Abstract
Phenylketonuria (PKU) is a rare genetic disorder in which the amino acid phenylalanine cannot be sufficiently metabolised. Although a build-up of phenylalanine causes irreversible cognitive impairment, this can be prevented through a strict, lifelong diet restricted in natural protein. Despite the severe consequences of poor metabolic control, many children and adolescents have phenylalanine levels above their recommended limits. This systematic review was the first to examine studies reporting demographic and/or psychosocial influences on blood phenylalanine levels, with the aim to identify factors that were robustly linked with metabolic control. Four electronic databases were searched, yielding 1,808 articles. Articles were included if they reported a statistical examination of the association between one or more demographic or psychosocial factor(s) and metabolic control (as measured by blood phenylalanine concentration) for children and adolescents with PKU. Twenty-nine studies were selected for inclusion, which examined a range of child, parent and family factors related to blood phenylalanine levels. The most reproducible association was with child age, with metabolic control worsening with increasing age. This suggests that interventions promoting treatment adherence would be particularly beneficial for adolescents. There was a paucity of studies in some areas, and the quality of included studies varied; therefore, the conclusions of this review are preliminary. Research recommendations focus on promoting the growth of the evidence-base to support clinical practice.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Phenylketonuria (PKU, OMIM 261600) is a rare genetic disorder with an incidence of approximately 1 in 10,000 (Donlon et al. 2004). Due to a deficiency in the enzyme phenylalanine hydroxylase, the amino acid phenylalanine (phe) cannot be sufficiently metabolised. PKU is primarily seen in a so-called ‘classic’ form in which the level of blood phe is above 1,200 μmol/L, as well in a milder variant, the prevalence of which is unknown, in which the blood phe level is between 600–1,200 μmol/L. In both cases, but to a necessarily greater degree in the case of classic PKU, phe builds up in the body, causing severe and irreversible cognitive impairment. However, this can be prevented to a considerable degree by a strict, lifelong, natural protein-restricted diet with amino acid supplements (see Koch et al. 2002). Although the aim of dietary treatment is to maintain blood phe concentrations within an acceptable target range, which is monitored via frequent blood samples, currently there is no universally accepted range, with different countries, and different clinics within countries, using varied management guidelines (Ahring et al. 2009; Feilliet et al. 2010).
Poor metabolic control in children and adolescents is associated with increased cognitive difficulties and poorer academic achievement (e.g., Azen et al. 1991; Chang et al. 2000). A meta-analysis of 40 studies by Waisbren et al. (2007) showed a 1.3–3.1 point reduction in Intelligence Quotient (IQ) for each 100 μmol/L increase in phe concentration. Furthermore, elevated phe levels have been associated with increased behavioural difficulties (Anjema et al. 2011; Smith and Knowles 2000) and poorer psychological wellbeing (Brumm et al. 2010; Clacy et al. 2014), with a hypothesised biological basis of these difficulties due to raised phe levels. Given the severe consequences of poor treatment adherence, it might be expected that very few children and adolescents have poor metabolic control. However, research indicates that this is not the case, with many children and adolescents having phe levels above the recommended range (Levy and Waisbren 1994; MacDonald et al. 2010, 2012; Walter et al. 2002; Walter and White 2004). For example, in a study with 330 patients, a quarter of 0–9 year-olds, half of 10–14 year-olds, and more than three-quarters of 15–19 year-olds had phe levels above their maximum recommended limits (Walter et al. 2002).
These issues can be considered within the broader context of treatment adherence in long-term treatments and/or chronic conditions (Horne et al. 2005; Haynes et al. 2008).
Despite the recognised difficulties with metabolic control, very few studies have examined interventions to improve treatment adherence, and those that have are limited and mainly uncontrolled (e.g., MacDonald et al. 2010). To inform interventions, it is necessary to identify the factors that affect treatment adherence for children and adolescents, and whether certain groups are at greater risk of poor metabolic control. A narrative review by MacDonald et al. (2010) highlighted a number of influences on dietary adherence, including patient age, social pressures, educational achievement of carers, and level of family cohesion [marital/cohabiting status of parents (Olsson et al. 2007)]. However, to date, there has been no systematic review of the factors affecting metabolic control for children and adolescents with PKU.
The aim of this review was to identify factors that were robustly linked with treatment adherence by examining studies reporting a statistical examination of the association between demographic and/or psychosocial factors relating to children and adolescents with PKU and their families, and metabolic control, as assessed by blood phe concentration.
Method
Search Strategy
A systematic search of Ovid Medline, PsychInfo, Embase, and EBSCO CINAHL was performed on 11th December 2015. Search terms were Phenylketonuria AND adheren* OR diet* OR treatment OR complian* OR control OR phenylalanine OR outcome* OR concordan*. Search limitations included English language, children and adolescents (0–18 years) and years 1985–2015.
