113
Buchsbaum RJ, Connolly BA, Feig LA (2002) Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol Cell Biol 22:4073–4085
Fan WT, Koch CA, de Hoog CL, Fam NP, Moran MF (1998) The exchange factor Ras-GRF2 activates Ras-dependent and Rac-dependent mitogen-activated protein kinase pathways. Curr Biol 8:935–938
Kiyono M, Satoh T, Kaziro YG (1999) Protein beta gamma subunit-dependent Rac-guanine nucleotide exchange activity of Ras-GRF1/CDC25(Mm). Proc Natl Acad Sci U S A 96:4826–4831
Freedman TS et al (2006) A Ras-induced conformational switch in the Ras activator Son of sevenless. Proc Natl Acad Sci U S A 103:16692–16697
Martegani E et al (1992) Cloning by functional complementation of a mouse cDNA encoding a homologue of CDC25, a Saccharomyces cerevisiae RAS activator. EMBO J 11:2151–2157
Wei W, Schreiber SS, Baudry M, Tocco G, Broek D (1993) Localization of the cellular expression pattern of cdc25NEF and ras in the juvenile rat brain. Brain Res Mol Brain Res 19:339–344
Krapivinsky G et al (2003) The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 40:775–784
Giese KP et al (2001) Hippocampus-dependent learning and memory is impaired in mice lacking the Ras-guanine-nucleotide releasing factor 1 (Ras-GRF1). Neuropharmacology 41:791–800
Fernandez-Medarde A et al (2002) Targeted disruption of Ras-Grf2 shows its dispensability for mouse growth and development. Mol Cell Biol 22:2498–2504
Font de Mora J et al (2003) Ras-GRF1 signaling is required for normal beta-cell development and glucose homeostasis. EMBO J 22:3039–3049
Ruiz S, Santos E, Bustelo XR (2007) RasGRF2, a guanosine nucleotide exchange factor for Ras GTPases, participates in T-cell signaling responses. Mol Cell Biol 27:8127–8142
Cai D, Choi PS, Gelbard M, Meyerson M (2019) Identification and characterization of oncogenic SOS1 mutations in lung adenocarcinoma. Mol Cancer Res 17:1002–1012
Swanson KD et al (2008) SOS1 mutations are rare in human malignancies: implications for Noonan syndrome patients. Genes Chromosomes Cancer 47:253–259
Maurer T et al (2012) Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci U S A 109:5299–5304
Sun Q et al (2012) Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated activation. Angew Chem Int Ed Engl 51:6140–6143
Burns MC et al (2014) Approach for targeting Ras with small molecules that activate SOS-mediated nucleotide exchange. Proc Natl Acad Sci U S A 111:3401–3406
Howes JE et al (2018) Small molecule-mediated activation of RAS elicits biphasic modulation of phospho-ERK levels that are regulated through negative feedback on SOS1. Mol Cancer Ther 17:1051–1060
Burns MC et al (2018) High-throughput screening identifies small molecules that bind to the RAS:SOS:RAS complex and perturb RAS signaling. Anal Biochem 548:44–52
Patgiri A, Yadav KK, Arora PS, Bar-Sagi D (2011) An orthosteric inhibitor of the Ras-Sos interaction. Nat Chem Biol 7:585–587
Leshchiner ES et al (2015) Direct inhibition of oncogenic KRAS by hydrocarbon-stapled SOS1 helices. Proc Natl Acad Sci U S A 112:1761–1766
Nichols RJ et al (2018) RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat Cell Biol 20:1064–1073
Dance M, Montagner A, Salles JP, Yart A, Raynal P (2008) The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal 20:453–459
Depeille P et al (2015) RasGRP1 opposes proliferative EGFR-SOS1-Ras signals and restricts intestinal epithelial cell growth. Nat Cell Biol 17:804–815
Hartzell C et al (2013) Dysregulated RasGRP1 responds to cytokine receptor input in T cell leukemogenesis. Sci Signal 6:ra21
Ksionda O et al (2015) RasGRP1 overexpression in T-ALL increases basal nucleotide exchange on Ras rendering the Ras/PI3K/Akt pathway responsive to protumorigenic cytokines. Oncogene 35(28):3658–3668
Rambaratsingh RA, Stone JC, Blumberg PM, Lorenzo PS (2003) RasGRP1 represents a novel non-protein kinase C phorbol ester signaling pathway in mouse epidermal keratinocytes. J Biol Chem 278:52792–52801
Oki-Idouchi CE, Lorenzo PS (2007) Transgenic overexpression of RasGRP1 in mouse epidermis results in spontaneous tumors of the skin. Cancer Res 67:276–280
Yang D et al (2010) RasGRP3 contributes to formation and maintenance of the prostate cancer phenotype. Cancer Res 70:7905–7917
Yang D et al (2011) RasGRP3, a Ras activator, contributes to signaling and the tumorigenic phenotype in human melanoma. Oncogene 30(45):4590–4600
Chen X et al (2017) RasGRP3 mediates MAPK pathway activation in GNAQ mutant uveal melanoma. Cancer Cell 31:685–696 e686
