Abstract
The immune system protects us from enormously diverse microbial pathogens but needs to be tightly regulated to avoid deleterious immune-mediated inflammation and tissue damage. A wide range of molecular determinants and cellular components work in concert to control the magnitude and duration of a given immune response. In the past decade, microRNAs (miRNAs), a major class of small non-coding RNA species, have been extensively studied as key molecular players in immune regulation. In this chapter, we will discuss how miRNAs function as negative regulators to restrict innate and adaptive immune responses. Moreover, we will review the current reports regarding miRNAs in human immunological diseases. Finally, we will also address the emerging roles of other non-coding RNAs, long non-coding RNAs (lncRNAs) in particular, in the regulation of the immune system.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Since the first microRNA (miRNA), lin-4, was discovered in Caenorhabditis elegans by the laboratories of Victor Ambros and Gary Ruvkun in 1993 (Lee et al. 1993; Wightman et al. 1993), these small non-coding RNA species have been extensively studied for their roles in post-transcriptional regulation of gene expression. Like other protein encoding genes, primary miRNA transcripts are first transcribed in the nucleus but are sequentially processed by a microprocessor complex, which contains the RNase III Drosha and the double-stranded RNA binding protein DGCR8 in mammals and Pasha in other species, into a characteristic ~60 nucleotide stem-loop structure, named precursor-miRNA (pre-miRNA) (Lee et al. 2003; Han et al. 2006; Denli et al. 2004; Gregory et al. 2004). The pre-miRNAs are then exported via exportin 5 (Lund et al. 2004; Yi et al. 2003) into the cytoplasm, where the hairpin of pre-miRNA is further processed by the evolutionarily conserved RNase III enzyme Dicer to generate mature miRNAs (Bernstein et al. 2001; Hutvagner et al. 2001). These mature miRNAs are capable of being incorporated into the RNA-induced silencing complex (RISC) where they interact with the core component Argonaute protein (Hutvagner and Zamore 2002; Lingel et al. 2003; Liu et al. 2004). Once assembling with mature miRNA and engaging the targets, active RISCs recognize complementary messenger RNA (mRNA) transcripts for degradation or translational silencing (Jing et al. 2005; Song et al. 2004; Liu et al. 2004). By modulating the expression level of their target genes, miRNAs have been found to regulate almost all biological processes including cell growth, differentiation, and apoptosis in both plants and animals (Ameres and Zamore 2013). As is the case for non-immune cells, the role of miRNA-mediated gene regulation in the immune system in recent years has become a focus of intense investigation.
2 miRNAs in the Immune System
Even though our understanding of miRNA biogenesis and its overall cellular function was initially established based on discoveries in nematodes and plants (Lee et al. 1993; Baulcombe 2004), accumulating evidence has demonstrated that miRNAs also have a crucial role in controlling all aspects of immune responses (Mehta and Baltimore 2016). The importance of miRNA in immune cells was first demonstrated in studies where key components in miRNA biogenesis were disrupted. To this end, conditional deletion of Dicer during early B cell development leads to a dramatic block at the pro- to pre-B transition (Koralov et al. 2008). Subsequent studies using mice with Dicer ablated specifically in peripheral activated B cells further demonstrated that miRNAs are also required for germinal center B cell formation and the generation of the antibody diversity (Xu et al. 2012). Interestingly, unlike what has been reported in B cell differentiation, Dicer ablation in early T cell progenitors did not exhibit any substantial alterations in thymic T cell development with the exception of reduced thymic cellularity (Cobb et al. 2005). Nevertheless, the specificity of the role of miRNA in peripheral T cells is much more apparent, as T cells lacking Dicer failed to differentiate into multiple helper T cell lineages and exhibited aberrant effector T cell function (Muljo et al. 2005). Moreover, when Dicer or Drosha ablation was restricted to regulatory T (Treg) cell lineage, mice developed highly aggressive autoimmunity comparable to those devoid of a functional Foxp3 gene (Zhou et al. 2008; Liston et al. 2008; Chong et al. 2008), suggesting an indispensable role of miRNA in controlling Treg cell biology.
To date, more than hundreds of miRNAs have been reported to be differentially expressed in immune cells. Distinct miRNA signatures not only were found in individual immune cell lineages but could also be detected in the same cell subsets that are in different developmental stages. For example, whereas miR-139 is highly expressed in Pro- and Pre-B cells, elevated levels of miR-28, miR-320 and miR-148a are detected in germinal center (GC) B cells and plasma cells, respectively (Kuchen et al. 2010). Moreover, expression of miRNAs in immune cells can also be dynamically regulated in response to a variety of stimuli, such as antigens recognized by T or B cell receptors, proinflammatory cytokines, and microbial components that trigger Toll-like receptors (O’Connell et al. 2007; Taganov et al. 2006; Cobb et al. 2006). To this end, a recent study reported that T cell activation induces proteasome-mediated degradation of Argonaute, and subsequently causes a global down-regulation of mature miRNAs (Bronevetsky et al. 2013). It was suggested that activation-induced miRNA down-regulation confers effector functions to helper T cells via relaxing the repression of genes that direct T cell differentiation. Finally, hierarchical clustering analysis of miRNA profiling clearly separated cells of the immune system from other tissues. Taken together, these results implied certain miRNAs might play a specific role in controlling development and effector functions of the immune system (Kuchen et al. 2010), and that miRNAs need to be tightly regulated as aberrant expression of miRNAs often leads to dysregulated innate and adaptive immunity.