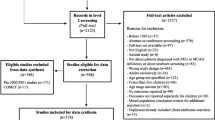
Figure 1 presents an outline of the search process based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al. 2009). Studies were included if they (1) reported a statistical examination of the association between one or more demographic or psychosocial factor(s) and metabolic control (as measured by blood phe concentration) for children and adolescents with PKU, (2) were in the English language, and (3) were published in a peer-reviewed journal between 1985–2015. Case series and review papers were excluded.
Titles and abstracts were screened for inclusion by the first author and relevant abstracts were selected for full-text review (n = 62). Full text articles were assessed for eligibility and excluded if they did not meet the inclusion criteria. Any uncertainty about eligibility was resolved via discussion with another author. Six full text articles could not be accessed via inter-library loan, internet search, or by contacting authors via email. Twenty-eight papers were excluded: 16 did not examine the association between one or more demographic or psychosocial factor(s) and metabolic control and 12 reported a relationship but did not examine the association(s) statistically. Reference lists of included papers were manually examined, yielding one additional paper; thus, 29 studies were included. Data were extracted and entered into a database by the first author (see Supplementary Table 1).
Quality Assessment
The Quality Assessment Tool for Studies with Diverse Designs (QATSDD) was used to assess study quality (Sirriyeh et al. 2012). The QATSDD has shown good reliability and validity and was chosen due to the diverse methodologies of the included studies.
The 14 QATSDD items relating to quantitative studies were rated on a 4-point scale from ‘not at all’ (0) to ‘complete’ (3). The item scores were summed to provide a total score, with a maximum score of 42 (see Supplementary Table 2). The first author rated all studies, and another author independently rated five studies (17%) to determine inter-rater reliability, which was good (κ = 0.71).
Results
Twenty-nine studies were included in this review, representing 1,784 participants with PKU (including children, adolescents and adults, see Supplementary Table 1 for participant age ranges). Sample sizes ranged from 13 to 167 participants and the sample characteristics that were reported varied greatly. Most studies provided information on patient age and sex, but few provided further details, such as socioeconomic status and ethnicity, alongside country of completion, with the most common country being the USA (n = 11). Of the 29 studies, 19 were cross-sectional, 7 were longitudinal, and 3 were intervention studies. Two of the intervention studies were pre-post designs with no control group (Gleason et al. 1992; Singh et al. 2000), and one was a randomised controlled trial (Durham-Shearer et al. 2008), with participants allocated to the intervention (educational resource) or control group (no educational resource). Whilst all studies statistically examined associations between demographic or psychosocial factors and blood phe levels, many studies (n = 13) did not have this as their primary objective.
Quality Ratings
Quality ratings ranged from 14 to 34 (% of maximum score range 33–81%), with a mean of 26 (61%). Reasons for low ratings included having weak references to theory, limited rationale for choice of data collection tools, limited assessment of the reliability and validity of measurement tools, minimal evidence of user involvement in design, and minimal discussion of study limitations. Several studies also had small sample sizes, very few provided evidence that the sample size was considered in terms of analysis, and some did not provide a clear rationale for choice of analytic method. Only one study (Hood et al. 2014) provided separate effect sizes for significant findings and further inspection of the reported results identified that some post-hoc effect sizes could be computed for significant findings in five studies (Fehrenbach and Peterson 1989; Gleason et al. 1992; Arnold et al. 1998; Weglage et al. 1999; Griffiths et al. 2000) but that this was not possible for four studies (Verkerk et al. 1994; Griffiths et al. 2000; Singh et al. 2000; Crone et al. 2005) due to the lack of relevant statistical information data in the papers. In all other cases, there were either no significant findings or correlational analyses were used. Therefore, it was decided that a comprehensive meta-analysis would be neither appropriate nor feasible and individual effect sizes (d) were reported where possible, following the convention of reporting small (d = 0.2), medium (d = 0.5) and large (d = 0.8) effect sizes (Cohen 1988).
Finally, on observation, an association between study quality and year of publication or methodological design was not evident. As this is the first review of the influence of psychosocial and demographic factors on metabolic control for children and adolescents, all studies were retained to provide a comprehensive overview of the available research.
Study Findings
Studies were grouped according to whether they examined the influence of child, parent or other family factors on metabolic control: 24 assessed child factors, 14 parent factors, and 8 reported on other family factors (see Supplementary Table 3).
Child Factors
Of 16 studies that examined the association between child age and metabolic control, 7 used correlational analyses, with 6 reporting a positive correlation (Al-Qadreh et al. 1998; Freehauf et al. 2013; McMurry et al. 1992; Schulz and Bremer 1995; VanZutphen et al. 2007; Vilaseca et al. 2010), and 1 reported no significant correlation (Arnold et al. 1998). Five Pearson’s r coefficients ranged from 0.35–0.64, and one Spearman’s rho coefficient was 0.62 (Freehauf et al. 2013). However, the positive correlation reported by Vilaseca et al. (2010); Pearson’s r = 0.63 might be partially explained by an increase in target blood phe level for 6–12 year-olds (<600 μmol/L) compared to under 6-year-olds (<480 μmol/L). An additional study (Crone et al. 2005) found a quadratic rather than linear association between age and metabolic control, with blood phe increasing after 13 years.
