Abstract
Aim: To compare the measurement of total body water (TBW) and fat-free mass (FFM) using the criterion method of deuterium dilution space (2H2O) with bioelectrical impedance analysis (BIA) using a portable QuadScan 4000, Bodystat® in children and adolescents with phenylketonuria (PKU).
Methods: Sixteen patients with PKU, median age is 12.5 (range 5–20.6) years, were recruited into this cross-sectional study. TBW was measured by both deuterium dilution and BIA on the same occasion as per a standard protocol. FFM was estimated from predictive equations.
Results: There was no significant difference between TBWDeut and TBWBIA (p = 0.344) or FFMDeut and FFMBIA (p = 0.111). TBWDeut and TBWBIA were highly correlated (r = 0.990, p < 0.0001), as were FFMDeut and FFMBIA (r = 0.984, p < 0.0001). Bland-Altman plots demonstrated that there was no proportional bias between the criterion method, TBWDeut, and the test method TBWBIA, in estimating TBW (β = −0.056, adjusted r 2 = 0.069, p = 0.169) or FFM (β = −0.089, adjusted r 2 = 0.142, p = 0.083).
Conclusion: Our results suggest that when compared with the criterion method, the QuadScan 4000, Bodystat® can reliably be used to predict TBW and FFM in patients with PKU. We suggest that due to the portability and non-invasive approach, this method can reliably be used to monitor body composition in the outpatient clinic setting, to further improve the monitoring and assessment of nutritional status in PKU.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Bioelectrical impedance analysis
- Body composition
- Deuterium dilution
- Fat-free mass
- Phenylketonuria
- Total body water
Introduction
Phenylketonuria (PKU; MIM ID #261600) is a rare inborn error of protein metabolism. Lifelong goals of management are to maintain blood phenylalanine (Phe) levels within a recommended target range associated with optimal neurocognitive outcome and maintain normal growth and development (Singh et al. 2016; van Spronsen et al. 2017). This requires adherence to a diet low in natural protein and supplemented with phe-free L-amino-acid-based formula, to meet estimated protein and micronutrient requirements (van Spronsen et al. 2017). The dietary alterations involved may increase the risk of decreased liner growth (Dobbelaere et al. 2003; Aldámiz-Echevarría et al. 2014), increased prevalence of overweight (Scaglioni et al. 2004; Burrage et al. 2012) with changes in body composition such as higher percentage of body fat (Albersen et al. 2010).
The measurement of body composition is a valuable tool in the evaluation of the effects of modified diets, and in particular protein modified diets, on somatic development (Huemer et al. 2007). The value of body composition measurement in patients with PKU, in addition to other anthropometric parameters, including BMI and waist circumference, is now acknowledged (Albersen et al. 2010; MacDonald et al. 2011). Of the four body compartments used to assess body composition, fat, water, mineral and protein (dry lean mass), water is the largest component (Wells and Fewtrell 2006). Measuring additional components of body composition beyond just body fat mass is becoming progressively more important in clinical practice with increasing recognition of their effect on health outcomes (Wells and Fewtrell 2006). Multicompartment models, such as dual-energy X-ray absorptiometry (DEXA), that measure body composition are most accurate with good acceptability of measurements but are expensive, require exposure to radioactivity, are used predominantly in specialist research and do not specifically measure total body water (Wells and Fewtrell 2006). Deuterium dilution is a criterion or reference method to measure total body water (TBW) (TBWDeut), and subsequently fat-free mass (FFM) (FFMDeut) can be derived by using well-validated predictive equations (International Atomic Energy Agency 2010). This method is highly technical in its application and as such is not practical as a routine bedside method of measuring body composition and remains a research tool.
Currently there is no agreed or validated method of measuring body composition in PKU, and several methods have been reported, including bioelectrical impedance analysis (BIA) (Dobbelaere et al. 2003; Rocha et al. 2013b), body air-displacement plethysmography using a Bod PodTM (Albersen et al. 2010), anthropometric skinfold measurements (Allen et al. 1996) and total body electrical conductivity (TOBEC) (Huemer et al. 2007). More recently, it has been recommended that methods such as BIA could be used to monitor longitudinal changes in body compartments in PKU, due to the ease and speed in performing this assessment in the clinical setting (Rocha et al. 2016). However, to date the method of BIA has not been validated for use in children with PKU.
