Abstract
Acute intermittent porphyria (AIP), variegate porphyria (VP), and hereditary coproporphyria (HCP) are caused by mutations in the hydroxymethylbilane synthase (HMBS), protoporphyrinogen oxidase (PPOX), and coproporphyrinogen oxidase (CPOX) genes, respectively. This study aimed to identify mutations in seven Bulgarian families with AIP, six with VP, and one with HCP. A total of 33 subjects, both symptomatic (n = 21) and asymptomatic (n = 12), were included in this study. The identification of mutations was performed by direct sequencing of all the coding exons of the corresponding enzymes in the probands. The available relatives were screened for the possible mutations. A total of six different mutations in HMBS were detected in all seven families with AIP, three of which were previously described: c.76C>T [p.R26C] in exon 3, c.287C>T [p.S96F] in exon 7, and c.445C>T [p.R149X] in exon 9. The following three novel HMBS mutations were found: c.345-2A>C in intron 7–8, c.279-280insAT in exon 7, and c.887delC in exon 15. A total of three different novel mutations were identified in the PPOX gene in the VP families: c.441-442delCA in exon 5, c.917T>C [p.L306P] in exon 9, and c.1252T>C [p.C418R] in exon 12. A novel nonsense mutation, c.364G>T [p.E122X], in exon 1 of the CPOX gene was identified in the HCP family. This study, which identified mutations in Bulgarian families with AHP for the first time, established seven novel mutation sites. Seven latent carriers were also diagnosed and, therefore, were able to receive crucial counseling to prevent attacks.
Competing interests: None declared
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute Intermittent Porphyria
- Acute Intermittent Porphyria
- Variegate Porphyria
- Protoporphyrinogen Oxidase
- Hereditary Coproporphyria
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Acute intermittent porphyria (AIP) (OMIM 176000), variegate porphyria (VP) (OMIM 176200), and hereditary coproporphyria (HCP) (OMIM 121300) are autosomal dominant, low-penetrant inborn errors of the heme biosynthesis pathway that result in the decreased activity of hydroxymethylbilane synthase (HMBS) (EC 4.3.1.8), protoporphyrinogen oxidase (PPOX) (EC 1.3.3.4), and coproporphyrinogen oxidase (CPOX) (EC 1.3.3.3), respectively.
AIP, VP, and HCP present with clinically identical recurrent neurovisceral attacks. Additionally, erosive bullous cutaneous lesions and hyperpigmentation on sun-exposed areas are more common in VP than in HCP (Sassa 2006). The acute attacks include three major classes of symptoms: gastrointestinal, neurological, and psychiatric. These symptoms are represented by severe abdominal pains, motor neuropathy, depression, and psychosis. The most common factor triggering these attacks is the use of numerous porphyrinogenic drugs. Infections, alcohol, a low-calorie diet, and natural sex hormones fluctuations in women, related to menstrual cycle and pregnancy can also provoke attacks (Kappas et al. 1995).
The diagnosis of acute porphyric attack is based on both clinical manifestations and typical biochemical abnormalities. The main laboratory finding is the dramatic increase of the porphyrin precursors porphobilinogen (PBG) and δ-aminolevulinic acid (ALA) in urine. The exact distinction between the three different diseases requires measuring the urinary and fecal porphyrin excretion patterns, which are characteristic for each enzymatic defect. A fluorescence scan of native plasma is also an important diagnostic criterion, presenting (or not) a characteristic peak for each entity (Sassa 2006; Hift et al. 2004). Decreased levels of HMBS activity in AIP, PPOX activity in PV, and CPOX activity in HCP clarify the diagnosis in the proband and establish the enzymatic defect in the latent carriers (Meyer et al. 1972; Deybach et al. 1981). Unfortunately, when measuring enzymatic activities, results similar to the reference values may bring in uncertainty in the precise diagnosis of the latent carriers (Mustajoki 1981). Thus, the optimal strategy for the detection of these individuals includes the implementation of molecular genetic methods (Whatley et al. 2009).