One study (Freehauf et al. 2013) found a positive correlation between age and blood phe for over 12-year-olds (Spearman’s rho = 0.48), but not for under 12-year-olds. When using a measure of difference score between phe level and target level, a significant correlation remained with age for over 12-year-olds (Spearman’s rho = 0.48), indicating a progressive reduction of metabolic control from adolescence. If this is the case, this could explain the lack of a significant correlation found in Arnold et al.’s (1998) study, which examined the association between age and blood phe in 1–8-year-olds. Nevertheless, in a sample of 8–19-year-olds, age was not significantly associated with metabolic control when phe was dichotomised into poor and good control (Olsson et al. 2007). However, as only a small proportion had poor control (n = 14), there might not have been sufficient statistical power to identify an association.
Two studies (Griffiths et al. 2000; Viau et al. 2011) found that blood phe levels significantly increased year-by-year with age, and eight studies found that blood phe was significantly higher for those above 6 years of age (Gokmen-Ozel et al. 2008; Vilaseca et al. 2010), 8 years (Al-Qadreh et al. 1998), 10 years (Hood et al. 2014), 12 years (McMurry et al. 1992; Vilaseca et al. 2010), and 14 years (Weglage et al. 1999). However, in Gokmen-Ozel et al.’ (2008) study, the increase in phe for over 6-year-olds compared to under 6-year-olds might be partially explained by an increase in target phe levels from age 6.
Two studies examined the proportion of children who achieved good metabolic control and found contrasting results: Vilaseca et al. (2010) discovered that the proportion with good control decreased with increasing age (from under 6 years, to 6–12 years, to over 12 years), but Hartnett et al. (2013) found no significant difference between under 6 and 6–12-year-olds, whilst Cotugno et al. (2011) observed that more over 10-year-olds achieved target phe levels than under 10-year-olds, apparently contradicting studies showing reduced metabolic control with age.
Three studies (Hartnett et al. 2013; Hood et al. 2014; Viau et al. 2011) examined the relationship between age and variability in phe levels, but their findings showed no significant associations.
Sex
There was no significant relationship with blood phe levels (Al-Qadreh et al. 1998; Freehauf et al. 2013; Vilaseca et al. 2010) and the proportion achieving target levels (Cotugno et al. 2011) did not significantly differ between male and female children, and sex did not predict phe levels (Olsson et al. 2007; Verkerk et al. 1994). Olsson et al. (2007) noted that sex had a borderline statistical significance in a subgroup of children whose parents had not separated, with a tendency toward lower phe levels in female children. In addition, in a sample of 6–17-year olds, the proportion with 70% or more of their phe levels within target range was significantly higher for females than males (MacDonald et al. 2011).
Child Knowledge
Three studies evaluated the impact of adolescent treatment programmes designed to improve treatment adherence (Durham-Shearer et al. 2008; Gleason et al. 1992; Singh et al. 2000). Gleason et al. (1992) identified that post-intervention improvements in knowledge were accompanied by reductions in blood phe level; however, other treatment factors, including motivational techniques, might have led to reduced levels rather than improved knowledge. Two studies (Durham-Shearer et al. 2008; Singh et al. 2000) reported that post-intervention improvements in PKU knowledge were not accompanied by sustained reductions in blood phe. However, the intervention studies were limited by small sample sizes (n ranged from 16–32), and did not examine the direct association between PKU knowledge and metabolic control. An additional study (Bekhof et al. 2003) found that knowledge of PKU did not significantly predict blood phe levels.
Other Child Factors
In their examination of the association between metabolic control and child attributional style, Antshel et al. (2004) measured using locus of control ratings for vignettes describing a young person with behavioural dysregulation or academic difficulties. Locus of control ratings were significantly correlated with blood phe level (Pearson’s r = 0.61 for behavioural dysregulation vignettes and 0.43 for academic difficulties vignettes), with higher blood phe associated with a higher external locus of control. They suggested that children with a higher internal locus of control assumed more personal responsibility for treatment adherence, resulting in better metabolic control, or that children with a higher external locus of control felt more powerless over their condition, leading to reduced treatment adherence.
In their intervention study examining the associations between metabolic control, attitudes, and health beliefs relating to PKU, Singh et al. (2000) found that post-intervention improvements in attitudes and health beliefs (assessed by questionnaires based on previous research) were not accompanied by sustained reductions in blood phe levels.
Finally, Ievers-Landis et al. (2005) examined the associations between adherence strategies, perceived strategy effectiveness, perceived problem frequency and difficulty (assessed by semi-structured interview and Likert scales), and metabolic control. Higher child perceived strategy effectiveness was associated with lower blood phe levels (Pearson’s r = −0.68), but perceived problem frequency and difficulty were not significantly associated with blood phe levels. In addition, children who used strategies coded as maladaptive for treatment adherence (e.g., avoiding problems) had higher blood phe levels than those who did not use maladaptive strategies.
