Abstract
The choroid plexus comprises of a monolayer of tightly connected epithelial cells that form an important physical, enzymatic, and immunologic barrier, called the blood–cerebrospinal fluid (CSF) barrier. It is a highly vascularized structure located in the brain ventricles and plays a key role in maintaining brain homeostasis by producing CSF.
During aging, the morphology and normal function of the choroid plexus is compromised. Different alterations of the choroid plexus have been reported such as atrophy of the choroid plexus epithelial cells, decreased CSF production and secretion, decreased CSF clearance and absorption resulting in reduced clearance of toxic compounds, reduced enzymatic and metabolic activity, loss of barrier integrity, and insufficient distribution of nutrients. The described degeneration of the structure and function of the choroid plexus can result in multiple brain deficits and contribute to cognitive deterioration. In fact, these alterations of the choroid plexus are even more prominent in age-related neurodegenerative diseases including late-onset Alzheimer’s disease. A better understanding of the alterations in structure, activity, and function of the choroid plexus epithelial cells during aging and how the choroid plexus is implicated in aging and age-associated neurological diseases might reveal novel strategies to combat age-related cognitive decline and age-related neurological disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Aging is a complex, multifactorial process influenced by many unknown genetic and environmental factors. It is associated with progressive decline in normal cell and organ functioning. An important hallmark of aging is ‘inflamm-aging’, a state of chronic, low-grade inflammation, caused by an elevated concentration of inflammatory markers in the circulation (Calder et al. 2017). The balance between pro- and anti-inflammatory cytokines in the healthy adult brain is shifted with aging towards a pro-inflammatory state (Franceschi 2007). This immunological fragile state makes the aged brain more susceptible to diseases, infection, and stress, which might even influence the onset of age-related neurodegenerative brain diseases (Franceschi and Campisi 2014; Gorle et al. 2016).
With life expectancy exponentially increasing, age-related diseases will become an emerging epidemic and a tremendous public health issue due to the high costs of dementia care. Numbers are predicted to increase to 152 million in 2050 and there are over 9.9 million new cases of dementia each year worldwide (Report 2018), in addition no treatment to reverse or halt disease progression exists. Increasing evidence indicates that degeneration of the choroid plexus can result in brain deficits and contribute to cognitive impairment. Therefore, extensive insights in the aging choroid plexus are essential in understanding age-associated neurodegenerative and neuroinflammatory disorders and pave new ways for therapy.
The role of the choroid plexus in health and disease is being increasingly recognized and it has been reported to play a central role during aging (Gorle et al. 2016; Vandenbroucke 2016; Marques et al. 2017). The choroid plexus is a highly vascularized brain structure, consisting of a monolayer of choroid plexus epithelial cells firmly interconnected by tight junctions (De Bock et al. 2014), that form one of the brain barriers, called the blood-cerebrospinal fluid (CSF) barrier. Together with other brain barriers, the blood-CSF barrier assures a balanced and well-controlled micro-environment in the central nervous system (CNS), providing protection against external insults such as toxins, infectious agents, and peripheral blood fluctuations (Gorle et al. 2016). The choroid plexus produces CSF and receives input from both circulatory, autonomic and immune system. It can respond as a key regulator to local changes of different physiological signals by changing its secretome, including proteins (Marques and Sousa 2015; Silva-Vargas et al. 2016) and extracellular vesicles (EVs) (Balusu et al. 2016b). The normal functioning of the choroid plexus is severely affected during aging and this dysfunction is even aggravated in age-related neurodegenerative brain diseases like Alzheimer’s disease (Balusu et al. 2016a). Understanding how the function and activity of the choroid plexus is altered in aging might lead to the identification of strategies to attenuate aging-associated cognitive decline and related diseases (Baruch et al. 2014; Vandenbroucke 2016; Gorle et al. 2016).
9.2 Morphological Changes of the Choroid Plexus Epithelium Upon Aging
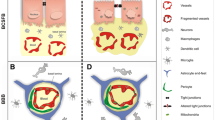
Several reports have been published describing the morphological alterations of the choroid plexus epithelium upon aging (Fig. 9.1), which is comparable to other secretory epithelia. Across species epithelial atrophy and weight increase have been observed (Wen et al. 1999), however slightly different modifications have been described according to species.
Schematic representation of the changes at the choroid plexus during aging. Several morphological changes are observed at the choroid plexus: the choroid plexus epithelial cells are flattened with an irregular nucleus and shortened microvilli, more Biondi rings and lipofuscin are present, and the basement membrane is thickened and contains fragmented vessels, collagen fibers, hyaline bodies, calcifications, and psammomas. Functionally multiple alterations were shown: increased cerebrospinal fluid (CSF)/serum albumin ratio, reduced metabolic activity, decreased extracellular vesicles (EVs) in the CSF, increased levels of lactate and vasopressin, and altered immune cell recruitment (linked with changes in the interferon (IFN) balance). Key: CSF: cerebrospinal fluid, EV: extracellular vesicles, IFN: interferon, IL: interleukin, LDH: lactate dehydrogenase, SDH: succinate dehydrogenase
In humans, the height of the epithelial cells decreases with approximately 11% during life and the cells become more flattened (Serot et al. 2000). The aged cell cytoplasm contains protein inclusions called Biondi ring tangles. In addition, the presence of lipofuscin deposits can be found. Since this age pigment is a product of lipid peroxidation by free oxygen radicals it will probably alter the cell functioning (ZS-Nagy et al. 1995). The nuclei become more irregular in elderly and have a flattened shape (Serot et al. 2000, 2001). Moreover, the epithelial basement membrane has been reported to become thicker with aging. Also the stroma of the aged choroid plexus is thicker and contains collagen fibers, hyaline bodies, calcifications, and psammomas (i.e. dystrophic calcifications) (Eriksson and Westermark 1986; Jovanovic et al. 2004; Sturrock 1988; Wen et al. 1999). An age-associated increase in size and volume density of the psammoma bodies has been described (Zivkovic et al. 2017). The arterial walls become thicker, especially the media and adventitia, while the blood vessel volume density decreases and elastic fibers are fragmented (Serot et al. 2000; Shuangshoti and Netsky 1970; Zivkovic et al. 2017), resulting in a reduced contact area between the blood and the choroid plexus epithelium.
Rodent models show similar epithelial disruptions of the choroid plexus epithelium compared to humans (Serot et al. 2001; Sturrock 1988). In elderly rats the epithelial cells lose height, approximately 15%, and become more flattened. The cells show an irregular, elongated nucleus and shortened microvilli, causing a decrease in the choroid plexus epithelium-CSF contact area. Lipid vacuoles are present in the cytoplasm of the choroidal epithelial cells. Irregular fibrosis has been described in the stroma of elderly rats together with thickening of the basement membranes (Serot et al. 2001; Sturrock 1988).
Age-associated reduction in contact area between blood-choroid plexus epithelium and choroid plexus epithelium-CSF due to morphological alterations, together with the changes in choroidal proteins involved in CSF production (Masseguin et al. 2005), negatively influence the CSF production (Vandenbroucke 2016). These morphological changes will result in functional alterations, which may consequently have an impact on brain homeostasis.
9.3 Functional Alterations of the Choroid Plexus in Aging
9.3.1 CSF Dynamics
9.3.1.1 CSF Production and Secretion
One of the major functions of the choroid plexus is CSF production and secretion. CSF flows from the choroid plexus through the ventricular system to the subarachnoid space and continues to the spinal column. The classic theory suggests that CSF flow is pulsatile and generated by cardiac pulsations and pulmonary respiration (Khasawneh et al. 2018; Sakka et al. 2011). CSF not only provides mechanical support to the brain (Segal 2000) but also helps to remove toxic catabolites of the brain metabolism (Brown et al. 2004). Furthermore, CSF can be considered as a route of communication within the brain as it carries hormones, growth factors, and neurotransmitters between different areas of the brain (Kaur et al. 2016; Marques et al. 2011; Silva-Vargas et al. 2016; Preston 2001; Brown et al. 2004; Strazielle and Ghersi-Egea 2000).
