Abstract
Although genetic transfer between viruses and vertebrate hosts occurs less frequently than gene flow between bacteriophages and prokaryotes, it is extensive and has affected the evolution of both parties. With retroviruses, the integration of proviral DNA into chromosomal DNA can result in the activation of adjacent host gene expression and in the transduction of host transcripts into retroviral genomes as oncogenes. Yet in contrast to lysogenic phage, there is little evidence that viral oncogenes persist in a chain of natural transmission or that retroviral transduction is a significant driver of the horizontal spread of host genes. Conversely, integration of proviruses into the host germ line has generated endogenous retroviral genomes (ERV) in all vertebrate genomes sequenced to date. Some of these genomes retain potential infectivity and upon reactivation may transmit to other host species. During mammalian evolution, sequences of retroviral origin have been repurposed to serve host functions, such as the viral envelope glycoproteins crucial to the development of the placenta. Beyond retroviruses, DNA viruses with complex genomes have acquired numerous genes of host origin which influence replication, pathogenesis and immune evasion, while host species have accumulated germline sequences of both DNA and RNA viruses. A codicil is added on lateral transmission of cancer cells between hosts and on migration of host mitochondria into cancer cells.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 A Year of Virological Anniversaries
In addition to Peter Vogt’s 50 years on the Editorial Board of this journal, 2017 is a year to acknowledge several remarkable anniversaries in virology and mobile genetic elements. Exactly 300 years ago, the Western world was introduced to immunization against smallpox by Lady Mary Wortley Montagu and 40 years ago, total eradication of this scourge was achieved (Weiss and Esparza 2015). One hundred years ago, Félix d’Hérelle isolated and propagated bacteriophage (d’Herelle 1917). Fifty years ago, Lynn Margulis (Sagan 1967) postulated that eukaryotic cells evolved by inclusion into the host cell of a number of previously independent microbes that became self-replicating endosymbionts which I shall discuss at the end of this article. This year also marks a personal 50th anniversary since I published my first virological paper (Weiss 1967) with a finding that was simultaneously observed by Peter Vogt (1967), and which led to the discovery of endogenous retroviruses. Three years later, I joined Peter Vogt’s laboratory for 21 months which I regard as the most formative period in my career. Thus it is a great privilege for me to contribute to this volume honoring Peter’s immense contributions.
1.1 Bacteriophage and Prokaryote Transduction
Bacteriophage lysis was first observed in 1915 by the British microbiologist (Twort 1915) as a filterable anti-bacterial factor. Twort’s findings were not conclusive and the Nobel laureate, Jules Bordet, considered that the lytic effect was due to an enzyme rather than a transmissible agent (Bordet and Ciuca 1922; Summers 1999). However, while investigating dysentery among the allied troops, the French-Canadian microbiologist at Institut Pasteur, Félix d’Hérelle, observed lysis of Shigella dysenteriae by a replicating filterable agent (d’Herelle 1917). Whether d’Hérelle was aware of Twort’s report is not known but he did not cite it. D’Hérelle was the first to establish multi-passage phage cultures and to quantitatively measure the extraordinary high titers of infection. He coined the terms bacteriophage for the agent and plaque for the clear areas of lysis in the bacterial films on the sloping agar plates of his cultures. He was convinced that bacteriophage could be used to cure sepsis and exaggerated his success in so doing (Summers 1999). Owing to the difficulties other investigators had in attempts to treat infection (not least because the host bacteria tended to become resistant), the use of bacteriophage to treat infections fell out of fashion in the West, although it continued to be promoted in the Soviet Union. Today, phage therapy is experiencing a renaissance (Cisek et al. 2017).
D’Hérelle’s discovery 100 years ago eventually opened the field of molecular genetics following World War II, led by Max Delbrück and called the Phage Group (Cairns et al. 2007; Summers 1999). Lysogeny was not a particular focus of the Phage Group but was later investigated at the Institute Pasteur led by André Lwoff, whose discovery of transduction of host genes mediated by lysogenic phage (Lwoff 1953) not only became a useful tool in molecular genetics and gene regulation (Ptashne 2004), but together with plasmid transfer was also revealed to be a major means of exchange of host sequences among prokaryotes (Lane 2015). In the 40 years since the discovery of the Archaea (Woese and Fox 1977), the monophyletic origin of life forms on this planet have been confirmed for the evolutionary roots of prokaryotes, but the enormous extent of horizontal exchange confounds attempts to draw accurate phylogenetic trees with linear pedigrees within the major realms of Archea and Bacteria (Choi and Kim 2007; Martin 2011; Lane 2015).
Phage studies continue to be of great relevance to modern molecular biology. They serve as models for evolutionary theory and real-time experiments (Stern and Sorek 2011; Refardt et al. 2013). The discovery of DNA restriction enzymes came out of host range restriction of phage (Loenen et al. 2014), and the CRSPR-Cas9 mechanism of gene editing also has its origins in the natural control of invading phage by bacterial cells (Hartmann 2017).
1.2 The Debt of Tumor Virus Research to Phage Genetics
The linear pedigree of tumor virus research is much clearer than that of prokaryote evolution referred to above! At Caltech in the 1950s, Renato Dulbecco adapted the bacterial lawn culture practices of the Phage Group to the recently established technique of animal cell monolayers. He developed plaque assays for lytic viruses (Dulbecco 1952) and transformation assays for DNA tumor viruses (Vogt and Dulbecco 1960). His associate, Harry Rubin investigated Rous sarcoma virus (RSV) research and with his student, Howard Temin, developed a quantitative transformation assay for RSV (Temin and Rubin 1958). After Rubin moved to Berkeley, Peter Vogt joined his laboratory and detected a non-transforming, replication-competent Rous associated virus (RAV) in stocks of RSV (Rubin and Vogt 1962).
Temin (1960) observed variants of Rous transformed cells which had an elongated, fusiform morphology which bred true according to the variant of RSV used to infect them (Fig. 1) and were later shown to carry mutations within the C-terminus of the src gene (Rohrschneider and Reynolds 1985). This finding led Temin (1962) further noted a distinction between cell transformation and viral replication. He proposed that there was genetic information carried by the virus which persisted in the host cells and affected their phenotype, which he compared to lysogenic prophage (Sankaran 2014). In the same year, Jan Svoboda (who sadly died earlier this year) observed that rats non-productively inoculated at birth with RSV still contained the virus in adult tumors which lent support to the persistence of a latent genome (Svoboda 1960). One year later, however, Crawford and Crawford (1961) demonstrated that the genome of RSV was composed of RNA, not DNA. Temin went on to postulate his provirus hypothesis, proposing that RSV forms a DNA copy of the genome in the infected cell which integrates into the host genome (Temin 1964). Although indirect evidence supported the provirus hypothesis, it remained speculative until the discovery of reverse transcriptase by Temin and Mizutani (1970) and Baltimore (1970).
1.3 Fifty-Five Years of Peter Vogt’s Contributions to Retroviruses and Oncogenes
Although Peyton Rous discovered his eponymous virus over 100 years ago (Rous 1911) and the transforming function could be titrated on the chorioallantoic membrane of chick eggs (Keogh 1938), it was not until the quantitative virus assay in vitro was established by Temin and Rubin (1958) that RNA tumor research really blossomed (Weiss and Vogt 2011). Peter’s first paper on RSV showed that the Bryan high titer strain of RSV virus used at that time in the USA actually comprised two components, a virus that transformed chick embryo fibroblasts and also an avian leukosis virus (Rubin and Vogt 1962). This finding led directly to the discovery that this strain of RSV was defective for replication, missing the gene env encoding the envelope glycoproteins (Hanafusa et al. 1963). These glycoproteins were provided by the accompanying leukosis virus or ‘helper’ virus (Hanafusa et al. 1963) which determined the specificity of antibody neutralization (Ishizaki and Vogt 1966) and of host range through the use of different cell surface receptors (Vogt and Ishizaki 1965). However, strains of RSV used in European laboratories were non-defective, possessing the src gene in addition to replication genes. The B77, Carr-Zilber, Prague, and Schmidt-Ruppin strains were derived from RSV that had been used to induce non-productive tumors in rats and rescued by inoculation into chickens or co-cultivation with permissive chicken cells (Svoboda 1966). The genome structures (Vogt and Hu 1977) were later depicted as:
- Replication-competent leukosis virus::
-
LTR-gag-pol-env-LTR
- Replication-defective RSV::
-
LTR-gag-pol-src-LTR
- Replication-competent RSV::
-
LTR-gag-pol-env-src-LTR
From these seminal studies, the distinction between replication genes and oncogenes came to light (Toyoshima and Vogt 1969; Duesberg and Vogt 1970; Martin 1970) culminating in the demonstration of the host origin of oncogenes (Stehelin et al. 1976), and the identification of the Src protein (Brugge and Erikson 1977) as the first example of a tyrosine kinase (Hunter and Sefton 1980). Other retroviral oncogenes similarly have a cellular origin. This fascinating story has been reviewed by Steven Martin (2004), Peter Vogt (2012), Klaus Bister (2015), and the recent memoir on tumor viruses and cancer cell biology by Harold Varmus (2017).
In the same year that Peter Vogt was appointed to the Editorial Board of CTMI, I published my first virological paper on the release of infectious RSV particles possessing novel envelope properties in the apparent absence of a helper virus (Weiss 1967). To my surprise but gratification, a similar paper was published by Peter a month later Vogt (1967). I was still a doctoral student of Michael Abercombie who discovered contact inhibition but was not a virologist, and my mentor in Abercrombie’s laboratory, Warren Levinson, had returned to San Francisco. I felt rather nervous that my findings seemed to contradict what was known about the defectiveness of RSV and Peter’s paper helped me to gain confidence in my own findings. Moreover, Peter’s was the more elegant study because he showed that the virus which he called RSV(0) more readily infected Japanese quail cells rather than chick cells. It represented the first demonstration of what later became known for murine leukemia viruses as xenotropism (Levy 1978). Our 1967 papers provided the first step in evidence that led to the elucidation of endogenous retroviruses, a story I have told elsewhere (Weiss 2006).