At least 600 different mutations in the HMBS, PPOX, and CPOX genes have been identified so far (www.hgmd.cf.ac.uk). Most of these mutations are specific to one or a few families, although a founder effect has been clearly demonstrated for both AIP and VP (Thunnel et al. 2006; Meissner et al. 1996). Most AIP, VP, and HCP carriers are heterozygotes. Mutations are heterogeneous and are comprised of single nucleotide substitutions, small insertions and deletions. Recently, large insertions/deletions have been described in the HMBS, PPOX, and CPOX genes (Whatley et al. 2009; Barbaro et al. 2013). In Bulgaria, during a 50-year period as the sole porphyria service at University Hospital “Saint Ivan Rislki” Sofia, 35 families with AIP, 20 with VP, and 2 with HCP have been diagnosed, treated, and followed up. However, the molecular analysis of the patients with AIP, VP, and HCP has not yet been performed. The aim of this study was to identify mutations in the HMBS, PPOX, and CPOX genes in Bulgarian families with acute hepatic porphyrias.
Materials and Methods
Patients
Seven independent index cases with AIP were included. The diagnosis was based on clinical symptoms and increased urinary PBG and ALA values. Decreased levels of HMBS activity in erythrocytes and the absence of a plasma fluorescence peak at 624–627 nm confirmed the diagnosis of AIP. Six independent index cases with VP were also studied. The diagnosis of these patients included the evaluation of the clinical symptoms, a typical plasma fluorescence peak at 624–627 nm, elevated urinary PBG and ALA levels during acute attacks and increased stool porphyrins, with a predominance of protoporphyrin over coproporphyrin in the cases with cutaneous symptoms. One patient with HCP was included. The diagnosis was based on the symptoms that occurred during acute attack, increased urinary PBG and ALA levels, markedly increased total porphyrins in the urine, and a plasma fluorescence peak at 618 nm. Molecular analysis of the HMBS, PPOX, and CPOX genes confirmed the precise diagnosis. These 14 probands were diagnosed, treated, and followed up in the Porphyria Unit of “Saint Ivan Rilski” University Hospital Sofia. The precise places of birth and the pedigree trees of the patients were determined, with no apparent signs of consanguinity. Once the diagnosis of AIP was confirmed, the HMBS activity was evaluated in the available asymptomatic family members. A total of 33 individuals, including the probands and asymptomatic relatives, from 15 families with AIP, VP, and HCP gave their written consent to participate in this study, which was approved by the Ethics Committee.
Methods
Biochemical Measurements
PBG and ALA levels in the urine were measured according to the method described by Mauzarell and Granick (1956). Urinary and fecal porphyrins were assessed according to the method of Rimington (1971). Total fecal total porphyrin levels were measured according to the method of Lockwood et al. (1985). Total porphyrin levels in urine were evaluated according to our modification and optimization of the method described by Deacon and Elder (2001). HMBS activity in the erythrocytes was determined according to the method described by Adjarov et al. (1994). Plasma fluorescence scanning was performed on a Perkin–Elmer fluorescence spectrophotometer MPF 43, with an excitation wavelength of 398 nm and an emission spectrum from 580 to 700 nm.
Identification of Mutations
Genomic DNA was isolated from peripheral whole blood samples using the innuPREP Blood DNA Midi kit (Analytik Jena Life Science, Germany) according to the manufacturer’s protocol. PCR amplification of exons 1 to 15 for HMBS, exons 1 to 13 for PPOX, and exons 1 to 7 for CPOX, with corresponding flanking intron–exon boundaries, was performed; the primers and PCR conditions are available upon request. The PCR products were automatically sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit and a 3500xL Genetic Analyzer (Applied Biosystems, Foster City, USA).
To prove the rarity of the identified novel missense mutations, the corresponding exons of the HMBS and PPOX genes were screened in 96 control DNA samples. In silico prediction of the pathogenicity of these mutations was determined by the HumVar score using the PolyPhen-2 tool (http://genetics.bwh.harvard.edu/pph2/).