The adult brain contains a constant volume of 150 ml CSF, of which 25 ml in the brain ventricles and 125 ml in the subarachnoid compartments. The total CSF production is about 500 ml per day in healthy individuals at a rate of about 0.3–0.4 ml per minute and is completely replaced about four times a day (Brown et al. 2004; Khasawneh et al. 2018). CSF is for 99% composed of water with the remaining 1% accounted for by proteins, ions, neurotransmitters, and glucose. Ion concentrations of Na+, Cl−, and Mg2+ are higher in CSF than the levels in plasma, while K+ and Ca2+ concentrations are lower (Bulat and Klarica 2011; Sakka et al. 2011). The majority of the total CSF volume (60–90%) is being produced and secreted by the choroid plexus epithelium, the remaining CSF originates from the brain interstitial fluid, ependyma, and cerebral capillaries (Redzic and Segal 2004; Sakka et al. 2011). CSF secretion by the choroid plexus is dependent on active translocation of ions and water from the basolateral membrane to the cytoplasm and subsequently across the apical membrane into the brain ventricles (Brown et al. 2004). Transport of Na+, Cl−, K+ , and HCO3 − takes place via different transporters present on the choroid plexus epithelium. Na+ and Cl− are transported into the epithelial cells by Cl−/HCO3 − and Na+ linked Cl−/HCO3 − transporters present on the basolateral surface (Lindsey et al. 1990). Translocation of these ions creates an osmotic gradient which drives water transport facilitated by aquaporins (AQP) on the epithelial surface (Liddelow 2015). At the apical surface, the Na+, K+-ATPase plays an important role creating an osmotic gradient which facilitates transfer of various molecules in and out the choroid plexus epithelium (Pershing and Johanson 1982; Plotkin et al. 1997; Redzic and Segal 2004; Speake et al. 2001; Johanson et al. 2008). Besides the Na+, K+-ATPase transporter, also the electrogenic sodium-bicarbonate cotransporter (NBCe2) facilitates Na+ ion transport into the CSF. Interestingly, a knockout of this NBCe2 cotransporter resulted in significant remodeling of choroid plexus epithelium including abnormal mitochondrial distribution, cytoskeletal protein expression, CSF electrolyte imbalance, and neurological impairment (Kao et al. 2011), reflecting the importance of cotransporter in the normal physiology of the nervous system (Christensen et al. 2018).
In elderly, the CSF production is reduced as shown in multiple studies in human, rat, and sheep (Table 9.1). The reduced expression of choroidal proteins involved in CSF secretion such as carbonic anhydrase II and AQP1 have been described in aging rat models and sheep. In addition, Na+, K+-ATPase mRNA levels decrease with age (Chen et al. 2009; Kvitnitskaia-Ryzhova and Shkapenko 1992; Masseguin et al. 2005). Next to a decreased CSF production rate, the mean CSF pressure declines steadily after the age of 50. In comparison to a 20–49 year old group, the 50–54 age group showed a reduction of 2.5% and this even increased to 13% in individuals older than 70 years of age (Fleischman et al. 2012).
9.3.1.2 CSF Absorption
The site of CSF absorption is still a point of discussion in the research field. For decades it was believed that CSF returns to the venous blood in the brain sinuses through the arachnoid villi and granulations (Kida et al. 1988). These arachnoid granulations are projections of the arachnoid membrane into the dural venous sinuses. The driving force of the absorption of CSF into the venous bloodstream is a difference in fluid pressure between the subarachnoid space and the venous system. As such, fluid is driven out of the granulations into the circulation (Damkier et al. 2013). In addition, CSF absorption sites have been identified on meningeal recesses of spinal and cranial nerve roots, particularly the trigeminal and cochlear nerve (Sakka et al. 2011). Recently, dynamic imaging suggested that lymphatic outflow might be the major outflow route for CSF. Using non-invasive imaging techniques, the authors were able to demonstrate that tracers added to the CSF rapidly reach the lymph nodes using perineural routes through the foramina of the skull to finally reach the peripheral blood (Ma et al. 2017; Proulx et al. 2017). Interestingly, this lymphatic outflow system showed significant decline in aged mice (Ma et al. 2017).
9.3.1.3 CSF Turnover and Circulation
Moderate brain tissue atrophy that occurs during healthy aging, leading to an increase in total cranial CSF compartment and volume, affects the turnover or replacement time of CSF (Table 9.2) (Preston 2001). The decreased production and secretion of CSF together with increased CSF volume results in a longer CSF turnover with age. These observations have been confirmed by reduced clearance of radio-iodinated human serum albumin from the brain in individuals around 62 years of age (Henriksson and Voigt 1976). Similarly, reduced clearance of 3H-polyethylene glycol and 125I-Amyloid beta (Aβ) (1–40) was observed in older rats (Preston 2001). Cross-sectional studies in healthy humans show a doubling of CSF volume between the age of 30 and 70 years (Foundas et al. 1998; Matsumae et al. 1996a). In elderly humans, CSF turnover is reduced to two times daily in comparison to three to four times in young adults (Chiu et al. 2012; Johanson et al. 2008). In humans, additional factors have been described that contribute to the reduced CSF turnover, including increased resistance for CSF drainage by fibrosis present in the arachnoid membranes and an increase in central venous pressure, both noted to be increased in normal aging (Preston 2001; Bellur et al. 1980; Rubenstein 1998).
The diminished CSF production, secretion, and reduced CSF turnover rate might have serious complications. In senescence, alterations in CSF composition due to reduced turnover could bring about inadequate distribution of nutritive components and trophic factors, and the diminished CSF clearance leads to accumulation of toxic compounds and waste products from the brain (Marques et al. 2017; Preston 2001). Both will result in increased cellular stress and changes in the cerebral metabolism and blood flow, disrupting cognitive and motor functions (Rubenstein 1998), eventually influencing age-related cognitive decline and development of age-associated neurological diseases (Emerich et al. 2005). In addition, adult neural stem cells contact the CSF in the ventricular-subventricular stem cell niche of which the lateral choroid plexus is an important component. This implicates that secreted factors and toxic compounds accumulated due to diminished CSF clearance can impact the neural stem cells, which are especially sensitive to age-related changes (Silva-Vargas et al. 2016).
CSF production, clearance rate, and CSF flow are altered during aging, thereby affecting brain homeostasis. Interestingly, a highly organized pattern of ependymal cilia is responsible for the transport of CSF in the ventricles of the mouse brain. Coordinated cilia beating patterns collectively give rise to a network of fluid flows that allow for precise CSF directional flow, which may control substance distribution in the ventricle. A cilia-based switch was discovered that reliably and periodically alters the flow pattern and may control substance distribution in the ventricle (Faubel et al. 2016). However, it remains to be determined whether changes in beating patterns occur in aging and whether this affects the distribution of components throughout the brain. Peak CSF volume flow is altered in aging and higher aqueductal peak CSF flow velocities were described in elderly healthy volunteers (Gideon et al. 1994). In addition, the total cerebral blood flow decreases with aging and consequently CSF stroke volumes (i.e. mean volume of CSF passing through the aqueduct during both systole and diastole) and pulsations were significantly reduced in elderly (Stoquart-Elsankari et al. 2007).
9.3.1.4 Choroid Plexus Biochemistry
Choroid plexus functioning and metabolism are largely energy-dependent. All the homeostatic and secretory functions of the choroid plexus are linked to energy dependent mechanisms, explaining the huge number of mitochondria in the choroid plexus epithelial cells. Morphometric studies in different model organisms indicate that mitochondria constitute 10–14% of the choroid plexus cytoplasm (Cornford et al. 1997). In addition, proteome analysis in rats identified a total of 1400 proteins in the choroid plexus of which a high percentage (33.5%) are mapped to metabolism, e.g. several enzymes like hydrolases, oxidoreductases, and transferases. The presence of a substantial number of mitochondrial proteins in the proteome analyses suggests a high mitochondrial density (Sathyanesan et al. 2012). Aging however leads to a reduced metabolic activity of the choroid plexus epithelial cells, as demonstrated by in vitro choroid plexus cultures (Emerich et al. 2007). Additionally, the number of epithelial cells deficient in cytochrome C oxidase has been shown to be increased with age (Cottrell et al. 2001).
The mammalian brain depends on glucose as main energy source and a continuous supply is essential to sustain neural activity (Siesjo 1978; Simpson et al. 2007). Glucose provides energy for physiological brain functioning (biosynthesis of neurotransmitters, maintenance of action potentials, information processing) by oxidative metabolism and tight regulation of the glucose metabolism is necessary (Mergenthaler et al. 2013). Glucose transporter proteins transfer glucose from the blood circulation to the brain. The blood-CSF barrier expresses GLUT1 (Redzic 2011; Serot et al. 2003; Simpson et al. 2007). Disruption of the glucose metabolism and the pathways involved in glucose delivery can have pathophysiological consequences and lead to brain diseases. There is compelling evidence that the aging tissue is unable to maintain appropriate energy output. During aging, the expression of enzymes necessary for anaerobic respiration and oxidative phosphorylation, such as lactate dehydrogenase (LDH) and succinate-dehydrogenase (SDH), are diminished and consequently energy production in choroid plexus epithelial cells decreases (Fig. 9.1) (Emerich et al. 2005; Ferrante and Amenta 1987; Gorle et al. 2016). Both LDH and SDH play a key role in glucose metabolism and show a major reduction with age, respectively 9 and 26% (Ferrante and Amenta 1987; Preston 2001). Impairment of glucose dependent energy transduction mechanisms may influence the functional activity of the choroid plexus epithelial cells (Ferrante and Amenta 1987). In addition, in humans CSF levels of lactate increase with age (Fig. 9.1). Since CSF lactate and brain lactate concentration correlate closely, this might suggest a decline in the efficiency of glucose metabolism in brain tissue (Yesavage et al. 1982).