2 Acquisition of Host Genes by Viruses
2.1 Oncogenesis by Simple Retroviruses and Transduction of Oncogenes
Leukemogenic retroviruses lacking oncogenes integrate at near-random sites mainly in ‘open’ regions of host chromosomal DNA. Among the millions of cells infected, some proviruses integrate next to host proto-oncogenes and activate their expression through promoter and enhancer sequences in the retroviral long terminal repeat. The first to be defined was c-myc in avian lymphoid leukosis (Hayward et al. 1981). The transduction of cellular oncogenes begins in this way (Vogt 2012; Varmus 2017).
With avian lymphoid leukosis, activation of c-myc appears to be sufficient for leukemogenesis of B cells in the bursa of Fabricius. However, with gamma-retroviruses such as murine and feline leukemia viruses, the emergence of transformed leukemic cells requires more complex recombination events between infectiously transmitted retroviruses and related endogenous genomes before the recombinant retrovirus activates cellular oncogenes (Rosenberg and Jolicoer 1997). For example, in cats, feline leukaemia virus subtype A (FeLV-A) is the major transmissible agent, but in most cats that develops leukemia, recombination with an endogenous retroviral genome encoding a subtype B env must occur (Roy-Burman 1995). Multiple interactions occur between exogenous and endogenous LTRs and env regions giving rise to variants with different pathogenic attributes (Neil et al. 1991; Bolin and Levy 2011; Stewart et al. 2011).
The various types of retroviral oncogenes show no structural similarities and they act at many sites in cell signaling and regulation, including the transcriptional regulators myc and jun studied by Peter Vogt (Stefan and Bister 2017). Thus the transduction of oncogenes by retroviruses does not depend on sequence homology but rather on read-through transcription and packaging of RNA transcripts into virus particles, followed by reverse transcription and integration into the next infected cell via normal replicative events (Vogt 2012). Apart from the European strains of RSV passed through mammals referred to above, oncogene-bearing retroviruses appear to be replication-defective and have been rescued through aberrant splicing. All that is required in the transcript is for the virion packaging signal the 5′ end of gag to be contiguous with the host sequence.
There appears to be little selective advantage to the virus to carry oncogenes, other than that tumor cells tend to be more permissive to retrovirus replication. In fact, there is scant evidence that onc-bearing viruses are naturally transmitted from host to host unless they come to the attention of pathologists and virologists who deliberately propagate them. The exception is the cyclin oncogene of replication-competent epsilon-retroviruses in fish, but it is probably not of host origin (Rovnak and Quackenbush 2010). Oncogene transduction appears to be restricted to the ‘simple’ retroviruses, alpha- and gamma-retroviruses. It remains a puzzle why cellular gene incorporation into and beta-, delta-, spuma- and lenti-viruses has not been found to date.
Host oncogenes have been transduced by retroviruses in many species including primates (Thielen et al. 1971). Outbred animals such as cats and chickens as well as inbred strains of mice give rise to onc-bearing viruses. Towards the end of my period in Peter Vogt’s laboratory, I spent two weeks at a chicken abattoir harvesting solid tumors that the veterinary inspectors identified along the processing line. Approximately one tumor per 400 chickens was found, a high number considering that they were broiler fowl less than six months old. My time with Peter came to an end before I was able to establish what proportion of the tumor-bearing chickens were infected with avian leukosis and whether the tumors had acquired transduced oncogenes. Indeed, there is little quantitative evidence to this day on oncogene transduction in any animal species although Miles and Robinson (1985) detected frequent transduction of the c-erbB oncogene in chicks with erythroblastosis following experimental inoculation of RAV-1. Further novel oncogenes came to light in avian sarcomas, not least Peter’s discovery of jun (Cavalieri et al. 1985; Maki et al. 1987), a key player in transcriptional regulation (Vogt 2002). Overall, the study of retroviral oncogenes had an immense impact because so many oncogenes found in human cancer were first identified in animal retroviruses (Varmus 2017).
2.2 Why Is Retroviral Transduction not a Major Driver of Virus or Host Evolution?
It is clear that retroviruses are equipped both to transduce host genes and to infect the germ lines of the same and foreign species. It is therefore germane to ask why retroviruses have not become generalized transducing agents by horizontal transfer to new hosts. Since alpha- and gamma-retroviruses readily acquire diverse oncogenes, they must surely pick up other gene transcripts even though they will not be detected through the clonal expansion of tumor cells. If lysogenic bacteriophages are a driving force in prokaryotic evolution, why don’t retroviruses serve a similar function in vertebrate evolution?
The first consideration is that almost all oncogene-bearing retroviruses of birds and mammals are replication-defective and therefore need helper viruses to continue successive rounds of replication. However, the defective retroviruses should be able to propagate in vivo and spread to new hosts if the concentration of helper virus is sufficient. After all, infection by hepatitis delta virus and adeno-associated viruses rely on helper viruses, and plant partitiviruses with split genomes packaged in different particles are successful pathogens. Even if defective onc-bearing retroviruses do not spread infectiously through a host population, it would need only one round of integration into a germ cell for hereditary transduction to be accomplished. Moreover, if there were strong selective forces benefitting the host for transduction, replication-competent transducing viruses would probably emerge, like replication-competent strains of RSV.
A second point is that if transduction occurred only within the same host species, there would be little selective pressure for horizontal transmission because it would not present an advantageous alternative genetic exchange to sexual reproduction. But the widespread phenomenon of xenotropism and infection of unrelated germ-lines discussed in Sect. 3.3 below shows that retroviruses could potentially be highly successful in effecting lateral transfer of host sequences.
A third, a more serious limitation, is that retroviral transduction involves reverse transcription of spliced or partially spliced RNA transcripts. Therefore sequences introduced into a new host would represent pseudogenes, as mediated by retrotransposons. There may be difficulty in controlling expression of the transduced gene if it lacked regulatory sequences and micro-RNA sequences normally present in the intron (Roy and Gilbert 2006). However, introns can be gained during evolution (Yenerall and Zhou 2012) so that this hurdle is not insurmountable.
It is possible that we have not looked in sufficient depth for evidence of horizontal gene transfer by retroviruses. If it does occur between distantly related hosts one would expect whole genome sequence analysis to reveal genes or pseudogenes with greater homology to the species whence the retrovirus came than to pre-existing homologs in its adopted host.
2.3 Incorporation of Host Genetic Sequences into Complex Viruses
When retroviral oncogenes were first shown to be derived from host genes (Stehelin et al. 1976), the gene content of viruses with large, complex genomes was not known. With the advent of whole genome sequencing and bioinformatics, it became possible to interrogate the genomes of large DNA viruses for sequences that have been hi-jacked from ancient and recent hosts and repurposed (exapted) to fine tune the virus’s life style, whether it be replication, latency or immune evasion. Krupovic and Koonin (2017) argue that such basic components of viruses as capsid proteins originally evolved from host cells, but here I wish to examine acquisitions from the host that were imported into virus genomes long after viruses became established as replicating entities.
Herpesviruses, Poxviruses and Polydnaviruses of insects carry numerous genes originally derived from their hosts. Throughout virus-host evolution, viruses possessing a substantial packaging capacity have incorporated host genes and modified them to serve viral functions (Haig 2001). Although poxviruses replicate in the cytoplasm, that has not prevented them adopting multiple host genes (Bugert and Darai 2000; Odom et al. 2009; Austin et al. 2010). Trafficking of DNA between nucleus and cytoplasm is mediated by Importin 7 (Danoya et al. 2013), which may aid genetic exchange between virus and host. Some of the viral genes have introns and probably represent the horizontal transfer of host DNA rather than RNA transcripts that have been reverse transcribed.
The extent of host gene ‘invasion’ of large DNA viruses can be seen by comparing the differences between viruses of a single group that separated millions of years ago. For example, the two human gamma-herpesviruses, Epstein-Barr virus and Kaposi’s sarcoma virus, have homologies among their core replication genes but extensive differences in the genes acquired from the host. Comparative genome analysis across the various human herpesviruses reveals multiple host contributions during virus evolution (Holzerlandt et al. 2002) as depicted in Fig. 2. Immunomodulatory genes are essential to the natural history of viruses in vivo, but tend to be lost during extensive passage in vitro, as seen for standard laboratory strains of Cytomegalovirus (Cunningham et al. 2010) and in the evolution of modified Vaccinia Ankara from wild type Vaccinia virus (Volz and Sutter 2017). Several of the acquired genes of oncogenic herpesviruses may play a role in oncogenesis, such as the chemokine homologs (v-mip) that we studied in Kaposi’s sarcoma herpesvirus (Boshoff et al. 1997).
Genes in human herpesviruses with human homologs. Some genes are common to all types of human herpesvirus whereas others have been acquired individually. Bars are color coded according to functional class: green, DNA replication; dark blue, nucleotide repair/metabolism; light blue, enzyme; purple, gene expression regulation; yellow, glycoprotein; red, host-virus interaction; and black, unknown. Diagonal lines within a box indicate two gene copies (per viral genome); vertical lines, three copies; and horizontal lines, up to 10 copies (adapted with permission from Holzerlandt et al. 2002)
MicroRNA sequences are present in some viruses (Guo and Steitz 2014) and their precursors presumably were derived from cellular sequences. Viruses also alter host miRNA functions to their own advantage. For instance, Epstein-Barr virus EBNA3A and EBNA3C proteins affect regulation of c-myc in B-cells by inducing miR-221/miR-222 expression in transformed B-cells (Bazot et al. 2015).
The remarkable evolution of polydnaviruses in parasitic wasps further exemplifies the two-way genetic exchange between virus and host (Herniou et al. 2013). These large DNA viruses become endogenous in their hosts (Strand and Burke 2014) and probably act as endosymbionts (Herniou et al. 2013). The genetic exchange between amoebae and their intracellular viral and bacterial parasites (or endosymbionts) is also extraordinary. The ‘giant’ Mimiviruses of amoebae contain numerous genes of host origin as well as being themselves prey to smaller viruses, the so-called virophages (Colson et al. 2017). Perhaps there is something special about amoebae because Legionella species (which are intracellular parasites of amoebae as well as occasionally colonizing alveolar macrophages in humans) also acquire hundreds of host genes (Gomez-Valero et al. 2013; Burstein et al. 2016). Conversely, some hosts can acquire whole or partial viral and bacterial genomes, such as the transfer of endosymbiont Wolbachia to insect and nematode nuclear genomes (Dunning Hotopp et al. 2007).
