Abstract
The threat of novel influenza viruses emerging into the human population from animal reservoirs, as well as the short duration of protection conferred by licensed vaccines against human seasonal strains has spurred research efforts to improve upon current vaccines and develop novel therapeutics against influenza viruses. In recent years these efforts have resulted in the identification of novel, highly conserved epitopes for neutralizing antibodies on the influenza virus hemagglutinin protein, which are present in both the stalk and globular head domains of the molecule. The existence of such epitopes may allow for generation of novel therapeutic antibodies, in addition to serving as attractive targets of novel vaccine design. The aims of developing improved vaccines include eliciting broader protection from drifted strains, inducing long-lived immunity against seasonal strains, and allowing for the rational design of vaccines that can be stockpiled for use as pre-pandemic vaccines. In addition, an increased focus on influenza virus vaccine research has prompted an improved understanding of how the immune system responds to influenza virus infection.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Influenza A viruses circulate annually among human populations and are a source of significant morbidity and mortality. It is estimated that 10–20 % of the US population is infected with influenza viruses Influenza each year, and as many as 49,000 associated deaths occur annually (Glezen and Couch 1978; Fox et al. 1982; Thompson et al. 2004; CDC 2010). Correspondingly, a recent study indicated that approximately 20 % of the UK population may be infected annually (Hayward et al. 2014). In addition to the disease burden caused by seasonal human influenza virus lineages, zoonotic infections, against which there is an absence of immunity in the human population, occasionally result in the emergence of pandemic strains (Neumann et al. 2009). The emergence of the (pandemic) H1N1 subtype influenza virus from the swine reservoir in 2009 (Itoh et al. 2009) and human infections by the avian H5N1 and H7N9 viruses further highlight the potential risk posed by animal reservoirs of influenza A viruses.
Although a limited number of therapeutic drugs are available to combat influenza (Muthuri et al. 2014), they are typically costly, and public health measures that rely on single drug therapy suffer from the potential emergence of drug resistant strains of influenza viruses Influenza (Hayden and Hay 1992; Samson et al. 2013). Thus, the development of multiple therapeutic drugs, especially those that target distinct aspects of the viral lifecycle, are of critical importance.
The most cost-effective strategy to protect against influenza -related disease is vaccination. Current vaccination approaches are effective (Tricco et al. 2013; McNeil et al. 2014), particularly among healthy adult individuals and children (Osterholm et al. 2012), but are hampered by the need to achieve a close match between circulating strains and those included in the vaccine. Due to the rapid and unpredictable antigenic drift of seasonal influenza viruses, such a match is often difficult to attain. The possibility of a zoonotic influenza A virus entering the human population and establishing a novel human lineage represents a further challenge to current vaccination strategies. Since it is impossible to predict which subtype and which strain will cause the next influenza pandemic, current strain-specific approaches will fall short in a pandemic setting. Moreover, current influenza vaccines are recognized to be less effective in high-risk groups, such as the elderly, the very young, and the immune-compromised (Osterholm et al. 2012; Beyer et al. 2013).
Rational attempts to improve influenza vaccines would benefit from a better understanding of the immunological correlates of protection from influenza disease, and the mechanisms underlying such protection. The detailed mechanisms by which the immune system responds to influenza viruses Influenza infection, or vaccination, are remarkably poorly understood and most likely vary depending upon how viral antigen is presented to immune cells (Bucasas et al. 2011; Li et al. 2013).
The best-established correlate of protection from disease following inactivated influenza virus vaccination measures serum-mediated viral hemagglutination inhibition (HAI) of red blood cells: an HAI titer of approximately 1/40 correlates well with protection from disease in humans (Ellebedy and Webby 2009; Katz et al. 2011). Although the HAI assay detects classical neutralizing antibodies that block receptor binding, several alternative immune mechanisms that interfere with influenza virus replication have been described. These include antibodies that inhibit viral fusion with the cellular membrane (Ekiert et al. 2009; Wang et al. 2010b; Tan et al. 2012; Brandenburg et al. 2013), mucosal antibodies (Renegar and Small 1991; Seibert et al. 2013), complement pathways (Terajima et al. 2011; Co et al. 2012; Dilillo et al. 2014), and antibody dependent cellular cytotoxicity (Jegaskanda et al. 2013; Dilillo et al. 2014). The correlation between these alternative modes of in vitro neutralization and protection from disease in vivo are not well studied. This raises the possibility that vaccination strategies may be developed to exploit previously unappreciated protection modalities. To this end, advances in the characterization of novel conserved and protective epitopes present in the hemagglutinin protein have provoked interest in the field. This chapter focuses on studies examining neutralizing antibodies directed against conserved regions of the influenza viruses Influenza , and approaches designed to utilize these epitopes in novel vaccination strategies. If successful, such vaccines stand to increase protection from drifted strains, thereby reducing the need for annual vaccination. Moreover, broadly protective vaccines would prove highly valuable against pandemic strains.