Parent Factors
Three studies examined the association between parent income and metabolic control. Whilst Griffiths et al. (2000) found that chief earner income was positively correlated with blood phe, two other studies (MacDonald et al. 2008; Reber et al. 1987) failed to identify a significant correlation between metabolic control and income. Of the three studies examining the association between parent employment or occupational status and metabolic control, only Alaei et al. (2011) noted that children with employed parents had significantly lower blood phe levels than children with unemployed parents. Employment status was not significantly associated with metabolic control in MacDonald et al.’s (2008) study and occupational level did not predict blood phe in Verkerk et al.’s (1994) study.
Five studies examined the association between parents’ educational level and metabolic control. According to Reber et al. (1987) and MacDonald et al. (2008), blood phe was not significantly associated with parents’ level of education. Alaei et al. (2011) found that metabolic control was not significantly different for parents with different educational levels. Although Olsson et al. (2007) noted that parental educational level did not predict phe levels dichotomised into good and poor control, Shulman et al. (1991) identified that children’s concurrent phe level was correlated with maternal (Pearson’s r = −0.27) and paternal education (r = −0.28), with higher education associated with lower blood phe.
Three studies examined the association between parent knowledge of PKU (using questionnaires based on previous research) and metabolic control. Whilst Gokmen-Ozel et al. (2008) found a significant negative correlation between maternal exchange knowledge score and blood phe level (Pearson’s r = −0.17), total knowledge scores were not significantly associated with metabolic control. Similarly, MacDonald et al. (2008) reported that mother’s total knowledge of PKU was not associated with phe level, nor was their ability to calculate exchanges or estimate the number of phe exchanges in food portions. Although Bekhof et al. (2003) noted that higher parent knowledge predicted lower blood phe levels, this association disappeared when other confounders were adjusted for (pre-treatment phe, dietary phe tolerance, parent age, parent educational level, and ethnicity).
Fehrenbach and Peterson (1989) examined the associations between parent problem-solving skills, parenting strategies, and metabolic control. Children with good metabolic control had parents who produced a higher number and higher quality of verbal responses to PKU problem scenarios than children with poor metabolic control. Furthermore, those with good metabolic control had families that were organised in a more hierarchical manner with more firmly fixed rules. However, Ievers-Landis et al. (2005) observed that parents using strategies coded as “authoritarian” had children with higher phe levels than those who did not. In addition, higher parent perceived strategy effectiveness was associated with lower phe levels (Pearson’s r = −0.64), and higher ratings of problem frequency (r = 0.55), problem difficulty (r = 0.79) and affective intensity (r = 0.61) were associated with higher phe levels.
Crone et al. (2005) examined the associations between parent attitudes, subjective norms, self-efficacy and metabolic control. Children’s blood phe levels were lower when parents’ experiences were that their child adhered well to the diet, even if their phe levels were sometimes too high (attitudes), and when parents answered that having their child eat the synthetic protein substitute was easy (self-efficacy). However, blood phe levels were higher when parents answered that their relatives did not agree when their child deviated from the diet (subjective norm).
Antshel et al. (2004) examined the association between metabolic control and parent attributional style. The latter was explored by asking parents to rate their perceived ‘locus of control’ (Rotter 1966) (i.e. the degree to which a person perceives events to be under their own control [internal locus of control] or under the control of other people or events [external locus of control]) in a series of written vignettes describing a young person with PKU presenting with behavioural dysregulation or academic difficulties. The subsequent locus of control ratings was significantly correlated with blood phe level (Pearson’s r = 0.69 for behavioural dysregulation vignettes and 0.52 for academic difficulties vignettes), with higher blood phe associated with a higher external locus of control. Antshel and colleagues suggested that parents with a higher external locus of control felt more powerless in relation to their child’s condition, leading to reduced efforts in supporting treatment adherence.
Finally, two studies examined the relationship between parental distress and metabolic control, and found that parental distress, parenting-related stress, and marital satisfaction were not significantly associated with phe levels (Reber et al. 1987), and level of external stress was not significantly different for those with good and poor control (Fehrenbach and Peterson 1989).
Other Demographic Factors
Two studies from the Netherlands examined the association between parental country of origin and metabolic control (Crone et al. 2005; Verkerk et al. 1994). Children with parents who had emigrated from a different country had higher blood phe levels than children with Dutch parents, possibly because of barriers to accessing health care services for some immigrants, such as language difficulties. In their study of the relationship between parent age and metabolic control, MacDonald et al. (2008) found no significant association.