Results and Discussion
Detailed clinical, biochemical, and genetic data are presented in Table 1 for the AIP families and in Table 2 for the VP and HCP families. In three of our female AIP patients, the attacks were related to the patient’s menstrual cycle. Infections and/or medications played a triggering role in three AIP and four VP cases, as well as in the HCP patient. Nine subjects with AIP manifested with one or two acute attacks. Some patients (family I-F, family II-P2 and II-Sc1, family IV-P4 and family V-P5) suffered chronic symptoms, including fatigue, lower back pain, paresthesia in the lower limbs, and depression. The proband P2 had suffered from two unrecognized acute attacks and had residual paresis at the time of presentation in our clinic. She also had chronic neurological symptoms when Cimetidine treatment was applied. During the 6 months course of Cimetidine administration, a reduction in porphyrin precursors levels and clinical improvement was achieved. Family V-P5 suffered from one acute attack following infection and antibiotic treatment after surgery. She also had chronic neurological symptoms. Cimetidine was administered after the acute onset, but over the first weeks the pains worsened and no biochemical improvement was noticed. Both acute and cutaneous symptoms were present in four symptomatic VP patients, and only acute symptoms were present in one patient and only cutaneous symptoms in four patients. All VP patients manifested with a single acute attack, and only family III-So exhibited chronic symptoms similar to those observed in AIP. The majority of our VP patients presented with severe photodermatosis alone or accompanying acute onset, with a frequency similar to that reported in South Africa and Western Europe (Whatley et al. 1999). The patient with HCP manifested with acute symptoms only. During onset, all of the patients had markedly increased levels of PBG and ALA. In all of the investigated AIP probands, except for one (family VI-P6), the HMBS activity in the erythrocytes was decreased. In P6, the HMBS activity was measured during acute onset, when transitory normal levels could be expected. Heme-arginate is not available in Bulgaria; therefore, all patients with acute onset, except for one, were treated with i.v. glucose infusions (from 200 to 500 g/day according to the severity of the attack) or Cimetidine until the clinical and biochemical parameters improved.
A total of six different mutations in HMBS were detected in all seven families with AIP, three of which were previously described as single nucleotide substitutions: c.76C > T [p.R26C] in exon 3, c.287C > T [p.S96F] in exon 7, and c.445C > T [p.R149X] in exon 9; the other three mutations were newly detected. The novel ones included a single nucleotide change, a small insertion and a single nucleotide deletion: c.345-2A > C in intron 7–8, c.279-280insAT in exon 7, and c.887delC in exon 15. Overall, there were two missense, one nonsense, one splice-site, and two frameshift mutations. The alterations identified in the Bulgarian patients were heterogeneous, as previously reported in many other populations (Whatley et al. 2009; Puy et al. 1997).
Three of the substitutions identified in the Bulgarian AIP patients have been reported in various ethnic populations. The mutations identified in families I-P1 (p.R26C) and IV-P4 (p.R149X) were initially identified in Finish patients (Kauppinen et al. 1995). p.R26C has been subsequently reported in Slavic (Hrdinka et al. 2006), Spanish (To-Figueras et al. 2006), French (Puy et al. 1997), Chinese (Yang et al. 2008), and Venezuelan populations (Paradisi and Arias 2010). The p.R149X mutation was found to be one of the relatively prevalent mutations (approximately 5%) in a large cohort of 109 mutation-positive AIP families (Puy et al. 1997). It has been shown that the R26 and R149 residues are located in the substrate-binding site and are crucial for enzymatic activity (Llewellyn et al. 1998; Gill et al. 2009). AIP family IV is of particular interest due to its gypsy origin. The gypsy people represent the largest minority in Bulgaria, and the search for the p.R149X substitution in this population deserves further attention.
In families VI-P6 and VII-P7, an identical p.S96F missense mutation was found in the HMBS gene; this mutation was first described by Kaupinnen et al. (2002). There was no significant difference in the clinical phenotype of the two probands; both patients presented with a single acute attack that was triggered by hormonal changes during their twenties. At present, both patients are symptom-free.