BIA is a rapid, non-invasive, safe and inexpensive method to estimate body composition via accurate estimation of total body water (Böhm and Heitmann 2013; Mulasi et al. 2015). BIA methodology measures impedance to the flow of electrical current through the water component of body cells and uses empirical linear regression models to measure TBW and predict FFM. It offers the advantage of relative simplicity in obtaining results repeatedly with an instrument that is both functionally robust and physically portable. However, there are limitations in its use, particularly in populations with abnormal hydration status and/or ‘body geometry’ (Mulasi et al. 2015), and it is important that this method be applied critically with consideration of factors that might lead to variable results (Jackson et al. 2013).
The purpose of this study was to compare the performance of a multi-frequency BIA machine (QuadScan 4000, Bodystat®) to measure TBW (TBWBIA) and FFM (FFMBIA), compared with the criterion method, deuterium dilution, in a group of patients with PKU.
Participants and Methods
This study was approved by the RCH Human Research Ethic Committee: HREC #32056D.
Sixteen patients with PKU (seven males, nine females) were recruited after signed consents were obtained from parents and/or participants. All participants had early and continuous treatment with a low-phe diet and phe-free amino acid formula. Patients over 4 years of age and who were continent of urine and who had no known co-morbidities that may affect hydration stats were considered eligible. In this cross-sectional study, all measurements (anthropometric, BIA and urine for deuterium dilution analysis) were collected on the same day for individual patients. Patients were well with no sign of illness or infection. Urine samples were collected, and measurements were taken and recorded by a single experienced practitioner (ME) using a standardised operating procedure. Data were collected between July 2016 and March 2017. Deuterium dilution analysis was performed by a trained technician (KN).
Anthropometry and BIA Measurements
Patients were instructed to eat and drink normally the day prior to measurement but fast from food and fluids from bedtime until the morning of the measurements. These occurred in the patient’s home and close to usual waking time after they had voided their bladder. Height was measured to the nearest 0.1 cm using a stadiometer, and weight was measured to the nearest 0.1 kg using a digital weight measuring scale. Participants were in light clothing with no shoes. All anthropometric measurements were expressed as age- and gender-specific z-scores, using the epidemiological software package Epi Info (version 3.5.1), based on the Centres for Disease Control and Prevention (Atlanta, GA) 2002 reference database.
Body composition was then measured by BIA in patients lying in the supine position and with electrodes in the tetrapolar (wrist-ankle) arrangement using the multi-frequency BIA analyser QuadScan 4000, Bodystat® (Isle of White, United Kingdom LTD.) as per the manufacturer’s instructions. This analyser measures impedance at 5-, 50-, 100-, and 200-kHz and uses the 50-kHz frequency to predict the value of TBW and FFM. An undisclosed proprietary equation developed by the manufacturer calculated TBW. The BIA analyser measures FFM using predictive linear regression equations including the equation of Houtkooper for children (Houtkooper et al. 1992). Measurements were taken in duplicate over approximately 1 min. After the measurements, all impedance, water values and lean weight (FFM) values were recorded.
Criterion Method: Deuterium Isotopic Dilution
TBW was measured using the deuterium dilution technique according to the International Atomic Energy Agency standard procedures (International Atomic Energy Agency 2010). The baseline fasting spot urine sample was collected for determination of background isotope enrichment. Participants were then provided a dose of 1:10 dilution of deuterium oxide (99.8 atom % excess; Sercon Ltd., Crewe, UK) following the recommended doses for participants of different body weights. The bottle containing the dose was rinsed with 50 mL tap water to ensure no labelled water remained in the bottle. Patients were advised to drink, eat and move normally after samples had been collected but avoid exercise. A spot mid flow urine sample was collected at 5 h, and total urine output was measured from dosing with the isotope until the end of the 5-h equilibration period. Equilibration is the process whereby the deuterium oxide is evenly mixed throughout the body water resulting in all compartments of body water containing equal concentrations of deuterium. The spot urine samples were stored frozen at −20°C for batch analysis.
Analysis of deuterium enrichment was determined with an Isoprime Dual Inlet Isotope Ratio Mass Spectrometer (Isoprime, Manchester, UK) coupled in-line with a Multiprep-Gilson Autosampler. Hydrogen analyses were completed by an overnight equilibration with hydrogen gas at 40°C using Hokko coils. All samples were analysed in duplicate, and laboratory standards were calibrated using the international standards USGS45, USGS46, and GFLES-4. Results were reported in ‰ (delta per mil units) relative to Standard Mean Ocean Water (SMOW). TBW was calculated assuming that the deuterium oxide space is 4.1% higher than TBW due to exchange of hydrogen with non-aqueous hydrogen in the body. TBW was then converted to fat-free mass using Lohman’s age- and sex-specific ‘constants’ for the hydration of fat-free mass (Lohman 1993).