Other Family Factors
Four studies examined the associations between family composition factors and metabolic control. Children with separated or divorced parents were more likely to have higher phe levels than children with married or cohabitant parents (Alaei et al. 2011; Olsson et al. 2007), but family size/number of children was not associated with metabolic control (Alaei et al. 2011; MacDonald et al. 2008). Whilst Alaei et al. (2011) found that blood phe was positively correlated with the number of children with PKU (strength of correlation not reported), Crone et al. (2005) noted that the number of children with PKU did not predict phe level. Three older studies specifically examined the association between family cohesion [i.e. marital/cohabiting status of parents] and metabolic control using parent questionnaires. Two studies used the Family Adaptability and Cohesion Evaluation Scale (Reber et al. 1987; Shulman et al. 1991) and one used the Family Environment Scale (Fehrenbach and Peterson 1989). Although Shulman et al. (1991) reported that lower blood phe level was moderately associated with higher paternal and maternal family cohesion scores (Pearson’s r = −0.34; −0.36, respectively), Reber et al. (1987) and Fehrenbach and Peterson (1989) found no significant association with metabolic control. Finally, Freehauf et al. (2013) found no significant association between distance from home to clinic and metabolic control.
Discussion
This systematic review examined 29 identified studies reporting a statistical examination of the association between one or more demographic or psychosocial factor(s) and metabolic control (as measured by blood phe concentration) for children and adolescents with PKU. Only studies reporting statistical analyses were included in order to identify the factors most robustly linked with metabolic control and the QATSDD was found to be a valid tool for assessing the methodological quality of the studies included in the current review. In summary, the included studies examined a range of child, parent and family factors and indicated some strong associations with blood phe levels. However, there were some areas of investigation with a paucity of studies, highlighting a need for further research in this area.
This review suggests that the most reproducible factor associated with blood phe level currently is child age. Sixteen studies examined child age, with the majority finding a progressive reduction in metabolic control with age, and some suggesting that this occurred from adolescence. Reported correlations between age and metabolic control ranged from 0.35–0.64, indicating moderate to large associations. A similar influence of age has been found in children with diabetes (Neylon et al. 2013), implying that this association may be common in other metabolic disorders. With increasing age, it is likely that increased independency from the family and heightened social pressures, for example, around food and lifestyle, contribute to reduced dietary adherence (Levy and Waisbren 1994). It was noted that all 16 studies reporting an association with age did not examine this as their primary aim, suggesting that demographic data are frequently examined in health-related studies as a secondary objective.
Following age, the next most reproducible factor was child sex, with six studies indicating no association with sex and one study finding that more females had 70% or more of their phe levels within target range than males (MacDonald et al. 2011). In this study, the endpoint measure of 70% or more levels within target range might have allowed greater sensitivity with regard to identifying more subtle differences in metabolic control between males and females.
Due to the small numbers of studies examining other child, parent, and family factors, it is difficult to draw firm conclusions regarding their influence. However, regarding child factors, the available research indicated that blood phe level was not associated with child knowledge of PKU, attitudes, health beliefs, perceived problem frequency, or perceived problem difficulty. Conversely, blood phe level showed moderate to large correlations with child attributional style, a strong correlation with perceived strategy effectiveness, and was significantly different for those using maladaptive and non-maladaptive strategies.
Regarding parent and family factors, the available findings indicated that metabolic control was associated with parenting strategies, attitudes, subjective norms, self-efficacy, perceived strategy effectiveness, perceived problem frequency, perceived problem difficulty, attributional style, affective intensity of problems, country of origin, and marital status. Conversely, metabolic control was not associated with or inconsistently associated with parent knowledge of PKU, parent age, parent distress, family size, number of children with PKU in the family, family cohesion, geographic proximity to clinic, and socioeconomic factors, such as parent income, education, and occupation. The inconsistencies in findings between studies could be a result of different study methodologies, measures and cohort characteristics. For example, in relation to socioeconomic factors, participants from different countries may experience different levels of social inequality, and therefore factors such as unemployment may have a greater impact on treatment adherence and availability of low protein foods and food substitutes in some countries than others.
Limitations
Whilst this review identified a number of factors related to metabolic control, it was difficult to draw firm conclusions due to both a paucity of studies in some areas of investigation and some inconsistent findings. Furthermore, the strength of conclusions that can be drawn is limited by the varied quality of the included studies. As highlighted by the QATSDD ratings, a number of studies had small sample sizes with no evidence of consideration of the sample size in terms of analysis, meaning that the power of some studies could have been limited. However, it should be noted that the potential recruitment pool of young people with PKU is small due to the rarity of the condition, and hence small sample sizes are common. Nevertheless, as studies often provided scarce sample descriptions and the majority used cross-sectional methodology, it is difficult to draw conclusions about cause and effect influences on metabolic control. Finally, as there was limited availability of articles during the search process (six full-text articles were not available), it is unknown whether these would have met the inclusion criteria and contributed to the results and conclusions of this review.
