Abstract
Cold-adapted Ann Arbor based live attenuated influenza vaccine (LAIV) has been available in the USA since 2003. The vaccine is efficacious against influenza infection. Features of LAIV include: easy administration suitable for mass immunization, cross-reactivity to drifted strains for broader coverage, and establishment of herd immunity for control of influenza spread. Annual seasonal LAIV now contains four strains against influenza A H1N1, H3N2, influenza B-Victoria, and B-Yamagata lineages that are co-circulating in humans. LAIV played a significant role in protecting the public from the 2009 H1N1 pandemic and has been evaluated for pandemic preparedness. Pandemic vaccines including influenza H2, H5, H6, H7, and H9 subtypes have been produced and evaluated in preclinical and small-scale phase I clinical studies. This review summarizes the current status and perspectives of seasonal and pandemic LAIV.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Influenza Virus
- Vaccine Virus
- Influenza Vaccine
- Highly Pathogenic Avian Influenza
- Live Attenuate Influenza Vaccine
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Influenza viruses cause significant morbidity and mortality, leading to more than 100,000 hospitalizations and 3,000–49,000 deaths annually (Thompson et al. 2003). Influenza viruses undergo constant genetic drift resulting in emergent antigenic variants that can escape immunity to HA and NA antigens of previously circulating strains. Therefore, influenza vaccines must be updated annually to match the contemporary strains in order to provide optimal protection. Trivalent LAIV (T/LAIV, H1N1, H3N2, one B strain) in a frozen formulation was approved by the US FDA in 2003 for healthy persons 5–49 years of age and the next generation of a liquid formulation was approved for 2–49 year olds in 2007. LAIV is the first new influenza vaccine, as well as the first nasally administered vaccine of any kind for human use, in the USA since the introduction of injectable trivalent influenza vaccine (TIV) in the 1940s (Grabenstein et al. 2006). The formulation of the vaccine was recently changed from trivalent to quadrivalent to account for the epidemiology of B strains in circulation. Two antigenically distinct lineages of influenza B viruses have circulated globally since 1985 and vaccines against one lineage do not offer cross-protection against the other lineage (Rota et al. 1990). In order to provide broader coverage of influenza B viruses an additional B strain is now included in the vaccine. T/LAIV was therefore discontinued in 2013 with the approval and marketing of a quadrivalent LAIV (Q/LAIV, H1N1, H3N2, two B strains).
The development of LAIV is the culmination of over 40 years of collaborative research and development between University of Michigan and scientists from the National Institutes of Health (NIH) and the biopharmaceutical industry (Wyeth, Aviron, and MedImmune). Following the approval in the USA in 2003, T/LAIV was approved in Israel, South Korea, United Arab Emirates, Mexico, and Macau for individuals 2–49 years of age, in Canada for individuals 2–59 years of age and in the European Union for individuals 2–17 years of age. In June 2014, the Advisory Committee on Immunization Practices (ACIP) made a preferential recommendation of LAIV for healthy children ages 2 to 8 years old in the USA.
2 Development of Cold-Adapted Ann Arbor Donor Viruses
LAIV is developed based on two cold-adapted master donor viruses, A/Ann Arbor/6/60 (H2N2) for influenza A vaccines (MDV-A) and B/Ann Arbor/1/66 for influenza B vaccines (MDV-B). Each donor virus donates the cold adapted (ca), temperature sensitive (ts), and attenuated (att) phenotype to the 6:2 reassortant vaccine viruses that contain six internal protein gene segments of MDV and the HA and NA surface proteins of the wild-type (wt) influenza virus. Both MDV-A and MDV-B were developed by Dr. John Maassab at the University of Michigan in the 1960s (Maassab 1967). Influenza A/Ann Arbor/6/60 was subjected to serial in vitro passage at gradually reduced temperature in primary chicken kidney cells (PCKC), 2 passages (2x) at 36–37 °C, 7x at 33 °C, 7x at 30 °C, 7x at 25 °C, 6x plaquing at 25 °C and 3x amplification in embryonated chicken eggs. Influenza B/Ann/Arbor/1/66 was passaged less extensively, two passages (2x) at 36–37 °C, 2x at 33 °C, 5x at 27 °C, 6x at 25 °C, 7x plaquing at 25 °C, and 3x amplification in eggs. During cold passage, the MDV-A and MDV-B acquired a number of genetic changes in multiple gene segments, which confer the ca, ts, and att phenotypes that can be imparted to 6:2 reassortant vaccine strains. The ca phenotype reflects efficient viral replication at a lower temperature of 25 °C, while most wt influenza viruses do not replicate well at this temperature. The ts phenotype reflects restricted replication at a higher temperature at which most wt viruses can replicate well. The shut-off temperature of MDV-A and MDV-B is different: 39 °C for MDV-A and 37 °C for MDV-B. The att phenotype can be measured in the ferret model, viral replication is detected in the nasal tissues but not in the lungs of ferrets intranasally infected with the vaccine virus. The ca/ts/att phenotypes provide safety features of the reassortant vaccine strains that can replicate in the cooler nasal tissues but not at the higher core body temperature of the lungs.
3 Genetic Basis of ca/ts/att Phenotypes of the Vaccine Donor Viruses
3.1 MDV-A
By comparing viral genomic sequences between the wt and ca A/Ann Arbor/6/60 strains and introduction of each of the mutations individually and combination, the amino acids that confer the ts phenotype have been precisely mapped to three residues (E391, G581, T661) in PB1, one residue (S265) in PB2 and one residue in NP (G34) proteins (Table 1) (Jin et al. 2003). These five loci in the PB1, PB2, and NP genes also confer the att phenotype (Jin et al. 2004). The ca phenotype of MDV-A could not be experimentally mapped as the available wt A/Ann Arbor/6/60 strain had been passaged extensively in tissue culture and also grows well at 25 °C. Since the ts and att phenotypes are specified by five residues in three gene segments, the chance for the vaccine virus to revert to wt phenotype is extremely low, explaining genetic stability of the vaccine strains.
Limited studies have been conducted to understand the molecular mechanism of these loci in specifying viral phenotypes. By minigenome analysis, the five loci have been shown to greatly reduce viral RNA-dependent polymerase activity of AA ca at the nonpermissive temperature of 39 °C (Jin et al. 2004). During viral infection in vitro, the vaccine virus can initiate single cycle replication, but multicycle viral replication at 39 °C is significantly reduced. vRNA synthesis and translocation of viral RNP from nucleus to cytoplasm are reduced. In addition, incorporation of the M1 protein into virions is significantly reduced, resulting in irregular viral morphology (Chan et al. 2008).