Watanabe-Okochi N et al (2009) Possible involvement of RasGRP4 in leukemogenesis. Int J Hematol 89:470–481
Gasper R, Wittinghofer F (2019) The Ras switch in structural and historical perspective. Biol Chem 401:143–163
Wittinghofer A, Scheffzek K, Ahmadian MR (1997) The interaction of Ras with GTPase-activating proteins. FEBS Lett 410:63–67
Bernards A (2003) GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta 1603:47–82
Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB (1998) A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron 20:895–904
Hennig A, Markwart R, Esparza-Franco MA, Ladds G, Rubio I (2015) Ras activation revisited: role of GEF and GAP systems. Biol Chem 396:831–848
Erickson KE, Rukhlenko OS, Posner RG, Hlavacek WS, Kholodenko BN (2019) New insights into RAS biology reinvigorate interest in mathematical modeling of RAS signaling. Semin Cancer Biol 54:162–173
Brightman FA, Fell DA (2000) Differential feedback regulation of the MAPK cascade underlies the quantitative differences in EGF and NGF signalling in PC12 cells. FEBS Lett 482:169–174
Kholodenko BN (2000) Negative feedback and ultrasensitivity can bring about oscillations in the mitogen-activated protein kinase cascades. Eur J Biochem 267:1583–1588
Kamioka Y, Yasuda S, Fujita Y, Aoki K, Matsuda M (2010) Multiple decisive phosphorylation sites for the negative feedback regulation of SOS1 via ERK. J Biol Chem 285:33540–33548
Cirit M, Wang CC, Haugh JM (2010) Systematic quantification of negative feedback mechanisms in the extracellular signal-regulated kinase (ERK) signaling network. J Biol Chem 285:36736–36744
Holt KH, Kasson BG, Pessin JE (1996) Insulin stimulation of a MEK-dependent but ERK-independent SOS protein kinase. Mol Cell Biol 16:577–583
Corbalan-Garcia S, Yang SS, Degenhardt KR, Bar-Sagi D (1996) Identification of the mitogen-activated protein kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol Cell Biol 16:5674–5682
Rozakis-Adcock M, van der Geer P, Mbamalu G, Pawson T (1995) MAP kinase phosphorylation of mSos1 promotes dissociation of mSos1-Shc and mSos1-EGF receptor complexes. Oncogene 11:1417–1426
Douville E, Downward J (1997) EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene 15:373–383
Markevich NI, Hoek JB, Kholodenko BN (2004) Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J Cell Biol 164:353–359
Markevich NI et al (2004) Signal processing at the Ras circuit: what shapes Ras activation patterns? Syst Biol 1:104–113
Mues M, Roose JP (2016) Distinct oncogenic Ras signals characterized by profound differences in flux through the RasGDP/RasGTP cycle. Small GTPases 8:20–25
Lubeck BA et al (2015) Cutting edge: Codeletion of the Ras GTPase-activating proteins (RasGAPs) Neurofibromin 1 and p120 RasGAP in T cells results in the development of T cell acute lymphoblastic leukemia. J Immunol 195:31–35
Kazlauskas A, Ellis C, Pawson T, Cooper JA (1990) Binding of GAP to activated PDGF receptors. Science 247:1578–1581
Anderson D et al (1990) Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science 250:979–982
Hennig A et al (2016) Feedback activation of neurofibromin terminates growth factor-induced Ras activation. Cell Commun Signal 14:5
Taylor SJ, Shalloway D (1996) Cell cycle-dependent activation of Ras. Curr Biol 6:1621–1627
Gille H, Downward J (1999) Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem 274:22033–22040
Vasjari L, Bresan S, Biskup C, Pai G, Rubio I (2019) Ras signals principally via Erk in G1 but cooperates with PI3K/Akt for Cyclin D induction and S-phase entry. Cell Cycle 18:204–225
Downward J (1992) Regulatory mechanisms for ras proteins. BioEssays 14:177–184
Schlesinger TK, Demali KA, Johnson GL, Kazlauskas A (1999) Platelet-derived growth factor-dependent association of the GTPase-activating protein of Ras and Src. Biochem J 344(Pt 2):519–526
Liu Q et al (2005) CAPRI and RASAL impose different modes of information processing on Ras due to contrasting temporal filtering of Ca2+. J Cell Biol 170:183–190
Kwiatkowski DJ, Manning BD (2005) Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet 14 Spec No. 2:R251–R258
Im E et al (2002) Rheb is in a high activation state and inhibits B-Raf kinase in mammalian cells. Oncogene 21:6356–6365
Li Y, Inoki K, Guan KL (2004) Biochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activity. Mol Cell Biol 24:7965–7975
Hsu YC, Chern JJ, Cai Y, Liu M, Choi KW (2007) Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature 445:785–788