2 Neutralizing Antibodies Directed Against Conserved Regions of the influenza viruses HA
The development of multiple broadly neutralizing monoclonal therapeutic vaccine antibodies (bnAbs) targeting the influenza hemagglutinin is desirable for two reasons. Firstly, such drugs may be used as therapeutic agents, which could be administered singly, or as cocktails of multiple antibodies and/or other therapeutic drugs, potentially providing effective clinical protection from influenza . Secondly, the use of drug cocktails may prevent, or limit the emergence of drug resistant strains of virus into human populations. Two main classes of broadly neutralizing antibodies against conserved regions of the HA have been described: those directed against the conserved, membrane proximal stalk (or stem) domain of the HA, and those directed against the membrane distal receptor binding site (RBS).
2.1 Stalk-Reactive Neutralizing Monoclonal Antibodies
The earliest reports of hemagglutinin stalk-reactive antibodies relied on a sensitive radioimmunoprecipitation assay to identify antibodies present in rabbit immune sera, which mapped to the hemagglutinin HA2 domain, (Polakova et al. 1978; Russ et al. 1978a, b). In 1983, Graves and colleagues characterized immune sera obtained from mice vaccinated with inactivated whole influenza virus which had been acid- and DTT-treated to remove the globular head of the HA (Graves et al. 1983). While data from this study demonstrated that antigenic epitopes were preserved in the hemagglutinin in the absence of the globular head domain, virus-neutralizing activity was not observed, possibly due to the loss of conformation of the immunizing antigen.
The first virus neutralizing stalk-reactive monoclonal antibody (mAb) was isolated from a mouse using traditional hybridoma technology in 1993 by Okuno et al. (1993). This antibody, C179, has fusion inhibiting activity and is able to bind and neutralize a broad range of group 1 HA encoding viruses, including H1, H2, H5, and H6 subtype strains (Okuno et al. 1994; Smirnov et al. 1999). Development of powerful new technologies such as plasmablast sorting followed by single cell PCR, phage display libraries for antibody screening, and divergent technologies to recover antibody coding sequences from memory B-cells has aided in the isolation of more of these relatively rare antibodies. The first examples of bnAbs isolated using phage display screening and specific to the hemagglutinin of influenza virus were reported in 2008 (Kashyap et al. 2008). These recombinant antibodies were constructed from individual heavy and light chain sequences derived from B-cells of individuals who had survived infection with H5N1 virus. They were identified using a phage display screen that enriched for antibodies specific for influenza H5 subtype HA. One such bnAb (A06) exhibited in vitro neutralization of H5N1 and seasonal H1N1 subtype influenza viruses Influenza at concentrations of antibody as low as 1–10 ug/ml (Kashyap et al. 2008). Clone A06 was also effective in vivo against the H1N1 subtype 2009 pandemic influenza virus, conferring protection from death in mice treated either prophylactically, or therapeutically up to 3 days post infection (Kashyap et al. 2010).
Two further, well-characterized human mAbs also isolated using phage technology, CR6261 and F10, were described in 2008 and 2009, respectively (Throsby et al. 2008; Sui et al. 2009). Both mAbs neutralize a broad range of group 1 HAs. Structural characterizations revealed that the footprints of both antibodies localize to the stalk domain and overlap that of the murine mAb C179 (Ekiert et al. 2009; Sui et al. 2009; Dreyfus et al. 2013). The activity of a distinct broadly neutralizing Ab, 12D1, targets a micro-conformational epitope on the long alpha helix of the H3 stalk domain, demonstrating that stalk-reactive antibodies also exist for group 2 HAs (Wang et al. 2010b). Clone 12D1 was isolated from mice sequentially vaccinated with divergent H3 HAs that share conserved epitopes, an approach that was subsequently used to generate the pan-H1 stalk-reactive antibody 6F12 (Tan et al. 2012). A third, more membrane proximal epitope on the stalk domain was defined by the pan-group 2 antibody CR8020 (Ekiert et al. 2011). To date, stalk-reactive antibodies can be grouped into three categories: antibodies that broadly neutralize within a specific subtype , including 12D1 and 6F12; antibodies that broadly neutralize strains within group 1 (CR6261, F10 etc.) or group 2 (CR8020, CR8043 etc. Friesen et al. (2014)); and antibodies that are pan-HA reactive. The last group is currently limited to three characterized members, FI6 and 39.29, which exhibit binding to cross-group HAs of type A influenza viruses (Corti et al. 2011; Nakamura et al. 2013), and CR9114, which is reactive against HAs of both influenza A and B viruses (Dreyfus et al. 2012). However, the number of stalk-reactive antibodies described is growing rapidly. Several laboratories, including that of Patrick Wilson’s at the University of Chicago, have isolated, but not yet fully characterized, large numbers of antibodies belonging to each of the aforementioned categories (Corti et al. 2010; Wrammert et al. 2011; Li et al. 2012; Thomson et al. 2012; Nakamura et al. 2013; Whittle et al. 2014).