Different imaging methods have been developed to allow non-invasive brain measurements. Functional magnetic resonance spectroscopy (fMRS) is used for the measurement of metabolite concentrations in the human brain (Jahng et al. 2016). In addition, alterations of the glucose metabolism in the choroid plexus can be visualized and measured in vivo with dynamic fluorodeoxyglucose positron emission tomography (dynamic 18F-FDG-PET). By using this technique, the dynamic uptake of FDG in the choroid plexus and CSF can be measured over time. A recent study showed the presence of decreased glucose metabolism in Alzheimer’s disease patients. Conversely, dynamic uptake was higher in CSF for Alzheimer’s disease patients. The activity of the choroid plexus gradually decreases in patients with cognitive decline. This results in the disturbance of the glucose exchange at the blood-CSF barrier and alters the CSF-choroid plexus glucose equilibrium (Daouk et al. 2016).
9.3.1.5 Iron Metabolism
Iron is an essential element for different metabolic processes, tissue homeostasis, and brain functioning. However, in excessive amounts, iron becomes toxic for cells. Therefore, the iron delivery in the brain is strictly regulated through receptor mediated endocytosis of iron-bound transferrin by the blood-CSF barrier and the blood-brain barrier (Morris et al. 1992; Deane et al. 2004; Rouault et al. 2009; Hubert et al. 2019). During aging, decreased metabolic activity, increased oxidative stress, impaired barrier functioning, impaired protein secretion, and diminution of the CSF flow might all affect the iron metabolism and iron-mediated toxicity (Marques et al. 2009, 2007; Chen et al. 2012b). Moreover, pro-inflammatory cytokines like IL-6, which are increased in the blood with age, can influence the secretion of hepcidin by choroid plexus epithelial cells. Hepcidin is a central regulator of iron homeostasis and secretion is influenced through the Stat3 signal transduction pathway ( Chongbin et al. 2014; Chen et al. 2008; Villeda et al. 2011; Rouault et al. 2009; Hubert et al. 2019; Leitner and Connor 2012; Lu et al. 1995).
9.3.2 Growth Factors and Hormones Secreted by the Choroid Plexus
The choroid plexus is uniquely located at the interface between blood and CSF. It expresses many receptors for growth factors and hormones, such as growth hormone (GH), prolactin, corticotrophin-releasing hormone, vasopressin, and leptin, in order to respond to local and peripheral signals (Kaur et al. 2016; Marques et al. 2011; Silva-Vargas et al. 2016). In this way, the choroid plexus is a key component in neuroendocrine regulation, having an impact on hormonal signaling and in addition, also the choroid plexus functioning is regulated by a variety of hormones (Preston 2001). Several studies revealed that different hormones and neuropeptides might be actively processed by the choroid plexus, namely GH, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF1 and 2), and insulin-like growth factor binding protein 2 (IGFBP2) (Emerich et al. 2005; Holm et al. 1994; Nilsson et al. 1996; Vega et al. 1992). IGF2 plays an important role in cell growth, in development, and maintenance of the nervous system and regulates the functional plasticity of the adult brain (Lenoir and Honegger 1983; Mill et al. 1985; Mozell and Mcmorris 1991). The production of IGF partially depends on the presence of GH (Cohen et al. 1992). In elderly humans, reduced binding of GH to the choroid plexus has been reported, resulting in reduced activity of IGF2, which might have an impact on epithelial cell growth and repair (Nilsson et al. 1992; Preston 2001). Next to changes in IGF, in vitro studies showed that VEGF secretion in aged choroid plexus epithelial cells was reduced compared to young epithelial cells (Emerich et al. 2007).
Interestingly, the growth factors secreted by the choroid plexus into the CSF may be involved in the proliferation, differentiation, and survival of neural progenitor cells in the subventricular zone (Falcao et al. 2012; Lun et al. 2015). More recently, it was shown that the lateral ventricle choroid plexus affects the behavior of neural stem cells of the ventricular-subventricular zone by the secretion of several factors promoting for colony formation and proliferation (Silva-Vargas et al. 2016). The functional effect of the lateral ventricle secretome changes throughout life, with activated neural stem cells being especially sensitive to age-related changes. The lateral ventricle choroid plexus is an important compartment that contributes to the age-related changes of the ventricular-subventricular zone stem cells. Transcriptome analysis revealed two proteins, BMP5 and IGF1, that might play an important role in these age-dependent effects of the choroid plexus (Silva-Vargas et al. 2016). The expression of both proteins decreases with aging, BMP5 levels are lower in aged human CSF and systemic IGF levels decrease with aging (Baird et al. 2012; Bartke et al. 2013). Furthermore, a study revealed that implants of young choroid plexus in rats were potently neuroprotective, whereas the choroid plexus implants from aged rats were only modestly effective and less potent. This study links aging with a diminished neuroprotective capacity of the choroid plexus epithelial cells (Emerich et al. 2007).
Vasopressin is a neurohormone produced by the hypothalamus, involved in the regulation of blood pressure. A high density of Vasopressin receptors (V1) is present at the choroid plexus. The activation of the V1 receptors regulates CSF production by decreased choroidal blood flow or by the effect on the choroidal epithelial cells’ ion channels (Chodobski and Szmydynger-Chodobska 2001; Faraci et al. 1988). Vasopressin is able to reduce the efflux of Cl− ions by regulating the Na+, K+, 2Cl−cotransporter, and maintains the volume of the choroidal epithelial cells. Vasopressin levels in blood and CSF can vary substantially, and elevated levels of vasopressin have been found in the CSF of old rats and in the plasma of elderly humans (Fig. 9.1) (Frolkis et al. 1999). In addition to vasopressin, also angiotensin II and endothelin-1, secreted by the choroid plexus, can affect the choroidal blood flow and CSF secretion (Kaur et al. 2016). A reduced production and secretion of CSF could influence the delivery of many components to the brain and may interfere with the normal physiological pathways (Kaur et al. 2016; Preston 2001).
The Klotho protein is a transmembrane protein that was identified as aging-suppressor (Kuro et al. 1997). A defect in Klotho gene expression in mice accelerates aging-like phenotypes and results in a syndrome that resembles human aging including a short lifespan, impaired cognition (Uchida et al. 2001; Shiozaki et al. 2008), abnormal brain pathology, infertility, arteriosclerosis (Arking et al. 2003), skin atrophy, osteoporosis (Ogata et al. 2002), and emphysema (Kuro et al. 1997). Vice versa, the overexpression of Klotho in mice extends life span and improves memory (Kurosu et al. 2005; Li et al. 2019). Moreover, gene expression analysis of brain white matter in rhesus monkeys also indicated the implication of Klotho in the regulation of brain aging (Duce et al. 2008). In humans, a functional variant of the KLOTHO (KL) gene showed to be associated with high-density cholesterol, blood pressure, stroke, and longevity (Arking et al. 2002, 2005). The Klotho protein functions as a circulating hormone that represses intracellular signals of insulin and IGF1, an evolutionarily conserved mechanism for extending life span. In Klotho-deficient mice the disruption of insulin and IGF1 signaling lead to the improvement of aging-like phenotypes, suggesting that Klotho-mediated inhibition of insulin and IGF1 signaling contributes to its anti-aging properties (Kurosu et al. 2005). Klotho is predominantly secreted by the choroid plexus, the distal tubule cells of the kidney, and parathyroid glands; high levels of Klotho are expressed in the choroid plexus of juvenile and adult mice, humans, and mammals (Kuro 2010). Soluble Klotho has been demonstrated to be present in human CSF and serum (Imura et al. 2004; Semba et al. 2014). Aging is associated in mice with decreased klotho expression in the choroid plexus (Zhu et al. 2018). Moreover, CSF klotho concentrations are lower in older versus younger cognitive healthy individuals and in addition, CSF klotho concentrations are significantly lower in Alzheimer’s disease patients compared to adults without cognitive problems (Semba et al. 2014). Selective depletion of klotho in the choroid plexus triggered the expression of multiple proinflammatory factors and macrophage infiltration into the choroid plexus. Furthermore, experimental reduction of klotho in the choroid plexus demonstrated enhanced microglial activation in the hippocampus following peripheral stimulation with lipopolysaccharide (Zhu et al. 2018). These results suggest that klotho depletion from the choroid plexus could contribute to the age-dependent priming of microglia for activation by peripheral infections (Henry et al. 2009; Zhu et al. 2018). In primary macrophage cultures, Klotho suppressed the activation of the NLRP3 inflammasome by enhancing fibroblast growth factor (FGF)23 (Zhu et al. 2018). This suggests that Klotho controls the brain-immune systems interface in the choroid plexus. Moreover, Klotho depletion in aging or disease may weaken this barrier and promote immune-mediated neuropathogenesis (Zhu et al. 2018). In addition, Klotho secreted by the choroid plexus might enhance oligodendrocyte maturation and myelination of the CNS (Chen et al. 2013). In this way it may play a role in the prevention of myelin degeneration in the aging brain (Chen et al. 2013; Semba et al. 2014).