Statistical Analysis
Shapiro-Wilk’s test (p > 0.05) was used to explore data distribution. Normally distributed data were examined using Pearson correlation coefficient and Lin’s concordance coefficient to evaluate the relationship between TBW and FFM determined by the two methods. Paired samples t-test was used to evaluate the difference between the mean values of TBWBIA and TBWDeut and between the mean values of FFMBIA and FFMDeut. The Bland-Altman method was used to compare two measurements of the same variable and thus used to evaluate agreement between the TBWBIA and TBWDeut and between the FFMBIA and FFMDeut. This method calculated the mean difference between the two methods of measurement (the ‘bias’) and 95% limits of agreement as the mean difference (1.96 SD). A Bland-Altman plot was then constructed to explore the difference scores of the two measurements against the mean for each subject. To test for proportional bias, a linear regression of difference between measurements on the mean of the measurements was completed. Statistical analyses were performed using SPSS for Windows software version 23 (IBM, Illinois, Chicago, IL). Significance was set at p < 0.05. Data are expressed as mean (SD) and median (range).
Results
Participants were 16 patients with PKU (7 males, 9 females). Median age is 12.5 years (range 5–20.6 years). Participants’ anthropometric results are summarised in Table 1. Measurements of TBW and FFM taken from the duplicate BIA readings were identical in 14/16 participants and within 1% for 2/16 patients, and the mean of these values was used.
Total Body Water
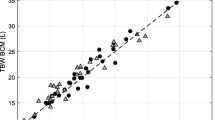
Values are summarised in Table 1. A Shapiro-Wilk’s test (p > 0.05) demonstrated that the TBWDeut (p = 0.098) and TBWBIA (p = 0.198) results were both normally distributed. When comparing the variance in TBW values between those obtained without correcting for urine output during the 5 h after deuterium dosing, i.e. ‘uncorrected’ and those values obtained after ‘correction’ for urine output, the difference observed was minimal (median of 1.5%; 0.3–6.6%). Given this small difference, uncorrected values were used in the subsequent analysis. Figure 1a depicts the relationship between TBWDeut and TBWBIA (r = 0.990, p < 0.0001). Lin’s concordance coefficient confirmed the significance of the correlation; Rc = 0.987, 95% CI [0.967–0.995]. One-sample t-test of the difference between TBWDeut and TBWBIA was not significant (p = 0.344). Paired samples t-test showed no significant difference between the means of the TBWDeut and the TBWBIA measurements (p = 0.344) (Table 1). Variability between TBWDeut and TBWBIA measurements for individuals showed a median of 4.32% with range 0.45–9.1%.
(a) Correlation between TBW calculated from BIA versus TBW calculated from deuterium dilution. (b) The solid line indicates the mean; the dashed lines represent the upper (mean + (1.96 SD)) and lower (mean − (1.96 SD)) levels of the 95% CI. Each dot represents the method difference versus the method mean for individuals
Bland-Altman analysis and subsequent plot of the difference between the TBW measurement and the mean of the TBW measurements is depicted in Fig. 1b. Results indicate that there was no significant proportional bias between the criterion method, TBWDeut, and the test method, TBWBIA, to measure TBW (β = −0.056, adjusted r 2 = 0.069, p = 0.169).
Fat-Free Mass Determination
Correlation analysis showed that FFM calculated from BIA correlated significantly with FFM calculated from TBWDeut using the equation FFM = TBW/Hydration coefficient (r = 0.984, p < 0.0001) (Fig. 2a). Lin’s concordance coefficient confirmed the significance of the correlation; Rc = 0.969, 95% CI [0.924–0.988]. One-sample t-test of the difference between FFMDeut and FFMBIA was not significant (p = 0.111). Paired samples t-test showed no significant difference between the means of the FFMDeut and the FFMBIA measurements (p = 0.111) (see Table 1).