Recommendations for Clinical Practice
This review indicates that certain groups of young people may be at higher risk of poor treatment adherence, particularly older children and adolescents. It is therefore important that clinicians and parents are aware of the tendency for worsening metabolic control with age and consider providing extra support to older children (around age 12 and above). Whilst PKU clinics routinely provide information about PKU and associated dietary treatment to young people and their carers, this review indicated that child knowledge of PKU was not associated with metabolic control, and parental knowledge was only weakly or inconsistently associated. Thus, treatment knowledge is necessary but not sufficient for dietary adherence. To date, there are no effective interventions to improve treatment adherence in PKU but current management guidelines for PKU in the UK (NSPKU 2014) recommend that services should be multi-disciplinary with a clinical psychologist to focus on, among other things, promoting ‘patient and parent motivation to comply with treatment’. On the basis of the current review, such work could usefully focus on child and parent attributional style, attitudes and self-efficacy.
Recommendations for Future Research
This review highlights that there is a paucity of studies examining the potential demographic or psychosocial influences on metabolic control for young people with PKU, which appears to be in contrast to other metabolic conditions, such as diabetes, where metabolic control has been associated with factors such as ethnicity, personality characteristics and coping style (Neylon et al. 2013). Whilst currently unexplored, it is possible that these factors also influence treatment adherence for children with PKU. One of the reasons for limited research in this field is the small sampling pool due to the rarity of the condition, which makes it difficult to recruit sufficient numbers of patients. It is therefore recommended that future studies promote increased recruitment by working more in partnership with clinicians, patients, carers and support groups (DeWard et al. 2014).
It is important that future studies provide more information about participant characteristics, such as socioeconomic details, particularly as these may impact treatment adherence outcomes. In addition, more longitudinal studies are needed to help ascertain cause and effect influences on metabolic control and to explore whether other variables mediate the associations between factors such as age and metabolic control, such as increased social pressures. Although this is currently an emerging evidence-base, further studies could design and examine interventions to improve treatment adherence, informed by the factors highlighted in this review.
A further area for future research would be to examine treatment adherence from a health economics perspective and incorporate cost-benefit analyses alongside psychological and physiological data.
It is also important to acknowledge that small sample size is likely to continue to be an issue in future research in this field (Griggs et al. 2009). Therefore, further research could be informed by recent methodological thinking, for example, Abrahamyan et al.’s (2014) toolkit for conducting clinical trials with rare disorders and proposals to incorporate Bayesian statistical techniques into such studies (Billingham et al. 2001).
Conclusion
This review was the first to systematically examine studies reporting a statistical examination of the association between demographic or psychosocial factors and metabolic control for children and adolescents with PKU. Findings suggested that the most reproducible association was with child age, with control worsening with increasing age. Whilst a number of other factors were associated with blood phe levels, the evidence-base was small with some methodological limitations, and therefore the conclusions of this review are preliminary. This review highlights a paucity of research examining many demographic or psychosocial influences on metabolic control for young people with PKU. Research recommendations are therefore targeted towards promoting the growth of the evidence-base to support clinical practice.
References
Abrahamyan L, Diamond IR, Johnson SR, Feldman BM (2014) A new toolkit for conducting clinical trials in rare disorders. J Popul Ther Clin Pharmacol 21(1):e66–e78
Ahring K, Bélanger-Quintana A, Dokoupil K et al (2009) Dietary management practices in phenylketonuria across European Centres. Clin Nutr 28:231–236
Alaei M, Asadzadeh-Totonchi G, Gachkar L, Farivar S (2011) Family social status and dietary adherence of patients with phenylketonuria. Iran J Pediatr 21:379–384
Al-Qadreh A, Schulpis KH, Athanasopoulou H et al (1998) Bone mineral status in children with phenylketonuria under treatment. Acta Paediatr 87:1162–1166
Anjema K, van Rijn M, Verkerk PH et al (2011) PKU: high plasma phenylalanine concentrations are associated with increased prevalence of mood swings. Mol Genet Metab 104:231–234
Antshel KM, Brewster S, Waisbren SE (2004) Child and parent attributions in chronic pediatric conditions: phenylketonuria (PKU) as an exemplar. J Child Psychol Psychiatry 45:622–630