3.2 MDV-B
The ca/ts/att phenotypes of MDV-B have been mapped by reverse genetics (RG) (Chen et al. 2008; Hoffmann et al. 2005). The ts loci are specified by three amino acids in the PA and NP proteins: M431 in the PA, A114, and H410 in the NP (Table 1). These three residues and Q159 and V183 residues in the M gene segment contribute to the att phenotype. Five residues in three segments confer the ca phenotype: R630 in PB2, M431 in the PA, A114, H410, and T509 in the NP. A total of seven loci distributed in four gene segments of MDV-B control the ca/ts/att phenotypes, making the vaccine donor genetically stable. These loci not only reduce viral polymerase function but also affect virus assembly and release at the restricted temperature (Chan et al. 2008).
4 LAIV by Reverse Genetics and Yearly LAIV Production Process
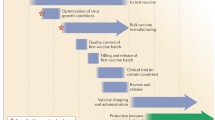
LAIV is manufactured in specific pathogen free (SPF) embryonated chicken eggs. Human influenza viruses normally do not replicate well in eggs and viral replication in eggs often results in mutations in the HA receptor binding region. The quasispecies of HA sequence variations from egg adaptation have different abundance and biological impact. While certain changes improve viral growth in eggs without affecting viral antigenicity and immunogenicity, other changes are not suitable for a vaccine. It is therefore critical to evaluate multiple candidate vaccines in order to select an appropriate HA variant for vaccine production. The application of reverse genetics (RG) technology to the production of 6:2 reassortant vaccine viruses has revolutionized the vaccine seed production process. Traditional classical reassortment, which is a very time consuming and unpredictable method, has been replaced by the use of RG for LAIV production since the 2008–2009 influenza season. The 6:2 reassortant vaccine virus contains six internal protein gene segments from MDV-A or MDV-B and the surface HA and NA glycoprotein gene segments of a wt virus (Fig. 1).
During vaccine seed production, each candidate vaccine variant is evaluated for yield in eggs, antigenicity is assessed by reactivity with a reference antiserum from ferrets immunized with wt virus, and immunogenicity is assessed by examining serum antibody levels achieved in ferrets following intranasal administration of vaccine virus variants. Table 2 summarizes the amino acid sequence changes that have been frequently detected in the HA and their impact on viral biological activities (for a recent review, please see Jin and Chen 2014). Most of the changes do not affect viral antigenicity and immunogenicity, however, several changes identified in the HA have been shown to reduce viral antigenicity or to render vaccine virus less immunogenic (Chen et al. 2010a, b).
Once a LAIV seed is selected, the vaccine virus is purified by one round of limited dilution in eggs to produce a Master Virus Seed (MVS) that is used for bulk production of monovalent vaccine. Each vaccine lot is subjected to a lot release test to ensure that no adventitious agents are present. The four vaccine viruses, H1N1, H3N2, B-Yamagata, and B-Victoria, are formulated to contain 107.0±0.5 FFU (fluorescent focus units)/strain in 0.2-mL in a nasal sprayer. If a new vaccine virus component is incorporated into the annual seasonal vaccine, the vaccine is tested in a small-scale safety trial prior to final release of the vaccine for widespread use.
5 Preclinical Studies of LAIV
Extensive studies have been performed to evaluate vaccine viruses for their attenuated replication, immunogenicity, and protection against wt influenza virus challenge infection in the ferret model. Vaccine viruses have been shown to be able to replicate in the nasal turbinates (NT) but not in the lungs of vaccinated ferrets. Although vaccine strains in general elicit hemagglutination inhibition (HAI) antibodies at levels lower than that induced by homologous wt virus (MedImmune data), they provide significant protection against replication of homologous challenge virus in the upper and lower respiratory tract (LRT) of ferrets.
Ferret studies have been conducted to compare T/LAIV with Q/LAIV in protection against wt challenge infection to address vaccine interference (Bandell et al. 2011). Q/LAIV is comparable to T/LAIV in vaccine-induced protective immune responses against wt virus replication in the upper and LRT of vaccinated ferrets (Fig. 2). The vaccine strains A/California/7/2009 (A/CA/09, H1N1), A/Perth/16/2009 (A/Perth/09, H3N2), B/Brisbane/60/2008 (B/Bris/08, Victoria lineage), and B/Wisconsin/1/2010 (B/Wis/10, B/Yamagata lineage) had comparable immunogenicity given in either Q/LAIV or T/LAIV formulation, elicited robust antibody responses to each vaccine component and fully protected ferrets from wt virus challenge in the lungs after two doses of vaccine. Each vaccine strain also offered significant protection from wt virus replication in the NT. These studies indicate that LAIV strains in multivalent vaccine formulations do not demonstrate evidence of viral or immune interference affecting efficacy of each vaccine component.
Challenge virus titers in NT and lung of ferrets vaccinated with Q/LAIV or T/LAIV. Ferrets were vaccinated with sucrose phosphate (SP) buffer, T/LAIV1 (A/CA/09, A/Perth/09, B/Bris/08), T/LAIV2 (A/CA/09, A/Perth/09, B/Wis/10), or Q/LAIV (A/CA/09, A/Perth/09, B/Bris/08 and B/Wis/10) in two doses one month apart. The ferrets were challenged with indicated honologous wt viruses with the exception of the H3N2 A/Rhode Island/2010 strain as wt A/Perth/09 did not replicate well in ferret lungs. Viral titers in the NT and lung tissues were determined by 50 % egg infectious dose (EID50) assay. The limit of detection of the assay was 1.5 log10EID50/g
6 Clinical Studies of LAIV
Clinical studies of Ann Arbor (AA) ca-based LAIV strains in the USA include monovalent, bivalent including two type A strains, trivalent including H1N1, H3N2 and a B strain, and quadrivalent including two type A and two B strains (for review see Murphy and Coelingh 2002). T/LAIV has been evaluated in more than 73 clinical research trials completed worldwide in >141,000 people ranging in age from 6 weeks to >90 years. Approximately 80 million doses have been distributed for commercial use since the initial US licensure in 2003 up through March 2014 (LAIV Scientific Product Monograph 2013–2014, MedImmune, Gaithersburg, MD).
6.1 Safety
LAIV is generally well tolerated and safe (reviewed by Ambrose et al. 2011). The vaccine viruses infect and replicate in cells lining the nasopharynx of the recipient to induce immunity, but are not able to replicate in the LRT due to their ts and att phenotypes. The most common solicited adverse reactions are runny nose or nasal congestion (ages 2 to 49 years), fever >100 °F (ages 2–6 years), and sore throat (18–49 years). The rate of headache and tiredness in LAIV recipients is higher than in a placebo control group but is similar to TIV recipients (Baxter et al. 2012a, b; Toback et al. 2013). One study showed that LAIV was associated with an increased rate of all-cause hospitalization among children aged 6–11 months and an increased rate of medically attended wheezing in children aged 6–23 months (Belshe et al. 2007). For this reason, LAIV is not approved for children younger than 24 months of age.