Novel substitutions in the HMBS gene that resulted in frameshift and splice-site alterations were identified in families II, III, and V. A small insertion (c.279-280insAT in exon 7) was identified in the II-P2 proband, resulting in a frameshift mutation that led to the formation of premature stop codon after the incorporation of 24 different amino acid residues, compared to the reference transcript. A small out-of-frame deletion (c.887delC) was identified in the III-P3 family, and this mutation led to a premature stop codon after 3 amino acids, compared to the reference sequence. The nucleotide change revealed in family V-P5 (c.345-2A>C in intron 7) affected the invariant AG acceptor splice site and possibly interfered with mRNA processing. At the same position, a different nucleotide change (c.345-2 A>G) has been reported in Swedish patients, listed in a table (Floderus et al. 2002). These three alterations have not been reported in reference databases (such as dbSNP, HGMD, 1KG, and ESP). To exclude the possibility of the novel mutations being SNPs, 96 control DNA samples were screened, and no samples revealed the presence of the novel mutations of the HMBS gene (c.345-2A>C in intron 7, c.279-280insAT in exon 7, and c.887delC in exon 15). The above finding suggests that these mutations are pathogenic. RNA analysis is needed to prove the pathogenicity of the c.345-2A > C change.
After detecting specific mutations in the families, the available family members (n = 4 symptomatic and n = 5 asymptomatic) were screened for the presence of the identified mutations (Table 1). All symptomatic relatives harbored the corresponding family-specific nucleotide changes. Among the five asymptomatic subjects three were mutation positive, including family II-M, VI-D, and VII-D subjects. Subject II-M was a postmenopausal female with a low level of HMBS activity in the erythrocytes and concomitant diabetes mellitus type II. Even if the diabetes has been treated, a slightly elevated serum glucose levels could exert a protective effect against overt disease (Andersson and Lithner 2001). Subjects VI-D and VII-D, females presently aged 21 and 15 years, respectively, whose HMBS activity levels in the erythrocytes were unavailable, shared identical causative mutations, which facilitated genetic counseling to prevent acute attacks. The HMBS levels in the four mutation negative subjects were within the normal range in two cases and were unavailable in the other two cases.
A total of three different novel substitutions in the PPOX gene were found in all six families with VP: c.441-442delCA in exon 5, c.917T>C [p.L306P] in exon 9, and c.1252T>C [p.C418R] in exon 12. In total, two missense mutations and one small deletion were observed in the VP families. All of the probands from families I, II, III, IV, V, and VI had positive plasma scan at 626 nm, increased levels of urinary porphyrins and their precursors PBG and ALA. Probands from families II, III, IV, and VI had increased fecal porphyrins levels as well. A small out-of-frame deletion (c.441-442delCA) was found in family I-P1; this mutation led to a premature stop codon after the introduction of nine different amino acids. Families II-P2, III-P3, and IV-P4 shared an identical mutation (c.917T>C) that resulted in a leucine to proline substitution at position 306 (p.L306P). The V-P5 and VI-P6 probands harbored an identical change (c.1252T>C) that led to the replacement of a cysteine with proline in codon 418 (p.C418R). These three novel alterations have not been reported in reference databases (such as dbSNP, HGMD, 1KG, and ESP) and were not detected in the 96 control DNA samples. Residues L306 and C418 are located in the highly conserved FAD-binding domain (Qin et al. 2011), and these alterations most likely disrupt this interaction. The HumVar scores of 1.00 for p.L306P and 0.921 for p.C418R predicted that