Arnold GL, Kramer BM, Kirby RS et al (1998) Factors affecting cognitive, motor, behavioral and executive functioning in children with phenylketonuria. Acta Paediatr 87:565–570
Azen CG, Koch R, Friedman EG et al (1991) Intellectual development in 12-year-old children treated for phenylketonuria. Am J Dis Child 145:35–39
Bekhof J, Van Spronsen FJ, Crone MR et al (2003) Influence of knowledge of the disease on metabolic control in phenylketonuria. Eur J Pediatr 162:440–442
Billingham L, Malottki K, Pritchard M, Steven N (2001) Trials in rare diseases: the need to think differently. Trials 12(Suppl 1):A107
Brumm VL, Bilder D, Waisbren SE (2010) Psychiatric symptoms and disorders in phenylketonuria. Mol Genet Metab 99:59–63
Chang PN, Gray RM, O’Brien LL (2000) Patterns of academic achievement among patients treated early with phenylketonuria. Eur J Pediatr 159:96–99
Clacy A, Sharman R, McGill J (2014) Depression, anxiety, and stress in young adults with phenylketonuria: associations with biochemistry. J Dev Behav Pediatr 35:388–391
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale, NJ
Cotugno G, Nicolo R, Cappelletti S et al (2011) Adherence to diet and quality of life in patients with phenylketonuria. Acta Paediatr 100:1144–1149
Crone MR, Van Spronsen FJ, Oudshoorn K et al (2005) Behavioural factors related to metabolic control in patients with phenylketonuria. J Inherit Metab Dis 28:627–637
DeWard SJ, Wilson A, Bausell H et al (2014) Practical aspects of recruitment and retention in clinical trials of rare genetic diseases: the phenylketonuria (PKU) experience. J Gene Couns 23:20–28
Donlon J, Levy H, Scriver C (2004) Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver BA, Beaudet AL, Sly WS, Valle D, Vogelstein B, Childs B (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York
Durham-Shearer SJ, Judd PA, Whelan K, Thomas JE (2008) Knowledge, compliance and serum phenylalanine concentrations in adolescents and adults with phenylketonuria and the effect of a patient-focused educational resource. J Hum Nutr Diet 21:474–485
Fehrenbach AM, Peterson L (1989) Parental problem-solving skills, stress, and dietary compliance in phenylketonuria. J Consult Clin Psychol 57:237–241
Feilliet F, van Spronsen FJ, MacDonald A et al (2010) Challenges and pitfalls in the management of phenylketonuria. Pediatrics 126:333–341
Freehauf C, Van Hove JL, Gao D, Bernstein L, Thomas JA (2013) Impact of geographic access to care on compliance and metabolic control in phenylketonuria. Mol Genet Metab 108:13–17
Gleason LA, Michals K, Matalon R, Langenberg P, Kamath S (1992) A treatment program for adolescents with phenylketonuria. Clin Pediatr 31:331–335
Gokmen-Ozel H, Kucukkasap T, Koksal G et al (2008) Does maternal knowledge impact blood phenylalanine concentration in Turkish children with phenylketonuria? J Inherit Metab Dis 31:S213–S217
Griffiths PV, Demellweek C, Fay N et al (2000) Wechsler subscale IQ and subtest profile in early treated phenylketonuria. Arch Dis Child 82:209–215
Griggs RC, Batshaw M, Dunkle M et al (2009) Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab 96:20–26
Hartnett C, Salvarinova-Zivkovic R, Yap-Todos E et al (2013) Long-term outcomes of blood phenylalanine concentrations in children with classical phenylketonuria. Mol Genet Metab 108:255–258
Haynes RB, Ackloo E, Sahota N et al (2008) Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2(2):CD000011
Hood A, Grange DK, Christ SE et al (2014) Variability in phenylalanine control predicts IQ and executive abilities in children with phenylketonuria. Mol Genet Metab 111:445–451
Horne R, Weinman J, Barber N et al (2005) Concordance, adherence and compliance in medicine taking. Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R & D (NCCSDO). NCCSDO, London
Ievers-Landis CE, Hoff AL, Brez C et al (2005) Situational analysis of dietary challenges of the treatment regimen for children and adolescents with phenylketonuria and their primary caregivers. J Dev Behav Pediatr 26:186–193
Koch R, Burton B, Hoganson G, Peterson R et al (2002) Phenylketonuria in adulthood: a collaborative study. J Inherit Metab Dis 25:333–346
Levy HL, Waisbren SE (1994) PKU in adolescents: rationale and psychosocial factors in diet continuation. Acta Paediatr Suppl 407:92–97
MacDonald A, Davies P, Daly A et al (2008) Does maternal knowledge and parent education affect blood phenylalanine control in phenylketonuria? J Hum Nutr Diet 21:351–358
MacDonald A, Gokmen-Ozel H, van Rijn M, Burgard P (2010) The reality of dietary compliance in the management of phenylketonuria. J Inherit Metab Dis 33:665–670
MacDonald A, Nanuwa K, Parkes L et al (2011) Retrospective, observational data collection of the treatment of phenylketonuria in the UK, and associated clinical and health outcomes. Curr Med Res Opin 27:1211–1222
MacDonald A, van Rijn M, Feillet F et al (2012) Adherence issues in inherited metabolic disorders treated by low natural protein diets. Ann Nutr Metab 61:289–295
McMurry MP, Chan GM, Leonard CO, Ernst SL (1992) Bone mineral status in children with phenylketonuria – relationship to nutritional intake and phenylalanine control. Am J Clin Nutr 55:997–1004
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269