6.2 Transmission and Genetic Stability
Vaccine viruses can be cultured from nasal secretions in the first few days after vaccination. The relationship of viral replication in a vaccine recipient to transmission of vaccine viruses to other individuals has not been well established. LAIV is poorly transmissible because most infection is not symptomatic and this decreases the likelihood of viral spread via cough or sneezing. In addition, levels of vaccine virus replication in nasal tissues are much lower than wt virus even in seronegative children making its spread to contacts very inefficiently (Murphy and Coelingh 2002). In studies performed to date, viruses shed from vaccine recipients have been consistently phenotypically and genotypically stable, maintaining the ca, ts, and att phenotypes (Cha et al. 2000; Vesikari et al. 2006). LAIV has been shown to be poorly transmissible to spouses, roommates, and household members under a variety of circumstances in small clinical trials (Murphy and Coelingh 2002). Eighty percent of trivalent LAIV recipients who were 6–36 month old children in a day care setting, shed at least one vaccine strain, with a mean duration of shedding of 7.6 days ranging from 1 to 21 days (Vesikari et al. 2006). Transmission of vaccine viruses from vaccine recipients to placebo subjects was a rare event. The ca and ts phenotypes were preserved in all recovered viruses tested (n = 135 tested of 250 strains isolated at the local laboratory). The probability of a young child acquiring vaccine virus after close contact with a single trivalent LAIV vaccinee in a day care setting was 0.58 % (95 % CI: 0, 1.7) based on the Reed Frost model (Longini et al. 1982). With documented transmission of type B virus in one placebo subject and possible transmission of type A virus in four placebo subjects, the maximum probability of acquiring a transmitted vaccine virus was estimated to be 2.4 %.
6.3 Efficacy
The efficacy and effectiveness of an influenza vaccine can be evaluated by three criteria: (1) comparison of culture-positive influenza infection rates, which are most feasible in young children because they readily shed influenza virus (i.e., vaccine efficacy); (2) a 4-fold antibody increase from baseline levels, which is subject to inherent bias from prior vaccine or natural disease exposure, and therefore is a method of limited value; or (3) observations of clinical events or “medically attended acute respiratory illness” (MAARI) which is a less specific endpoint than culture-confirmed influenza illness, and results in effectiveness point estimates that are significantly lower than efficacy estimates. Adults shed virus in low quantity and for a short duration and thus clinical trials in adults are more commonly conducted using clinical endpoints (Belshe et al. 2004). LAIV efficacy trials in the pediatric population consist of nine controlled studies comprising over 20,000 infants and toddlers, children, and adolescents, during seven influenza seasons (summarized in LAIV Scientific Product Monograph 2013–2014, MedImmune, Gaithersburg, MD). Four placebo-controlled studies were conducted to include revaccination in a second season. Overall, LAIV has an efficacy of 62–93 % against antigenically matched strains and 49–93 % against all strains in children 15–71 months of age. LAIV has demonstrated superiority compared to TIV in 3 active-controlled studies in children. LAIV is about 44 % (range 34.7–52.7 %) better than TIV for matched strains and 31.9–54.9 % better than TIV for all strains. In one of the largest field efficacy trials (MI-CP111), LAIV was shown to be more efficacious than inactivated TIV in children 6–59 months of age (Belshe et al. 2007). Because of the higher efficacy of LAIV in children, LAIV is preferentially recommended in the UK, Germany, Israel, Canada, and Sweden for children of various age groups (Ambrose et al. 2012), and recently for 2–8 year old children in the USA.
LAIV efficacy in adults has been demonstrated in two efficacy trials (Nichol et al. 1999b; Treanor et al. 2000). In the first trial, LAIV was shown to reduce laboratory-documented influenza illness by 85 % compared to TIV in a challenge study conducted in healthy adults 18–41 years of age who were presumed to be susceptible to at least one strain included in the vaccine based on prevaccination antibody titers (Treanor et al. 2000). In the second, larger trial, LAIV recipients exhibited significant reduction in days of febrile illness, missed work, health care provider visits, and antibiotic usage. The efficacy of LAIV and TIV can be affected by a number of factors, such as the age and health of the vaccine recipients and the extent of antigenic similarity between the vaccine strains and circulating strains. In a study conducted by Monto et al. (Monto et al. 2009), LAIV was 50 % less efficacious than TIV in reduction of laboratory-confirmed influenza during the 2008–2009 influenza season when an H3N2 virus was the predominant circulating strain. Based on a subgroup analysis of subjects 50–64 years of age in the study by Nichol et al. (1999a), LAIV was not approved for this age group in the USA. A later study showed that LAIV offered statistically significant protection against culture-confirmed influenza in adults ≥60 years of age (De Villiers et al. 2009).
6.4 Immunogenicity
The immunogenicity of 19 different LAIV strains was studied over a period of 25 years at various locations and in different populations (Murphy and Coelingh 2002), and annual commercial vaccines have been evaluated over the past 10 years. Protection against influenza generally correlates with serum IgG hemagglutination-inhibiting antibodies (HAI), especially in seronegative children. After two doses of LAIV, children who were presumed to be susceptible to at least one strain included in the vaccine based on prevaccination antibody titers, mounted an adequate HAI response (>90 % seroconverted to type A/H3 and B strains, and 60–90 % to type A/H1 strain) (Belshe et al. 1998, 2000; Zangwill 2003). Antibodies persisted for 5–8 months after vaccination with LAIV, and protection generally persisted for at least 1 year (Zangwill 2003). In a study of young children, protective efficacy lasted for the duration of the influenza season and as late as 5.5–13 months after the second dose (Ambrose et al. 2008; Tam et al. 2007). In adults, the serologic response has been less robust (<35 % for A/H3 and B and 60–90 % for A/H1), and the correlates of immunity may be related to other immune responses (Gorse et al. 1995; Tomoda et al. 1995; Zangwill 2003). LAIV may be more effective than IIV in inducing a nasal IgA response that is important for viral clearance and recovery, whereas IIV vaccine more consistently elicits serum HA antibodies in adults (Beyer et al. 2002; Cox et al. 2004; Renegar et al. 2004). The role of cell-mediated immune responses in the protection of young children against influenza was studied in a large randomized, double-blind, placebo-controlled dose-ranging efficacy trial with 2,172 children of 6 to <36 months old in Philippines and Thailand (Forrest et al. 2008). LAIV was found to elicit substantial CMI responses as measured by interferon-gamma ELISPOT assay that correlated with protection. Another study conducted in children showed that LAIV induced cell-mediated responses including CD4(+), CD8(+), and γδ T cells that are relevant for broadly protective heterosubtypic immunity (Hoft et al. 2011). In a study conducted in young adults, although TIV induced higher levels of vaccine-specific plasmablasts and plasmablast-derived polyclonal antibodies (PPAb) than LAIV, LAIV induced a greater vaccine-specific IgA plasmablast response as well as a greater plasmablast response to the conserved influenza nucleoprotein and better cross-reactivity to heterologous strains than TIV (Sasaki et al. 2014).