3 Acquisition of Viral Genes by Hosts
3.1 Viral Genomes in Host DNA: Retroviruses, DNA Viruses and RNA Viruses
Whole genome sequencing has led us to appreciate that every vertebrate species examined contains multiple endogenous retroviral genomes (ERV) (Stoye 2012; Hayward et al. 2015; Magiorkinis et al. 2015). As displayed in Fig. 3, approximately 8% of human DNA is derived from germ-line infection by retroviruses (Griffiths 2001) and a larger proportion of our genome is represented by retrotransposons such as LINE elements (Cordaux and Batzer 2009; Rebollo et al. 2012). Rather than being relics of an earlier RNA world converted into genes, one may view ERV as ‘fossils’ of highly evolved, sophisticated viruses because they have the hallmarks of having been acquired by infection (Belshaw et al. 2004).
Proportion of transposable elements in the human genome. Lines and Sines are retrotransposons (adapted with permission from Cordaux and Batzer 2009)
With the detection of endogenous spumavirus genomes in multiple hosts (Ruboyianes and Worobey 2016) and the discovery of an endogenous single copy delta-retrovirus genome in bats (Farkašová et al. 2017), it is now apparent that all seven genera of retroviruses have endogenous counterparts. The defective delta-retrovirus is distantly related to human T-cell leukemia virus and bovine leukosis virus and probably diverged over 25 million years ago, while the foamy viruses entered the germ-line even earlier.
Some retroviruses are promiscuous in integration so long as DNA is on hand. In addition to insertion into the host chromosomal genome, avian reticuloendotheliosis virus (REV) also integrates into the genomes of DNA viruses co-infecting the same chickens and turkeys, namely Marek’s disease herpesvirus (Isfort et al. 1992) and fowlpox virus (Hertig et al. 1997). REV is a gamma-retrovirus of mammalian origin with related viruses endogenous in mongoose species. It has invaded birds and avian DNA viruses in the recent evolutionary past (Etienne and Emerman 2013). Niewiadomska and Gifford (2013) argue REV was introduced iatrogenically from mammals to chickens and turkeys through administration of live attenuated viral vaccines. Although 25 years have passed since the initial report of REV integration in a DNA viral genome (Isfort et al. 1992), I am not aware of integration of other retroviruses into DNA viral genomes, so this may be a special property of REV.
Retroviruses are not the only viruses to invade the host genome. It has become evident that some DNA herpesvrisues and hepadnaviruses endogenize in host genomes. Mendelian inheritance of the beta-herpesvirus, human herpes virus 6 (HHV-6) was first reported by Daibata et al. (1999). Chromosomally integrated HHV-6 is found in about 1% of the human Caucasian population (Pellet et al. 2012; Hill et al. 2016) and debate continues whether these endogenous genomes are linked to disease. An endogenous gamma-herpesvirus has similarly been detected in the prosimian tarsier (Aswad and Katzourakis 2014). Avian hepadnaviruses such as duck hepatitis virus are rapidly evolving DNA viruses with a reverse transcription step in their life cycle. Gilbert and Feschotte (2010) detected an endogenous hepadnavirus in the zebra finch which appears to have colonized the passerine germline nearly 20 million years ago. Beyond viruses, there is evidence of bacterial genomes becoming incorporated into the genomes of multicellular eukaryotes (Dunning Hotopp et al. 2007).
The presence of chromosomally integrated DNA that is complementary to gene sequences of RNA viruses was first reported by Zhdanov (1975) for measles virus and then forgotten for 20 years until recalled (Weiss and Kellam 1997) when Klenerman et al. (1997) detected cDNA of hepatitis C virus, soon followed by the discovery of bornavirus and filovirus sequences (Belyi et al. 2010; Horie et al. 2010; Taylor et al. 2010). The reverse transcription and integration is linked to LINE retroposon elements (Belyi et al. 2010). In an experimental study of vesicular stomatitis virus infection in vitro, the synthesis and integration of cDNA was dependent on expression of LINE-1 (Shimizu et al. 2014).
Germline inserts of RNA virus genome fragments were first detected for bornaviruses (Belyi et al. 2010; Horie et al. 2010) and filoviruses related to Marburg and Ebola viruses (Taylor et al. 2010). The ability to search whole animal and human genomes for homologs of viruses has revealed complementary DNA sequences related to RNA viruses in the germline (Ferschotte and Gilbert 2012). Bornavirus cDNA is widely dispersed in animal genomes (Horie et al. 2013). Whether they play a functional role for the host is not known, but endogenous bornavirus sequences are expressed in human tissues and they may inhibit exogenous infection (Honda and Tomonaga 2016), like the immunity to superinfection exerted by some endogenous retroviruses referred to below. It remains questionable whether bornavirus is currently infectious for humans as clinical reports of detection may result from expression of endogenous bornavirus sequences. Overall, the accumulation of viral sequences in host germline DNA is a much broader phenomenon than the endogenous retroviruses with which we have long been familiar.
3.2 Evolutionary Dynamics of Exogenous and Endogenous Retroviruses
It is customary to view parasite-host relations as an ‘evolutionary arms race’, the term coined by van Valen (1973) when he introduced the predator-prey ‘Red Queen’ hypothesis: “It takes all the running you can do to keep in the same place,” the Red Queen tells Alice in Through the Looking Glass, “If you want to get somewhere else, you must run at least twice as fast as that”. Rapidly replicating pathogens can outpace their hosts in immune escape and the development of drug resistance.
There is, however, a stark difference between the replication rate of exogenous viruses and that of their endogenous counterparts, as noted by Lee et al. (2013) for retroviruses and by Gilbert and Feschotte (2010) for avian hepadnavirus. Exogenous retroviruses undergo millions of replication cycles, including the reverse transcription step which is not subject to repair of errors, whereas endogenous retroviruses are by definition part of the host and replicate at the pace of the host germline, at less than 1000th the rate of virus replication (Aiewsakun and Katzourakis 2015). Retroviruses parked in the genome of one host may act as a hidden reservoir for infection to another.
Potentially pathogenic ERV and retrotransposons are controlled by host intracellular restriction factors (Sanz-Ramos and Stoye 2013; Goodier 2016). In addition, the Env glycoprotein of ERV can itself act as a restriction factor against exogenous infection by blocking receptors (Malfavon-Borja and Feschotte 2015), a phenomenon that we first reported for avian ERV (Payne et al. 1971). However, such restrictions often do not apply if the virus gains access to an unrelated host species and it is clear that replication-competent ERVs can make large leaps to infect distant host species. For instance, the baboon ERV that horizontally crossed hosts to become an ERV (RD114) in cats (Benveniste and Todaro 1974) retains its ability to replicate and to re-infect cats in vivo (Shimode et al. 2015). This phenomenon of xenotropism led us to examine the potential infection hazard of porcine ERV in pig-to-human xenotransplantation (Patience et al. 1997; Le Tissier et al. 1997) although fortunately human infection has not been reported to date.
Another interesting example of cross-species infection by replication-competent ERVs are the gamma-retroviruses related to gibbon ape leukemia virus (GALV). Isolates of GALV were made among captive lar gibbons in Thailand (Kawakami et al. 1972, 1978) although this exogenous retrovirus has not been recorded in wild gibbons. ERV sequences related to GALV were detected in the DNA of several species of South-East Asian rodents (Benveniste et al. 1977), including one in Australia and Indonesia (Simmons et al. 2014; Alfano et al. 2016). The koala retrovirus (KoRV) is also closely related to GALV (Hanger et al. 2000). It was first observed in 1988 (Canfield et al. 1988) but analysis of preserved taxidermy specimens indicates that KoRV has been present in koalas for at least 120 years (Avila-Arcos et al. 2013) and possibly much longer (Ishida et al. 2015). KoRV is associated with leukemia (Canfield et al. 1988; Tarlinton et al. 2006). The current leukemia epidemic appears to be related to an envelope variant (KoRV-B or KoRV-J) co-existing with the original form (KoRV-A) (Shojima et al. 2013; Xu et al. 2013). KoRV-A utilizes the Pit-1 cell surface receptor like GALV and FeLV-B, whereas KoRV-J utilises a thiamine transport receptor like FeLV-A (Shojima et al. 2013).
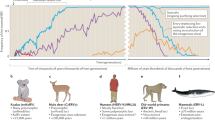
It thus appears that an ERV of rodents has crossed host species at least twice, to become an exogenous pathogenic retrovirus in both gibbons and koalas. Moreover, KoRV is in the process of becoming endogenized in the koala germ line (Tarlinton et al. 2006; Ishida et al. 2015). Since many rodent species of the family Muridae carry related ERVs (Alfano et al. 2016), we may be confident that the precursor of GALV evolved in rodents and subsequently spread horizontally to the koala and the ape (Fig. 4).
Endogenous retrovirus in one host species can act as a ‘reservoir’ for millions of years and emerge to invade unrelated hosts. Gamma-retroviruses resident as mendelian genomes in South-East Asian rodents have transferred horizontally to lar gibbons (GALV) and to Koalas (KoRV). KoRV is becoming endogenous in its new host. Based on findings reported by Benveniste et al. (1977), Kawakami et al. (1978) and Tarlington et al. (2006)
3.3 Pathogenic and Beneficial Attributes of Endogenous Retroviruses
ERVs and other retrotransposons are open to natural selection operating on the host which presents a two-edged sword. On one side, integration and expression of certain ERV is associated with disease; on the other, the presence of transposable elements may be of overall benefit to the host population (Rebollo et al. 2012; Babaian and Mager 2016). There has been much discussion about both deleterious and beneficial effects of ERVs (Moyes et al. 2007; Stoye 2012) and new ERV insertions may disrupt essential host genes (Chuong et al. 2017). Recently integrated ERVs can be oncogenic, contributing to leukemia in mice and cats and to mammary cancer in mice (Rosenberg and Jolicoeur 1997; Ross 2010).
Whether human ERV sequences (HERV) play a causative role in human cancers is less definitive (Magiorkinis et al. 2015; Babaian and Mager 2016; Kassiotis and Stoye 2017). Beta-retrovirus HERV-K (HML2) genomes have been implicated in testicular tumors, melanoma and breast cancer. Certain HERV-K loci are more highly expressed in tumor tissue, but that does not provide conclusive evidence for causality; tumor cells impose fewer restrictions on HERV-K so that its expression may be a consequence rather than a cause of the malignancy.