Neylon OM, O’Connell MA, Skinner TC, Cameron FJ (2013) Demographic and personal factors associated with metabolic control and self-care in youth with type 1 diabetes: a systematic review. Diabetes Metab Res Rev 29:257–272
NSPKU (National Society for PKU) (2014) Management of phenylketonuria: a consensus document for the diagnosis and management of children, adolescents and adults with phenylketonuria (PKU). The National Society for Phenylketonuria (UK) Ltd, Purley
Olsson GM, Montgomery SM, Alm J (2007) Family conditions and dietary control in phenylketonuria. J Inherit Metab Dis 30:708–715
Reber M, Kazak AE, Himmelberg P (1987) Phenylalanine control and family functioning 48 in early-treated phenylketonuria. J Dev Behav Pediatr 8:311–317
Rotter JB (1966) Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr 80:1–28
Schulz B, Bremer HJ (1995) Nutrient intake and food consumption of adolescents and young adults with phenylketonuria. Acta Paediatr 84:743–748
Shulman S, Fisch RO, Zempel CE et al (1991) Children with phenylketonuria: the interface of family and child functioning. J Dev Behav Pediatr 12:315–321
Singh RH, Kable JA, Guerrero NV et al (2000) Impact of a camp experience on phenylalanine levels, knowledge, attitudes, and health beliefs relevant to nutrition management of phenylketonuria in adolescent girls. J Am Diet Assoc 100:797–803
Sirriyeh R, Lawton R, Gardner P, Armitage G (2012) Reviewing studies with diverse designs: the development and evaluation of a new tool. J Eval Clin Pract 18:746–752
Smith I, Knowles J (2000) Behaviour in early treated phenylketonuria: a systematic review. Eur J Pediatr 159:89–93
VanZutphen K, Packman S et al (2007) Executive functioning in children and adolescents with phenylketonuria. Clin Genet 72:13–18
Verkerk PH, Van Spronsen FJ, Van Houten M et al (1994) Predictors of mean phenylalanine levels during the first five years of life in patients with phenylketonuria who were treated early. Acta Paediatr Suppl 83:70–72
Viau KS, Wengreen HJ, Ernst SL et al (2011) Correlation of age-specific phenylalanine levels with intellectual outcome in patients with phenylketonuria. J Inherit Metab Dis 34:963–971
Vilaseca MA, Lambruschini N, Gomez-Lopez L et al (2010) Quality of dietary control in phenylketonuric patients and its relationship with general intelligence. Nutr Hosp 25:60–66
Waisbren SE, Noel K, Fahrbach K, Levy H (2007) Phenylalanine blood levels and clinical outcomes in phenylketonuria: a systematic literature review and meta-analysis. Mol Genet Metab 92:63–70
Walter JH, White FJ (2004) Blood phenylalanine control in adolescents with phenylketonuria. Int J Adolesc Med Health 16:41–45
Walter JH, White FJ, Hall SK et al (2002) How practical are recommendations for dietary control in phenylketonuria? Lancet 360:55–57
Weglage J, Pietsch M, Denecke J et al (1999) Regression of neuropsychological deficits in early-treated phenylketonurics during adolescence. J Inherit Metab Dis 22:693–705
Acknowledgements
We would like to express our sincere gratitude to Katie Carpenter for her assistance with this literature review.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: BOLI-D-16-00290R3
Synopsis
This systematic review identified that whilst a range of demographic and psychosocial variables were associated with metabolic control for children with phenylketonuria, the most reproducible association was with child age.
Compliance with Ethics Guidelines
Conflict of Interest
Emma Medford, Dougal Hare and Anja Wittkowski declare that they have no conflicts of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, revised in 2013. However, as this article does not contain any studies with human or animal subjects performed by any of the authors, informed consent was not required.
Details of the Contributions of Individual Authors
Emma Medford contributed to identifying the review question, planning the search process and reporting, carrying out all aspects of the method section, and writing the majority of the article.
Dougal Hare contributed to identifying the review question, analysing and interpreting the results and to finalising this article.
Anja Wittkowski contributed to identifying the review question, planning the search process and reporting, screening articles, and finalising this article.
Electronic Supplementary Material
Supplementary Table 1
Methodological characteristics of included studies and factors examined for association with treatment adherence (DOCX 33 kb)
Supplementary Table 2
Overview of QATSDD item scores per study (DOCX 42 kb)
Supplementary Table 3
Study findings separated by child, parent and other family factors (DOCX 55 kb)
Rights and permissions
Copyright information
© 2017 Society for the Study of Inborn Errors of Metabolism (SSIEM)
About this chapter
Cite this chapter
Medford, E., Hare, D.J., Wittkowski, A. (2017). Demographic and Psychosocial Influences on Treatment Adherence for Children and Adolescents with PKU: A Systematic Review. In: Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V. (eds) JIMD Reports, Volume 39. JIMD Reports, vol 39. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2017_52
Download citation
DOI: https://doi.org/10.1007/8904_2017_52
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-57576-5
Online ISBN: 978-3-662-57577-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)