9.3.3 Barrier Permeability and Transport by the Choroid Plexus
The blood-CSF barrier ensures a stable, balanced, and well-controlled micro-environment of the brain, which is necessary for proper functioning of the CNS. Transport across the barrier is restricted by tight junctions between the choroid plexus epithelial cells and require transporter and receptor systems in a directional way (Redzic 2011; Saunders et al. 2013). The choroid plexus produces CSF by passive filtration of fluid across the fenestrated capillaries and regulated secretion of molecules across the choroid plexus epithelial cells (Brinker et al. 2014), together with active production of molecules by the choroid plexus epithelial cells (Thouvenot et al. 2006). Dysregulation of choroid plexus transporters and tight junction complexes subsequently reflects into CSF compositional changes. Several studies have reported a compromised blood-CSF barrier in response to inflammatory signals (Brkic et al. 2015; Marques and Sousa 2015; Vandenbroucke et al. 2012). As described previously, aging is associated with morphological changes of the choroid plexus epithelial cells and is in addition associated with a state of low grade, chronic inflammation or inflamm-aging which might lead to the loss of the barrier function at the choroid plexus. Loss of barrier integrity might result in leakage of components from the blood circulation into the CSF, thus changing CSF composition. In agreement with this, a study performed by Chen et al., showed increased blood-CSF permeability for proteins upon aging in sheep. However, no complete disruption of the barrier is present since the passage of larger molecules was still prevented (>109.51–120 kDa) (Chen et al. 2009, 2012a). Studies conducted in healthy elderly individuals report only small changes in CSF composition: the concentration of molecules including Transthyretin (TTR) (Serot et al. 2003; Kleine et al. 1993b), alpha2-macroglobulin (Garton et al. 1991; Kleine et al. 1993b), and IgG (Blennow et al. 1993a; Garton et al. 1991; Kleine et al. 1993b; Chen et al. 2018) increases slightly with age. The CSF versus serum albumin ratio is used to evaluate blood-CSF barrier functioning and an increased variability in the CSF/serum albumin ratio has been observed from the age of 45 years, indicating that the blood-CSF barrier is compromised in elderly humans (Fig. 9.1) (Blennow et al. 1993a, b, c). However, it is difficult to determine whether this is the result of increased blood-CSF barrier permeability or altered clearance of the proteins (Preston 2001; Serot et al. 2003, 1997). Often these elevated levels are interpreted as blood-CSF barrier integrity loss.
Choroid plexus epithelial cells are able to secrete EVs, including exosomes, into the CSF as a mechanism of blood-brain communication (Balusu et al. 2016b). EVs are membrane-derived vesicles that can enclose specific repertoires of proteins, lipids, and RNA molecules (Van Niel et al. 2018; Mathieu et al. 2019) and are able to transport these molecules both to adjacent and distant cells (Baixauli et al. 2014; Mittelbrunn and Sanchez-Madrid 2012; Paolicelli et al. 2018). In the CNS, EVs have shown to mediate intercellular communication over long range distances and are believed to be important for the cross-talk between neurons and glial cells in the brain (Paolicelli et al. 2018). Inflammation, which is also present in the aging brain, was shown to induce an increase in EV production, together with an altered EV content (Balusu et al. 2016b). The number of EVs present in the CSF declines in elderly humans and their miRNA content changes throughout life (Fig. 9.1) (Tietje et al. 2014). However, the size of the EVs and their size distribution did not change during aging (Tietje et al. 2014; Yang et al. 2015). EVs in the CSF can be produced by different cell types and no data is currently available on EV production by the choroid plexus epithelial cells during aging. However, if affected, this might have consequences for the nutrient delivery to the brain. As an example, exosomes, a specific type of EVs, which are secreted via the fusion of multivesicular bodies with the plasma membrane, are important for the delivery of folate, an important vitamin for the brain, across the choroid plexus epithelial cells into the CSF (Grapp et al. 2013).
9.4 Immune Cell Trafficking at the Choroid Plexus
Migration of immune cells into brain tissue and (limited) inflammatory reactions are fundamental mechanisms to sustain normal physiology, immune surveillance, host defense, and learning processes (Engelhardt and Coisne 2011; Garner et al. 2006; Ransohoff and Engelhardt 2012; Galea et al. 2007). However, these mechanisms are tightly controlled by the presence of different brain barriers. The blood-CSF barrier is perfectly located at the interface between blood and CSF to provide active immune surveillance and serves as active and selective gate for immune cell trafficking (Demeestere et al. 2015). The tightly connected choroidal epithelium limits paracellular transport of not only molecules, but also immune cells. Additionally, the choroid plexus contains fenestrated capillaries, allowing free communication between the stroma and peripheral blood (Demeestere et al. 2015). The choroid plexus stroma contains a large population of macrophages, dendritic cells, CD3+ and CD4+ T cells, and CX3CR1hi Ly6Clow monocytes (Shechter et al. 2013). Macrophages at the apical side of the choroid plexus are called epiplexus or Kolmer cells (Maslieieva and Thompson 2014) and are thought to contribute to the immune component of the blood-CSF barrier. In healthy conditions, the CSF contains CD4+ T cells, natural killer cells, and B cells (Ransohoff and Engelhardt 2012). Although leukocytes enter the CSF, in steady state conditions they do not invade the brain parenchyma (Shechter et al. 2013). Leukocyte infiltration however is modulated in response to disease or trauma like meningitis, multiple sclerosis, or peripheral inflammation. The cells can transmigrate from the blood across the fenestrated endothelium to enter the stroma matrix. After travelling through the stroma of the choroidal cells, the immune cells can, in response to specific triggers, cross the choroid plexus epithelium and enter the CSF where they are able to skew toward specific effector responses, including regulatory T cells, T helper 2 cells, and alternatively activated macrophages (Shechter et al. 2013). The CD4+ T cells present in the CSF are distinct from the T cell populations in the blood circulation and brain parenchyma, indicating that the influx of T cells via the choroid plexus into the CSF is highly regulated (Engelhardt and Ransohoff 2012). After entering the CSF, leukocytes might be able to cross the ependymal cell layer and migrate further into the brain parenchyma under inflammatory conditions or might travel to the arachnoidea via the CSF flow.
The expression of adhesion molecules, chemokines, and chemokine receptors control leukocyte trafficking across the blood-CSF barrier. Inflammation causes the upregulation of adhesion molecules in the choroid plexus epithelial cells, such as intercellular adhesion molecule 1 (ICAM-1), vascular cellular adhesion molecule 1 (VCAM-1), and mucosal vascular addressin cell adhesion molecule 1 (MADCAM-1) (Endo et al. 1998). The expression of cell adhesion molecules at the brain barriers could possibly be increased during aging because of the pro-inflammatory state of the brain. The choroid plexus also produces cytokines (e.g. interleukin-1β (Il-1β) and tumor necrosis factor α (TNFα)) and chemokines (e.g. C-X-C motif chemokine ligand (CXCL10) and monocyte chemoattractant protein 1 (MCP1)). These cyto- and chemokines are necessary for the activation and/or recruitment of immune cells during systemic inflammation (Demeestere et al. 2015). Interestingly, aging leads to an increased expression of Il-1β in the choroid plexus (Silva-Vargas et al. 2016).
In the healthy adult brain, a balance is present between pro- and anti-inflammatory cytokines, but Baruch and colleagues observed in the choroid plexus a shift towards a Th2-like pro-inflammatory state with increasing age (Baruch et al. 2013; Sparkman and Johnson 2008). This pro-inflammatory state is reflected by the reduced production of interferon (IFN)-γ and increased production of IL-4, which negatively affect brain functioning (Baruch et al. 2013). Mice lacking the IFNγ receptor show a decreased number of leukocytes in the CSF and premature cognitive decline (Baruch et al. 2013). A type I IFN signature was described in the aged choroid plexus (Baruch et al. 2014). In both mouse models and human samples, the choroid plexus showed an increased type I and decreased type II IFN dependent gene expression profile (Fig. 9.1). This type I IFN signature negatively influences type II IFN signaling, leading to a reduced expression of homing and trafficking molecules (Cd34, Madcam1, Ccl2, Cx3cr1, Cxcl13, Il2) that are required for leukocyte entry in the CSF during aging, eventually leading to increased brain inflammation and cognitive decline (Baruch et al. 2014; Kunis et al. 2013). Interestingly, blocking IFN type I signaling restored cognitive functioning and hippocampal neurogenesis and in addition was able to diminish astrogliosis and microgliosis, and increase the anti-inflammatory cytokine IL-10 in the hippocampus (Baruch et al. 2014). It was suggested that this IFN I signaling is a mechanism to attenuate neuroinflammation which eventually becomes detrimental to brain plasticity resulting in age-associated cognitive decline (Baruch et al. 2014). However, therapeutically targeting the IFN pathway could influence the immune surveillance at the choroid plexus, since a tight balance between type I IFNs and IFNγ is central in leukocyte entry and cognition (Deczkowska et al. 2016). The importance of this balance is reflected in the IFNβ treatment, which is used in the clinic to reduce clinical relapses in multiple sclerosis (Wingerchuk and Carter 2014). Patients treated with IFN experience several adverse effects including increased risk for developing depression, cognitive decline, and they develop Parkinson like symptoms (Manouchehrinia and Constantinescu 2012). Similarly, type I IFNs aggravate disease in multiple mouse models of Parkinson’s disease (Main et al. 2017).