Human endogenous retroviruses may be beneficial to the host through non-coding sequences providing new sources of gene regulation and by coding sequences providing useful proteins. For example, HERV-K is expressed in pre-implantation embryos and is linked with pluripotency but becomes transcriptionally silenced upon differentiation (Fuchs et al. 2013; Grow et al. 2015). HERV-H genomes and LINE elements are also tightly linked to transcriptional regulation in pluripotent cells and exert an influence on early development (Robbez-Masson and Rowe 2015; Schlesinger and Goff 2015). Modulation of expression of host enzymes by ERVs has also been turned to use by the host (Rebollo et al. 2012). For instance, the expression of human salivary amylase in the parotid gland is controlled by a novel HERV-E insertion in the primate lineage which became amplified in hominids (Ting et al. 1992). In the HERV-E LTR, the promoter and tissue-specific enhancers activate amylase expression which is otherwise restricted to the pancreas. It may have helped our forebears adapt from a diet mainly of fruit to one containing starch.
3.4 Role of Endogenous Retroviral Envelopes in the Placenta
The most striking example of ERVs becoming a benefit to their hosts is the role of Env in effecting cell fusion to form the syncytiotrophoblast of the mammalian placenta. The notion that HERV envelope might be involved in placental development interested me from the early 1990s, but was notably carried forward by the laboratories of Thierry Heidmann, John M McCoy and François-Loïc Cosset.
In the human placenta, we noted high expression of a defective HERV with an open reading frame for env, called ERV-3 (Boyd et al. 1993). ERV-3 expression was tightly linked to the syncytiotrophoblast (Fig. 3) and we postulated that a functional retroviral Env glycoprotein would be able to induce the cell-to-cell fusion (Venables et al. 1995). Indeed, Lin et al. (1999) demonstrated that transfection of ERV-3 into BeWo choriocarcinoma cells (a malignant version of cytotrophoblast) induced cell fusion and differentiation into syncytiotrophoblast. However, it was found that some humans lack the ERV-3 genome (de Parseval and Heidmann 1998) although they must have been gestated with a healthy placenta. Gorillas also lack ERV-3 (Hervé et al. 2004). But our hypothesis of ERV driving placental differentiation survived albeit involving different ERVs. Blond et al. (2000) and Mi et al. (2000) showed that HERV-W Env also induced the syncytiotrophoblast fusion. Lavillette et al. (2002) showed that HERV-W interacts with several amino-acid transporters already known to act as beta-retrovirus receptors to initiate fusion. Mi et al. (2000) coined a new term for the HERV-W envelope glycoprotein: syncytin (Figs. 3 and 5).
Endogenous Env glycproteins function in the human placenta as ‘syncytins’. a Indirect immunofluorescence of ERV-3 envelope glycoprotein in the syncytiotrophoblast of a full term human placenta (reproduced with permission from Venables et al. 1995); b Cos cells transfected with reverse orientation HERV-W syncytin 1 and c syncytium formation in Cos cells transfected by correctly oriented HERV-W syncytin 1 (reproduced with permission from Mi et al. 2000)
The syncytin story became grew more convoluted with the discovery of a second human Env glycoprotein, syncytin-2, encoded by HERV-FRD (Blaise et al. 2003). Low expression of syncytin 1 and 2 is correlated with poor placental development and pre-eclampsia (Vargas et al. 2011). Moreover, different orders of placental mammal employ the Env glycoproteins of quite distinct ERVs to induce cell fusion of the trophoblast (Lavialle et al. 2013) and even in a proto-placenta in the marsupial opossum (Cornelis et al. 2015).
If the evolution of the placenta was a monophyletic event, why have placental mammals repeatedly entrained different ERVs to effect trophoblast differentiation into a syncytium? Imakawa et al. (2015) postulate a ‘baton pass’ hypothesis, in which multiple successive ERV variants take over cell-fusion roles, resulting in variations in placental structures and enhanced reproductive success in placental mammals. They speculate that ERVs replaced more ancient mediators of trophoblast fusion. Imakawa et al. (2015) support this view with the observation that several of the ERVs encoding syncytins have become endogenous in their respective host genomes only within the past 12–80 million years, more recently than the evolution of the mammalian placenta. In humans, redundancy in retroviral-driven trophoblast fusion explains why ERV-3, HERV-W and HERV-FRD can each induce fusion of the cytotrophoblast to form the syncytiotrophoblast.
HERV Env may have a dual role in the placenta: to be locally immunosuppressive at the maternal-fetal interface (the syncytiotrophoblast) in order to protect the fetus from maternal rejection, as well as to induce the trophoblast fusion (Denner 2016). The immunosuppressive domain in the transmembrane envelope protein is present in ERV-3 and HERV-W env (Boyd et al. 1993; Lavialle et al. 2013). Syncytins have maintained their virus entry capacity. For instance, HERV-W Env functionally pseudotypes retroviral vectors (Blond et al. 2000). HERV-W is also expressed in multinuclear macrophages such as osteoclasts (Søe et al. 2009), but there is no evidence that other syncytial tissues (e.g., striated muscle) utilize ERV syncytins to effect cell fusion. Nonetheless, placental mammals have given a virtuoso performance playing on a keyboard of retrovirus envelope-cell surface receptor engagement to repurpose ERVs to provide fusion proteins beneficial to the host.
4 Endosymbiont Organelles in Eukaryotic Cells and Their Horizontal Transfer in Cancer
4.1 Evolution of Complex Cells and Their Organelles
The year in which Peter Vogt joined the Editorial Board of CTMI also marks the birth of the hypothesis that eukaryotic cells originated from the combination of prokaryotes to form complex cells containing organelles. This notion was postulated by Lynn Margulis, publishing under her first married name Sagan (1967); like many novel concepts was initially greeted with much skepticism (Margulis 2009). Lynn Margulis herself enjoyed stoking up controversies and she was attracted to the notion that HIV does not cause AIDS, holding that AIDS was nothing more than syphilis masquerading under a new name (Margulis et al. 2009).
Today, we accept that mitochondria, chloroplasts, cilia and the nuclear membrane had distinct origins and coalesced together to form eukaryotic cells (Lane 2014; Martin et al. 2015) although how this happened is open to debate (Baum and Baum 2014). During the long period of endosymbiosis, genes have switched location; for example, many genes involved in oxidative respiration appear to have transferred to the nuclear genome as the mtDNA episome became reduced (Allen 2003; Gershoni et al. 2009). If each kind of subcellular organelle in eukaryotes is monophyletic to what extent are they transferred laterally between species? In animals, the genomes of mitochondria have co-evolved with the nuclear genome because both types of genetic sequence have concordant phylogenies. Yet there is evidence of widespread lateral mobility of mitochondria and plastids in higher plants (Bergthorsson et al. 2003; Warren et al. 2016). This difference may be explained by mitochondrial variation driving evolution of sexes but also the germline-soma distinction that exists in metazoan animals but not in higher plants (Radzvilavicius et al. 2016).
Mitochondrial transfer between animal cells is illustrated by research into transmissible tumor cells in which I became involved a few years ago. My interest was aroused by a note published by Hayes et al. (1983) just before the discovery of HIV speculating—wrongly it turned out when a causative virus was discovered (Chang et al. 1994)—that canine transmissible venereal tumor (CTVT) might be a model for Kaposis’s sarcoma in AIDS.
4.2 Horizontal Spread of Cancer Cells
Certain transmissible tumors are spread from one individual to another not by an oncogenic virus but by the migration of the tumor cell itself. Common marker chromosomes indicated that the transmissible agent of CTVT is the cancer cell itself (Cohen 1985) and it was shown that a LINE-1 retrotransposon insertion near c-myc is unique to the tumor (Katzir et al. 1985; Amariglio et al. 1991). Using forensic DNA markers we confirmed the cellular transmission of CTVT, and demonstrated that the tumor represents a single clone which has colonized dogs worldwide (Murgia et al. 2006).
CTVT was first described in 1876 (Novinski 1876) and it played an important role in the early years of cancer research because it was the only tumor that could be experimentally transplanted from one animal to another before the developmental of inbred lines. We estimated that CTVT emerged in an ancient dog breed ~11,000 years ago (Murchison et al. 2014). Thus CTVT represents a naturally occurring cancer cell clone some 2000 times older than HeLa cells. It has undergone thousands of somatic mutations, deletions, amplifications and chromosome rearrangements since it first emerged (Murchison et al. 2014) and it is a useful tool to examine how diverse a tumor cell can be while retaining its proliferative properties. For a cellular parasite that no longer requires many host functions (e.g., a sense of smell), it is noteworthy that CTVT has not shown a massive reduction of the size compared to the canine genome. Since household genes are interspersed with specialty genes, any deletions of unnecessary genes would have to occur on a case by case basis.
The Devil facial tumor disease (DFDT) is also transmitted horizontally as a tumor cell (Pearse and Swift 2006; Murchison et al. 2012) in the Tasmanian Devil (Sarcophilus harrisii), an endangered marsupial species which has relatively low genetic diversity (Siddle and Kaufman 2015). There are two independent DFDT clones circulating in Devils (Pye et al. 2016) both of recent provenance. David Metzger in Stephen Goff’s laboratory has shown that some clam species are infested with clonal tumor cells (Metzger et al. 2015), including one in which the host species differs from that in which the tumor first arose (Metzger et al. 2016). The modes of transmission of these tumors differ: in dogs the tumor is mainly spread sexually, in Devils through biting, and among clams by filter feeding water containing tumor cells.
The emergence of transmissible tumor cells remains a rare phenomenon and we do not fully understand how the tumors evade the host immune response. The lack of major histocompatability antigens or their down regulation would promote the chance of tumor emergence (Murgia et al. 2006; Siddle and Kaufman 2015). Looking into the literature, however, I found only one example of ‘naturally’ transmissible tumors among inbred strains of laboratory rodents: a leukemia in Syrian hamsters which remarkably could be transmitted by mosquitoes (Banfield et al. 1965). In humans, there are several examples of horizontal tumor transmission from donors to immunosuppressed transplant recipients (Nalesnik et al. 2011). Leukemia has also been transmitted in utero between fetuses sharing a placenta (Greaves et al. 2003).