In contrast to canonical neutralizing HAI active antibodies that function by blocking the interactions between the RBS and the host receptor, stalk-reactive antibodies work through a plethora of mechanisms downstream of the initial binding event. First and foremost, stalk-reactive antibodies prevent fusion of the viral and endosomal membranes during acidification of the endosome (Ekiert et al. 2009; Wang et al. 2010b; Tan et al. 2012; Brandenburg et al. 2013). Antibodies bind to the HA outside of the cell and are then (likely) imported into the endosome together with the virus. To allow progression of the viral lifecycle, the HA must undergo a conformational change that triggers membrane fusion, upon acidification of the endosome. Stalk-reactive antibodies prevent this conformational step by locking the HA in the pre-fusion conformation. It has been shown that C179 interacts with about 70 % of the HA trimers of an influenza virion at neutralizing concentrations of as low as 25 µg/ml (Okuno et al. 1994). In addition, stalk-reactive antibodies are also able to inhibit viral egress and cleavage of HA by blocking access to the proteolytic cleavage site between HA1 and HA2 (Ekiert et al. 2009, 2011; Krammer and Palese 2013; Friesen et al. 2014). Finally, stalk-targeting antibodies can limit viral spread through complement dependent lysis and antibody-dependent cell mediated cytotoxicity (Terajima et al. 2011; Dilillo et al. 2014). Typically, such antibodies interact with antigen by binding to accessible hydrophobic pockets located within the HA stalk. Notably, a large majority of the stalk binding antibodies employ a heavy chain-directed interaction with HA in which hydrophobic residues present in an extended heavy chain complementarity determining region (HCDR) (typically, but not exclusively HCDR2 or 3) interact with hydrophobic residues present in the HA to fill a hydrophobic pocket (Table 1). Correct positioning of the extended loop is supported by further mutations present in the same and other CDRs. The germline heavy chain gene Vh1-69 appears to be frequently employed in the generation of antibodies that interact with epitopes in the HA stem, possibly due to the presence of several hydrophobic residues encoded in this gene. Since stalk-reactive antibodies have very broad therapeutic activity, they are currently being considered and tested as anti-viral therapeutics in humans. Furthermore, they serve as important tools for antibody -guided universal vaccine approaches (see Sect. 4 below).
2.2 Receptor Binding Site-Reactive Neutralizing Monoclonal Antibodies
The globular head region of HA is considered to be hypervariable at multiple sites (Gerhard et al. 1981; Caton et al. 1982; Brown et al. 1990), resulting in the antigenic drift that is responsible for the narrow strain specificity of currently licensed influenza vaccines. However, due to functional constraints associated with HA binding to the sialic acid receptor, there is a highly conserved region of the HA globular head which encompasses the RBS (Wilson et al. 1981; Weis et al. 1988). Advances in cloning and screening technologies have permitted the identification of increasing numbers of neutralizing monoclonal therapeutic vaccine antibodies specific to HA including a small number of broadly neutralizing antibodies that map to a previously unappreciated epitope close to and overlapping with the RBS of the protein (Ekiert et al. 2009; Yoshida et al. 2009; Krause et al. 2011; Ohshima et al. 2011; Whittle et al. 2011; Lee et al. 2012; Xu et al. 2013) (Table 1). These newly isolated antibodies may be of therapeutic interest, as they may confer significantly broader protection from influenza viruses Influenza infection and disease than -specific antibodies that interact with the conventional antigenic sites. These RBS-directed antibodies may also be refractory to virus-mediated escape from protection. Of further interest, such antibodies appear to possess several common features that may be associated with their reactivity. Several RBS-specific antibodies bind antigen predominantly through heavy chain directed interactions, and have unusually long, somatically hypermutated CDRs. Additionally, multiple studies have indicated that avidity may be an important mechanism that increases the neutralizing breadth of at least some of these antibodies (Ekiert et al. 2012; Lee et al. 2012).
The epitope(s) recognized by several RBS-targeting bnAbs include(s) a strictly conserved hydrophobic tryptophan residue within the floor of the RBS groove, at HA1 position 153. This amino acid residue appears critical in the binding of several of the recently described bnAbs to the HA protein.
One recent study has demonstrated that three human mAbs conferring atypically broad, pan-H2 subtype neutralization activity (Xu et al. 2013) similarly exploit aromatic residues in the CDRs of their respective heavy chains. These antibodies utilize the presence of other supporting changes from germline sequences in order to interact with the receptor binding pocket of the HA. The interactions are characterized by the extension of the heavy chain into the RBS pocket, resulting in an antibody–antigen interaction footprint of approximately 200 Å2 or more, out of a total antibody -antigen interaction of approximately 800 Å2. The hydrophobic residues on antibody and antigen interact by π-π stacking to fill a hydrophobic cavity in the RBS. Intriguingly, this raises the possibility that by focusing the interaction between HA and mAb on a few critical, highly conserved residues thus limiting the contribution of supporting interactions surrounding the RBS, such RBS-directed antibodies may limit the loss of binding upon subsequent genetic drift in the hypervariable antigenic sites of the HA. The downstream consequence of such binding would be greater broadly neutralizing activity. Antibody CO5, which binds and neutralizes multiple strains belonging to group one and two subtypes (Kashyap et al. 2008, 2010) has been shown to interact with the influenza HA via π-π stacking of HA1 TRP 153 with a hydrophobic residue in a CDR of the antibody heavy chain (Ekiert et al. 2012; Xu et al. 2013). mAbs CO5, 2G1, 8M2, 8F8, S139/1, CH65, and likely F045-092 and F026-427 (Ohshima et al. 2011; Whittle et al. 2011; Ekiert et al. 2012; Lee et al. 2012; Xu et al. 2013), (Table 1), also possess residues in the HCDR domains which interact with the RBS of hemagglutinin.