these mutations are most likely damaging. A small deletion (c.916_917delCT) in codon 306 has been described by Wiman et al. (2003), while no mutations have been reported in codon 418 so far. The available family members (n = 3 symptomatic and n = 7 asymptomatic) were screened for the presence of the corresponding nucleotide changes, and the details of these individuals are shown in Table 2. The symptomatic subjects III-So, IV-D, and V-Si harbored the family-specific nucleotide changes. Family III-So had an acute attack followed by chronic neurological symptoms and skin involvement and increased levels of PBG, ALA, total porphyrins in urine and feces. Family IV-D suffered from cutaneous lesions only. She had a plasma emission peak at 626 nm, normal PBG and ALA levels due to the lack of acute symptoms, and increased levels of urinary and fecal porphyrins. Family V-Si suffered from acute onset and cutaneous legions, unfortunately PBG and ALA levels shown in Table 2 were measured after the acute onset and were within normal range. However, plasma emission peak at 626 nm and increased total porphyrins in urine were observed even after the resolution of the attack. Among the seven asymptomatic subjects, four harbored the specific alterations, family I-D, II-So, IV-So, and V-Ni. Unfortunately, no biochemical data was available for the adolescent individuals I-D and V-Ni. Family II-So and IV-So had normal total porphyrins levels in urine and feces and urinary PBG and ALA levels. Plasma scan was also negative in both subjects. These results could be expected considering the fact that the significance of plasma emission peak at 626 nm is partly limited in the elderly asymptomatic carriers (IV-So). It is usually absent in asymptomatic children (II-So) (Hift et al. 2004). Unfortunately, PPOX activity could not be measured to check the cosegregation of the mutation and low enzymatic activity levels. Further analysis is needed to prove the pathogenicity of these alterations.
The majority of Bulgarian VP patients can be associated with 2 endemic regions. One of these regions is populated with ethnic Bulgarians, who are Muslim. These individuals reside in remote villages around the mountain town of Velingrad in the Rhodope mountains. Only 2 families, V and VI, from this region agreed to take part in this study, although more patients were invited. Most likely, the majority of VP patients share the identical p.C418R mutation due to consanguinity. The ancestors of families II, III, and IV originated from the small remote village of Buynovtsi, which is situated in the central Balkan mountains. Thus, a possible founder effect can also be anticipated for the p.L306P change. The endemic aggregation of families with VP and the distribution of the corresponding novel missense mutation also emphasize the pathogenic effect of these novel alterations.
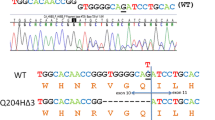
A novel nonsense mutation (c.364G>T [p.E122X]) was identified in the patient with HCP shortly after her first acute attack in 2013; the details of this patient can be found in Table 2. She had increased total porphyrins levels in urine and feces and urinary PBG and ALA. Plasma scan emission peak was at 618 nm. Cutaneous involvement was absent. The p.E122X mutation is located in exon 1 and would lead to an unstable and inactive CPOX protein, which is likely removed by proteolytic degradation. The CPOX activity measurements could not be performed to prove the cosegregation of the novel alteration and low enzymatic activity.
This is the first report to describe mutations in Bulgarian patients with AIP, VP, and HCP. We identified a total of seven novel mutations in these families. Seven latent gene carriers were also detected. The identification of the latent gene carriers can result in the prevention of acute attacks by avoiding the well-known exogenic triggering factors.