3 miRNAs as Negative Regulators of Immune Responses
3.1 miRNA in Adaptive Immunity
While studies of mice with B or T cell-specific deletion of the entire miRNA pathway seemed to suggest that miRNAs generally play a positive role in promoting adaptive immunity as discussed in the previous section, many miRNAs have been identified as important negative regulators in restricting B and T cell responses (Fig. 1). For example, miR-150, a miRNA that is predominantly expressed in mature B cells was shown to control multiple B cell populations through regulating the expression level of transcription factor c-Myb (Xiao et al. 2007). Genetic ablation of miR-150 resulted in the expansion of B1 cells, one of the subsets of mature B cells, in spleen and peritoneal cavity. While the numbers of follicular B cells were not significantly altered, upon immunization elevated antibody responses could be easily detected (Xiao et al. 2007). Compared to miR-150, miR-210, a miRNA that is highly induced upon B cell activation, appears to play an even larger role in functioning as a negative feedback regulator to restrain B cell responses; deletion of miR-210 leads to the development of age-associated autoantibodies (Mok et al. 2013). In contrast, while miR-142 was shown to target B cell-activating factor receptor (BAFF-R), a molecule that is critical for B cell proliferation and survival, hypogammaglobulinemia phenotype was detected in mice devoid of miR-142 despite having increased follicular B cell numbers (Kramer et al. 2015). Similarly, despite the fact that activation-induced cytidine deaminase (AID), a potent enzyme critical for somatic hypermutation and class-switch recombination (CSR), has been shown to be a bona fide miR-155 target where lack of AID regulation by miR-155 led to defective affinity maturation (Teng et al. 2008; Dorsett et al. 2008), miR-155-deficient mice actually exhibited reduced germinal center function and failed to generate high-affinity IgG1 antibodies (Rodriguez et al. 2007). Together, these results suggested that other miR-142 or miR-155 targets are likely responsible for their respective effects on humoral immunity and further demonstrate the complex nature of miRNA-mediated immune regulation.
Like B cell, activated T cells also express increased level of miR-155; mice devoid of miR-155 displayed increased lung airway remodeling, and miR-155-deficient CD4+ T cell cells are intrinsically biased toward Th2 differentiation in vitro. Mechanistically, it was shown that miR-155 can inhibit Th2 responses through modulating the level of c-Maf, a transcription factor known to promote Th2 immunity (Rodriguez et al. 2007). In addition to miR-155, we and others have recently demonstrated that miR-24 and miR-27, two members of the mi-R23 cluster family, collaboratively limit Th2 responses and associated immune pathology through targeting IL-4, GATA3 as well as other Th2-related genes in both direct and indirect manners (Cho et al. 2016; Pua et al. 2016). While miR-23 does not seem to play any role in Th2 regulation, it is indispensable for restraining activation-induced necrosis of CD4+ T cells by enforcing intracellular reactive oxygen species (ROS) equilibrium through targeting cyclophilin D, a regulator of ROS escape from mitochondria (Zhang et al. 2016). In addition to Th2 regulation, miRNAs have also been implicated in regulating other Th lineages. To this end, miR-29 was shown to control Th1 responses by repressing multiple genes associated with Th1 differentiation and function including both T-bet and Eomes, two transcription factors known to induce IFN-γ production and IFN-γ itself (Ma et al. 2011; Steiner et al. 2011). On the other hand, hypoxia-induced miR-210 was reported to negatively regulate Th17 responses through restricting the expression of HIF-1α, a key transcription factor that promotes Th17 polarization under limited oxygen (Wang et al. 2014). Similarly, Th17 differentiation and the resultant pathogenesis of experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis, could be suppressed by miR-20b via targeting RORγt and STAT3, two key Th17 transcription factors (Zhu et al. 2014). Finally, miR-146a has highly induced in T follicular helper (Tfh) cells, a specialized Th cell subset required for humoral immunity, and can act as a post-transcriptional brake to control Tfh cell and corresponding GC B cell responses by regulating ICOS-ICOSL axis (Pratama et al. 2015).
In addition to its role in Tfh cells, miR-146a has also been shown to function as a key molecular regulator to confer suppressor function to Treg cells. In the absence of miR-146a-mediated regulation of STAT1, mice succumbed to spontaneous IFN-γ-dependent Th1-mediated immunopathology (Lu et al. 2010). On the other hand, albeit dispensable for Treg cell suppressor function, Foxp3-dependent miR-155 ensures Treg cell competitive fitness through targeting SOCS1 (Lu et al. 2009, 2015). Moreover, our recent work also demonstrated that miR-27 controls multiple aspects of Treg cell biology and suggests that excessive expression of miR-27 in Treg cells resulted in a breakdown of Treg cell-mediated immunological tolerance (Cruz et al. 2017). Together with the aforementioned studies in which the entire miRNA pathway was ablated in Treg cells, these results suggested that miRNAs are able to mediate their regulatory effects on the immune system indirectly through maintaining optimal Treg cell function and homeostasis (Zhou et al. 2008; Liston et al. 2008; Chong et al. 2008).