The germline gene Vh1-69, which encodes the variable heavy region of human immunoglobulin genes, partially encodes three out of nine of these broadly neutralizing mAbs. The repeated use of the Vh1-69 gene may be due to the unique presence among variable heavy genes of two hydrophobic residues encoded in this germline sequence (Ile 53 and Phe 54). These hydrophobic residues may give Vh1-69 encoded mAbs a selective advantage in terms of initiating and subsequently increasing the strength of interactions with the hydrophobic pocket of the HA RBS through somatic hypermutation. However, it is important to keep in mind that alternative germline genes, for example Vh3-33 and Vh3-23, can also encode RBS-specific bnAbs. Such antibodies function, at least in some cases, through a mechanism similar to that of 2G1, except that the antigen-interacting hydrophobic residues present in their CDR domains are obtained through somatic hypermutation, as opposed to being encoded in the germline. The ability to generate bnAbs from multiple germline alleles is an important consideration when developing broadly protective vaccines, as a critical criterion for such strategies will be the ability to elicit neutralizing responses from a genetically diverse population.
2.3 Vestigial Esterase Domain-Reactive Neutralizing Monoclonal Antibodies
A third non-conventional, conserved neutralizing epitope in the influenza viruses Influenza H5 hemagglutinin and a cognate antibody were identified in 2013. The neutralizing mAb (H5M9) was crystallized in association with the HA, and the structure demonstrated that the antibody does not interfere with receptor binding. Instead H5M9 recognizes an epitope that includes amino acids 273–278 in the conserved vestigial esterase domain, as well as residues 53–62, 78–83a, and 117–119 of the HA molecule (Zhu et al. 2013). A second antibody (HA-7) generated against the H5 HA from A/Anhui/1/2005, that is specific for a putative overlapping epitope including HA residue E83a, was independently reported (Du et al. 2013). One possible explanation for the observed broadly neutralizing activity of these mAbs is that they inhibit the conformational rearrangement of the HA.
3 Induction of Stalk-Reactive Antibodies by Natural Infection and Standard Vaccination
Until recently, quantitative data about the frequency of stalk-reactive antibodies in serum was unavailable. Qualitative studies analyzing plasmablast and memory B-cells indicated that stalk-reactive antibodies could be found only occasionally in humans exposed to seasonal influenza viruses by vaccination, or in one cited study, infection (Throsby et al. 2008; Wrammert et al. 2008; Corti et al. 2010; Moody et al. 2011). However, a higher frequency of these antibodies was found after infection or vaccination with pandemic H1N1 (Wrammert et al. 2011; Li et al. 2012; Thomson et al. 2012). The first quantitative approach to measure stalk-reactive antibodies in human sera was made by Sui and colleagues using a competition-based assay involving the stalk-reactive antibody F10. These authors found a wide prevalence of F10-like antibodies in human sera, yet such antibodies exist at low levels. It is estimated that they account for approximately 0.001 % of total antibody (Sui et al. 2011). Using chimeric HAs that consisted of group 1 or group 2 stalks (H1 and H3 subtype respectively) and exotic head domains (Fig. 1) to which humans are naïve, stalk-reactive antibodies in sera were detected directly (Hai et al. 2012). Assays for detection of stalk-binding antibodies have been reported that use recombinant cHA in ELISA or other immuno-based assays (Tan et al. 2012) or by using cHA-expressing viruses that have irrelevant neuraminidases (usually N3) in neutralization assays. Using cHAs as substrate, Pica and colleagues demonstrated that individuals infected with pandemic H1N1 developed a strong response to the HA stalk domain (Pica et al. 2012). These data are consistent with studies performed in the Wilson and Schrader laboratories (Wrammert et al. 2011; Li et al. 2012; Thomson et al. 2012), which found that stalk-reactive antibodies represented an estimated 7 % of the total serum IgG of human individuals and also reported strong cross-reactivity to H5 HA. Since earlier qualitative studies found no or very low levels of stalk-reactive antibodies in individuals exposed to seasonal influenza viruses (Wrammert et al. 2008; Corti et al. 2010; Moody et al. 2011) (by vaccination and/or infection) it has been suggested that the unique combination of a conserved stalk to which people were already primed and a new head domain to which people were naive (the head domain of pH1N1 and sH1N1 share less than 70 % amino acid identity) specifically boosted stalk-reactive antibodies (Pica et al. 2012). The same phenomenon was also observed in the mouse model, where animals sequentially infected with two drifted sH1N1 strains developed significantly lower levels of stalk-reactive antibodies than animals sequentially infected with sH1N1 and pH1N1 strains (Krammer et al. 2012). Moreover, individuals exposed to the A/New Jersey/76 H1N1 swine influenza virus vaccine (in which the HA is only distantly related to seasonal H1N1 HA) showed higher levels of stalk-reactive