Abbreviations
- AHP:
-
Acute hepatic porphyrias
- AIP:
-
Acute intermittent porphyria
- ALA:
-
δ-Aminolevulinic acid
- CPOX:
-
Coproporphyrinogen oxidase
- HCP:
-
Hereditary coproporphyria
- HMBS:
-
Hydroxymethylbilane synthase
- PBG:
-
Porphobilinogen
- PPOX:
-
Protoporphyrinogen oxidase
- VP:
-
Variegate porphyria
References
Adjarov DG, Naydenova EN, Kerimova MA, Pentieva ED, Ivanova LB, Ivanova VA (1994) Influence of protein calorie malnutrition and fasting on the activities of δ-aminolevulinic acid dehydratase and porphobilinogen deaminase in rats. Exp Toxicol Pathol 46:199–202
Andersson C, Lithner F (2001) Diabetic metabolism protective in severe acute intermittent porphyria. Lakartidningen 98(51–52):5874–5876
Barbaro M, Kotajarvi M, Harper P, Floderus Y (2013) Partial protoporphyrinogen oxidase (PPOX) gene deletions, due to different Alu-mediated mechanisms, identified by MLPA analysis in patients with variegate porphyria. Orphanet J Rare Dis 8:13
Deacon AC, Elder GH (2001) Front line tests for the investigation of suspected porphyria. ACP Best Practice No 165. J Clin Pathol 54:500–507
Deybach JC, de Verneuil H, Nordmann Y (1981) The inherited enzymatic defect in porphyria variegata. Hum Genet 58(4):425–428
Floderus Y, Shoolingin-Jordan PM, Harper P (2002) Acute intermittent porphyria in Sweden. Molecular, functional and clinical consequences of some new mutations found in the porphobilinogen deaminase gene. Clin Genet 62(4):288–297
Gill R, Kolstoe S, Mohammed F et al (2009) Structure of human porphobilinogen deaminase at 2.8 A: the molecular basis of acute intermittent porphyria. Biochem J 420(1):17–25
Hift RJ, Davidson BP, van der Hooft C, Meissner DM, Meissner PN (2004) Plasma fluorescence scanning and fecal porphyrin analysis for the diagnosis of variegate poprhyria: precise determination of sensitivity and specificity with detection of protoporphyrinogen oxidase mutations as a reference standard. Clin Chem 50(5):915–923
Hrdinka M, Puy H, Martasek P (2006) Update in porphobilinogen deaminase gene polymorphisms and mutations causing acute intermittent porphyria: comparison with the situation in Slavic population. Physiol Res 55(2):S119–S136
Kappas A, Sassa S, Galbraith RA, Nordmann Y (1995) The porphyrias. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) Metabolic and molecular bases of inherited disease, 7th edn. McGraw-Hill, New York, vol 2, pp 2103–2159
Kauppinen R, Mustajoki S, Pihlaja H, Peltonen L, Mustajoki P (1995) Acute intermittent porphyria in Finland: 19 mutations in the porphobilinogen deaminase gene. Hum Mol Genet 4(2):215–222
Kaupinnen R, von und zu Fraunberg M (2002) Molecular and biochemical studies of acute intermittent porphyria in 196 patients and their families. Clin Chem 48(11):1891–1900
Llewellyn DH, Whatley S, Elder GH (1998) Acute intermittent porphyria caused by an arginine to histidine substitution (R26H) in the cofactor-binding cleft of porphobilinogen deaminase. Hum Mol Genet 2(8):1315–1316
Lockwood WH, Poulos V, Rossi E, Curnow DH (1985) Rapid procedure for fecal porphyrin assay. Clin Chem Jul 31(7):1163–1167
Mauzarell D, Granick S (1956) The occurrence and determination of δ-amino-levulinic acid and porphobilinogen in urine. J Biol Chem 219(1):435–446
Meissner PN, Dailey TA, HIft RJ, Ziman M, Corrigal AV, Roberts AG et al (1996) A R59W mutation in human protoporphyrinogen oxidase results in decreased enzyme activity and is prevalent in South Africans with variegate porphyria. Nat Genet 13(1):95–97
Meyer UA, Strand LJ, Doss M, Rees AC, Marver HS (1972) Intermittent acute porphyria – demonstration of a genetic defect in porphobilinogen metabolism. N Engl J Med 286(24):1277–1282
Mustajoki P (1981) Normal erythrocyte uroporphyrinogen I synthase in a kindred with acute intermittent porphyria. Ann Intern Med 95(2):162–166
Paradisi I, Arias S (2010) Marked geographic aggregation of acute intermittent porphyria families carrying mutation Q180X in Venezuelan populations, with description of further mutations. J Inherit Metab Dis. doi:10.1007/s10545-010-9228-x
Puy H, Deybach JC, Lamoril J et al (1997) Molecular epidemiology and diagnosis of PBG deaminase gene defects in acute intermittent porphyria. Am J Hum Genet 60(6):1373–1383