3.2 miRNA in Innate Immunity
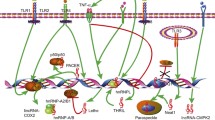
Similar to what was described in the adaptive immune system, significant progress has been made over the past decade in characterizing individual miRNAs that control the function of innate immune cells (Fig. 2). Among them, miRNA-mediated regulation of myeloid cell function is best characterized. Both miR-146 and miR-155 were identified in macrophages in response to LPS activation (Taganov et al. 2006; O’Connell et al. 2007). Between these two miRNA, miR-146a serves as a negative regulator to limit inflammatory responses by targeting TRAF6 and IRAK1 (Taganov et al. 2006; Boldin et al. 2011). During virus infection, miR-146a expression is also upregulated in macrophages in RIG-I-dependent manner, and its function negatively regulates type I interferon (IFN) production through repressing IRAK2 (Hou et al. 2009). While miR-155 is generally considered as a positive player in mediating inflammatory responses as it can directly repress SOCS1 and SHIP1 (Androulidaki et al. 2009; O’Connell et al. 2009), two known inhibitory molecules affecting multiple signaling pathways, miR-155 can also down-modulate TLR/IL-1 inflammatory pathway via targeting TAB2 (Ceppi et al. 2009). Moreover, protein kinase Akt1, which is activated by LPS in macrophages, positively regulates let-7e, a miRNA that can inhibit TLR4 expression to control endotoxin sensitivity and tolerance (Androulidaki et al. 2009). In addition to direct TLR4 targeting, miR-21, another miRNA induced by LPS can also act as a negative feedback regulator of TLR4 signaling by targeting PDCD4, a proinflammatory protein required for LPS-induced death (Sheedy et al. 2010). Finally, miRNAs can also restrict DC function by directly suppressing the production of proinflammatory cytokines. To this end, NOD2-induced miR-29 expression in human dendritic cells (DCs) was shown to inhibit IL-23 expression by repressing IL-12p40 directly and IL-23p19 indirectly via targeting ATF2 (Brain et al. 2013).
Besids myeloid cell subsets, much experimental evidence has also pointed to miRNAs as important negative regulators for other innate immune cells. For example, miR-223 has been shown to function as a cell intrinsic negative modulator of neutrophil activation and killing. Mice lack of miR-223 developed spontaneous inflammatory lung pathology and exhibited exaggerated tissue damage upon LPS treatment (Johnnidis et al. 2008). Moreover, miR-27a*, miR-378, and miR-30e have all been shown to act as negative regulators of NK cell cytotoxicity by silencing perforin and granzyme (GzmB) expression (Kim et al. 2011; Wang et al. 2012). Finally, miRNAs can also regulate immune responses by targeting in non-innate immune cells. To this end, miR-146a expression was shown to be induced by TLR2 stimulation in human keratinocytes. miR-146a can then serve as a potent negative feedback regulator to prevent further TLR2-induced inflammatory responses (Meisgen et al. 2014). Collectively, the aforementioned studies not only provide important insights into miRNA-mediated immune regulation but also offer the molecular basis to understand the precise role of miRNAs in the pathogenesis of human immunological diseases.
4 miRNAs in Human Immunological Diseases
As summarized in the previous sections, studies employing genetically manipulated mice with in vitro experimental approaches and in vivo disease models have helped us to gain valuable knowledge of miRNA function in regulating immune responses. However, it is also important to study miRNA in the context of human immunological diseases. Indeed, numerous studies have shown the correlation between the expression of several miRNAs and immune related disorders, such as autoimmunity, hypersensitive diseases, and hematopoietic malignancies.
4.1 miRNA in Autoimmunity and Hypersensitivity Diseases
Abnormal expression of miRNAs has been associated with many autoimmune diseases (Table 1). One of the best examples is systemic lupus erythematosus (SLE), a multifaceted autoimmune disease with a strong genetic predisposition, characterized by enhanced type I interferon signaling. To this end, it has been reported that peripheral blood mononuclear cells (PBMCs) isolated from SLE patients express reduced miR-146a. The amount of miR-146a was shown to negatively correlate with the clinical disease activity and type I interferon levels in patients. It was suggested that lack of miR-146a-mediated regulation of STAT1 and IRF5 led to the excessive production of type I interferon (Tang et al. 2009). Moreover, sequencing analysis of single-nucleotide polymorphisms (SNPs) in SLE patients identified a genetic variant in the miR-146a promoter region that is functionally significant in downregulating the expression of miR-146a by altering its binding affinity for Ets-1 (Luo et al. 2011). In addition to miR-146a, diminished expressions of both miR-125a and miR-155 were also reported in patients with SLE (Zhao et al. 2010; Lashine et al. 2015). While elevated level of RANTES in the absence of optimal miR-125a-mediated regulation was considered to promote the disease, increased expression of protein phosphatase 2A (PP2A) in juvenile SLE patients with reduced miR-155 was thought to be responsible for enhanced pathogenesis. On the other hand, elevated levels of miR-21 and miR-148a were detected in circulating CD4+ T cells from SLE patients. Both of these two miRNAs are able to upregulate autoimmune-associated methylation-sensitive genes such as CD70 and LFA-1 through promoting DNA hypomethylation and by repressing the expression of RASGRP1 and DNA methyltransferase 1 (DNMT1), respectively (Pan et al. 2010).
Like SLE, multiple sclerosis (MS) has also been linked to the aberrant expression of many miRNAs. For example, the expression level of miR-326 has been shown to be highly correlated with disease severity in MS patients (Du et al. 2009). Mechanistically, miR-326 promotes Th17 cell induction through targeting Ets-1, a known negative regulator of Th17 differentiation. Moreover, increased miR-27, miR-128, as well as miR-340, were detected in CD4+ T cells of patients with MS. It was shown that through repressing BMI1, a molecule that stabilizes GATA3, miR-27b inhibits Th2 differentiation and promotes proinflammatory Th1 autoimmune responses (Guerau-de-Arellano et al. 2011). Following up this study, miR-27 was further shown to dampen TGFβ signaling, leading to impaired Treg cells and enhanced susceptibility to developing multiple sclerosis (Severin et al. 2016). Besides these two autoimmune diseases, abnormal miRNA expression has also been associated with other autoimmune inflammation including rheumatoid arthritis (RA), psoriasis and type I diabetes (Li et al. 2010; Stanczyk et al. 2008; Lu et al. 2014; Sonkoly et al. 2007; Sebastiani et al. 2011).