antibodies than unvaccinated individuals (Miller et al. 2013b). In a longitudinal study that examined immunity to influenza virus in a sub-cohort of the Framingham heart study, researchers found that people exposed to H2N2 virus had higher group I stalk titers than individuals who had no HI antibodies against this virus subtype (Miller et al. 2013a). Since the stalk domain is relatively conserved among all group 1 HAs (H1, H5, H6, H8, H9, H11, H12, H13, H16, H17, and H18), it is likely that exposure to H2—which has a completely different head but a conserved stalk—boosted stalk-reactive antibody titers. The same study also reported that group 1 stalk-reactive antibodies are in general present at higher titers in humans than group 2 stalk-reactive antibodies (Miller et al. 2013a). This makes sense considering group 1 HA viruses have circulated as drifted strains of multiple subtypes in the human population during the last 100 years, with varying globular heads but relatively conserved stalk domains. In contrast, a single subtype of group 2 HA virus, H3N2, has circulated in humans over the same period of time (Fig. 2). However, there is also evidence that group 2 stalk-reactive antibodies, mainly induced by natural infection, are present at low levels in the population (Margine et al. 2013a). In a recent study it was shown that individuals infected with H7N9 virus—which has a conserved group 2 stalk but a head domain that is quite divergent from H3—had elevated levels of group 2 cross-reactivity likely based on stalk-reactive antibodies (Guo et al. 2014). Similarly, group 1 stalk-reactive antibodies could be isolated from humans infected with H5N1 influenza viruses Influenza or vaccinated with H5N1 hemagglutinin (Kashyap et al. 2008; Whittle et al. 2014). Based on these data it has been hypothesized that baseline levels of stalk-reactive antibodies are induced in the population primarily by natural infection, and that these antibodies can then be boosted by exposure to divergent HAs that share a conserved stalk domain with the priming virus. Such events have occurred in 1957, when people primed by H1N1 were subsequently exposed to H2N2 and again in 2009 when people primed by sH1N1 (and H2N2) were exposed to pH1N1 (Palese and Wang 2011). In each case, the virus circulating before the introduction of the novel pandemic virus disappeared, suggesting that elevated stalk-reactive antibody levels might play an important role in the elimination of seasonal influenza viruses from the human population (Palese and Wang 2011). The extinction of H2N2 in 1968 provides an exceptional case as its disappearance was likely caused by the induction of cross-reactive N2 NA antibodies by H3N2. Stalk-reactive antibodies are unlikely to be responsible for elimination of the preceding virus lineage in this example, since H2 and H3 belong to different HA groups and antibodies cross-reactive against group 1 and group 2 are extremely rare.
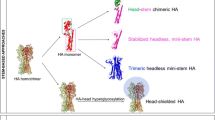
Schematic of a chimeric HA-based vaccination strategy. a Chimeric HAs are combinations of heterosubtypic head domains with stalk domains of H1 (Group 1) or H3 (Group 2) HAs. Here we show a chimeric H5/H3 HA which consists of the membrane distal head domain of H5 HA (red) and the membrane proximal stalk domain of H3 HA (green). A disulfide linkage between cysteines 52 and 277 (indicated in yellow) serves to demarcate the stalk and head domains. b Vaccination regimen based on chimeric HAs. Vaccination with chimeric H5/H3 HA induces a primary response against the H5 head and an almost undetectable response against the immunosubdominant H3 stalk domain. Upon sequential boosting with chimeric H7/H3 HA (H7 head (blue) on top of H3 stalk (green)) and chimeric H10/H3 HA (H10 head domain (golden) on top of an H3 stalk domain (green)), only a primary response is mounted against the heterosubtypic head domains but antibody titers against conserved epitopes in the H3 stalk domain are boosted. All structures are based on PDB# 1RU7 and were visualized in Protein Workshop. Modified from Krammer et al. (2014b)
Circulation of influenza virus strains in the human population since 1918. Influenza A and B viruses have circulated in humans continuously throughout the twentieth and twenty-first centuries. Multiple influenza A virus lineages have circulated during the same time period. H1N1 was introduced into the human population in 1918 and induced a baseline level of anti-group 1 stalk (Anti-G1 stalk) and anti-N1 NA antibody titers. Titers likely remained stable in the population till 1957 when H2N2 crossed the species barrier into humans. H1 and H2 HAs belong to group 1, with highly similar stalk domains but more divergent globular head domains. The introduction of H2N2 might therefore have boosted antibodies against conserved epitopes in the stalk domain which in turn contributed to the extinction of H1N1 in humans in 1957. Furthermore, the introduction of H2N2 would have induced baseline titers of anti-N2 NA antibodies in the population. H2N2 circulated from 1957 till 1968 when H3N2 was introduced. H3 HA is a group 2 HA, divergent from group 1 H2 HA, but the NA is highly related to the N2 of H2N2. The combination of novel HA with an NA for which humans were already primed might have boosted anti-N2 NA titers, facilitating the elimination of H2N2 from the population. After 1968 N2 NA titers may have declined to 1957–1968 levels due to population turnover. Also, introduction of H3N2 would have induced baseline antibody titers against the stalk of group 2 HA (anti-G2 stalk). N1 titers probably started to decline after 1957 due to population turnover but were brought back to baseline in 1977 when H1N1 was re-introduced. Similarly, anti-group 1 HA stalk titers probably started to decline on a population level back to the 1918–1957 baseline until 2009 when they were boosted by the 2009 pH1N1 virus