(a) Correlation between FFM calculated from BIA versus FFM calculated from deuterium dilution. (b) The solid line indicates the mean; the dashed lines represent the upper (mean + (1.96 SD)) and lower (mean − (1.96 SD)) levels of the 95% CI. Each dot represents the method difference versus the method mean for individuals
Bland-Altman analysis and plot is depicted in Fig. 2b. Results indicate no proportional bias between the deuterium dilution and BIA methods to measure FFM (β = −0.089, r 2 = 0.142, p = 0.083).
Discussion
Anthropometric assessment of height and weight and the subsequent calculation of BMI are valuable clinical tools that monitor growth against standards and allow screening for overweight. However, they are not sufficient on their own for the comprehensive assessment of nutritional status and body composition in health and disease (Battezzati et al. 2003). Longitudinal body composition monitoring in PKU in an outpatient setting may allow individualised nutritional management strategies based on lean body mass rather than the relatively blunt instrument of body weight (MacDonald et al. 2011). It also provides important information in relation to disorder-specific management strategies in the context of overall longer-term good health (Albersen et al. 2010) by enabling a better understanding of the course of an individual’s anthropometric and body composition profiles (Rocha et al. 2013a).
The implementation of a reliable, quick and easy method to measure body composition in the outpatient clinic setting would be advantageous for clinicians managing individuals with PKU. Bioelectrical impedance machines are very useful due to their non-invasive nature, safety, ease of use, portability and relatively low cost compared to other clinically available methods of measuring body composition (Mulasi et al. 2015). However, the proprietary and confidential nature of each manufacturer’s algorithm equations from which body compartments are derived make it essential that each machine is validated for use in a population of interest. In this study, we compared the performance of the QuadScan 4000, Bodystat® against a criterion method, deuterium dilution, to determine TBW in a group of 16 patients with PKU. As both TBW values determined by deuterium dilution and the impedance values determined by BIA can be used to calculate FFM (Cleary et al. 2008), we compared these methods for the estimation of FFM.
When comparing mean values of both methods, we found no significant difference between TBWDeut and TBWBIA and between FFMDeut and FFMBIA. We also show a significant correlation between TBWDeut and TBWBIA and between FFMDeut and FFMBIA. Bland-Altman analysis confirmed that there was no significant proportional bias between the methods (Martin Bland and Altman 1986), although there slight bias for TBWDeut to be greater than TBWBIA and for FFMBIA to be greater than FFMDeut. Consequently, we conclude that deuterium dilution and BIA using the QuadScan 4000, Bodystat® can be used to measure TBW and estimate FFM in patients with PKU.
We observed individual differences in measurements between TBWBIA and TBWDeut, and three of four participants with the greatest difference were 13- and 14-year-old males who are entering puberty and therefore likely to be undergoing a rapid change in body composition with changes in hydration status and increased FFM deposition. It is possible that because deuterium dose is based on body weight alone, this measurement was not precise enough to account for the potential body composition and hydration status changes in these boys. A study that included individuals of a similar age and pubertal stage may better address this potential issue. However, as the outliers in the difference between the measurements were minimal, this demonstrated a strength in the methods tested.
Other studies have reported differences in TBW values obtained for individuals from deuterium dilution and from BIA. In a BIA validation study performed in pregnant women with or without HIV infection, a systematic predictive bias was seen in TBW using BIA at each time point during the pregnancy despite TBWDeut and TBWBIA being highly correlated (Kupka et al. 2011).
We also show a strong and statistically significant correlation between FFM results obtained using both methods. We suggest, therefore, that the proprietary regression equations within the QuadScan 4000, Bodystat® analyser, which have been developed for the healthy population, are valid in individuals with PKU. It is possible that no predictive bias was seen between the methods in our study because our participants with PKU are free living with normal physical development.
While it has been shown that BIA alone can be used as a surrogate to measure FFM in a paediatric population (Pietrobelli et al. 2003), predictive equations used in BIA analysis have also been validated in several population groups including healthy children (Cordain et al. 1988; Ellis et al. 1999), overweight and obese children (Cleary et al. 2008) and young female gymnasts (Eckerson et al. 1997). Population-specific BIA equations have also been developed when required, such as race-combined equations for large epidemiological studies (Sun et al. 2003). A study of healthy individuals aged 4–24 years showed that while height2/impedance was a strong predictor of lean mass, some variability was observed in the younger years and older adolescent years, suggesting that no single BIA equation may be applied over all age groups (Montagnese et al. 2013).