7 Pandemic LAIV
There are 18 known HA and 11 known NA subtypes of influenza A viruses in nature; 16 HA and 9 NA subtypes have been isolated from waterfowl and shorebirds, and a variety of subtypes have been isolated from other animal species including pigs, horses, and dogs. Animal influenza viruses are the source from which novel HA and NA subtypes are introduced into the human population, by reassortment with other animal or human influenza viruses or direct infection of humans. In the last century, influenza pandemics occurred in 1918, 1957, and 1968 and the first pandemic of this century occurred in 2009. Each of these pandemics was associated with significant morbidity and mortality. Since 1997, animal H5N1, H6N1, H7N7, H7N3, H7N9, H9N2, and H10N8 viruses have caused human infections but have not spread efficiently from person to person. The pandemics and the sporadic emergence of animal viruses into humans underline the need for the generation of pandemic influenza vaccines and their evaluation in humans. The criteria for licensure of currently licensed inactivated influenza vaccines are protective antibodies directed primarily against the HA, the major protective antigen of the virus that induces neutralizing antibody and/or demonstrated efficacy.
LAIV have several attributes related to safety, immunogenicity, cross-protection against antigenic drift strains, high yield, and needle-free administration that make them attractive candidates for control of pandemic influenza. LAIV generally induce broadly cross-reactive protection (Coelingh et al. 2014; Murphy and Coelingh 2002), which may be a useful feature in the event of a pandemic if a vaccine generated from the emerging pandemic strain is not immediately available. Importantly, the infrastructure for manufacture and distribution of a LAIV exists. Therefore, the United States NIH and MedImmune undertook a joint effort to develop and evaluate LAIV bearing the HA and NA genes from animal influenza viruses on the MDV-A backbone as pandemic LAIV (pLAIV) candidates. Our approach includes: (1) generation of a pLAIV bearing an HA and appropriate NA from an animal influenza virus on the attenuated MDV-A background; (2) evaluation of the attenuation, immunogenicity, and protective efficacy of the candidate vaccine in animal models; (3) preparation and qualification of a clinical lot of each pandemic vaccine candidate; (4) evaluation of the safety, infectivity, and immunogenicity of each candidate in humans; (5) storage of human sera obtained from vaccinees to determine cross-reactivity with the newly emerged pandemic viruses; and (6) storage of seed viruses for use in the manufacture of vaccine to prevent disease caused by related pandemic viruses that do emerge such that vaccine manufacture can be initiated with pretested vaccines without delay.
A theoretical concern associated with the use of a pLAIV bearing genes derived from an animal influenza virus is the risk of reassortment of the vaccine virus with a circulating influenza virus, resulting in a novel subtype of influenza that could spread in the human population. Although such a reassortment event may not be of great significance in the face of widespread disease caused by a pandemic influenza strain, it would be an unfavorable outcome if the threatened pandemic did not materialize. This risk would be carefully considered by public health authorities before a decision is made to introduce a live attenuated vaccine in a threatened pandemic. With the exception of one H9N2 ca virus, the pLAIV viruses were generated by RG. The HAs of highly pathogenic avian influenza (HPAI) H5 and H7 viruses were modified to remove the multibasic amino acid cleavage motif that is a known virulence determinant for poultry.
7.1 Preclinical Studies
We have developed pLAIV candidates against 6 different subtypes (H1, H2, H5, H6, H7, and H9) (Chen et al. 2003, 2009a, b, 2011b, 2014; Joseph et al. 2008; Min et al. 2010; Suguitan et al. 2006). The genetic loci responsible for the ts and att phenotypes associated with the MDV-A were confirmed in each of the pLAIV viruses. The replication of the pLAIV viruses was evaluated in the respiratory tract of ferrets 3 days following intranasal administration of 107 TCID50 of viruses. As discussed previously, highly restricted replication in the LRT of ferrets defines the att phenotype of the pLAIV strains (Table 3). Some pLAIV viruses (H2, H5, and H7N3) replicated to high titer in the upper respiratory tract (URT) of ferrets, while others such as the H6N1, H7N7, and H9N2 vaccine viruses were restricted in replication in the URT. The pLAIV were also evaluated for replication in mice 2, 3, and 4 days following intranasal administration of 106 TCID50 of virus (Table 3). The body temperature of mice is 37 °C, which is below the shut-off temperature of the ca viruses. Therefore, although the replication of the pLAIV viruses in mice is restricted compared to the corresponding wt viruses, they are not as restricted in replication in mice as they are in ferrets that have a core body temperature of ~39 °C.
The immunogenicity of the pLAIV was evaluated in mice and ferrets by measuring the serum antibody response by HAI and/or microneutralization (MN) assays against homologous and heterologous viruses following intranasal administration of one or two doses of pLAIV; neutralizing antibody (MN) titers are reported in Tables 4 and 5. The pH1N1 LAIV that was used for vaccine production incorporated two mutations in the HA gene that improved the yield in eggs and immunogenicity in ferrets (Chen et al. 2010a). The homologous antibody response to one dose of vaccine in mice and ferrets ranged from poor (VN04 H5N1 and H7N7 vaccines) to robust (H6N1 and H9N2 vaccines) (Tables 4 and 5) but a correlation between the magnitude of the antibody response and replication of the pLAIV in mice or ferrets was not apparent (Tables 3, 4 and 5). Notably, in all cases, a second dose of vaccine boosted serum antibody responses against homologous and heterologous viruses.