In addition to autoimmunity, dysregulated miRNAs also contribute to the development or pathogenesis of hypersensitivity diseases like asthma. miRNA profiling analysis of human airway-infiltrating T cells revealed that miR-19a, a member of the miR-17-92 cluster, was greatly upregulated in CD4+ T cells isolated from asthmatic airways compared with cells from healthy subjects. Mechanistically, miR-19a was shown to repress multiple genes including PTEN, SOCS1, and TNFAIP3, leading to specific augmentation of Th2 responses and associated immune pathology (Simpson et al. 2014). Together, more and more studies have revealed a causative role of miRNA in the development of many human immunological diseases.
4.2 miRNA in Blood Cancer
As one of the major functions of miRNAs is to regulate cell differentiation and proliferation, it is not surprising that when dysregulated miRNAs can drive the development of malignancies of the immune system (Table 2). miRNAs can either act as tumor suppressors or function as oncomirs to promote or prevent tumorigenesis. As tumor suppressors, miR-15a and miR-16 were shown to inhibit the development of B cell chronic lymphocytic leukemias (B-CLL) through targeting Bcl-2, and that in more than 50% of B-CLL patients a region encoding miR-15a and miR-16 was found to be deleted (Cimmino et al. 2005; Calin et al. 2002). miR-28 was also identified as a tumor suppressor and is significantly downregulated in Burkitt lymphoma. Oncogene Myc was shown to negatively regulate miR-28 expression leading to the uncontrolled proliferation of certain B cell subsets (Schneider et al. 2014). Another example is miR-29b whose expression is deregulated in primary acute myelogeneous leukemia (AML). Restoration of miR-29b in AML cell lines was able to induce apoptosis and dramatically reduce tumorigenicity, pointing to a clear tumor suppressor role (Garzon et al. 2009).
In contrast to the aforementioned roles of miRNA in preventing tumorigenesis, many miRNAs also exhibit oncogenic activity in hematologic malignancies. For example, the miR-17-92 cluster, which is located in a region of DNA that is frequently amplified in human B cell lymphomas, has been shown to promote malignancy of immune cells (He et al. 2005; Tagawa and Seto 2005; Inomata et al. 2009; Lu et al. 2010). In addition to miR-17-92 cluster, miR-155, another well-characterized oncomir, has also been shown to be expressed at higher levels in many different types of B cell lymphomas including Hodgkin’s lymphoma, DLBCL, Burkitt’s lymphoma as well as NK cell lymphoma (Eis et al. 2005; Kluiver et al. 2005; van den Berg et al. 2003; Metzler et al. 2004; Yamanaka et al. 2009). On the other hand, miR-223 was shown to be upregulated in human T cell acute lymphoblastic leukemia (T-ALL) in a TAL1-dependent manner (Mansour et al. 2013). It is thought that miR-223 promotes T-ALL through repressing a tumor suppressor, FBXW7. Finally, elevated levels of the oncomir, miR-125b, has been reported in a variety of human neoplastic blood disorders and could potentially induce myeloid- and B cell leukemia by inhibiting IRF4 (So et al. 2014). The miRNA signatures identified from these clinical studies not only provide great value as prognostic parameters for cancer progression but might also serve as potential novel therapeutic targets to treat human hematopoietic malignancies.
5 Other Non-coding RNAs
Like miRNAs, many other non-coding RNA (ncRNA) species including long non-coding RNAs (lncRNAs) have also been identified as important gene regulators in the immune system (Fig. 3). Since the first lncRNA H19 was reported in 1990 (Brannan et al. 1990), extensive investigation in lncRNA-mediated gene regulation has demonstrated that lncRNAs can regulate gene expression in various biological processes, including immune responses (Ponting et al. 2009). LncRNAs are transcribed by RNA polymerase II, 5’-capped, polyadenylated, and undergo splicing similar to that for mRNAs (Guttman et al. 2009). They can function both in cis to regulate the gene expression in close genomic proximity at the site of transcription, or in trans to target distant transcriptional activators or repressors (Ponting et al. 2009). Moreover, since lncRNAs usually contain multiple modular domains that can either interact with proteins or form complementary pairs with nucleotides, these molecules could connect DNA, RNA, and proteins and be involved in nearly all stages of gene regulation (Guttman and Rinn 2012).
A Recent report suggests that up to two third of transcribed genes across all cell types in humans are classified as lncRNAs (Iyer et al. 2015). In T cells, a lncRNA, NRON (non-coding RNA, repressor of NFAT) was shown to form a large cytoplasmic RNA-protein scaffold complex that can repress the transcriptional activation of NFAT-responsive genes via regulating NFAT nuclear trafficking (Willingham et al. 2005; Sharma et al. 2011). Moreover, another lncRNA, lncRNA-CD244, whose expression has been shown to be driven by CD244 signaling upon Tuberculosis (TB) infection, is able to inhibit cytokine production by CD8+ T cells by mediating histone H3K27 trimethylation at promoter regions of IFN-γ and TNF-α (Wang et al. 2015). On the other hand, in Th17 cells, rather than inhibiting their effector function, a lncRNA, lncRNA Rmrp was shown to interact with a complex of RORγt and an RNA helicase, DDX5, to activate RORγt-dependent Th17-relative gene transcription (Huang et al. 2015).