4.3 Colonization of Cancer Cells by Host Mitochondria
When we attempted to determine the date the most recent common ancestor of the tumor cell clone we obtained contradictory data for nuclear microsatellite DNA and mitochondrial DNA, as the latter appeared to have diversified for a longer time. We tentatively suggested that the two major clades of mtDNA in CTVT might have distinct origins (Murgia et al. 2006), and this hypothesis was verified by Rebbeck et al. (2011). It is now apparent that host mtDNA was acquired at least five times during the passage of the tumor clone through countless canine hosts (Strakova et al. 2016). Mitochondrial function may be a driver in tumor progression and recombination between mtDNA genomes is found in some CTVT tumors (Strakova et al. 2016). If mutations in the mtDNA accrue during serial passage of the tumor, acquisition of mtDNA from the host may contribute to the fitness of the tumor to persist. Thus a tumor that has spread worldwide as a somatic cell parasite has itself been colonized by host mitochondria via lateral transfer.
It would be interesting to determine whether similar colonization of tumors by host mitochondria occurs in the other transmissible tumor cells that have recently come to light. In particular, the tumor that has colonized a different species of clam than that from which it arose (Metzger et al. 2016) would be suitable to look for evidence of cross-species transfer of mitochondria and other organelles.
Mitochondria have lost many of their genes to the nuclear genome (Lane 2010), perhaps mediated by Importin 7 (Danoya et al. 2013). Thus there are likely to be constraints on cytoplasmic organelles containing DNA to co-evolve with the nuclear genome, owing to interplay of non-coding RNA elements (Vendramin et al. 2017) and to protein complexes with components encoded by both genomes. Therefore lateral transfer of mitochondria to distantly related species is unlikely to occur. Horizontal spread of mitochondria does occur within individuals, however, and has been detected in cancer. In an experimental murine system using tumor cells devoid of mitochondria, tumor progression was activated upon transfer of mitochondria from host stroma (Tan et al. 2015). Human tumor cells may also acquire host mitochondria from endothelial cells (Pasquier et al. 2013). Tumor progression and relapse may sometimes involve colonization of tumor cells by ‘fitter’ mitochondria imported from the host.
5 Concluding Remarks
The horizontal exchange of genetic sequences is widespread in all forms of life. Viruses embed themselves in host DNA, and host genes are transferred to viruses. Endogenous retroviruses can lie ‘dormant’ in the germ line for millions of years and re-emerge as replication-competent viruses infecting distantly related species. Host cells can emerge as transmissible malignant clones, survive longer than any other vertebrate somatic cells, and be colonized in turn by mitochondria from new hosts many transplant generations later. There is far more fluidity of genomes and cells within the eukaryotic world than we imagined 50 years ago.
References
Aiewsakun P, Katzourakis A (2015) Endogenous viruses: connecting recent and ancient viral evolution. Virology 479–480:26–37. doi:10.1016/j.virol.2015.02.011
Alfano N, Michaux J, Morand S, Aplin K, Tsangaras K, Löber U, Fabre PH, Fitriana Y, Semiadi G, Ishida Y, Helgen KM, Roca AL, Eiden MV, Greenwood AD (2016) Endogenous gibbon ape leukemia virus identified in a rodent (Melomys burtoni subsp.) from Wallacea (Indonesia). J Virol 90:8169–8180. doi:10.1128/JVI.00723-16
Allen JF (2003) Why chloroplasts and mitochondria contain genomes. Comp Funct Genomics 4:31–36. doi:10.1002/cfg.245
Amariglio EN, Hakim I, Brok-Simoni F, Grossman Z, Katzir N, Harmelin A, Ramot B, Rechavi G (1991) Identity of rearranged LINE/c-MYC junction sequences specific for the canine transmissible venereal tumor. Proc Natl Acad Sci U S A 88:8136–8139
Aswad A, Katzourakis A (2014) The first endogenous herpesvirus, identified in the tarsier genome, and novel sequences from primate rhadinoviruses and lymphocryptoviruses. PLoS Genet 10:e1004332
Austin L, Hughes AL, Irausquin S, Friedman R (2010) The evolutionary biology of poxviruses. Infect Genet Evol 10:50. doi:10.1016/j.meegid.2009.10.001
Ávila-Arcos MC, Ho SY, Ishida Y, Nikolaidis N, Tsangaras K, Hönig K, Medina R, Rasmussen M, Fordyce SL, Calvignac-Spencer S, Willerslev E, Gilbert MT, Helgen KM, Roca AL, Greenwood AD (2013) One hundred twenty years of koala retrovirus evolution determined from museum skins. Mol Biol Evol 30:299–304
Babaian A, Mager DL (2016) Endogenous retroviral promoter exaptation in human cancer. Mob DNA 7:24. doi:10.1186/s13100-016-0080-x
Baltimore D (1970) RNA-dependent DNA polymerase of RNA tumour viruses. Nature 226:1209–1211
Banfield WG, Woke PA, Mackay CM, Cooper HL (1965) Mosquito transmission of a reticulum cell sarcoma of hamsters. Science 148:1239–1240
Baum DA, Baum B (2014) An inside-out origin for the eukaryotic cell. BMC Biol 12: 76. doi: 10.1186/s12915-014-0076-2
Bazot Q, Paschos K, Skalska L, Kalchschmidt JS, Parker GA, Allday MJ (2015) Epstein-Barr virus proteins EBNA3A and EBNA3C together induce expression of the oncogenic microRNA cluster miR-221/miR-222 and ablate expression of its target p57KIP2. PLoS Pathog 11:e1005031. doi:10.1371/journal.ppat.1005031
Belshaw R, Pereira V, Katzourakis A, Talbot G, Paces J, Burt A, Tristem M (2004) Long-term reinfection of the human genome by endogenous retroviruses. Proc Natl Acad Sci U S A 101:4894–4899
Belyi V, Levine AJ, Skalka AM (2010) Unexpected inheritance: multiple integrations of ancient bornavirus and ebolavirus/marburgvirus sequences in vertebrate genomes. PLoS Pathog 6:e1001030
Benveniste RE, Todaro GJ (1974) Evolution of C-type viral genes: inheritance of acquired viral genes. Nature 252:456–459
Benveniste RE, Callahan R, Sherr CJ, Chapman V, Todaro GJ (1977) Two distinct endogenous type C viruses isolated from the Asian rodent Mus cervicolor: conservation of virogene sequences in related rodent species. J Virol 21:849–862
Bergthorsson U, Adams KL, Thomason B, Palmer JD (2003) Widespread horizontal transfer of mitochondrial genes in flowering plants. Nature 424:197–201
Bister K (2015) Discovery of oncogenes: the advent of molecular cancer research. Proc Natl Acad Sci U S A. 112:15259–15260. doi:10.1073/pnas.1521145112
Blaise S, de Parseval N, Bénit Land Heidmann T (2003) Genome wide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc Natl Acad Sci U S A. 100:13013–13018
Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset FL (2000) An envelope glycoprotein of the human endogenous retrovirus HERV-W expressed in the human placenta and fuses cells expressing the D-type mammalian retrovirus receptor. J Virol 74:3321–3329
Bolin LL, Levy LS (2011) Viral determinants of FeLV infection and pathogenesis: lessons learned from analysis of a natural cohort. Viruses 3:1681–1698. doi:10.3390/v3091681
Bordet J, Ciuca MC (1922) Concerning the theories of the so-called ‘bacteriophage’. Br Med J 2:296
Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, Schweickart VL, Siani MA, Sasaki T, Williams TJ, Gray PW, Moore PS, Chang Y, Weiss RA (1997) Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science 278:290–294
Boyd MT, Bax CMR, Bax BE, Bloxam DL, Weiss RA (1993) The human endogenous retrovirus ERV-3 is upregulated in differentiating placental trophoblast cells. Virology 196:905–909
Brugge JS, Erikson RL (1977) Identification of a transformation-specific antigen induced by by an avian sarcoma virus. Nature 269:346–348
Bugert JJ, Darai G (2000) Poxvirus homologues of cellular genes. Virus Genes 21:111–133
Burstein D, Amaro F, Zusman T, Lifshitz Z, Cohen O, Gilbert JA, Pupko T, Shuman HA, Segal G (2016) Genomic analysis of 38 Legionella species identifies large and diverse effector repertoires 48: 167–175. doi:10.1038/ng.3481
Cairns J, Stent GS, Watson JD (2007) Phage and the origins of molecular biology. Cold Spring Harbor Laboratory Press, New York
Canfield PJ, Sabine JM, Love DN (1988) Virus particles associated with leukaemia in a koala. Aust Vet J 65:327–328
Cavalieri F, Ruscio T, Tinoco R, Benedict S, Davis C, Vogt PK (1985) Isolation of three new avian sarcoma viruses: ASV 9, ASV 17, and ASV 25. Virology 143:680–683
Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 265: 1865–1869
Choi IG, Kim SH (2007) Global extent of horizontal gene transfer. Proc Natl Acad Sci U S A 104:4489–4494. doi:10.1073/pnas.0611557104
Chuong EB, Elde NC, Feschotte C (2017) Regulatory activities of transposable elements: from conflicts to benefits. Nat Rev Genetics 18:71–86. doi:10.1038/nrg.2016.139
Cisek AA, Dabrowska I, Gregorczyk KP, Wyzewski Z (2017) Phage therapy in bacterial infections treatment: one hundred years after the discovery of bacteriophages. Curr Microbiol 74:277–283. doi:10.1007/s00284-016-1166-x