Rehmann H et al (2008) Biochemical characterisation of TCTP questions its function as a guanine nucleotide exchange factor for Rheb. FEBS Lett 582:3005–3010
Rubio I, Wetzker R (2000) A permissive function of phosphoinositide 3-kinase in Ras activation mediated by inhibition of GTPase-activating proteins. Curr Biol 10:1225–1228
Downward J, Graves JD, Warne PH, Rayter S, Cantrell DA (1990) Stimulation of p21ras upon T-cell activation. Nature 346:719–723
Downward J, Graves J, Cantrell D (1992) The regulation and function of p21ras in T cells. Immunol Today 13:89–92
Zhang K, Papageorge AG, Lowy DR (1992) Mechanistic aspects of signaling through Ras in NIH 3T3 cells. Science 257:671–674
Cui Y et al (2019) The NF2 tumor suppressor merlin interacts with Ras and RasGAP, which may modulate Ras signaling. Oncogene 38:6370–6381
Marshall CB et al (2012) Probing the GTPase cycle with real-time NMR: GAP and GEF activities in cell extracts. Methods 57:473–485
Maertens O, Cichowski K (2014) An expanding role for RAS GTPase activating proteins (RAS GAPs) in cancer. Adv Biol Regul 55:1–14
Revencu N et al (2008) Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat 29:959–965
Lapinski PE et al (2012) RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice. J Clin Invest 122:733–747
Marshall CJ (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179–185
Muroya K, Hattori S, Nakamura S (1992) Nerve growth factor induces rapid accumulation of the GTP-bound form of p21ras in rat pheochromocytoma PC12 cells. Oncogene 7:277–281
Traverse S, Gomez N, Paterson H, Marshall C, Cohen P (1992) Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J 288(Pt 2):351–355
Roberts AE et al (2007) Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet 39:70–74
Marino B, Digilio MC, Toscano A, Giannotti A, Dallapiccola B (1999) Congenital heart diseases in children with Noonan syndrome: an expanded cardiac spectrum with high prevalence of atrioventricular canal. J Pediatr 135:703–706
Sharland M, Burch M, McKenna WM, Paton MA (1992) A clinical study of Noonan syndrome. Arch Dis Child 67:178–183
Witt DR et al (1988) Bleeding diathesis in Noonan syndrome: a common association. Am J Med Genet 31:305–317
Sharland M, Patton MA, Talbot S, Chitolie A, Bevan DH (1992) Coagulation-factor deficiencies and abnormal bleeding in Noonan’s syndrome. Lancet 339:19–21
Schubbert S et al (2006) Germline KRAS mutations cause Noonan syndrome. Nat Genet 38:331–336
Pandit B et al (2007) Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet 39:1007–1012
Tartaglia M et al (2001) Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet 29:465–468
Legius E et al (2002) PTPN11 mutations in LEOPARD syndrome. J Med Genet 39:571–574
Digilio MC et al (2002) Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet 71:389–394
Aoki Y et al (2005) Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet 37:1038–1040
Tidyman WE, Rauen KA (2008) Noonan, Costello and cardio-facio-cutaneous syndromes: dysregulation of the Ras-MAPK pathway. Expert Rev Mol Med 10:e37
Denayer E et al (2008) Mutation analysis in Costello syndrome: functional and structural characterization of the HRAS p.Lys117Arg mutation. Hum Mutat 29:232–239
Yoon G, Rosenberg J, Blaser S, Rauen KA (2007) Neurological complications of cardio-facio-cutaneous syndrome. Dev Med Child Neurol 49:894–899
Rodriguez-Viciana P et al (2006) Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science 311:1287–1290
Anastasaki C, Estep AL, Marais R, Rauen KA, Patton EE (2009) Kinase-activating and kinase-impaired cardio-facio-cutaneous syndrome alleles have activity during zebrafish development and are sensitive to small molecule inhibitors. Hum Mol Genet 18:2543–2554
Acknowledgments
We thank Anna Hupalowska for creating the illustrations. Our activities are supported by grants from the NIH-NIAID (R01-AI104789 and P01-AI091580), NIH-NCI (R01—CA187318), the NIH-NHLBI (R01—HL120724), the Alex’ Lemonade Stand Foundation (Innovator Award), and the Mark Foundation for Cancer Research (Momentum Award).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Kulhanek, K.R., Roose, J.P., Rubio, I. (2021). Regulation of the Small GTPase Ras and Its Relevance to Human Disease. In: Rubio, I., Prior, I. (eds) Ras Activity and Signaling. Methods in Molecular Biology, vol 2262. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1190-6_2
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1190-6_2
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1189-0
Online ISBN: 978-1-0716-1190-6
eBook Packages: Springer Protocols