As for the role of lncRNAs in the innate immune cells, a TLR signaling induced intergenic lncRNA, lncRNA-Cox2, was reported to repress the expression of many critical inflammatory genes in macrophages through forming a complex with heterogeneous nuclear ribonucleoprotein (hnRNP) A/B and A2/B1 (Carpenter et al. 2013). In addition to lncRNA-Cox2, lncRNA-EPS was also recently identified as a key regulator in controlling macrophage inflammatory responses. Mice with lncRNA-EPS deficiency exhibited enhanced inflammation and lethality upon LPS challenges. Mechanistically, lncRNA-EPS was shown to limit the expression of immune response genes (IRGs) by controlling nucleosome positioning through interacting with hnRNP L (Atianand et al. 2016).
Finally, the link between lncRNA and human autoimmune diseases has also been demonstrated. Genome-wide association studies (GWAS) of celiac disease patients identified five SNPs in a region encoding a lncRNA, lnc13. Biopsies from celiac disease patients appeared to have substantially lower amounts of lnc13 compared with healthy donors. Further studies have shown that lnc13 is primarily expressed in the nucleus of human macrophages from the lamina propria. Like the aforementioned lncRNAs, lnc13 was also shown to repress many inflammatory genes through interaction with a hnRNP, hnRNP D in particular, as well as Hdac1 and chromatin. It was thus suggested that decreased levels of lnc13 in intestinal tissue from patients with celiac disease likely contributes to the observed inflammation in this autoimmune disorder (Castellanos-Rubio et al. 2016). Despite the great efforts made to study lncRNA biology, unlike miRNAs, it remains a major challenge to functionally evaluate a lncRNA as the sequence of the transcript lends no insight into how it may actually work within a given cell type. Nevertheless, it is evident that lncRNAs exhibit important regulatory functions in controlling immune responses as well as other biological processes.
6 Concluding Remarks
Over a decade of intense scrutiny into the role of miRNAs in the immune system, there is little doubt that miRNAs function as crucial gene modulators that would impact almost all facets of immune responses in both physiological and pathological settings. While miRNAs do not completely turn off (or in some cases, turn on) the expression of their targets, they can act by repressing genes involved in positive-feedback regulatory circuits or by regulating a set of genes that are in a shared pathway or protein complex. As such, even relatively small changes in gene expression introduced by miRNAs could cause major biological consequences. From impairment of immune functions to the pathogenesis of a variety of immunological diseases, the fact that dysregulation of individual miRNAs in the immune system has repeatedly been demonstrated to have profound physiological effects further supports this notion. Moreover, beyond gaining further molecular insights into miRNA-mediated gene regulation in immunological research, recent advances in modulating miRNA function by miRNA mimics or antisense oligonucleotides have shown promise in miRNA-targeted therapeutics. With increasing knowledge of miRNA biology and the development of novel approaches for efficient delivery of miRNA modifying agents to specific immune cell subsets, we are confident that manipulating miRNA pathways will soon become a viable option to treat a wide array of human immunological diseases.
References
Ameres SL, Zamore PD (2013) Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol 14:475–488
Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C (2009) The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 31:220–231
Atianand MK, Hu W, Satpathy AT, Shen Y, Ricci EP, Alvarez-Dominguez JR, Bhatta A, Schattgen SA, McGowan JD, Blin J, Braun JE, Gandhi P, Moore MJ, Chang HY, Lodish HF, Caffrey DR, Fitzgerald KA (2016) A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation. Cell 165:1672–1685
Baulcombe D (2004) RNA silencing in plants. Nature 431:356–363
Bernstein E, Caudy AA, Hammond SM, Hannon GJ (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363–366
Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D (2011) miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med 208:1189–1201
Brain O, Owens BM, Pichulik T, Allan P, Khatamzas E, Leslie A, Steevels T, Sharma S, Mayer A, Catuneanu AM, Morton V, Sun MY, Jewell D, Coccia M, Harrison O, Maloy K, Schonefeldt S, Bornschein S, Liston A, Simmons A (2013) The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity 39:521–536
Brannan CI, Dees EC, Ingram RS, Tilghman SM (1990) The product of the H19 gene may function as an RNA. Mol Cell Biol 10:28–36
Bronevetsky Y, Villarino AV, Eisley CJ, Barbeau R, Barczak AJ, Heinz GA, Kremmer E, Heissmeyer V, McManus MT, Erle DJ, Rao A, Ansel KM (2013) T cell activation induces proteasomal degradation of argonaute and rapid remodeling of the microRNA repertoire. J Exp Med 210:417–432
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM (2002) Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 99:15524–15529
Carpenter S, Aiello D, Atianand MK, Ricci EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB, O’Neill LA, Moore MJ, Caffrey DR, Fitzgerald KA (2013) A long noncoding RNA mediates both activation and repression of immune response genes. Science 341:789–792