This study is limited by the relatively small number of participants and their wide age range, which included adolescents likely to be experiencing pubertal body composition changes. Ongoing evaluation of BIA methodology to measure body composition could be done to ensure that predictive equations using raw impedance values to estimate FFM can be applied across all ages. With more data, it may be possible to develop PKU-specific equations if required in the future. Further study may also produce predictive equations for other metabolic disorders requiring dietary modification.
In summary, our results show no differences between the criterion deuterium dilution method and BIA in measuring TBW and predicting FFM in a group of children and adolescents with PKU. We suggest that BIA using QuadScan 4000, Bodystat® can be used to measure body composition in the outpatient clinic setting, to further improve the assessment of nutritional outcomes for patients with PKU.
References
Albersen M, Bonthuis M, de Roos N et al (2010) Whole body composition analysis by the BodPod air-displacement plethysmography method in children with phenylketonuria shows a higher body fat percentage. J Inherit Metab Dis 33:283–288
Aldámiz-Echevarría L, Bueno MA, Couce ML et al (2014) Anthropometric characteristics and nutrition in a cohort of PAH-deficient patients. Clin Nutr 33:702–717
Allen JR, Baur LA, Waters DL et al (1996) Body protein in prepubertal children with phenylketonuria. Eur J Clin Nutr 50:178–186
Battezzati A, Bertoli S, Testolin C, Testolin G (2003) Body composition assessment: an indispensable tool for disease management. Acta Diabetol 40:S151–S153
Böhm A, Heitmann BL (2013) The use of bioelectrical impedance analysis for body composition in epidemiological studies. Eur J Clin Nutr 67:S79–S85
Burrage LC, McConnell J, Haesler R et al (2012) High prevalence of overweight and obesity in females with phenylketonuria. Mol Genet Metab 107:43–48
Cleary J, Daniells S, Okely AD, Batterham M, Nicholls J (2008) Predictive validity of four bioelectrical impedance equations in determining percent fat mass in overweight and obese children. J Am Diet Assoc 108:136–139
Cordain L, Whicker RE, Johnson JE (1988) Body composition determination in children using bioelectrical impedance. Growth Dev Aging 52:37–40
Dobbelaere D, Michaud L, Debrabander A et al (2003) Evaluation of nutritional status and pathophysiology of growth retardation in patients with phenylketonuria. J Inherit Metab Dis 26:1–11
Eckerson JM, Evetovich TK, Stout JR et al (1997) Validity of bioelectrical impedence equations for estimating fat-free weight in high school female gymnasts. Med Sci Sports Exerc 29:962–968
Ellis KJ, Shypailo RJ, Wong WW (1999) Measurement of body water by multifrequency bioelectrical impedance spectroscopy in a multiethnic pediatric population. [Erratum appears in Am J Clin Nutr 2000 Jun;71(6):1618]. Am J Clin Nutr 70:847–853
Houtkooper LB, Going SB, Lohman TG, Roche AF, Van Loan M (1992) Bioelectrical impedance estimation of fat-free body mass in children and youth: a cross-validation study. J Appl Physiol 72:366–373
Huemer M, Huemer C, Moslinger D, Huter D, Stockler-Ipsiroglu S (2007) Growth and body composition in children with classical phenylketonuria: results in 34 patients and review of the literature. J Inherit Metab Dis 30:694–699
International Atomic Energy Agency (2010) Introduction to body composition assessment using the deuterium dilution technique with analysis of urine samples by isoptope ratio mass spectrometry. International Atomic Energy Agency, Division of Human Health, Vienna
Jackson AA, Johnson M, Durkin K, Wootton S (2013) Body composition assessment in nutrition research: value of BIA technology. Eur J Clin Nutr 67:S71–S78
Kupka R, Manji K, Wroe E et al (2011) Comparison of isotope dilution with bioelectrical impedance analysis among HIV-infected and HIV-uninfected pregnant women in Tanzania. Int J Body Compos Res 9:1–10
Lohman T (1993) Advances in body composition assessment. Human Kinetics Publishers, Champaign
MacDonald A, Rocha JC, Rijn M, Feillet F (2011) Nutrition in phenylketonuria. Mol Genet Metab 104:S10–S18
Martin Bland J, Altman D (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327:307–310
Montagnese C, Williams JE, Haroun D, Siervo M, Fewtrell MS, Wells JCK (2013) Is a single bioelectrical impedance equation valid for children of wide ranges of age, pubertal status and nutritional status? Evidence from the 4-component model. Eur J Clin Nutr 67:S34–S39