It remains to be determined whether loss of barrier integrity is responsible for immune cell trafficking across the blood-CSF barrier. Independent of barrier impairment, other mechanisms might determine the leukocyte migration across the blood-CSF barrier. Nitric oxide, a negative regulator of leukocyte trafficking has been found to be upregulated at the choroid plexus during aging (Baruch et al. 2015). Additionally, transcellular migration events of leukocytes occurring in close proximity of the tight junctions have been undervalued and might have been mistaken for paracellular migration (Phillipson et al. 2008; Wewer et al. 2011; Wolburg et al. 2005). Steinmann et al. were able to demonstrate transcellular migration of leukocytes across the blood-CSF barrier after bacterial infection as well as T-cell transmigration after viral stimulation (Steinmann et al. 2013; Wewer et al. 2011). Moreover, polymorphonuclear (PMN) and monocytes differentially migrate in a human blood-CSF barrier model (Steinmann et al. 2013).
9.5 Choroid Plexus and Neurodegenerative Diseases
Interestingly, all the age-related morphological changes in choroid plexus structure described above, such as the flattening of epithelium, thickening of basement membrane, and lipofuscin deposits, are significantly more prominent in neurodegenerative diseases.
The most prevalent neurodegenerative disease, Alzheimer’s disease, is characterized by the decline of memory and other cognitive functions. It is a progressive deteriorating disease, eventually leading to loss of autonomy and ultimately patients require full-time medical care (Jost and Grossberg 1995). Pathologically, Alzheimer’s disease is defined by severe neuronal loss, resulting in the loss of brain volume, which is most pronounced around the medial temporal lobe areas, and particularly in the hippocampus. Furthermore, the aggregation of beta-amyloid (Aβ) in extracellular senile plaques and formation of intraneuronal neurofibrillary tangles consisting of hyperphosphorylated tau protein have been identified to play a major role in Alzheimer’s disease pathogenesis (Braak and Braak 1991).
Ultrastructural changes are similar to those described in aging namely epithelial cell atrophy. In humans, the cells decrease in height with approximately 22% compared to healthy controls (Serot et al. 2000, 2003). The cytoplasm of the epithelial cells contains multiple lipofuscin and Biondi tangles (Miklossy et al. 1998). The basement membrane of the epithelium is thickened and irregular. Apical microvilli become irregular and fibrotic (Serot et al. 2000; Jellinger 1976). The stroma contains calcifications and psammomas, hyaline bodies, and thickened vessel walls (Serot et al. 2003).
TTR, a highly expressed protein at the choroid plexus, is a carrier for the thyroid hormones, but also has the ability to bind to Aβ and prevents the aggregation and deposition of Aβ plaques in the brain (Marques et al. 2013; Schwarzman et al. 1994). Studies reporting the production and secretion of TTR by the choroid plexus during aging show conflicting data: both an increase and decrease of TTR in the CSF has been described (Kleine et al. 1993a; Redzic et al. 2005; Serot et al. 1997). The blood-CSF barrier expresses several transporter systems including low-density lipoprotein receptor-related protein 1 (LRP1), receptor for advanced glycation end products (RAGE), receptor glycoprotein 330/megalin (LRP2), and Pgp. The LRP and Pgp receptors are responsible for the receptor-mediated efflux of Aβ from the brain, while RAGE mediates the influx of Aβ into the brain (Marques et al. 2013; Storck et al. 2016). Expression of LRP2 in the choroid plexus is decreased during aging, but an increase of LRP1 and Pgp is observed as well as no difference in RAGE expression (Gorle et al. 2016; Pascale et al. 2011).
Several studies have reported the beneficial effect of the choroid plexus on the rejuvenation of damaged brain regions because of the production of neurotrophic factors (Thanos et al. 2010; Borlongan et al. 2004a, b; Bolos et al. 2014). Choroid plexus epithelial cells treated in vitro with Aβ peptide lead to increased proliferation and differentiation of neuronal progenitor cells (Bolos et al. 2014). Moreover, transplantation of healthy choroid plexus epithelial cells into the brain of an Alzheimer’s disease mouse model induced a significant reduction in brain Aβ and tau levels and improved memory of the animals (Bolos et al. 2014). Also in other neurodegenerative diseases, such as Huntington’s disease choroid plexus cell transplantation studies showed successful results (Emerich and Borlongan 2009).
9.6 Conclusions
The choroid plexus, that contains the blood-CSF barrier, accomplishes important functions in the CNS and actively contributes to brain homeostasis. The choroid plexus is able to respond to changes both in the periphery and the brain parenchyma. However, during aging, the morphology and normal functioning of the choroid plexus is severely compromised. Alterations in brain barrier transport mechanisms, CSF production and clearance, receptor-mediated signaling, enzymatic and metabolic activity, loss of barrier integrity, and insufficient distribution of nutrients have an effect on brain functioning and might influence cognitive performance. Understanding how the blood-CSF barrier is altered in aging and how it can contribute to these age-associated diseases, might lead to novel strategies to attenuate aging-associated cognitive decline and related diseases.
References
Albeck MJ, Skak C, Nielsen PR, Olsen KS, Borgesen SE, Gjerris F (1998) Age dependency of resistance to cerebrospinal fluid outflow. J Neurosurg 89:275–278
Arking DE, Krebsova A, Macek M Sr, Macek M Jr, Arking A, Mian IS, Fried L, Hamosh A, Dey S, Mcintosh I, Dietz HC (2002) Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A 99:856–861
Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, Becker LC, Dietz HC (2003) KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet 72:1154–1161
Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC (2005) Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res 96:412–418
Baird GS, Nelson SK, Keeney TR, Stewart A, Williams S, Kraemer S, Peskind ER, Montine TJ (2012) Age-dependent changes in the cerebrospinal fluid proteome by slow off-rate modified aptamer array. Am J Pathol 180:446–456
Baixauli F, Lopez-Otin C, Mittelbrunn M (2014) Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol 5:403
Balusu S, Brkic M, Libert C, Vandenbroucke RE (2016a) The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: more than just a barrier. Neural Regen Res 11:534–537
Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K, Brkic M, Demeestere D, Vanhooren V, Hendrix A, Libert C, Vandenbroucke RE (2016b) Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med 8:1162–1183
Barkhof F, Kouwenhoven M, Scheltens P, Sprenger M, Algra P, Valk J (1994) Phase-contrast cine MR imaging of normal aqueductal CSF flow. Effect of aging and relation to CSF void on modulus MR. Acta Radiol 35:123–130
Bartke A, Sun LY, Longo V (2013) Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev 93:571–598
Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, Mirlas-Neisberg N, Cardon M, Vaknin I, Cahalon L, Berkutzki T, Mattson MP, Gomez-Pinilla F, Friedman N, Schwartz M (2013) CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci U S A 110:2264–2269
Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M (2014) Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346:89–93
Baruch K, Kertser A, Porat Z, Schwartz M (2015) Cerebral nitric oxide represses choroid plexus NFkappaB-dependent gateway activity for leukocyte trafficking. EMBO J 34:1816–1828
Bellur SN, Chandra V, Mcdonald LW (1980) Arachnoidal cell hyperplasia. Its relationship to aging and chronic renal failure. Arch Pathol Lab Med 104:414–416
Blennow K, Fredman P, Wallin A, Gottfries CG, Karlsson I, Langstrom G, Skoog I, Svennerholm L, Wikkelso C (1993a) Protein analysis in cerebrospinal fluid. II. Reference values derived from healthy individuals 18–88 years of age. Eur Neurol 33:129–133
Blennow K, Fredman P, Wallin A, Gottfries CG, Langstrom G, Svennerholm L (1993b) Protein analyses in cerebrospinal fluid. I. Influence of concentration gradients for proteins on cerebrospinal fluid/serum albumin ratio. Eur Neurol 33:126–128
Blennow K, Fredman P, Wallin A, Gottfries CG, Skoog I, Wikkelso C, Svennerholm L (1993c) Protein analysis in cerebrospinal fluid. III. Relation to blood-cerebrospinal fluid barrier function for formulas for quantitative determination of intrathecal IgG production. Eur Neurol 33:134–142
Bolos M, Antequera D, Aldudo J, Kristen H, Bullido MJ, Carro E (2014) Choroid plexus implants rescue Alzheimer’s disease-like pathologies by modulating amyloid-beta degradation. Cell Mol Life Sci 71:2947–2955
Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF (2004a) CNS grafts of rat choroid plexus protect against cerebral ischemia in adult rats. Neuroreport 15:1543–1547
Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF (2004b) Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke 35:2206–2210
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Brinker T, Stopa E, Morrison J, Klinge P (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11:10
Brkic M, Balusu S, Van Wonterghem E, Gorle N, Benilova I, Kremer A, Van Hove I, Moons L, De Strooper B, Kanazir S, Libert C, Vandenbroucke RE (2015) Amyloid beta oligomers disrupt blood-CSF barrier integrity by activating matrix metalloproteinases. J Neurosci 35:12766–12778
Brown PD, Davies SL, Speake T, Millar ID (2004) Molecular mechanisms of cerebrospinal fluid production. Neuroscience 129:957–970
Bulat M, Klarica M (2011) Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res Rev 65:99–112
Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Dore J, Franceschi C, Lehtinen MJ, Recker T, Salvioli S, Visioli F (2017) Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev 40:95–119
Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW (2008) Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun 22:301–311
Chen RL, Kassem NA, Redzic ZB, Chen CP, Segal MB, Preston JE (2009) Age-related changes in choroid plexus and blood-cerebrospinal fluid barrier function in the sheep. Exp Gerontol 44:289–296
Chen CP, Chen RL, Preston JE (2012a) The influence of ageing in the cerebrospinal fluid concentrations of proteins that are derived from the choroid plexus, brain, and plasma. Exp Gerontol 47:323–328
Chen X, Guo C, Kong J (2012b) Oxidative stress in neurodegenerative diseases. Neural Regen Res 7:376–385
Chen CD, Sloane JA, Li H, Aytan N, Giannaris EL, Zeldich E, Hinman JD, Dedeoglu A, Rosene DL, Bansal R, Luebke JI, Kuro OM, Abraham CR (2013) The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci 33:1927–1939
Chen CPC, Preston JE, Zhou S, Fuller HR, Morgan DGA, Chen R (2018) Proteomic analysis of age-related changes in ovine cerebrospinal fluid. Exp Gerontol 108:181–188
Chiu C, Miller MC, Caralopoulos IN, Worden MS, Brinker T, Gordon ZN, Johanson CE, Silverberg GD (2012) Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS 9:3
Chodobski A, Szmydynger-Chodobska J (2001) Choroid plexus: target for polypeptides and site of their synthesis. Microsc Res Tech 52:65–82
Chongbin L, Rui W, Chunyan W, Hu C, Qifeng D (2014) Altered hepcidin expression is part of the choroid plexus response to IL-6/Stat3 signaling pathway in normal aging rats. Bioenergetics 3:2
Christensen HL, Barbuskaite D, Rojek A, Malte H, Christensen IB, Fuchtbauer AC, Fuchtbauer EM, Wang T, Praetorius J, Damkier HH (2018) The choroid plexus sodium-bicarbonate cotransporter NBCe2 regulates mouse cerebrospinal fluid pH. J Physiol 596:4709–4728
Cohen P, Ocrant I, Fielder PJ, Neely EK, Gargosky SE, Deal CI, Ceda GP, Youngman O, Pham H, Lamson G et al (1992) Insulin-like growth factors (IGFs): implications for aging. Psychoneuroendocrinology 17:335–342
Cornford EM, Varesi JB, Hyman S, Damian RT, Raleigh MJ (1997) Mitochondrial content of choroid plexus epithelium. Exp Brain Res 116:399–405
Cottrell DA, Blakely EL, Johnson MA, Ince PG, Borthwick GM, Turnbull DM (2001) Cytochrome c oxidase deficient cells accumulate in the hippocampus and choroid plexus with age. Neurobiol Aging 22:265–272
Cutler RW, Page L, Galicich J, Watters GV (1968) Formation and absorption of cerebrospinal fluid in man. Brain 91:707–720
Damkier HH, Brown PD, Praetorius J (2013) Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93:1847–1892
Daouk J, Bouzerar R, Chaarani B, Zmudka J, Meyer ME, Baledent O (2016) Use of dynamic (18) F-fluorodeoxyglucose positron emission tomography to investigate choroid plexus function in Alzheimer’s disease. Exp Gerontol 77:62–68
De Bock M, Vandenbroucke RE, Decrock E, Culot M, Cecchelli R, Leybaert L (2014) A new angle on blood-CNS interfaces: a role for connexins? FEBS Lett 588:1259–1270
Deane R, Zheng W, Zlokovic BV (2004) Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. J Neurochem 88:813–820
Deczkowska A, Baruch K, Schwartz M (2016) Type I/II interferon balance in the regulation of brain physiology and pathology. Trends Immunol 37:181–192
Demeestere D, Libert C, Vandenbroucke RE (2015) Clinical implications of leukocyte infiltration at the choroid plexus in (neuro)inflammatory disorders. Drug Discov Today 20:928–941
Duce JA, Podvin S, Hollander W, Kipling D, Rosene DL, Abraham CR (2008) Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia 56:106–117
Emerich DF, Borlongan CV (2009) Potential of choroid plexus epithelial cell grafts for neuroprotection in Huntington’s disease: what remains before considering clinical trials. Neurotox Res 15:205–211
Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV, Thanos CG (2005) The choroid plexus in the rise, fall and repair of the brain. BioEssays 27:262–274
Emerich DF, Schneider P, Bintz B, Hudak J, Thanos CG (2007) Aging reduces the neuroprotective capacity, VEGF secretion, and metabolic activity of rat choroid plexus epithelial cells. Cell Transplant 16:697–705
Endo H, Sasaki K, Tonosaki A, Kayama T (1998) Three-dimensional and ultrastructural ICAM-1 distribution in the choroid plexus, arachnoid membrane and dural sinus of inflammatory rats induced by LPS injection in the lateral ventricles. Brain Res 793:297–301
Engelhardt B, Coisne C (2011) Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS 8:4
Engelhardt B, Ransohoff RM (2012) Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol 33:579–589
Eriksson L, Westermark P (1986) Intracellular neurofibrillary tangle-like aggregations. A constantly present amyloid alteration in the aging choroid plexus. Am J Pathol 125:124–129
Falcao AM, Marques F, Novais A, Sousa N, Palha JA, Sousa JC (2012) The path from the choroid plexus to the subventricular zone: go with the flow! Front Cell Neurosci 6:34
Faraci FM, Mayhan WG, Farrell WJ, Heistad DD (1988) Humoral regulation of blood flow to choroid plexus: role of arginine vasopressin. Circ Res 63:373–379
Faubel R, Westendorf C, Bodenschatz E, Eichele G (2016) Cilia-based flow network in the brain ventricles. Science 353:176–178
Ferrante F, Amenta F (1987) Enzyme histochemistry of the choroid plexus in old rats. Mech Ageing Dev 41:65–72
Fleischman D, Berdahl JP, Zaydlarova J, Stinnett S, Fautsch MP, Allingham RR (2012) Cerebrospinal fluid pressure decreases with older age. PLoS One 7:e52664
Foundas AL, Zipin D, Browning CA (1998) Age-related changes of the insular cortex and lateral ventricles: conventional MRI volumetric measures. J Neuroimaging 8:216–221
Franceschi C (2007) Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev 65:S173–S176
Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S4–S9
Frolkis VV, Kvitnitskaya-Ryzhova TY, Dubiley TA (1999) Vasopressin, hypothalamo-neurohypophyseal system and aging. Arch Gerontol Geriatr 29:193–214
Galea I, Bechmann I, Perry VH (2007) What is immune privilege (not)? Trends Immunol 28:12–18
Garner CC, Waites CL, Ziv NE (2006) Synapse development: still looking for the forest, still lost in the trees. Cell Tissue Res 326:249–262
Garton MJ, Keir G, Lakshmi MV, Thompson EJ (1991) Age-related changes in cerebrospinal fluid protein concentrations. J Neurol Sci 104:74–80
Gideon P, Thomsen C, Stahlberg F, Henriksen O (1994) Cerebrospinal fluid production and dynamics in normal aging: a MRI phase-mapping study. Acta Neurol Scand 89:362–366
Gorle N, Van Cauwenberghe C, Libert C, Vandenbroucke RE (2016) The effect of aging on brain barriers and the consequences for Alzheimer’s disease development. Mamm Genome 27:407–420
Grapp M, Wrede A, Schweizer M, Huwel S, Galla HJ, Snaidero N, Simons M, Buckers J, Low PS, Urlaub H, Gartner J, Steinfeld R (2013) Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun 4:2123
Henriksson L, Voigt K (1976) Age-dependent differences of distribution and clearance patterns in normal RIHSA cisternograms. Neuroradiology 12:103–107
Henry CJ, Huang Y, Wynne AM, Godbout JP (2009) Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun 23:309–317
Holm NR, Hansen LB, Nilsson C, Gammeltoft S (1994) Gene expression and secretion of insulin-like growth factor-II and insulin-like growth factor binding protein-2 from cultured sheep choroid plexus epithelial cells. Brain Res Mol Brain Res 21:67–74
Hubert V, Chauveau F, Dumot C, Ong E, Berner LP, Canet-Soulas E, Ghersi-Egea JF, Wiart M (2019) Clinical imaging of choroid plexus in health and in brain disorders: a mini-review. Front Mol Neurosci 12:34
Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y (2004) Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565:143–147
Jahng GH, Oh J, Lee DW, Kim HG, Rhee HY, Shin W, Paik JW, Lee KM, Park S, Choe BY, Ryu CW (2016) Glutamine and glutamate complex, as measured by functional magnetic resonance spectroscopy, alters during face-name association task in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 52:145–159
Jellinger K (1976) Neuropathological aspects of dementias resulting from abnormal blood and cerebrospinal fluid dynamics. Acta Neurol Belg 76:83–102
Johanson CE, Duncan JA 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD (2008) Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5:10
Jost BC, Grossberg GT (1995) The natural history of Alzheimer’s disease: a brain bank study. J Am Geriatr Soc 43:1248–1255
Jovanovic I, Stefanovic N, Antic S, Ugrenovic S, Djindjic B, Vidovic N (2004) Morphological and morphometric characteristics of choroid plexus psammoma bodies during the human aging. Ital J Anat Embryol 109:19–33
Kao L, Kurtz LM, Shao X, Papadopoulos MC, Liu L, Bok D, Nusinowitz S, Chen B, Stella SL, Andre M, Weinreb J, Luong SS, Piri N, Kwong JM, Newman D, Kurtz I (2011) Severe neurologic impairment in mice with targeted disruption of the electrogenic sodium bicarbonate cotransporter NBCe2 (Slc4a5 gene). J Biol Chem 286:32563–32574
Kaur C, Rathnasamy G, Ling EA (2016) The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol 75:198–213
Khasawneh AH, Garling RJ, Harris CA (2018) Cerebrospinal fluid circulation: what do we know and how do we know it? Brain Circ 4:14–18
Kida S, Yamashima T, Kubota T, Ito H, Yamamoto S (1988) A light and electron microscopic and immunohistochemical study of human arachnoid villi. J Neurosurg 69:429–435
Kleine TO, Hackler R, Lutcke A, Dauch W, Zofel P (1993a) Transport and production of cerebrospinal fluid (CSF) change in aging humans under normal and diseased conditions. Z Gerontol 26:251–255
Kleine TO, Hackler R, Zofel P (1993b) Age-related alterations of the blood-brain-barrier (bbb) permeability to protein molecules of different size. Z Gerontol 26:256–259
Kunis G, Baruch K, Rosenzweig N, Kertser A, Miller O, Berkutzki T, Schwartz M (2013) IFN-gamma-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain 136:3427–3440
Kuro OM (2010) Klotho. Pflugers Arch 459:333–343
Kuro OM, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, Mcguinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro OM (2005) Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833
Kvitnitskaia-Ryzhova T, Shkapenko AL (1992) A comparative ultracytochemical and biochemical study of the ATPases of the choroid plexus in aging. Tsitologiia 34:81–87
Leitner DF, Connor JR (2012) Functional roles of transferrin in the brain. Biochim Biophys Acta 1820:393–402
Lenoir D, Honegger P (1983) Insulin-like growth factor I (IGF I) stimulates DNA synthesis in fetal rat brain cell cultures. Brain Res 283:205–213
Li D, Jing D, Liu Z, Chen Y, Huang F, Behnisch T (2019) Enhanced expression of secreted alpha-klotho in the hippocampus alters nesting behavior and memory formation in mice. Front Cell Neurosci 13:133
Liddelow SA (2015) Development of the choroid plexus and blood-CSF barrier. Front Neurosci 9:32
Lindsey AE, Schneider K, Simmons DM, Baron R, Lee BS, Kopito RR (1990) Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci U S A 87:5278–5282
Lu J, Kaur C, Ling EA (1995) Expression and upregulation of transferrin receptors and iron uptake in the epiplexus cells of different aged rats injected with lipopolysaccharide and interferon-gamma. J Anat 187(Pt 3):603–611
Lun MP, Johnson MB, Broadbelt KG, Watanabe M, Kang YJ, Chau KF, Springel MW, Malesz A, Sousa AM, Pletikos M, Adelita T, Calicchio ML, Zhang Y, Holtzman MJ, Lidov HG, Sestan N, Steen H, Monuki ES, Lehtinen MK (2015) Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J Neurosci 35:4903–4916
Ma Q, Ineichen BV, Detmar M, Proulx ST (2017) Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 8:1434
Main BS, Zhang M, Brody KM, Kirby FJ, Crack PJ, Taylor JM (2017) Type-I interferons mediate the neuroinflammatory response and neurotoxicity induced by rotenone. J Neurochem 141:75–85
Manouchehrinia A, Constantinescu CS (2012) Cost-effectiveness of disease-modifying therapies in multiple sclerosis. Curr Neurol Neurosci Rep 12:592–600
Marques F, Sousa JC (2015) The choroid plexus is modulated by various peripheral stimuli: implications to diseases of the central nervous system. Front Cell Neurosci 9:136
Marques F, Sousa JC, Correia-Neves M, Oliveira P, Sousa N, Palha JA (2007) The choroid plexus response to peripheral inflammatory stimulus. Neuroscience 144:424–430
Marques F, Falcao AM, Sousa JC, Coppola G, Geschwind D, Sousa N, Correia-Neves M, Palha JA (2009) Altered iron metabolism is part of the choroid plexus response to peripheral inflammation. Endocrinology 150:2822–2828
Marques F, Sousa JC, Coppola G, Gao F, Puga R, Brentani H, Geschwind DH, Sousa N, Correia-Neves M, Palha JA (2011) Transcriptome signature of the adult mouse choroid plexus. Fluids Barriers CNS 8:10
Marques F, Sousa JC, Sousa N, Palha JA (2013) Blood-brain-barriers in aging and in Alzheimer’s disease. Mol Neurodegener 8:38
Marques F, Sousa JC, Brito MA, Pahnke J, Santos C, Correia-Neves M, Palha JA (2017) The choroid plexus in health and in disease: dialogues into and out of the brain. Neurobiol Dis 107:32–40
Maslieieva V, Thompson RJ (2014) A critical role for pannexin-1 in activation of innate immune cells of the choroid plexus. Channels (Austin) 8:131–141
Masseguin C, Lepanse S, Corman B, Verbavatz JM, Gabrion J (2005) Aging affects choroidal proteins involved in CSF production in Sprague-Dawley rats. Neurobiol Aging 26:917–927
Mathieu M, Martin-Jaular L, Lavieu G, Thery C (2019) Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21:9–17
Matsumae M, Kikinis R, Morocz I, Lorenzo AV, Albert MS, Black PM, Jolesz FA (1996a) Intracranial compartment volumes in patients with enlarged ventricles assessed by magnetic resonance-based image processing. J Neurosurg 84:972–981
Matsumae M, Kikinis R, Morocz IA, Lorenzo AV, Sandor T, Albert MS, Black PM, Jolesz FA (1996b) Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging. J Neurosurg 84:982–991
May C, Kaye JA, Atack JR, Schapiro MB, Friedland RP, Rapoport SI (1990) Cerebrospinal fluid production is reduced in healthy aging. Neurology 40:500–503
Mergenthaler P, Lindauer U, Dienel GA, Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36:587–597
Miklossy J, Kraftsik R, Pillevuit O, Lepori D, Genton C, Bosman FT (1998) Curly fiber and tangle-like inclusions in the ependyma and choroid plexus--a pathogenetic relationship with the cortical Alzheimer-type changes? J Neuropathol Exp Neurol 57:1202–1212
Mill JF, Chao MV, Ishii DN (1985) Insulin, insulin-like growth factor II, and nerve growth factor effects on tubulin mRNA levels and neurite formation. Proc Natl Acad Sci U S A 82:7126–7130
Mittelbrunn M, Sanchez-Madrid F (2012) Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol 13:328–335
Morris CM, Keith AB, Edwardson JA, Pullen RG (1992) Uptake and distribution of iron and transferrin in the adult rat brain. J Neurochem 59:300–306
Mozell RL, Mcmorris FA (1991) Insulin-like growth factor I stimulates oligodendrocyte development and myelination in rat brain aggregate cultures. J Neurosci Res 30:382–390
Nilsson C, Lindvall-Axelsson M, Owman C (1992) Neuroendocrine regulatory mechanisms in the choroid plexus-cerebrospinal fluid system. Brain Res Brain Res Rev 17:109–138
Nilsson C, Hultberg BM, Gammeltoft S (1996) Autocrine role of insulin-like growth factor II secretion by the rat choroid plexus. Eur J Neurosci 8:629–635
Ogata N, Matsumura Y, Shiraki M, Kawano K, Koshizuka Y, Hosoi T, Nakamura K, Kuro OM, Kawaguchi H (2002) Association of klotho gene polymorphism with bone density and spondylosis of the lumbar spine in postmenopausal women. Bone 31:37–42
Paolicelli RC, Bergamini G, Rajendran L (2018) Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405:148–157
Pascale CL, Miller MC, Chiu C, Boylan M, Caralopoulos IN, Gonzalez L, Johanson CE, Silverberg GD (2011) Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS 8:21
Pershing LK, Johanson CE (1982) Acidosis-induced enhanced activity of the Na-K exchange pump in the in vivo choroid plexus: an ontogenetic analysis of possible role in cerebrospinal fluid pH homeostasis. J Neurochem 38:322–332
Phillipson M, Kaur J, Colarusso P, Ballantyne CM, Kubes P (2008) Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS One 3:e1649
Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E (1997) Expression of the Na(+)-K(+)-2Cl- cotransporter BSC2 in the nervous system. Am J Phys 272:C173–C183
Preston JE (2001) Ageing choroid plexus-cerebrospinal fluid system. Microsc Res Tech 52:31–37