Qin X, Tan Y, Wang L et al (2011) Structural insight into human variegate porphyria disease. FASEB J 25:653–664
Rimington C (1971) Quantitative determination of porphobilinogen and porphyrins in urine and porphyrins in faeces and erythrocytes. Assoc Clin Pathol Broadsheet 70:1–9
Sassa S (2006) Modern diagnosis and management of the porphyrias. Br J Haematol 135(3):281–292
Thunnel S, Floderus Y, Henrichson A, Harper P (2006) Porphyria in Sweden. Physiol Res 55(Suppl 2):S109–S118
To-Figueras J, Badenas C, Carrera C et al (2006) Genetic and biochemical characterization of 16 acute intermittent porphyria cases with a high prevalence of R173W mutation. J Inherit Metab Dis 29(4):580–585
Whatley SD, Puy H, Morgan R et al (1999) Variegate porphyria in Western Europe: identification of PPOX gene mutations in 104 families, extent of allelic heterogeneity, and absence of correlation between phenotype and type of mutation. Am J Hum Genet 65(4):984–994
Whatley SD, Mason NG, Woolf JR, Newcombe RG, Elder GH, Badminton MN (2009) Diagnostic strategies for autosomal dominant acute porphyrias: retrospective analysis of 467 unrelated patients referred for mutational analysis of the HMBS, CPOX, or PPOX gene. Clin Chem 55(7):1406–1414
Wiman A, Harper P, Floderus Y (2003) Nine novel mutations in the protoporphyrinogen oxidase gene in Swedish families with variegate porphyria. Clin Genet 64(2):122–130
Yang CC, Kuo HC, You HL et al (2008) HMBS mutations in Chinese patients with acute intermittent porphyria. Ann Hum Genet 72(Pt 5):683–686
Acknowledgements
We would like to thank all of the families that participated in this study. We would also like to gratefully acknowledge Prof. Dimcho Adjarov for the fruitful discussions and suggestions, as well as for supplying the clinical and biochemical data used in this study. Mrs. Doroteya Leonkeva, Mrs. Kumi Kato, and Ms. Yoko Tateda are also gratefully acknowledged for their skillful technical assistance. We acknowledge the support from Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Eva Morava, MD PhD
Appendices
Take-Home Message
This is the first report to describe mutations in Bulgarian patients with acute hepatic porphyrias, and a total of seven novel mutations were identified in this study.
References to Electronic Databases
MIM 176000; OMIM 176200; OMIM 121300; EC 4.3.1.8; EC 1.3.3.4; EC 1.3.3.3; HMBS; PPOX; CPOX; HMBS Ensembl Accession number ENST00000442944; PPOX Ensembl Accession number ENST00000367999; CPOX Ensembl Accession number ENST00000264193; www.hgmd.cf.ac.uk
Compliance with Ethics Guidelines
Conflict of Interest
Sonya Dragneva, Monika Szyszka-Niagolov, Aneta Ivanova, Lyudmila Mateva, Rumiko Izumi, Yoko Aoki, and Yoichi Matsubara declare that they have no conflicts of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Details of the Contribution of Individual Authors
Sonya Dragneva: involved in design, analysis, and interpretation of data, drafted the initial version of the manuscript; Monika Niagolov: involved in conception and design and critical revision of the manuscript; Aneta Ivanova: involved in conception, design, and critical revision of the manuscript; Lyudmila Mateva: involved in conception, design, and critical revision the manuscript; Rumiko Izumi: involved in analysis and interpretation of data and critical revision of the manuscript; Yoko Aoki: involved analysis and interpretation of data and critical revision of the manuscript; Yoichi Matsubara (Guarantor): involved in analysis and interpretation of data and critical revision of the manuscript
Rights and permissions
Copyright information
© 2014 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Dragneva, S. et al. (2014). Seven Novel Mutations in Bulgarian Patients with Acute Hepatic Porphyrias (AHP). In: Zschocke, J., Gibson, K., Brown, G., Morava, E., Peters, V. (eds) JIMD Reports Volume 16. JIMD Reports, vol 16. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2014_320
Download citation
DOI: https://doi.org/10.1007/8904_2014_320
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-44586-0
Online ISBN: 978-3-662-44587-7
eBook Packages: MedicineMedicine (R0)