Protective efficacy of pLAIVs was assessed by determining the ability of one or two doses of intranasally administered vaccine to protect mice from lethal challenge with wt virus or to prevent replication of wt challenge virus in the URT and LRT of mice and ferrets (Tables 4 and 5). Even vaccines that were poorly immunogenic (e.g., VN04 H5N1 and H7N7 vaccines) provided complete protection from lethal challenge following one dose of vaccine (Min et al. 2010; Suguitan et al. 2006). Two doses of the pLAIVs provided complete protection from replication of homologous wt challenge viruses in the URT and LRT of mice and LRT of ferrets; challenge virus titers in the URT of ferrets were reduced compared to mock-immunized animals but protection in the URT was not always complete (Tables 4 and 5). A single dose of vaccine conferred partial to complete protection from challenge virus replication; higher serum antibody titers (MN >80 and HAI of 320) were associated with complete protection. The VN04 H5N1 and H7N7 vaccines that failed to induce a robust antibody response conferred only partial protection following a single dose of vaccine, with a reduction in titer of challenge virus in the range of 40 to 1500-fold compared to mock-immunized animals (Min et al. 2010; Suguitan et al. 2006).
Because it is not possible to predict which strain within a subtype will cause a pandemic and influenza viruses continue to evolve in nature, we evaluated the antibody response and protection from challenge with heterologous viruses. In fact, the data confirmed our hypothesis that a pLAIV would elicit a cross-reactive antibody response against other strains from the same subtype. Two doses of pLAIV elicited cross-reactive antibodies and provided near complete protection from replication of heterologous wt viruses in the URT and LRT of mice and LRT of ferrets (Tables 4 and 5).
Another important caveat of preclinical evaluation of pLAIVs is that the studies are generally carried out in influenza-naïve unprimed animals while humans with a prior history of influenza infection or vaccination are immunologically primed. This difference was striking in the H1N1 pandemic in 2009: although people younger than 60 years of age were seronegative to the H1N1pdm virus, all but the youngest children responded to a single dose of inactivated H1N1pdm vaccine while unprimed animals required two doses of inactivated vaccine. We investigated the ability of wt seasonal H1N1 virus, seasonal H1N1 LAIV and seasonal TIV to prime mice for an antibody and cellular immune response to a single dose of H1N1pdm LAIV (Chen et al. 2011a) and found that prior exposure to live seasonal H1N1 virus, wt, or LAIV, primed mice but seasonal TIV did not. While two doses of H1N1pdm LAIV induced a robust neutralizing serum antibody response, mice that were primed with seasonal H1N1 infection or seasonal LAIV followed by one dose of H1N1pdm LAIV were equally well protected from challenge, in the absence of neutralizing serum antibody (Chen et al. 2011a). Cheng et al. compared TIV and LAIV in ferrets and found that LAIV was superior to TIV in inducing influenza-specific immunity in naïve ferrets (Cheng et al. 2013). Although both types of vaccines induced comparable humoral immune responses in previously primed ferrets, only LAIV provided partial protection from heterologous seasonal H1N1 virus challenge (Cheng et al. 2013). The findings in LAIV-immunized naïve ferrets may explain the efficacy of LAIV in young children.
7.2 Clinical Studies
The pLAIVs described above have been evaluated in phase I clinical trials in small cohorts of healthy adults younger than 50 years of age (Karron et al. 2009a, b; Talaat et al. 2009, 2011, 2012). In order to minimize the risk of reassortment between a pLAIV bearing novel HA and NA genes and circulating human influenza viruses, the clinical trials are conducted in an inpatient unit during months when seasonal influenza activity is not detected, typically between April and December. Vaccine recipients are admitted to the inpatient unit a day prior to vaccine administration in case they are incubating an intercurrent respiratory virus infection and also to determine whether they can adjust to a 9–10 day stay in the inpatient unit. The studies are conducted as open-label inpatient trials with all participants receiving vaccine. Vaccine is administered intranasally as a nasal spray except for the H9N2 ca vaccine that was administered as nose drops. Participants are examined daily while on the inpatient unit by a health care provider. After discharge from the inpatient unit, vaccinees are asked to return to the outpatient clinic. At each visit, staff obtain vital signs, review interim histories, and obtain blood and nasal wash (NW) samples for antibody testing (Coelingh et al. 2014).
Vaccine safety is assessed through daily examinations and infectivity is assessed by viral culture and by realtime reverse transcription-polymerase chain reaction testing of NW specimens. Immunogenicity is assessed by measuring HAI antibodies, neutralizing antibodies, and IgG or IgA antibodies to recombinant HA in serum or NW. As reviewed by Coelingh et al. (2014), the pLAIVs were restricted in replication and were variably and generally not immunogenic in terms of antibody responses in humans. These findings were unexpected because it was anticipated that adults receiving pLAIVs bearing novel HA and NA proteins would respond to the vaccines like children receiving seasonal LAIV, shedding vaccine virus for several days, and developing serum and/or NW antibody responses. However, the H5N1 pLAIV appears to have established long term immune memory because when H5N1 pLAIV vaccine recipients were re-called and given a dose of unadjuvanted inactivated subunit vaccine, a rapid robust and high quality antibody response was detected (Talaat et al. 2014). This observation is being explored further in a series of ongoing clinical trials that will provide insights into the durability of pLAIV-mediated immune memory by determining the optimal interval between the LAIV and inactivated vaccine.
8 Mediators and Correlates of Protection
While serum HAI antibody is a correlate of protection for inactivated influenza virus vaccines, it is not an absolute correlate of protection for LAIV. In addition to serum antibody responses, seasonal LAIVs induce mucosal and cell-mediated immunity (Hoft et al. 2011). Although protective efficacy and effectiveness of seasonal LAIV have been repeatedly demonstrated in preclinical and clinical studies, an immune correlate of protection has not been identified. As reviewed by Bandell et al. (2011), seasonal LAIV was efficacious even in the absence of a rise in serum HAI antibody.
8.1 Studies in Animal Models
The role of humoral and cellular immune mediators and distribution of immune effectors induced by eight different LAIVs at mucosal and systemic sites were evaluated in mice (Lau et al. 2011). All LAIVs tested induced robust systemic immune responses but variable pulmonary immunity. The magnitude of lung immunity, including pulmonary IgA antibody and memory CD8+ T lymphocytes, induced by the vaccines depended on the replication efficiency of the LAIVs and the induction of cytokines/chemokines in the lungs. Both cellular and humoral immunity contribute to the protection provided by LAIV; the relative contribution of the two effector arms in viral clearance depends on the location and the rate of replication of a particular vaccine virus. The relevance of these findings for the human experience was not clear because LAIVs do not replicate in the LRT of humans. Therefore, an upper respiratory tract immunization (URTI) model was developed in mice to mimic the human situation. This permitted assessment of the protective efficacy of an H5N1 LAIV against highly pathogenic H5N1 virus challenge in the absence of significant pulmonary immunity (Lau et al. 2012). The experiments demonstrated that cellular immunity in the lungs is essential for protection against lethal wild-type H5N1 challenge, whereas influenza-specific serum ELISA antibodies and splenic influenza-specific CD8+ CTLs made little contribution to this protection. Optimal protection against wild-type virus challenge requires maturation of humoral responses, with the development of neutralizing activity. Passive transfer of postvaccination serum to naïve mice demonstrated that the magnitude of the humoral response and access of antibodies to the respiratory tract are equally important determinants of protection (Lau et al. 2012).