Castellanos-Rubio A, Fernandez-Jimenez N, Kratchmarov R, Luo X, Bhagat G, Green PH, Schneider R, Kiledjian M, Bilbao JR, Ghosh S (2016) A long noncoding RNA associated with susceptibility to celiac disease. Science 352:91–95
Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P (2009) MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci USA 106:2735–2740
Cho S, Wu CJ, Yasuda T, Cruz LO, Khan AA, Lin LL, Nguyen DT, Miller M, Lee HM, Kuo ML, Broide DH, Rajewsky K, Rudensky AY, Lu LF (2016) miR-23 approximately 27 approximately 24 clusters control effector T cell differentiation and function. J Exp Med 213:235–249
Chong MM, Rasmussen JP, Rudensky AY, Littman DR (2008) The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J Exp Med 205:2005–2017
Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM (2005) miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 102:13944–13949
Cobb BS, Nesterova TB, Thompson E, Hertweck A, O’Connor E, Godwin J, Wilson CB, Brockdorff N, Fisher AG, Smale ST, Merkenschlager M (2005) T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med 201:1367–1373
Cobb BS, Hertweck A, Smith J, O’Connor E, Graf D, Cook T, Smale ST, Sakaguchi S, Livesey FJ, Fisher AG, Merkenschlager M (2006) A role for Dicer in immune regulation. J Exp Med 203:2519–2527
Cruz LO, Hashemifar SS, Wu CJ, Cho S, Nguyen DT, Lin LL, Khan AA, Lu LF (2017) Excessive expression of miR-27 impairs Treg-mediated immunological tolerance. J Clin Invest 127:530–542
Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ (2004) Processing of primary microRNAs by the microprocessor complex. Nature 432:231–235
Dorsett Y, McBride KM, Jankovic M, Gazumyan A, Thai TH, Robbiani DF, di Virgilio M, B RS-M, Heidkamp G, Schwickert TA, Eisenreich T, Rajewsky K, Nussenzweig MC (2008) MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity 28:630–638
Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G (2009) MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol 10:1252–1259
Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, Lund E, Dahlberg JE (2005) Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci USA 102:3627–3632
Garzon R, Heaphy CE, Havelange V, Fabbri M, Volinia S, Tsao T, Zanesi N, Kornblau SM, Marcucci G, Calin GA, Andreeff M, Croce CM (2009) MicroRNA 29b functions in acute myeloid leukemia. Blood 114:5331–5341
Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R (2004) The microprocessor complex mediates the genesis of microRNAs. Nature 432:235–240
Guerau-De-arellano M, Smith KM, Godlewski J, Liu Y, Winger R, Lawler SE, Whitacre CC, Racke MK, Lovett-Racke AE (2011) Micro-RNA dysregulation in multiple sclerosis favours pro-inflammatory T-cell-mediated autoimmunity. Brain 134:3578–3589
Guttman M, Rinn JL (2012) Modular regulatory principles of large non-coding RNAs. Nature 482:339–346
Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES (2009) Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458:223–227
Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, Sohn SY, Cho Y, Zhang BT, Kim VN (2006) Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 125:887–901
He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM (2005) A microRNA polycistron as a potential human oncogene. Nature 435:828–833
Hou J, Wang P, Lin L, Liu X, Ma F, An H, Wang Z, Cao X (2009) MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol 183:2150–2158
Huang W, Thomas B, Flynn RA, Gavzy SJ, Wu L, Kim SV, Hall JA, Miraldi ER, Ng CP, Rigo F, Meadows S, Montoya NR, Herrera NG, Domingos AI, Rastinejad F, Myers RM, Fuller-Pace FV, Bonneau R, Chang HY, Acuto O, Littman DR (2015) DDX5 and its associated lncRNA Rmrp modulate TH17 cell effector functions. Nature 528:517–522
Hutvagner G, Zamore PD (2002) A microRNA in a multiple-turnover RNAi enzyme complex. Science 297:2056–2060
Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD (2001) A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293:834–838
Inomata M, Tagawa H, Guo YM, Kameoka Y, Takahashi N, Sawada K (2009) MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood 113:396–402
Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, Poliakov A, Cao X, Dhanasekaran SM, Wu YM, Robinson DR, Beer DG, Feng FY, Iyer HK, Chinnaiyan AM (2015) The landscape of long noncoding RNAs in the human transcriptome. Nat Genet 47:199–208
Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, di Padova F, Lin SC, Gram H, Han J (2005) Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120:623–634
Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD (2008) Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451:1125–1129
Kim TD, Lee SU, Yun S, Sun HN, Lee SH, Kim JW, Kim HM, Park SK, Lee CW, Yoon SR, Greenberg PD, Choi I (2011) Human microRNA-27a* targets Prf1 and GzmB expression to regulate NK-cell cytotoxicity. Blood 118:5476–5486
Kluiver J, Poppema S, de Jong D, Blokzijl T, Harms G, Jacobs S, Kroesen BJ, van den Berg A (2005) BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J Pathol 207:243–249
Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N, Rajewsky K (2008) Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell 132:860–874
Kramer NJ, Wang WL, Reyes EY, Kumar B, Chen CC, Ramakrishna C, Cantin EM, Vonderfecht SL, Taganov KD, Chau N, Boldin MP (2015) Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood 125:3720–3730