4 Stalk-Based Universal Influenza Virus Constructs
4.1 Stalk-Based Vaccine Constructs Lacking the Hemagglutinin Globular Head
As the globular head domain of the influenza HA possesses the immunodominant epitopes of the protein, early attempts to induce antibodies specific for the stalk domain focused on generating antigens in which the globular head domain was absent. Graves and colleagues used a whole influenza virus which had been chemically treated to remove the globular head of the HA as an immunogen in mice. This strategy succeeded in eliciting serum antibodies directed towards the stem domain (Graves et al. 1983). In 1993, Okuno et al. (1993) identified a mAb (C179) which was directed against the stalk region of the HA and conferred protection from challenge with an H1 subtype influenza virus in mice (Okuno et al. 1994). Sagawa et al. (1996) transfected a headless construct of an H2 HA into cells and when these same transfected cells were used to immunize mice partial protection following viral challenge with an H1N1 virus was observed. Similarly, a headless HA construct based on the A/PR/8/34 HA generated stalk-specific sera possessing pan-H1 subtype cross-reactivity in ELISA assays. Immunization with such constructs protected mice from lethal challenge (Steel et al. 2010). Interestingly, although ELISA titers to the homologous protein (PR8) were higher in animals vaccinated with full length hemagglutinin than those who received headless HA, the antibody titers to heterologous and heterosubtypic HA protein in ELISA assay were lower in those mice vaccinated with full length hemagglutinin (Steel et al. 2010).
A neutralizing epitope present in the alpha-helical portion of the influenza hemagglutinin HA2 domain (amino acids 76–130 (HA2)) and recognized by the neutralizing mAb 12D1 (Fig. 3) has been identified by Wang et al. (2010b). When this epitope was used to immunize mice, animals were protected from lethal challenge with either H3N2 or H5N1 subtype influenza viruses (Wang et al. 2010a). Two distinct epitopes in the hemagglutinin stem domain have additionally been identified through crystallographic studies that employed stem-directed mAbs in complex with the hemagglutinin molecule (Fig. 3) (Corti and Lanzavecchia 2013; Subbarao and Matsuoka 2013).
The three main epitopes on the HA stalk domain. a shows the structure of H3 HA (green) in combination with mAb CR8020 (Fab, purple). The footprint of CR8020 represents so far the most membrane proximal epitope on the stalk and is shared with CR8043. b The structure of H3 HA (green) with mAb CR9114 (Fab, purple). CR9114 uses a footprint that overlaps with most isolated stalk-reactive antibodies including CR6261, F10, FI6, and C179. c The epitope of mAb 12D1 (in red, here shown on an H3 HA) is so far the most membrane distal epitope on the stalk domain. Structures are based on PDB# 3SDY (a, c) and 4FQY (b) and were visualized in Protein Workshop
While it has been established that headless HA vaccination strategies can lead to the generation of higher levels of cross-reactive serum antibodies than identical vaccination regimens using full length HA antigen (Steel et al. 2010), headless hemagglutinin based approaches suffer considerably from inaccurate protein folding and poor antigen expression (Steel et al. 2010). In this regard, recent studies from Lu et al. (2014) and Bommakanti et al. (2010, 2012) aimed to improve the expression of the HA2 domain. Experiments by Lu et al demonstrated that a rationally engineered headless HA molecule that retains the antigenically conserved sites on the HA stalk can be expressed at very high concentration and be recognized by conformationally dependent stem-specific antibodies. The HA antigen has amino acid changes introduced into the construct, which alter intramolecular electrostatic interactions and reorganize intramolecular disulfide bonds, leading to expression of a stabilized protein (Lu et al. 2014). Bommakanti and colleagues similarly demonstrated stabilization of the stem domains of both group 1 and group 2 HA molecules through the introduction of mutations that replaced solvent exposed hydrophobic patches with polar residues. These regions were revealed by removal of the globular head. Working in the mouse model, the authors went on to demonstrate that this stabilized construct could protect animals from both lethal homologous and heterologous challenge (Bommakanti et al. 2010, 2012). Advances leading to the stable expression of structurally accurate headless HA antigens stand to increase the protective potential of vaccines based on headless HA approaches.