Cohen D (1985) The canine transmissible venereal tumor: a unique result of tumor progression. Adv Cancer Res 43:75–112
Colson P, La Scola B, Levasseur A, Caetano-Anollés G, Raoult D (2017) Mimivirus: leading the way in the discovery of giant viruses of amoebae. Nat Rev Microbiol 15:243–254. doi:10.1038/nrmicro.2016.197
Cornelis G, Vernochet C, Carradec Q, Souquere S, Mulot B, Catzeflis Nilsson MA, Menzies BR, Renfree MB, Pierron G, Zeller U, Heidmann O, Dupressoir A, Heidmann T (2015) Retroviral envelope gene captures and syncytin exaptation for placentation in marsupials. Proc Natl Acad Sci U S A. 112:E487–E496. doi:10.1073/pnas.1417000112
Cordaux R, Batzer MA (2009) The impact of retrotransposons on human genome evolution. Nat Rev Genet 10:691–703. doi:10.1038/nrg2640
Crawford LV, Crawford EM (1961) The properties of Rous sarcoma virus purified by density gradient centrifugation. Virology 13:227–232
Cunningham C, Gatherer D, Baluchova K, Dargan DJ, Thomson M, Griffiths PD, Wilkinson GWG, Schulz TF, Davison AJ (2010) Sequences of complete human cytomegalovirus genomes from infected cell cultures and clinical specimens. J Gen Virol 91:605–615. doi:10.1099/vir.0.015891-0
Daibata M, Taguchi T, Nemoto Y, Taguchi H, Miyoshi I (1999) Inheritance of chromosomally integrated human herpesvirus 6 DNA. Blood 94:1545–1549
Danoya A, Wang T, Keshavarz-Moore E, Fassati A, Chain BM (2013) Importin-7 mediates nuclear trafficking of DNA in mammalian cells. Traffic 14:165–175
Denner J (2016) Expression and function of endogenous retroviruses in the placenta. APMIS 124:31–43. doi:10.1111/apm.12474
d’Herelle F (1917) An invisible microbe that is antagonistic to the dysentery Bacillus. Compt Rend Acad Sci Paris 165:373–375
de Parseval N, Heidmann T (1998) Physiological knockout of the envelope gene of the single-copy ERV-3 human endogenous retrovirus in a fraction of the Caucasian population. J Virol 72:3442–3445
Duesberg PH, Vogt PK (1970) Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A 67:1673–1680
Dulbecco R (1952) Production of plaques in monolayer tissue cultures by single particles of an animal virus. Proc Natl Acad Sci U S A 38:747–752
Dunning Hotopp JC, Clark ME, Oliveira DC, Foster JM, Fischer P, Muñoz Torres MC, Giebel JD, Kumar N, Ishmael N, Wang S, Ingram J, Nene RV, Shepard J, Tomkins J, Richards S, Spiro DJ, Ghedin E, Slatko BE, Tettelin H, Werren JH (2007) Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 317:1753–1756
Etienne L, Emerman M (2013) The mongoose, the pheasant, the pox, and the retrovirus. PLoS Biol 11:e1001641. doi:10.1371/journal.pbio.1001641
Farkašová H, Hron T, Pačes J, Hulva P, Benda P, Gifford RJ, Elleder D (2017) Discovery of an endogenous Deltaretrovirus in the genome of long-fingered bats (Chiroptera: Miniopteridae).Proc Natl Acad Sci U S A. 2017 Mar 9. pii: 201621224. doi:10.1073/pnas.1621224114
Feschotte C, Gilbert C (2012) Endogenous viruses: insights into viral evolution and impact on host biology. Nat Rev Genet 13:283–296. doi:10.1038/nrg3199
Fuchs NV, Loewer S, Daley GQ, Izsvák Z, Löwer J, Löwer R (2013) Human endogenous retrovirus K (HML-2) RNA and protein expression is a marker for human embryonic and induced pluripotent stem cells. Retrovirology 10:115. doi:10.1186/1742-4690-10-115
Gershoni M, Templeton AR, Mishmar D (2009) Mitochondrial bioenergetics as a major motive force of speciation. BioEssays 31:642–650. doi:10.1002/bies.200800139
Gilbert C, Feschotte C (2010) Genomic fossils calibrate the long-term evolution of hepadnaviruses. PLoS Biol 8:e1000495
Gomez-Valero L, Buchrieser C. (2013) Genome dynamics in Legionella: the basis of versatility and adaptation to intracellular replication. Cold Spring Harb Perspect Med 3: pii: a009993. doi:10.1101/cshperspect.a009993
Goodier JL (2016) Restricting retrotransposons: a review. Mobile. DNA 7:16. doi:10.1186/s13100-016-0070
Greaves MF, Maia AT, Wiemel JL, Ford AM (2003) Leukemia in twins: lessons in natural history. Blood 102:2321–2333
Griffiths D (2001) Endogenous retroviruses in the human genome sequence. Genome Biol 2: reviews 1017.1-reviews 1017.5
Grow EJ, Flynn RA, Chavez SL, Bayless NL, Wossidlo M, Wesche D, Martin L, Ware C, Blish CA, Chang HY, Reijo Pe RA, Wysocka J (2015) Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells. Nature 522: 221–225. doi: 10.1038/nature14308
Guo YE, Steitz JA (2014) Virus meets host microRNA: the destroyer, the booster, the hijacker. Mol Cell Biol 34:3780–3787
Haig DM (2001) Subversion and piracy: DNA viruses and immune evasion. Res Vet Sci 70:205–219
Hanafusa H, Hanafusa T, Rubin H (1963) The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A 49:572–580
Hanger JJ, Bromham LD, McKee JJ, O’Brien TM, Robinson WF (2000) The nucleotide sequence of koala (Phascolarctos cinereus) retrovirus: a novel type C endogenous virus related to gibbon ape leukemia virus. J Virol 74:4264–4272
Hartmann G (2017) Nucleic Acid immunity. Adv Immunol 133:121–169. doi:10.1016/bs.ai.2016.11.001
Hayes HM, Biggar RJ, Pickle LW, Hoover R, Toft JD (1983) Canine transmissible venereal tumor: a model for Kaposi’s sarcoma? Am J Epidemiol 117:108–109
Hayward A, Cornwallis CK, Jern P (2015) Pan-vertebrate comparative genomics unmasks retrovirus macroevolution. Proc Natl Acad Sci U S A 112:464–469
Hayward WS, Neel BG, Astrin SM (1981) Activation of a cellular onc gene by promoter insertion ALV-induced lymphoid leukosis. Nature 290:475–480
Herniou EA, Huguet E, Thézé J, Bézier A, Periquet G, Drezen JM (2013) When parasitic wasps hijacked viruses: genomic and functional evolution of polydnaviruses. Philos Trans R Soc Lond B 368:20130051. doi:10.1098/rstb.2013.0051
Hertig C, Coupar BE, Gould AR, Boyle DB (1997) Field and vaccine strains of fowlpox virus carry integrated sequences from the avian retrovirus, reticuloendotheliosis virus. Virolgy 235:367–376
Hervé CA, Forrest G, Löwer R, Griffiths DJ, Venables PJ (2004) Conservation and loss of the ERV3 open reading frame in primates. Genomics 83:940–943
Hill JA, Ruth HallSedlak R, Magaret A, Huang ML, Zerr DM, Jerome KR, Boeckh M (2016) Efficient identification of inherited chromosomally integrated human herpesvirus 6 using specimen pooling. J Clin Virol 77:71–76
Holzerlandt R, Orengo C, Kellam P, Albà MM (2002) Identification of new herpesvirus gene homologs in the human genome. Genome Res 12:1739–1748. doi:10.1101/gr.334302
Honda T, Tomonaga K (2016) Endogenous non-retroviral RNA virus elements evidence a novel type of antiviral immunity. Mob Genet Elements 6:e1165785. doi:10.1080/2159256X.2016.1165785
Horie M, Honda T, Suzuki Y, Kobayashi Y, Daito T, Oshida T, Ikuta K, Jern P, Gojobori T, Coffin JM, Tomonaga K (2010) Endogenous non-retroviral RNA virus elements in mammalian genomes. Nature 463:84–87. doi:10.1038/nature08695
Horie M, Kobayashi Y, Suzuki Y, Tomonaga K (2013) Comprehensive analysis of endogenous bornavirus-like elements in eukaryote genomes. Phil Trans R Soc B 368:20120499. doi:10.1098/rstb.2012.0499
Hunter T, Sefton BM (1980) Transforming gene product of Rous sarcoma virus phoshorylates tyrosine. Proc Natl Acad Sci U S A 77:1311–1315
Imakawa K, Nakagawa S, Miyazawa T (2015) Baton pass hypothesis: successive incorporation of unconserved endogenous retroviral genes for placentation during mammalian evolution. Genes Cells 10:771–778
Isfort RJ, Jones D, Kost R, Witter R, Kung H-J (1992) Retrovirus insertion into herpesvirus in vitro and in vivo. Proc Natl Acad Sci U S A 89:991–995
Ishida Y, Zhao K, Greenwood AD, Roca AL (2015) Proliferation of endogenous retroviruses in the early stages of a host germ line invasion. Mol Biol Evol 32:109–120
Ishizaki R, Vogt PK (1966) Immunological relationships among envelope antigens of avian tumor viruses. Virology 30:375–387
Kassiotis G, Stoye JP (2017) Making a virtue of necessity: the pleiotropic role of human endogenous retroviruses in cancer. Phil Trans R Soc B (in press)
Katzir N, Rechavi G, Cohen JB, Unger T, Simoni F, Segal S, Cohen D, Givol D (1985) “Retroposon” insertion into the cellular oncogene c-myc in canine transmissible venereal tumor. Proc Natl Acad Sci U S A 82:1054–1058. doi:10.1073/pnas.82.4.1054
Kawakami TG, Huff SD, Buckley PM, Dungworth DL, Synder SP, Gilden RV (1972) C-type virus associated with gibbon lymphosarcoma. Nat New Biol 235:170–171
Kawakami TG, Sun l, McDowell TS (1978) Natural transmission of gibbon leukemia virus. J Natl Cancer Inst 61:1113–1115
Keogh EV (1938) Ectodermal lesions produced by the virus of Rous sarcoma. Brit J Exp Pathol 19:1–9
Klenerman P, Hengartner H, Zinkernagel RM (1997) A non-retroviral RNA virus persists in DNA form. Nature 390: 298–301
Krupovic M, Koonin EV (2017) Multiple origins of viral capsid proteins from cellular ancestors. Proc Natl Acad Sci U S A 114:E2401–E2410. doi:10.1073/pnas.1621061114
Lane N (2010) Life ascending: the ten great inventions of evolution. Profile Books, London
Lane N (2014) Bioenergetic constraints on the evolution of complex life. Cold Spring Harb Perspect Biol 6:a015982. doi:10.1101/cshperspect.a015982