In a recent study comparing LAIV and TIV in ferrets, while TIV only induced immune responses in primed ferrets, LAIV induced influenza-specific antibody and T cell responses in both naïve and primed ferrets (Cheng et al. 2013). In addition to influenza-specific serum IgA and IgG antibodies, CD4+ and CD8+ T cells were demonstrated in the circulation and in paratracheal lymph nodes and the latter correlated with protection from challenge virus replication in the URT (Cheng et al. 2013).
8.2 Studies in Humans
Immunity to influenza A viruses in humans is conferred primarily by antibodies directed at the HA and NA glycoproteins. Both serum IgG and mucosal IgA antibodies can independently contribute to resistance to influenza virus in humans (Clements et al. 1986), with serum IgG antibodies providing protection primarily to the LRT and IgA antibodies providing protection primarily to the URT (reviewed in Murphy and Coelingh 2002). Inactivated influenza vaccines induce higher titers of serum antibodies than LAIV in primed individuals, but LAIV are more efficient in inducing mucosal IgA antibody responses (Clements et al. 1986). Thus, serum HAI antibody is not an absolute correlate of protection for LAIV (Ambrose et al. 2008; Edwards et al. 1994; Treanor et al. 1999). Instead, NW IgA induced by LAIV or natural infection is associated with resistance to reinfection. LAIV viruses are shed for a longer duration and to higher titers in immunologically naive, seronegative children than in seronegative adults who have previous experience with homotypic influenza viruses (Murphy and Coelingh 2002). Homotypic immunity induced by prior infection with a drift variant restricts replication of LAIVs in seronegative adults or children to lower titer and for a shorter duration (reviewed in Murphy and Coelingh 2002). In seropositive adults and children, generally only the subset of vaccinees with low preexisting nasal HA-specific IgA antibody titer become infected and they shed a very small quantity of virus.
Although the contribution of CD8+ T cells to protection from influenza virus infection in humans is less clear than in mice and ferrets, the induction of influenza-specific CD4+, CD8+, and γδ T cells by LAIVs has been demonstrated in children (He et al. 2006; Hoft et al. 2011). In a large field study of seasonal LAIV administered to children, >100 spot forming cells in an interferon-gamma (IFN-γ) ELIspot assay was associated with vaccine efficacy (Forrest et al. 2008). In a prospective study in the UK during the 2009 pandemic, Sridhar et al. found that in the absence of cross-reactive neutralizing antibodies, CD8+ T cells specific to conserved viral epitopes correlated with cross-protection against symptomatic influenza. Higher frequencies of preexisting T cells to conserved CD8 epitopes were found in individuals who developed less severe illness, with total symptom score having the strongest inverse correlation with the frequency of IFN-γ+ interleukin-2- CD8+ T cells (Sridhar et al. 2013).
A robust Type I (IFNγ) memory response was observed including production of cytokines (GM-CSF, IL-1β, IFNα, IL-6) and chemokines (CCL5 and CXCL8 early and CCL2 and CXCL10 late) involved in T cell activation and recruitment, when peripheral blood mononuclear cells from LAIV recipients were cultured with LAIV (Lanthier et al. 2011). Influenza and respiratory syncytial virus infections have been shown to elicit different innate immune signatures by microarray and transcriptomics studies (Herberg et al. 2013), suggesting that LAIVs may also be associated with an innate immune signature. LAIV induced higher expression of type I IFN and interferon stimulated genes (ISGs) than TIV in young children where their genome-wide transcript profiles in whole blood were compared at 7 days following vaccination with LAIV or TIV (Zhu et al. 2010); some of these changes may serve as biomarkers of early responses to LAIV.
9 Future Directions
Live attenuated influenza vaccines are important public health tools for the prevention of seasonal and pandemic influenza. The ability to rapidly produce a vaccine for use in the event of a pandemic requires appropriate infrastructure and capacity that are built on experience with seasonal influenza vaccine. For both seasonal LAIV and pLAIV, the identification of a biomarker that is a reliable immune correlate of protection is a high priority, because this will allow a vaccine against a novel influenza virus to be licensed rapidly in the event of a pandemic threat. The observation that pLAIVs induce long-term immune memory requires further investigation and the feasibility of implementing this immunization strategy needs to be assessed.
Abbreviations
- LAIV :
-
Live attenuated influenza vaccine
- T/LAIV :
-
Trivalent live attenuated influenza vaccine
- Q/LAIV :
-
Quadrivalent live attenuated influenza vaccine
- pLAIV :
-
Pandemic live attenuated influenza vaccine
- TIV:
-
Trivalent inactivated influenza vaccine
- IIV:
-
Inactivated influenza vaccine
- MDV-A:
-
Master donor virus for influenza A vaccines
- MDV-B:
-
Master donor virus for influenza B vaccines
- ca :
-
Cold adapted
- ts :
-
Temperature sensitive
- att :
-
Attenuated
- wt :
-
Wild-type
- RG:
-
Reverse genetics
- PCKC:
-
Primary chicken kidney cells
- NT:
-
Nasal turbinates
- HAI:
-
Hemagglutination inhibition
- NW:
-
Nasal wash
References
Ambrose CS, Luke C, Coelingh K (2008) Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influenza Other Respir Viruses 2:193–202
Ambrose CS, Wu X, Knuf M, Wutzler P (2012) The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta-analysis of 8 randomized controlled studies. Vaccine 30:886–892
Ambrose CS, Yi T, Falloon J (2011) An integrated, multistudy analysis of the safety of Ann Arbor strain live attenuated influenza vaccine in children aged 2–17 years. Influenza Other Respir Viruses 5:389–397
Bandell A, Woo J, Coelingh K (2011) Protective efficacy of live-attenuated influenza vaccine (multivalent, Ann Arbor strain): a literature review addressing interference. Expert Rev Vaccines 10:1131–1141
Baxter R, Toback SL, Sifakis F, Hansen J, Bartlett J, Aukes L, Lewis N, Wu X, Ambrose CS (2012a) A postmarketing evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in adults 18–49 years of age. Vaccine 30:3053–3060
Baxter R, Toback SL, Sifakis F, Hansen J, Bartlett J, Aukes L, Lewis N, Wu X, Ambrose CS (2012b) A postmarketing evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in children 5 through 17 years of age. Vaccine 30:2989–2998
Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM (2007) Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 356:685–696
Belshe RB, Gruber WC, Mendelman PM, Cho I, Reisinger K, Block SL, Wittes J, Iacuzio D, Piedra P, Treanor J, King J, Kotloff K, Bernstein DI, Hayden FG, Zangwill K, Yan L, Wolff M (2000) Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr 136:168–175
Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, Bernstein DI, Hayden FG, Kotloff K, Zangwill K, Iacuzio D, Wolff M (1998) The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med 338:1405–1412
Belshe RB, Nichol KL, Black SB, Shinefield H, Cordova J, Walker R, Hessel C, Cho I, Mendelman PM (2004) Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5–49 years. Clin Infect Dis. 39:920–927
Beyer WE, Palache AM, de Jong JC, Osterhaus AD (2002) Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine 20:1340–1353
Cha TA, Kao K, Zhao J, Fast PE, Mendelman PM, Arvin A (2000) Genotypic stability of cold-adapted influenza virus vaccine in an efficacy clinical trial. J Clin Microbiol 38:839–845
Chan W, Zhou H, Kemble G, Jin H (2008) The cold adapted and temperature sensitive influenza A/Ann Arbor/6/60 virus, the master donor virus for live attenuated influenza vaccines, has multiple defects in replication at the restrictive temperature. Virology 380:304–311
Chen GL, Lamirande EW, Cheng X, Torres-Velez F, Orandle M, Jin H, Kemble G, Subbarao K (2014) Evaluation of three live attenuated h2 pandemic influenza vaccine candidates in mice and ferrets. J Virol 88:2867–2876
Chen GL, Lamirande EW, Jin H, Kemble G, Subbarao K (2009a) Safety, immunogencity, and efficacy of a cold-adapted A/Ann Arbor/6/60 (H2N2) vaccine in mice and ferrets. Virology 398:109–114
Chen Z, Santos C, Aspelund A, Gillim-Ross L, Jin H, Kemble G, Subbarao K (2009b) Evaluation of live attenuated influenza a virus H6 vaccines in mice and ferrets. J Virol 83:65–72
Chen GL, Lau YF, Lamirande EW, McCall AW, Subbarao K (2011a) Seasonal influenza infection and live vaccine prime for a response to the 2009 pandemic H1N1 vaccine. Proc Natl Acad Sci USA 108:1140–1145
Chen GL, Min JY, Lamirande EW, Santos C, Jin H, Kemble G, Subbarao K (2011b) Comparison of a live attenuated 2009 H1N1 vaccine with seasonal influenza vaccines against 2009 pandemic H1N1 virus infection in mice and ferrets. J Infect Dis 203:930–936
Chen H, Matsuoka Y, Swayne D, Chen Q, Cox NJ, Murphy BR, Subbarao K (2003) Generation and characterization of a cold-adapted influenza A H9N2 reassortant as a live pandemic influenza virus vaccine candidate. Vaccine 21:4430–4436
Chen Z, Aspelund A, Kemble G, Jin H (2008) Molecular studies of temperature-sensitive replication of the cold-adapted B/Ann Arbor/1/66, the master donor virus for live attenuated influenza FluMist vaccines. Virology 380:354–362
Chen Z, Wang W, Zhou H, Suguitan AL Jr, Shambaugh C, Kim L, Zhao J, Kemble G, Jin H (2010a) Generation of live attenuated novel influenza virus A/California/7/09 (H1N1) vaccines with high yield in embryonated chicken eggs. J Virol 84:44–51
Chen Z, Zhou H, Jin H (2010b) The impact of key amino acid substitutions in the hemagglutinin of influenza A (H3N2) viruses on vaccine production and antibody response. Vaccine 28:4079–4085
Cheng X, Zengel JR, Suguitan AL Jr, Xu Q, Wang W, Lin J, Jin H (2013) Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J Infect Dis 208:594–602
Clements ML, Betts RF, Tierney EL, Murphy BR (1986) Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol 24:157–160
Coelingh K, Talaat KR, Luke CJ, Jin H, Karron RA (2014) Development of live attenuated influenza vaccines against pandemic influenza strains. Expert Rev Vaccines 7:855–871
Cox RJ, Brokstad KA, Ogra P (2004) Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 59:1–15
De Villiers PJ, Steele AD, Hiemstra LA, Rappaport R, Dunning AJ, Gruber WC, Forrest BD, Network LEST (2009) Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older. Vaccine 28:228–234
Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF (1994) A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis 169:68–76
Forrest BD, Pride MW, Dunning AJ, Capeding MR, Chotpitayasunondh T, Tam JS, Rappaport R, Eldridge JH, Gruber WC (2008) Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol 15:1042–1053
Gorse GJ, Campbell MJ, Otto EE, Powers DC, Chambers GW, Newman FK (1995) Increased anti-influenza A virus cytotoxic T cell activity following vaccination of the chronically ill elderly with live attenuated or inactivated influenza virus vaccine. J Infect Dis 172:1–10
Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ (2006) Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev 28:3–26
He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM (2006) Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol 80:11756–11766
Herberg JA, Kaforou M, Gormley S, Sumner ER, Patel S, Jones KD, Paulus S, Fink C, Martinon-Torres F, Montana G, Wright VJ, Levin M (2013) Transcriptomic profiling in childhood H1N1/09 influenza reveals reduced expression of protein synthesis genes. J Infect Dis 208:1664–1668
Hoffmann E, Mahmood K, Chen Z, Yang CF, Spaete J, Greenberg HB, Herlocher ML, Jin H, Kemble G (2005) Multiple gene segments control the temperature sensitivity and attenuation phenotypes of ca B/Ann Arbor/1/66. J Virol 79:11014–11021
Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, Gerber MA, Bernstein DI, Newman F, Graham I, Anderson EL, Belshe RB (2011) Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 204:845–853
Jin H, Chen Z (2014) Production of live attenuated influenza vaccines against seasonal and pandemic influenza viruses. Curr Opin Virol 6:34–39
Jin H, Lu B, Zhou H, Kemble G (2004) Genetic studies of FluMist influenza vaccines derived from cold adapted A/Ann Arbor/6/60, options for the control of inluenza V. Elsevier, Okinawa. In: Kawaoka Y (ed), Int Congr Ser 1263, pp 153–156
Jin H, Lu B, Zhou H, Ma C, Zhao J, Yang CF, Kemble G, Greenberg H (2003) Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology 306:18–24
Joseph T, McAuliffe J, Lu B, Vogel L, Swayne D, Jin H, Kemble G, Subbarao K (2008) A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology 378:123–132
Karron RA, Callahan K, Luke C, Thumar B, McAuliffe J, Schappell E, Joseph T, Coelingh K, Jin H, Kemble G, Murphy BR, Subbarao K (2009a) A live attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults. J Infect Dis 199:711–716