Kuchen S, Resch W, Yamane A, Kuo N, Li Z, Chakraborty T, Wei L, Laurence A, Yasuda T, Peng S, Hu-Li J, Lu K, Dubois W, Kitamura Y, Charles N, Sun HW, Muljo S, Schwartzberg PL, Paul WE, O’Shea J, Rajewsky K, Casellas R (2010) Regulation of microRNA expression and abundance during lymphopoiesis. Immunity 32:828–839
Lashine YA, Salah S, Aboelenein HR, Abdelaziz AI (2015) Correcting the expression of miRNA-155 represses PP2Ac and enhances the release of IL-2 in PBMCs of juvenile SLE patients. Lupus 24:240–247
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425:415–419
Li J, Wan Y, Guo Q, Zou L, Zhang J, Fang Y, Zhang J, Zhang J, Fu X, Liu H, Lu L, Wu Y (2010) Altered microRNA expression profile with miR-146a upregulation in CD4 + T cells from patients with rheumatoid arthritis. Arthritis Res Ther 12:R81
Lingel A, Simon B, Izaurralde E, Sattler M (2003) Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature 426:465–469
Liston A, Lu LF, O’Carroll D, Tarakhovsky A, Rudensky AY (2008) Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med 205:1993–2004
Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ (2004) Argonaute2 is the catalytic engine of mammalian RNAi. Science 305:1437–1441
Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, Yoshimura A, Baltimore D, Rudensky AY (2010) Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell 142:914–929
Lu MC, Yu CL, Chen HC, Yu HC, Huang HB, Lai NS (2014) Increased miR-223 expression in T cells from patients with rheumatoid arthritis leads to decreased insulin-like growth factor-1-mediated interleukin-10 production. Clin Exp Immunol 177:641–651
Lu LF, Thai TH, Calodo DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB., Lee H, Yoshimura A, Rajewsky K, Rudensky AY (2009) Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immun 30:80–91
Lu LF, Gasteiger G, Yu IS, Chaudhry A, Hsin JP, Lu Y, Bos PD, Lin LL, Zawislak CL, Cho S, Sun JC, Leslie CS, Lin SW, Rudensky AY (2015) A single miRNA-mRNA interaction affects the immune response in a context- and cell-type-specific manner. Immun. 43:52–64
Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U (2004) Nuclear export of microRNA precursors. Science 303:95–98
Luo X, Yang W, Ye DQ, Cui H, Zhang Y, Hirankarn N, Qian X, Tang Y, Lau YL, de Vries N, Tak PP, Tsao BP, Shen N (2011) A functional variant in microRNA-146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLoS Genet 7:e1002128
Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, Hua M, Li N, Yao H, Cao X (2011) The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol 12:861–869
Mansour MR, Sanda T, Lawton LN, Li X, Kreslavsky T, Novina CD, Brand M, Gutierrez A, Kelliher MA, Jamieson CH, von Boehmer H, Young RA, Look AT (2013) The TAL1 complex targets the FBXW7 tumor suppressor by activating miR-223 in human T cell acute lymphoblastic leukemia. J Exp Med 210:1545–1557
Mehta A, Baltimore D (2016) MicroRNAs as regulatory elements in immune system logic. Nat Rev Immunol 16:279–294
Meisgen F, Xu Landen N, Wang A, Rethi B, Bouez C, Zuccolo M, Gueniche A, Stahle M, Sonkoly E, Breton L, Pivarcsi A (2014) MiR-146a negatively regulates TLR2-induced inflammatory responses in keratinocytes. J Invest Dermatol 134:1931–1940
Metzler M, Wilda M, Busch K, Viehmann S, Borkhardt A (2004) High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer 39:167–169
Mok Y, Schwierzeck V, Thomas DC, Vigorito E, Rayner TF, Jarvis LB, Prosser HM, Bradley A, Withers DR, Martensson IL, Corcoran LM, Blenkiron C, Miska EA, Lyons PA, Smith KG (2013) MiR-210 is induced by Oct-2, regulates B cells, and inhibits autoantibody production. J Immunol 191:3037–3048
Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K (2005) Aberrant T cell differentiation in the absence of Dicer. J Exp Med 202:261–269
O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D (2007) MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA 104:1604–1609
O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D (2009) Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA 106:7113–7118
Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, Li J, Zhou H, Tang Y, Shen N (2010) MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol 184:6773–6781
Ponting CP, Oliver PL, Reik W (2009) Evolution and functions of long noncoding RNAs. Cell 136:629–641
Pratama A, Srivastava M, Williams NJ, Papa I, Lee SK, Dinh XT, Hutloff A, Jordan MA, Zhao JL, Casellas R, Athanasopoulos V, Vinuesa CG (2015) MicroRNA-146a regulates ICOS-ICOSL signalling to limit accumulation of T follicular helper cells and germinal centres. Nat Commun 6:6436
Pua HH, Steiner DF, Patel S, Gonzalez JR, Ortiz-Carpena JF, Kageyama R, Chiou NT, Gallman A, de Kouchkovsky D, Jeker LT, McManus MT, Erle DJ, Ansel KM (2016) MicroRNAs 24 and 27 suppress allergic Inflammation and target a network of regulators of T Helper 2 cell-associated cytokine production. Immunity 44:821–832
Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A (2007) Requirement of bic/microRNA-155 for normal immune function. Science 316:608–611
Schneider C, Setty M, Holmes AB, Maute RL, Leslie CS, Mussolin L, Rosolen A, Dalla-Favera R, Basso K (2014) MicroRNA 28 controls cell proliferation and is down-regulated in B-cell lymphomas. Proc Natl Acad Sci USA 111:8185–8190
Sebastiani G, Grieco FA, Spagnuolo I, Galleri L, Cataldo D, Dotta F (2011) Increased expression of microRNA miR-326 in type 1 diabetic patients with ongoing islet autoimmunity. Diabetes Metab Res Rev 27:862–866