Lane N (2015) The vital question: why is life the way it is?. Profile Books, London
Lavillette D, Marin M, Ruggieri A, Mallet F, Cosset FL, Kabat D (2002) The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J Virol 76:6442–6652
Lavialle C, Cornelis G, Dupressoir A, Esnault C, Heidmann O, Vernochet C, Heidmann T (2013) Paleovirology of ‘syncytins’, retroviral env genes exapted for a role in placentation. Philos Trans R Soc Lond B 368:20120507. doi:10.1098/rstb.2012.0507
Lee A, Nolan A, Watson J, Tristem M (2013) Identification of an ancient endogenous retrovirus, predating the divergence of the placental mammals. Philos Trans R Soc Lond B Biol Sci 368:20120503
Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA (1997) Two sets of human-tropic pig retrovirus. Nature 389:681–682
Levy JA (1978) Xenotropic type C viruses. Curr Top Microbiol Immunol 79:111–213
Lin L, B. Xua B, Rote NS (1999) Expression of endogenous retrovirus ERV-3 induces differentiation in BeWo, a choriocarcinoma model of human placental trophoblast. Placenta 20:109–118
Loenen WAM, Dryden DTF, Raleigh EA, Geoffrey GG, Murray NE (2014) Highlights of the DNA cutters: a short history of the restriction enzymes. Nucleic Acids Res 42(1):3–19. doi:10.1093/nar/gkt990
Lwoff A (1953) Lysogeny. Bacteriol Rev 17:269–337
Magiorkinis G, Belshaw R, Katzourakis A (2015) ‘There and back again’: revisiting the pathophysiological roles of human endogenous retroviruses in the post-genomic era. Phil Trans R Soc B 368:20120504
Maki Y, Bos TJ, Davis C, Starbuck M, Vogt PK (1987) Avian sarcoma virus 17 carries the jun oncogene. Proc Natl Acad Sci U S A 84:2848–2852
Malfavon-Borja R, Feschotte C (2015) Fighting fire with fire: Endogenous retrovirus envelopes as restriction factors. J Virol 89:4047–4050
Margulis L, Maniotis A, MacAllister J, Scythes BO, Hall J et al (2009) Spirochete round bodies, syphilis, Lyme disease & AIDS: resurgence of “the great imitator”? Symbiosis 47:51–58
Margulis L (2009) Genome acquisition in horizontal gene transfer: symbiogenesis and macromolecular sequence analysis. Methods Mol Biol 532:181–191. doi:10.1007/978-1-60327-853-9_10
Martin GS (1970) Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature 227:1021–1023
Martin GS (2004) The road to Src. Oncogene 23:7910–7917
Martin WF (2011) Early evolution without a tree of life. Biol Direct 6:36
Martin WF, Garg S, Zimorski V (2015) Endosymbiotic theories for eukaryote origin. Phil Trans R Soc B 370:20140330. doi:10.1098/rstb.2014.0330
Metzger MJ Reinisch C, Sherry J, Goff SP (2015) Horizontal transmission of clonal cancer cells causes leukemia in soft-shell clams. Cell 161: 255–263
Metzger MJ, Villalba A, Carballal MJ, Iglesias D, Sherry J, Reinisch C, Muttray AF, Baldwin SA, Goff SP (2016) Widespread transmission of independent cancer lineages within multiple bivalve species. Nature 534:705–709
Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith JC, McCoy JM (2000) Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403:785–789
Miles BD, Robinson HL (1985) High-frequency transduction of c-erbB in avian leukosis virus-induced erythroblastosis. J Virol 54:295–303
Moyes D, Griffiths DJ, Venable PJ (2007) Insertional polymorphisms: a new lease of life for endogenous retroviruses in human disease. Trends Genet 23:326–333
Murchison EP, Schulz-Trieglaff OB, Ning Z, Alexandrov LB, Bauer MJ, Fu B, Hims M, Ding Z, Ivakhno S, Stewart C, Ng BL, Wong W, Aken B, White S, Alsop A, Becq J, Bignell GR, Cheetham RK, Cheng W, Connor TR, Cox AJ, Feng ZP, Gu Y, Grocock RJ, Harris SR, Khrebtukova I, Kingsbury Z, Kowarsky M, Kreiss A, Luo S, Marshall J, McBride DJ, Murray L, Pearse AM, Raine K, Rasolonjatovo I, Shaw R, Tedder P, Tregidgo C, Vilella AJ, Wedge DC, Woods GM, Gormley N, Humphray S, Schroth G, Smith G, Hall K, Searle SM, Carter NP, Papenfuss AT, Futreal PA, Campbell PJ, Yang F, Bentley DR, Evers DJ, Stratton MR (2012) Genome sequencing and analysis of the Tasmanian devil and its transmissible cancer. Cell 148:780–791
Murchison EP, Wedge DC, Alexandrov LB, Fu B, Martincorena I, Ning Z, Tubio JM, Werner EI, Allen J, De Nardi AB, Donelan EM, Marino G, Fassati A, Campbell PJ, Yang F, Burt A, Weiss RA, Stratton MR (2014) Transmissible dog cancer genome reveals the origin and history of an ancient cell lineage. Science 343:437–440
Murgia C, Pritchard KJ, Kim SK, Fassati A, Weiss RA (2006) Clonal origin and evolution of a transmissible cancer. Cell 126:477–487
Nalesnik MA, Woodle ES, DiMaio JM, Vasudev B, Teperman LW, Covington S, Taranto S, Gockerman JP, Shapiro R, Sharma V, Swinnen LJ, Yoshida A, Ison MG (2011) Donor-transmitted malignancies in organ transplantation: assessment of clinical risk. Amer J Transplant 11:1140–1147. doi:10.1111/j.1600-6143.2011.03565.x
Neil JC, Fulton R, Rigby M, Stewart M (1991) Feline leukemia virus: generation of pathogenic and oncogenic variants. Curr Top Microbiol Immunol 171:67–93
Niewiadomska AM, Gifford RJ (2013) The extraordinary evolutionary history of the reticuloendotheliosis viruses. PLoS Biol 11:e1001642
Novinski MA (1876) Zur Frage uber die Impfung der Krebsigen Geschwulste. Zentralbl Med Wissensch 14:790–791
Odom MR, Hendrickson RC, Lefkowitz EJ (2009) Poxvirus protein evolution: family wide assessment of possible horizontal gene transfer events. Virus Res 144:233–249. doi:10.1016/j.virusres.2009.05.006
Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N, Jacob A, Mirshahi M, Galas L, Rafii S, Le Foll F, Rafii A (2013) Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med 11:94. doi:10.1186/1479-5876-11-94
Patience C, Takeuchi Y, Weiss RA (1997) Infection of human cells by an endogenous retrovirus of pigs. Nat Medicine 3:282–286
Payne LN, Pani PK, Weiss RA (1971) A dominant epistatic gene which inhibits cellular susceptibility to RSV(RAV-O). J Gen Virol 13:455–462
PearseAM, Swift K (2006) Allograft theory: transmission of devil facial-tumour disease. Nature 439: 549
Pellett PE, Ablashi DV, Ambros PF, Agut H, Caserta MT, Descamps V, Flamand L, Gautheret-Dejean A, Hall CB, Kamble RT, Kuehl U, Lassner D, Lautenschlager I, Loomis KS, Luppi M, Lusso P, Medveczky PG, Montoya JG, Mori Y, Ogata M, Pritchett JC, Rogez S, Seto E, Ward KN, Yoshikawa T, Razonable RR Raymund R (2012) Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol 22:144–155
Ptashne M (2004) A genetic switch: phage lambda revisited, 3rd edn. Cold Harbor Spring Laboratory Press, New York. ISBN 978-0-87969-716-7
Pye RJ, Pemberton D, Tovar C, Tubio JM, Dun KA, Fox S, Darby J, Hayes D, Knowles GW, Kreiss A, Siddle HV, Swift K, Lyons AB, Murchison EP, Woods GM (2016) A second transmissible cancer in Tasmanian devils. Proc Natl Acad Sci U S A. 113(2):374–379. doi:10.1073/pnas.1519691113
Radzvilavicius AL, Hadjivasiliou Z, Pomiankowski A, Lane N (2016) Selection for mitochondrial quality drives evolution of the germline. PLoS Biol 14:e2000410. doi:10.1371/journal.pbio.2000410
Rebbeck CA, Leroi AM, Burt A (2011) Mitochondrial capture by a transmissible cancer. Science 331: 303
Rebollo R, Romanish MT, Mager DL (2012) Transposable elements: an abundant and natural source of regulatory sequences for host genes. Annu Rev Genet 46:21–42
Refardt D, Bergmiller T, Kümmerli R (2013) Altruism can evolve when relatedness is low: evidence from bacteria committing suicide upon phage infection. Proc R Soc B 280:20123035
Robbez-Masson L, Rowe HM (2015) Retrotransposons shape species-specific embryonic stem cell networks. Retrovirology 12:45. doi:10.1186/s12977-015-0173-5
Rohrschneider L, Reynolds S (1985) Regulation of cellular morphology by the Rous sarcoma virus src gene: analysis of fusiform mutants. Mol Cell Biol 5:3097–3107
Rosenberg N, Jolicoeur P (1997) Retroviral pathogenesis. In: Coffin JM, Hughes SH, Varmus HE (eds) Retroviruses. Cold Spring Harbor Laboratory Press, pp 475–585
Ross SR (2010) Mouse mammary tumor virus molecular biology and oncogenesis. Viruses 2:2000–2012
Rous P (1911) A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med 13: 397–411. doi:10.1084/jem.13.4.397
Rovnak J, Quackenbush SL (2010) Walleye dermal sarcoma virus: molecular biology and oncogenesis. Viruses 2:1984–1999
Roy SW, Gilbert W (2006). The evolution of spliceosomal introns: patterns, puzzles and progress. Nat Rev Genetics. 7: 211–221. doi:10.1038/nrg1807
Roy-Burman P (1995) Endogenous env elements: partners in generation of pathogenic feline leukemia viruses. Virus Genes 11:147–161
Rubin H, Vogt PK (1962) An avian leukosis virus associated with stocks of Rous sarcoma virus. Virology 17:184–194
Ruboyianes R, Worobey M (2016) Foamy-like endogenous retroviruses are extensive and abundant in teleosts. Virus Evol 2: vew032. doi: 10.1093/ve/vew03
Sagan L (1967) On the origin of mitosing cells. J Theoret Biol 14: 225–274. doi:10.1016/0022-5193(67)90079-3
Sankaran N (2014) When viruses were not in style: parallels in the histories of chicken sarcoma viruses and bacteriophages. Stud Hist Philos Biol Biomed Sci 48:189–199