Karron RA, Talaat K, Luke C, Callahan K, Thumar B, Dilorenzo S, McAuliffe J, Schappell E, Suguitan A, Mills K, Chen G, Lamirande E, Coelingh K, Jin H, Murphy BR, Kemble G, Subbarao K (2009b) Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine 27:4953–4960
Lanthier PA, Huston GE, Moquin A, Eaton SM, Szaba FM, Kummer LW, Tighe MP, Kohlmeier JE, Blair PJ, Broderick M, Smiley ST, Haynes L (2011) Live attenuated influenza vaccine (LAIV) impacts innate and adaptive immune responses. Vaccine 29:7849–7856
Lau YF, Santos C, Torres-Velez FJ, Subbarao K (2011) The magnitude of local immunity in the lungs of mice induced by live attenuated influenza vaccines is determined by local viral replication and induction of cytokines. J Virol 85:76–85
Lau YF, Wright AR, Subbarao K (2012) The contribution of systemic and pulmonary immune effectors to vaccine-induced protection from H5N1 influenza virus infection. J Virol 86:5089–5098
Longini IM Jr, Koopman JS, Monto AS, Fox JP (1982) Estimating household and community transmission parameters for influenza. Am J Epidemiol 115:736–751
Maassab HF (1967) Adaptation and growth characteristics of influenza virus at 25 degrees C. Nature 213:612–614
Min JY, Vogel L, Matsuoka Y, Lu B, Swayne D, Jin H, Kemble G, Subbarao K (2010) A live attenuated H7N7 candidate vaccine virus induces neutralizing antibody that confers protection from challenge in mice, ferrets, and monkeys. J Virol 84:11950–11960
Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, Rotthoff J, Boulton M, Victor JC (2009) Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 361:1260–1267
Murphy BR, Coelingh K (2002) Principles underlying the development and use of live attenuated cold-adapted influenza A and B virus vaccines. Viral Immunol 15:295–323
Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, Glezen WP, Wittes J (1999a) Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA 282:137–144
Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, Glezen WP, Wittes J (1999b) Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial [in process citation]. JAMA 282:137–144
Renegar KB, Small PA Jr, Boykins LG, Wright PF (2004) Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 173:1978–1986
Rota P, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K (1990) Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 175:59–68
Sasaki S, Holmes TH, Albrecht RA, Garcia-Sastre A, Dekker CL, He XS, Greenberg HB (2014) Distinct cross-reactive B-cell responses to live attenuated and inactivated influenza vaccines. JID. doi:10.1093/infdis/jiu190
Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A (2013) Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 19:1305–1312
Suguitan AL Jr, McAuliffe J, Mills KL, Jin H, Duke G, Lu B, Luke CJ, Murphy B, Swayne DE, Kemble G, Subbarao K (2006) Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med 3:e360
Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, Lamirande EW, Jin H, Coelingh KL, Murphy BR, Kemble G, Subbarao K (2009) A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a Phase I trial in healthy adults. Vaccine 27:3744–3753
Talaat KR, Karron RA, Liang PH, McMahon BA, Luke CJ, Thumar B, Chen GL, Min JY, Lamirande EW, Jin H, Coelingh KL, Kemble GW, Subbarao K (2012) An open-label phase I trial of a live attenuated H2N2 influenza virus vaccine in healthy adults. Influenza Other Respir Viruses 7:66–73
Talaat KR, Karron RA, Luke CJ, Thumar B, McMahon BA, Chen GL, Lamirande EW, Jin H, Coelingh KL, Kemble G, Subbarao K (2011) An open label Phase I trial of a live attenuated H6N1 influenza virus vaccine in healthy adults. Vaccine 29:3144–3148
Talaat KR, Luke CJ, Khurana J et al (2014) A live attenuated H5N1 vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis 209(12):1860–1869. doi:10.1093/infdis/jiu123
Tam JS, Capeding MR, Lum LC, Chotpitayasunondh T, Jiang Z, Huang LM, Lee BW, Qian Y, Samakoses R, Lolekha S, Rajamohanan KP, Narayanan SN, Kirubakaran C, Rappaport R, Razmpour A, Gruber WC, Forrest BD, Pan-Asian C-TPETN (2007) Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr Infect Dis J 26:619–628
Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K (2003) Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186
Toback SL, Ambrose CS, Eaton A, Hansen J, Aukes L, Lewis N, Wu X, Baxter R (2013) A postlicensure evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in children 24–59 months of age. Vaccine 31:1812–1818
Tomoda T, Morita H, Kurashige T, Maassab HF (1995) Prevention of influenza by the intranasal administration of cold-recombinant, live-attenuated influenza virus vaccine: importance of interferon-gamma production and local IgA response. Vaccine 13:185–190
Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, Singh S, Kinnersley N, Ward P, Mills RG (2000) Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 283:1016–1024
Treanor JJ, Kotloff K, Betts RF, Belshe R, Newman F, Iacuzio D, Wittes J, Bryant M (1999) Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 18:899–906
Vesikari T, Karvonen A, Korhonen T, Edelman K, Vainionpaa R, Salmi A, Saville MK, Cho I, Razmpour A, Rappaport R, O’Neill R, Georgiu A, Gruber W, Mendelman PM, Forrest B (2006) A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 25:590–595
Zangwill KM (2003) Cold-adapted, live attenuated intranasal influenza virus vaccine. Pediatr Infect Dis J 22:273–274
Zhu W, Higgs BW, Morehouse C, Streicher K, Ambrose CS, Woo J, Kemble GW, Jallal B, Yao Y (2010) A whole genome transcriptional analysis of the early immune response induced by live attenuated and inactivated influenza vaccines in young children. Vaccine 28:2865–2876
Acknowledgments
The seasonal influenza vaccine seed development program was conducted by the strain variant team of MedImmune at California. The pandemic vaccine program was conducted under a Cooperative Research and Development Agreement between MedImmune and NIH and was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH. We thank Dr. Zhongying Chen for providing data for Fig. 2 and Drs. Raburn Mallory and Kathy Coelingh for suggestions and comments.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Jin, H., Subbarao, K. (2014). Live Attenuated Influenza Vaccine. In: Oldstone, M., Compans, R. (eds) Influenza Pathogenesis and Control - Volume II. Current Topics in Microbiology and Immunology, vol 386. Springer, Cham. https://doi.org/10.1007/82_2014_410
Download citation
DOI: https://doi.org/10.1007/82_2014_410
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11157-5
Online ISBN: 978-3-319-11158-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)