Severin ME, Lee PW, Liu Y, Selhorst AJ, Gormley MG, Pei W, Yang Y, Guerau-De-arellano M, Racke MK, Lovett-Racke AE (2016) MicroRNAs targeting TGFbeta signalling underlie the regulatory T cell defect in multiple sclerosis. Brain 139:1747–1761
Sharma S, Findlay GM, Bandukwala HS, Oberdoerffer S, Baust B, Li Z, Schmidt V, Hogan PG, Sacks DB, Rao A (2011) Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc Natl Acad Sci USA 108:11381–11386
Sheedy FJ, Palsson-Mcdermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y, O’Neill LA (2010) Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 11:141–147
Simpson LJ, Patel S, Bhakta NR, Choy DF, Brightbill HD, Ren X, Wang Y, Pua HH, Baumjohann D, Montoya MM, Panduro M, Remedios KA, Huang X, Fahy JV, Arron JR, Woodruff PG, Ansel KM (2014) A microRNA upregulated in asthma airway T cells promotes TH2 cytokine production. Nat Immunol 15:1162–1170
So AY, Sookram R, Chaudhuri AA, Minisandram A, Cheng D, Xie C, Lim EL, Flores YG, Jiang S, Kim JT, Keown C, Ramakrishnan P, Baltimore D (2014) Dual mechanisms by which miR-125b represses IRF4 to induce myeloid and B-cell leukemias. Blood 124:1502–1512
Song JJ, Smith SK, Hannon GJ, Joshua-Tor L (2004) Crystal structure of Argonaute and its implications for RISC slicer activity. Science 305:1434–1437
Sonkoly E, Wei T, Janson PC, Saaf A, Lundeberg L, Tengvall-Linder M, Norstedt G, Alenius H, Homey B, Scheynius A, Stahle M, Pivarcsi A (2007) MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS ONE 2:e610
Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S, Kyburz D (2008) Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 58:1001–1009
Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CD, Matloubian M, Blelloch R, Ansel KM (2011) MicroRNA-29 regulates T-box transcription factors and interferon-gamma production in helper T cells. Immunity 35:169–181
Taganov KD, Boldin MP, Chang KJ, Baltimore D (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103:12481–12486
Tagawa H, Seto M (2005) A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia 19:2013–2016
Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, Huang X, Zhou H, de Vries N, Tak PP, Chen S, Shen N (2009) MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum 60:1065–1075
Teng G, Hakimpour P, Landgraf P, Rice A, Tuschl T, Casellas R, Papavasiliou FN (2008) MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity 28:621–629
van den Berg A, Kroesen BJ, Kooistra K, de Jong D, Briggs J, Blokzijl T, Jacobs S, Kluiver J, Diepstra A, Maggio E, Poppema S (2003) High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosomes Cancer 37:20–28
Wang P, Gu Y, Zhang Q, Han Y, Hou J, Lin L, Wu C, Bao Y, Su X, Jiang M, Wang Q, Li N, Cao X (2012) Identification of resting and type I IFN-activated human NK cell miRNomes reveals microRNA-378 and microRNA-30e as negative regulators of NK cell cytotoxicity. J Immunol 189:211–221
Wang H, Flach H, Onizawa M, Wei L, McManus MT, Weiss A (2014) Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol 15:393–401
Wang Y, Zhong H, Xie X, Chen CY, Huang D, Shen L, Zhang H, Chen ZW, Zeng G (2015) Long noncoding RNA derived from CD244 signaling epigenetically controls CD8+ T-cell immune responses in tuberculosis infection. Proc Natl Acad Sci USA 112:E3883–E3892
Wightman B, Ha I, Ruvkun G (1993) Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75:855–862
Willingham AT, Orth AP, Batalov S, Peters EC, Wen BG, Aza-Blanc P, Hogenesch JB, Schultz PG (2005) A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science 309:1570–1573
Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K (2007) MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell 131:146–159
Xu S, Guo K, Zeng Q, Huo J, Lam KP (2012) The RNase III enzyme Dicer is essential for germinal center B-cell formation. Blood 119:767–776
Yamanaka Y, Tagawa H, Takahashi N, Watanabe A, Guo YM, Iwamoto K, Yamashita J, Saitoh H, Kameoka Y, Shimizu N, Ichinohasama R, Sawada K (2009) Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood 114:3265–3275
Yi R, Qin Y, Macara IG, Cullen BR (2003) Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev 17:3011–3016
Zhang B, Liu SQ, Li C, Lykken E, Jiang S, Wong E, Gong Z, Tao Z, Zhu B, Wan Y, Li QJ (2016) MicroRNA-23a curbs necrosis during early T cell activation by enforcing intracellular reactive oxygen species equilibrium. Immunity 44:568–581
Zhao X, Tang Y, Qu B, Cui H, Wang S, Wang L, Luo X, Huang X, Li J, Chen S, Shen N (2010) MicroRNA-125a contributes to elevated inflammatory chemokine RANTES levels via targeting KLF13 in systemic lupus erythematosus. Arthritis Rheum 62:3425–3435
Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA (2008) Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med 205:1983–1991
Zhu E, Wang X, Zheng B, Wang Q, Hao J, Chen S, Zhao Q, Zhao L, Wu Z, Yin Z (2014) miR-20b suppresses Th17 differentiation and the pathogenesis of experimental autoimmune encephalomyelitis by targeting RORgammat and STAT3. J Immunol 192:5599–5609
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Wu, CJ., Lu, LF. (2017). MicroRNA in Immune Regulation. In: Yoshimura, A. (eds) Emerging Concepts Targeting Immune Checkpoints in Cancer and Autoimmunity. Current Topics in Microbiology and Immunology, vol 410. Springer, Cham. https://doi.org/10.1007/82_2017_65
Download citation
DOI: https://doi.org/10.1007/82_2017_65
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68928-9
Online ISBN: 978-3-319-68929-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)