Sanz-Ramos R, Stoye JP (2013) Capsid-binding retrovirus restriction factors: discovery, restriction specificity and implications for the development of novel therapeutics. J Gen Virol 94:2587–2598. doi:10.1099/vir.0.058180-0
Shimizu A, Nakatani Y, Nakamura T, Jinno-Oue A, Ishikawa O, Boeke JD, Takeuchi Y, Hoshino H (2014) Characterisation of cytoplasmic DNA complementary to non-retroviral RNA viruses in human cells. Sci Rep 4:5074. doi:10.1038/srep05074
Schlesinger S, Goff SP (2015) Retroviral transcriptional regulation and embryonic stem cells: war and peace. Mol Cell Biol 35:770–777
Shimode S, Nakagawa S, Miyazawa T (2015) Multiple invasions of an infectious retrovirus in cat genomes. Sci Rep 5:8164. doi:10.1038/srep08164
Shojima T, Yoshikawa R, Hoshino S, Shimode S, Nakagawa S, Ohata T, Nakaoka R, Miyazawa T (2013) Identification of a novel subgroup of koala retrovirus from koalas in Japanese zoos. J Virol 87:9943–9948
Siddle HV, Kaufman J (2015) Immunology of transmissible tumours. Immunology 144:11–20
Simmons G, Clarke D, McKe J, Young P, Meers J (2014) Discovery of a novel retrovirus sequence in an Australian native rodent (Melomys burtoni): a putative link between gibbon ape leukemia virus and koala retrovirus. PLoS ONE 9:e106954
Søe K, Andersen TL, Hobolt-Pedersen AS, Bjerregaard B, Larsson LI, Delaissé JM (2009) Involvement of human endogenous retroviral syncytin-1 in human osteoclast fusion. J Mol Biol 392:301–318
Stefan E, Bister K (2017) MYC and RAF: key effectors in cellular signaling and major drivers in human cancer. Curr Topics Microbiol Immunol (this Volume)
Stehelin D, Varmus HE, Bishop JM, Vogt PK (1976) DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature 260:170–173
Stern A, Sorek R (2011) The phage-host arms-race: shaping the evolution of microbes. BioEssays 33:43–51. doi:10.1002/bies.201000071
Stewart H, Jarrett O, Hosie MJ, Willett BJ (2011) Are endogenous feline leukemia viruses really endogenous? Vet Immunol Immunopathol 143:325–331
Stoye JP (2012) Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat Rev Microbiol 10:395–406
Strakova A, Ní Leathlobhair M, Wang GD, Yin TT, Airikkala-Otter I, Allen JL, Allum KM, Bansse-Issa L, Bisson JL, Castillo Domracheva A, de Castro KF, Corrigan AM, Cran HR, Crawford JT, Cutter SM, Delgadillo Keenan L, Donelan EM, Faramade IA, Flores Reynoso E, Fotopoulou E, Fruean SN, Gallardo-Arrieta F, Glebova O, Häfelin Manrique RF, Henriques JJ, Ignatenko N, Koenig D, Lanza-Perea M, Lobetti R, Lopez Quintana AM, Losfelt T, Marino G, Martincorena I, Martínez Castañeda S, Martínez-López MF, Meyer M, Nakanwagi B, De Nardi AB, Neunzig W, Nixon SJ, Onsare MM, Ortega-Pacheco A, Peleteiro MC, Pye RJ, Reece JF, Rojas Gutierrez J, Sadia H, Schmeling SK, Shamanova O, Ssuna RK, Steenland-Smit AE, Svitich A, Thoya Ngoka I, Vițălaru BA, de Vos AP, de Vos JP, Walkinton O, Wedge DC, Wehrle-Martinez AS, van der Wel MG, Widdowson SA, Murchison EP (2016) Mitochondrial genetic diversity, selection and recombination in a canine transmissible cancer. eLife 5: e14552. doi:10.7554/eLife.14552
Strand MR, Burke GR (2014) Polydnaviruses: nature’s genetic engineers. Annu Rev Virol 1:335–354. doi:10.1146/annurev-virology-031413-085451
Summers WC (1999) Felix d’Herelle and the origins of molecular biology. Yale University Press, New Haven
Svoboda J (1960) Presence of chicken tumour virus in the sarcoma of adult rat inoculated after birth with Rous sarcoma virus. Nature 186:980–981
Svoboda J (1966) Basic aspects of the interaction of oncogenic viruses with heterologous cells. Int Rev Exp Pathol 5:25–66
Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J Bajzikova M, Kovarova J, Peterka M, Yan B, Pesdar EA, Sobol M, Filimonenko A, Stuart S, Vondrusova M, Kluckova K, Sachaphibulkij K, Rohlena J, Hozak P, Truksa J, Eccles D, Haupt LM, Griffiths LR, Neuzil J, Berridge MV (2015) Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab 21:81–94
Tarlinton RE, Meers J, Young PR (2006) Retroviral invasion of the koala genome. Nature 442:79–81
Taylor DJ, Leach RW, Bruenn J (2010) Filoviruses are ancient and integrated into mammalian genomes. BMC Evol Biol 10:193. doi: 10.1186/1471-2148-10-193
Temin HM (1960) The control of cellular morphology on embryonic cells infected with Rous sarcoma virus in vitro. Virology 10:182–197
Temin HM (1962) Separation of morphological conversion and virus production in Rous sarcoma virus infection. Cold Spring Harb Symp Quant Biol 27:407–414
Temin HM (1964) Nature of the provirus of Rous sarcoma. Natl Cancer Inst Monog 17:557–570
Temin HM, Mizutani S (1970) RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 226:1211–1213
Temin HM, Rubin H (1958) Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology 6:669–688
Thielen GH, Goud D, Fowler M, Dungworth DL (1971) C-type virus in tumor tissue of a woolly monkey (Lagothrix spp) with fibrosarcoma. J Natl Cancer Inst 47:731–743
Ting CN, Rosenberg MP, Snow MP, Samuelson LC, Meisler MH (1992) Endogenous retroviral sequences are required for tissue-specific expression of a human salivary amylase gene. Genes Dev 6:1457–1465
Toyoshima K, Vogt PK (1969) Temperature sensitive mutants of an avian sarcoma virus. Virology 39:930–931
Twort FW (1915) An investigation on the nature of ultramicroscopic viruses. Lancet 2:1241–1243
Van Valen L (1973) A new evolutionary law. Evol Theor 1:1–30
Vargas A, Toufaily C, LeBellego F, Rassart É, Lafond J, Barbeau B (2011) Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod Sci 18:1085–1091
Varmus HE (2017) How tumor virology evolved into cancer biology and transformed oncology. Annu Rev Cancer Biol 1:11.1–11.18 doi:10.1146/annurev-cancerbio-050216-034315
Venables P, Brookes SM, Griffiths D, Weiss RA, Boyd MT (1995) Abundance of an endogenous retroviral envelope protein in placental trophoblasts suggests a biological function. Virology 211:589–592
Vendramin R, Marine JC, Leucci E (2017) Non-coding RNAs: the dark side of nuclear-mitochondrial communication. EMBO J pii: e201695546. doi:10.15252/embj.201695546
Vogt M, Dulbecco R (1960) Virus-cell interaction with a tumor-producing virus. Proc Natl Acad Sci U S A 46:365–370
Vogt PK (1967) A virus released by “nonproducing” Rous sarcoma cells. Proc Natl Acad Sci U S A 58:801–808
Vogt PK (2002) Fortuitous convergences: the beginnings of JUN. Nat Rev Cancer 2:465–469
Vogt PK (2012) Retroviral oncogenes: a historical primer. Nat Rev Cancer 12:639–648
Vogt PK, Hu SS (1977) The genetic structure of RNA tumor viruses. Annu Rev Genet 11:203–238
Vogt PK, Ishizaki R (1965) Reciprocal patterns of genetic resistance to avian tumor viruses in two lines of chickens. Virology 26:664–672
Volz A, Sutter G (2017) Modified Vaccinia Virus Ankara: history, value in basic research, and current perspectives for vaccine development. Adv Virus Res 97:187–243. doi:10.1016/bs.aivir.2016.07.001
Warren JM, Simmons MP, Wu Z, Sloan DB (2016) Linear plasmids and the rate of sequence evolution in plant mitochondrial genomes. Genome Biol Evol 8(2):364–374. doi:10.1093/gbe/evw003
Weiss RA (1967) Spontaneous virus production from “non-virus producing” Rous sarcoma cells. Virology 32:19–23
Weiss RA (2006) The discovery of endogenous retroviruses. Retrovirology 2006(3):67
Weiss RA, Esparza J (2015) The prevention and eradication of smallpox: a commentary on Sloane (1755) ‘An account of inoculation’. Philos Trans R Soc Lond B 370: pii: 20140378. doi:10.1098/rstb.2014.0378
Weiss RA, Kellam P (1997) Illicit viral DNA. Nature 390:235
Weiss RA, Vogt PK (2011) 100 years of Rous sarcoma virus. J Exp Med 208:2351–2355
Woese CR, Fox GE (1977) Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 74:5088–5090
Xu W, Stadler CK, Gorman K, Jensen N, Kim D, Zheng H, Tang S, Switzer WM, Pye GW, Eiden MV (2013) An exogenous retrovirus isolated from koalas with malignant neoplasias in a US zoo. Proc Natl Acad Sci U S A 110:11547–11552. doi:10.1073/pnas.1304704110
Yenerall P, Zhou L (2012) Identifying the mechanisms of intron gain: progress and trends. Biol Direct 7:29. doi:10.1186/1745-6150-7-29
Zhdanov VM (1975) Integration of viral genomes. Nature 256:471–473
Acknowledgements
I am indebted to my mentors early in my career, Warren Levinson, Jan Svoboda and Peter K Vogt for providing a stimulating intellectual environment combined with a rigorous approach to experimental research. I am grateful to Klaus Bister, Ariberto Fassati and Paul Kellam for constructive discussions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Weiss, R.A. (2017). Exchange of Genetic Sequences Between Viruses and Hosts. In: Hunter, E., Bister, K. (eds) Viruses, Genes, and Cancer. Current Topics in Microbiology and Immunology, vol 407. Springer, Cham. https://doi.org/10.1007/82_2017_21
Download citation
DOI: https://doi.org/10.1007/82_2017_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61803-6
Online ISBN: 978-3-319-61804-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)