Mulasi U, Kuchnia AJ, Cole AJ, Earthman CP (2015) Bioimpedance at the bedside. Nutr Clin Pract 30:180–193
Pietrobelli A, Andreoli A, Cervelli V, Carbonelli MG, Peroni DG, Lorenzo A (2003) Predicting fat-free mass in children using bioimpedance analysis. Acta Diabetol 40:s212–s215
Rocha JC, MacDonald A, Trefz F (2013a) Is overweight an issue in phenylketonuria? Mol Genet Metab 110:S18–S24
Rocha JC, Spronsen FJ, Almeida MF, Ramos E, Guimaraes JT, Borges N (2013b) Early dietary treated patients with phenylketonuria can achieve normal growth and body composition. Mol Genet Metab 110:S40–S43
Rocha JC, van Rijn M, van Dam E et al (2016) Weight management in phenylketonuria: what should be monitored. Ann Nutr Metab 68:60–65
Scaglioni S, Verduci E, Fiori L et al (2004) Body mass index rebound and overweight at 8 years of age in hyperphenylalaninaemic children. Acta Paediatr 93:1596–1600
Singh RH, Cunningham AC, Mofidi S et al (2016) Updated, web-based nutrition management guideline for PKU: an evidence and consensus based approach. Mol Genet Metab 118:72–83
Sun SS, Chumlea WC, Heymsfield SB et al (2003) Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr 77:331–340
van Spronsen FJ, van Wegberg AMJ, Ahring K et al (2017) Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. https://doi.org/10.1016/S2213-8587(16)30320-5
Wells JCK, Fewtrell MS (2006) Measuring body composition. Arch Dis Child 91:612–617
Acknowledgments
Analysis was undertaken at the Stable Isotope Geochemistry Laboratory, School of Earth and Environmental Sciences, and the University of Queensland, and we thank Kim Baublys and Wei Zhou for their assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Francois Feillet, MD, PhD
Appendices
Synopsis
No predictive bias existed when measuring total body water or fat-free mass by deuterium dilution and bioelectrical impedance analysis (BIA) using the QuadScan 4000, Bodystat®; we therefore suggest that this instrument could be used as a method to monitor body composition in the outpatient clinic setting in patients with phenylketonuria.
Contributions of Individual Authors
All authors contribute to the submitted paper: contributing content development, drafting and revising the manuscript and providing final approval of the version to be submitted:
-
1.
Maureen Evans has been responsible for conception and design of the project, data and sample collection, statistical analysis, researching and drafting of the manuscript through its various stages of development and completed the final version for submission.
-
2.
Kay Nguo was responsible for the analysis and interpretation of the deuterium dilution samples and revising the manuscript critically for content and format.
-
3.
Avihu Boneh was responsible for the interpretation of data and for revising the manuscript critically for content and format.
-
4.
Helen Truby was responsible for supervising the project, conception and design and revising the manuscript critically for content and format.
Corresponding Author
Maureen Evans, Department of Metabolic Medicine, Royal Children’s Hospital, Melbourne, Australia. Tel.: +61383416376. Email address: maureen.evans@rch.org.au.
Competing Interest Statement
No person was provided with an honorarium, grant, or other form of payment to conduct the study or produce the manuscript. Maureen Evans, Kay Nguo, Avihu Boneh and Helen Truby declare that they have no conflict of interest.
Details of Funding
This research did not receive any specific grant or sponsorship from funding agencies in the public, commercial or not-for-profit sectors.
Details of Ethics Approval
This study was approved by the RCH Human Research Ethics Committee: HREC #32056D.
Patient Consent Statement
This manuscript does not contain any personal information about patients. Informed consent was obtained for all study participants as a requirement of the RCH Human Research Ethics Committee process.
Rights and permissions
Copyright information
© 2017 Society for the Study of Inborn Errors of Metabolism (SSIEM)
About this chapter
Cite this chapter
Evans, M., Nguo, K., Boneh, A., Truby, H. (2017). The Validity of Bioelectrical Impedance Analysis to Measure Body Composition in Phenylketonuria. In: Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V. (eds) JIMD Reports, Volume 42. JIMD Reports, vol 42. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2017_75
Download citation
DOI: https://doi.org/10.1007/8904_2017_75
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-58364-7
Online ISBN: 978-3-662-58365-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)