4.2 Chimeric Hemagglutinin-Based Vaccine Constructs
An alternative strategy to induce stalk-reactive antibodies is based on the observation that sequential exposure to influenza viruses with divergent head but conserved stalk domains refocuses the immune response to the usually subdominant stalk. Vaccination with chimeric HAs exploits this approach. Chimeric HAs consist of H1 (group 1) or H3 (group 2) stalk domains combined with ‘exotic’ head domains, usually of avian origin (Hai et al. 2012). A conserved disulfide bond between cysteines 52 and 277 is used to demarcate the head and stalk domains. The region between these amino acids represents the head domain while the rest of the HA ectodomain is comprised of the stalk domain (N- and C-terminus of the HA1 and the ectodomain of HA2). The use of a heterologous head domain stabilizes structural epitopes in the stalk domain. Importantly, the stalk, in contrast to many headless HA approaches, is correctly folded and fully functional. In fact, influenza viruses Influenza expressing cHAs can be rescued and grown to titers comparable to wild type in embryonated eggs and cell culture (Hai et al. 2012). It was subsequently shown that sequential vaccination with different cHAs that have the same stalk but divergent heads induces high titers of stalk-reactive antibodies in mice and ferrets and broadly protects from lethal challenge with divergent group 1 and group 2 viruses including H5N1 and H7N9 viruses (Krammer and Palese 2013, 2014; Krammer et al. 2013, 2014a, b; Margine et al. 2013b). However, it is important to note that to date no cross-group protection has been observed (Krammer et al. 2013). Animals vaccinated with group 1 constructs failed to mount titers against the group 2 stalk and were not protected from group 2 (H3N2) virus challenge. This indicates that a successful cHA-based vaccine should minimally include three components: a group 1, a group 2 and an influenza B cHA (Krammer and Palese 2014). Chimeric HA based vaccine approaches have been successfully applied in the form of DNA vaccines, recombinant protein vaccines and viral vectors as well as in combination with several experimental adjuvants including oil-in water emulsions similar to the ones licensed for use in humans (Goff et al. 2013; Krammer and Palese 2013, 2014; Krammer et al. 2013, 2014a, b; Margine et al. 2013b). Vaccination with viruses expressing cHAs with exotic heads and N1 or N2 NAs may also enhance the immune response to the relatively conserved NAs. The resulting immune response, based on stalk-specific as well as NA-specific conserved epitopes may afford the cross protective responses necessary for a universal influenza virus vaccine.
4.3 Other Approaches
In addition to headless and chimeric HA constructs, a number of other strategies have been attempted to induce neutralizing antibodies to the HA stem. A vaccine based on the completely conserved fusion peptide was able to provide protection against homologous and heterosubtypic challenge (Janulíková et al. 2012). Furthermore, rationally designed nanoparticles based on the Flock House virus capsid have been used to elicit CR6261-like antibodies (Schneemann et al. 2012). A bacterial ferritin nanoparticle displaying HA trimers on its surface was also able to induce broadly reactive antibodies against both the head as well as the stalk domain of H1 and protected ferrets from heterologous H1N1 challenge (Kanekiyo et al. 2013). However, this approach was unable to elicit significant amounts of heterosubtypic immunity (Kanekiyo et al. 2013). Finally regimens using DNA or live virus priming followed by inactivated virus boosts are currently being investigated as novel strategies to increase broadly neutralizing antibody responses (Wei et al. 2010; Talaat et al. 2014).
5 Conclusions and Future Perspective
Recent developments in technologies that allow screening for and isolation of Abs derived from humans and animals exposed to influenza viruses Influenza antigens have expanded the extent of our knowledge on the repertoire of antibodies that are generated in response to influenza viruses. Examples of relatively rare, or previously unrecognized, classes of broadly neutralizing antibody have been identified, and common themes are emerging in relation to the mechanisms of neutralization of these antibodies. Biochemical, virological, as well as crystallography-based studies have allowed the detailed characterization of these novel antibodies, which map to conserved epitopes on group I or group II HA proteins (stem or globular head domains). These antibodies neutralize virus through distinct mechanisms, such as inhibition of receptor binding or fusion of the viral and cellular membranes. The availability of multiple classes of antibodies that neutralize virus through different mechanisms, as well as antibodies that function through a single mechanism but bind to the viral antigen using differing fine specificities, provide opportunities to develop multi-drug cocktails for use in humans.
Furthermore, the recent surge of interest in bnAbs, especially those antibodies that recognize the stalk domain of the hemagglutinin molecule, have stimulated research into the development of alternative influenza virus vaccines and vaccine regimens, in order to elicit greater cross protective responses to influenza virus. Considerable progress has been made towards demonstrating the potential utility of headless and chimeric hemagglutinin-based antigens, but further research is needed to understand how best to direct the antibody response to conserved epitopes and how to optimally elicit potent antibodies specific to such conserved epitopes. Efforts towards improved influenza vaccines designed to induce the broadest and most potently protective responses would greatly benefit from a better understanding of the immunological correlates of protection from disease. Taken together, the data reviewed here highlight the exciting advances being made to fulfill the vital and unmet need for improved influenza vaccines and vaccination practices.