Proulx ST, Ma Q, Andina D, Leroux JC, Detmar M (2017) Quantitative measurement of lymphatic function in mice by noninvasive near-infrared imaging of a peripheral vein. JCI Insight 2:e90861
Ransohoff RM, Engelhardt B (2012) The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 12:623–635
Redzic Z (2011) Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 8:3
Redzic ZB, Segal MB (2004) The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev 56:1695–1716
Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J (2005) The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol 71:1–52
Report WA (2018) World Alzheimer report
Rouault TA, Zhang DL, Jeong SY (2009) Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metab Brain Dis 24:673–684
Rubenstein E (1998) Relationship of senescence of cerebrospinal fluid circulatory system to dementias of the aged. Lancet 351:283–285
Sakka L, Coll G, Chazal J (2011) Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 128:309–316
Sathyanesan M, Girgenti MJ, Banasr M, Stone K, Bruce C, Guilchicek E, Wilczak-Havill K, Nairn A, Williams K, Sass S, Duman JG, Newton SS (2012) A molecular characterization of the choroid plexus and stress-induced gene regulation. Transl Psychiatry 2:e139
Saunders NR, Daneman R, Dziegielewska KM, Liddelow SA (2013) Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol Asp Med 34:742–752
Schwarzman AL, Gregori L, Vitek MP, Lyubski S, Strittmatter WJ, Enghilde JJ, Bhasin R, Silverman J, Weisgraber KH, Coyle PK et al (1994) Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A 91:8368–8372
Segal MB (2000) The choroid plexuses and the barriers between the blood and the cerebrospinal fluid. Cell Mol Neurobiol 20:183–196
Semba RD, Moghekar AR, Hu J, Sun K, Turner R, Ferrucci L, O’Brien R (2014) Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci Lett 558:37–40
Serot JM, Christmann D, Dubost T, Couturier M (1997) Cerebrospinal fluid transthyretin: aging and late onset Alzheimer’s disease. J Neurol Neurosurg Psychiatry 63:506–508
Serot JM, Bene MC, Foliguet B, Faure GC (2000) Morphological alterations of the choroid plexus in late-onset Alzheimer’s disease. Acta Neuropathol 99:105–108
Serot JM, Foliguet B, Bene MC, Faure GC (2001) Choroid plexus and ageing in rats: a morphometric and ultrastructural study. Eur J Neurosci 14:794–798
Serot JM, Bene MC, Faure GC (2003) Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci 8:s515–s521
Shechter R, London A, Schwartz M (2013) Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol 13:206–218
Shiozaki M, Yoshimura K, Shibata M, Koike M, Matsuura N, Uchiyama Y, Gotow T (2008) Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience 152:924–941
Shuangshoti S, Netsky MG (1970) Human choroid plexus: morphologic and histochemical alterations with age. Am J Anat 128:73–95
Siesjo BK (1978) Brain metabolism and anaesthesia. Acta Anaesthesiol Scand Suppl 70:56–59
Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F (2016) Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell 19:643–652
Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, Rubenstein E, Possin K, Saul TA (2001) The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology 57:1763–1766
Silverberg GD, Mayo M, Saul T, Rubenstein E, Mcguire D (2003) Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol 2:506–511
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791
Sparkman NL, Johnson RW (2008) Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation 15:323–330
Speake T, Whitwell C, Kajita H, Majid A, Brown PD (2001) Mechanisms of CSF secretion by the choroid plexus. Microsc Res Tech 52:49–59
Steinmann U, Borkowski J, Wolburg H, Schroppel B, Findeisen P, Weiss C, Ishikawa H, Schwerk C, Schroten H, Tenenbaum T (2013) Transmigration of polymorphnuclear neutrophils and monocytes through the human blood-cerebrospinal fluid barrier after bacterial infection in vitro. J Neuroinflammation 10:31
Stoquart-Elsankari S, Baledent O, Gondry-Jouet C, Makki M, Godefroy O, Meyer ME (2007) Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab 27:1563–1572
Storck SE, Meister S, Nahrath J, Meissner JN, Schubert N, Di Spiezio A, Baches S, Vandenbroucke RE, Bouter Y, Prikulis I, Korth C, Weggen S, Heimann A, Schwaninger M, Bayer TA, Pietrzik CU (2016) Endothelial LRP1 transports amyloid-beta(1-42) across the blood-brain barrier. J Clin Invest 126:123–136
Strazielle N, Ghersi-Egea JF (2000) Choroid plexus in the central nervous system: biology and physiopathology. J Neuropathol Exp Neurol 59:561–574
Sturrock RR (1988) An ultrastructural study of the choroid plexus of aged mice. Anat Anz 165:379–385
Thanos CG, Bintz B, Emerich DF (2010) Microencapsulated choroid plexus epithelial cell transplants for repair of the brain. Adv Exp Med Biol 670:80–91
Thouvenot E, Lafon-Cazal M, Demettre E, Jouin P, Bockaert J, Marin P (2006) The proteomic analysis of mouse choroid plexus secretome reveals a high protein secretion capacity of choroidal epithelial cells. Proteomics 6:5941–5952
Tietje A, Maron KN, Wei Y, Feliciano DM (2014) Cerebrospinal fluid extracellular vesicles undergo age dependent declines and contain known and novel non-coding RNAs. PLoS One 9:e113116
Uchida A, Komiya Y, Tashiro T, Yorifuji H, Kishimoto T, Nabeshima Y, Hisanaga S (2001) Neurofilaments of Klotho, the mutant mouse prematurely displaying symptoms resembling human aging. J Neurosci Res 64:364–370
Van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228
Vandenbroucke RE (2016) A hidden epithelial barrier in the brain with a central role in regulating brain homeostasis. Implications for aging. Ann Am Thorac Soc 13(Suppl 5):S407–S410
Vandenbroucke RE, Dejonckheere E, Van Lint P, Demeestere D, Van Wonterghem E, Vanlaere I, Puimege L, Van Hauwermeiren F, De Rycke R, Mc Guire C, Campestre C, Lopez-Otin C, Matthys P, Leclercq G, Libert C (2012) Matrix metalloprotease 8-dependent extracellular matrix cleavage at the blood-CSF barrier contributes to lethality during systemic inflammatory diseases. J Neurosci 32:9805–9816
Vega JA, Del Valle ME, Calzada B, Bengoechea ME, Perez-Casas A (1992) Expression of nerve growth factor receptor immunoreactivity in the rat choroid plexus. Cell Mol Biol 38:145–149
Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T (2011) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477:90–94
Wahlund LO, Almkvist O, Basun H, Julin P (1996) MRI in successful aging, a 5-year follow-up study from the eighth to ninth decade of life. Magn Reson Imaging 14:601–608
Wen GY, Wisniewski HM, Kascsak RJ (1999) Biondi ring tangles in the choroid plexus of Alzheimer’s disease and normal aging brains: a quantitative study. Brain Res 832:40–46
Wewer C, Seibt A, Wolburg H, Greune L, Schmidt MA, Berger J, Galla HJ, Quitsch U, Schwerk C, Schroten H, Tenenbaum T (2011) Transcellular migration of neutrophil granulocytes through the blood-cerebrospinal fluid barrier after infection with Streptococcus suis. J Neuroinflammation 8:51
Wingerchuk DM, Carter JL (2014) Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc 89:225–240
Wolburg H, Wolburg-Buchholz K, Engelhardt B (2005) Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol 109:181–190
Yang Y, Keene CD, Peskind ER, Galasko DR, Hu SC, Cudaback E, Wilson AM, Li G, Yu CE, Montine KS, Zhang J, Baird GS, Hyman BT, Montine TJ (2015) Cerebrospinal fluid particles in Alzheimer disease and parkinson Disease. J Neuropathol Exp Neurol 74:672–687
Yesavage JA, Holman CA, Sarnquist FH, Berger PA (1982) Elevation of cerebrospinal fluid lactate with aging in subjects with normal blood oxygen saturations. J Gerontol 37:313–315
Zhu L, Stein LR, Kim D, Ho K, Yu GQ, Zhan L, Larsson TE, Mucke L (2018) Klotho controls the brain-immune system interface in the choroid plexus. Proc Natl Acad Sci U S A 115:E11388–E11396
Zivkovic VS, Stanojkovic MM, Antic MM (2017) Psammoma bodies as signs of choroid plexus ageing - a morphometric analysis. Vojnosanit Pregl 74:1054–1059
ZS-Nagy I, Steiber J, Jeney F (1995) Induction of age pigment accumulation in the brain cells of young male rats through iron-injection into the cerebrospinal fluid. Gerontology 41(Suppl 2):145–158
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The American Physiological Society
About this chapter
Cite this chapter
Van Cauwenberghe, C., Gorlé, N., Vandenbroucke, R.E. (2020). Roles of the Choroid Plexus in Aging. In: Praetorius, J., Blazer-Yost, B., Damkier, H. (eds) Role of the Choroid Plexus in Health and Disease. Physiology in Health and Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-0716-0536-3_9
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0536-3_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-0716-0535-6
Online ISBN: 978-1-0716-0536-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)