Abbreviations
- bnAb:
-
Broadly neutralizing antibody
- CDR:
-
Complementarity determining region
- DTT:
-
Dithiothreitol
- H1, H2, H3, etc:
-
Hemagglutinin subtype 1, 2, 3, etc
- HA:
-
Hemagglutinin
- HA1:
-
Hemagglutinin subunit 1
- HA2:
-
Hemaggltinin subunit 2
- HAI:
-
Hemagglutination inhibition
- mAb:
-
Monoclonal antibody
- N1, N2, etc:
-
Neuraminidase subtype 1, 2, etc
- nAb:
-
Neutralizing antibody
- pH1N1:
-
2009 pandemic H1N1
- RBS:
-
Receptor binding site
- sH1N1:
-
Seasonal H1N1
References
Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus AD (2013) Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine 31:6030–6033. doi:S0264-410X(13)01339-X, 10.1016/j.vaccine.2013.09.063
Bommakanti G, Citron MP, Hepler RW et al (2010) Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc Natl Acad Sci USA 107:13701–13706. doi:10.1073/pnas.1007465107
Bommakanti G, Lu X, Citron MP et al (2012) Design of Escherichia coli-expressed stalk domain immunogens of H1N1 hemagglutinin that protect mice from lethal challenge. J Virol 86:13434–13444. doi:10.1128/JVI.01429-12
Brandenburg B, Koudstaal W, Goudsmit J et al (2013) Mechanisms of hemagglutinin targeted influenza virus neutralization. PLoS One 8:e80034. doi:10.1371/journal.pone.0080034
Brown LE, Murray JM, White DO, Jackson DC (1990) An analysis of the properties of monoclonal antibodies directed to epitopes on influenza virus hemagglutinin. Arch Virol 114:1–26
Bucasas KL, Franco LM, Shaw CA et al (2011) Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis 203:921–929. doi:10.1093/infdis/jiq156
Caton AJ, Brownlee GG, Yewdell JW, Gerhard W (1982) The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 31:417–427. doi:10.1016/0092-8674(82)90135-0
CDC (2010) Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep 59:1057–1062.
Co MD, Cruz J, Takeda A, Ennis FA, Terajima M (2012) Comparison of complement dependent lytic, hemagglutination inhibition and microneutralization antibody responses in influenza vaccinated individuals. Hum Vaccin Immunother 8:1218–1222. doi:10.4161/hv.21025
Corti D, Lanzavecchia A (2013) Broadly neutralizing antiviral antibodies. Annu Rev Immunol 31:705–742. doi:10.1146/annurev-immunol-032712-095916
Corti D, Suguitan AL Jr, Pinna D et al (2010) Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest 120:1663–1673. doi:10.1172/JCI41902
Corti D, Voss J, Gamblin SJ et al (2011) A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856. doi:10.1126/science.1205669
Dilillo DJ, Tan GS, Palese P, Ravetch JV (2014) Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 20:143–151. doi:10.1038/nm.3443
Dreyfus C, Laursen NS, Kwaks T et al (2012) Highly conserved protective epitopes on influenza B viruses. Science 337:1343–1348. doi:10.1126/science.1222908
Dreyfus C, Ekiert DC, Wilson IA (2013) Structure of a classical broadly neutralizing stem antibody in complex with a pandemic H2 influenza virus hemagglutinin. J Virol 87:7149–7154. doi:10.1128/JVI.02975-12
Du L, Jin L, Zhao G et al (2013) Identification and structural characterization of a broadly neutralizing antibody targeting a novel conserved epitope on the influenza virus H5N1 hemagglutinin. J Virol 87:2215–2225. doi:10.1128/JVI.02344-12
Ekiert DC, Bhabha G, Elsliger MA et al (2009) Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251. doi:10.1126/science.1171491
Ekiert DC, Friesen RH, Bhabha G et al (2011) A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333:843–850. doi:10.1126/science.1204839
Ekiert DC, Kashyap AK, Steel J et al (2012) Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 489:526–532. doi:10.1038/nature11414
Ellebedy AH, Webby RJ (2009) Influenza vaccines. Vaccine 27(Suppl 4):D65–D68. doi:S0264-410X(09)01214-6, 10.1016/j.vaccine.2009.08.038
Fox JP, Cooney MK, Hall CE, Foy HM (1982) Influenzavirus infections in Seattle families, 1975–1979. II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am J Epidemiol 116:228–242
Friesen RH, Lee PS, Stoop EJ et al (2014) A common solution to group 2 influenza virus neutralization. Proc Natl Acad Sci USA 111:445–450. doi:10.1073/pnas.1319058110
Gerhard W, Yewdell J, Frankel ME, Webster R (1981) Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature 290:713–717
Glezen WP, Couch RB (1978) Interpandemic influenza in the Houston area, 1974–1976. N Engl J Med 298:587–592. doi:10.1056/NEJM197803162981103
Goff PH, Eggink D, Seibert CW, Hai R, Martínez-Gil L, Krammer F, Palese P (2013) Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies. PLoS One 8:e79194. doi:10.1371/journal.pone.0079194
Graves PN, Schulman JL, Young JF, Palese P (1983) Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive HA2 determinants. Virology 126:106–116
Guo L, Zhang X, Ren L et al (2014) Human antibody responses to avian influenza A(H7N9) virus, 2013. Emerg Infect Dis 20:192–200. doi:10.3201/eid2002.131094
Hai R, Krammer F, Tan GS et al (2012) Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol 86:5774–5781. doi:10.1128/JVI.00137-12
Hayden FG, Hay AJ (1992) Emergence and transmission of influenza A viruses resistant to amantadine and rimantadine. Curr Top Microbiol Immunol 176:119–130
Hayward AC, Fragaszy EB, Bermingham A et al (2014) Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med 2(6):445–454. doi: 10.1016/52213-2600 (14) 70034-7
Itoh Y, Shinya K, Kiso M et al (2009) In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021–1025. doi:10.1038/nature08260
Janulíková J, Staneková Z, Mucha V, Kostolanský F, Varečková E (2012) Two distinct regions of HA2 glycopolypeptide of influenza virus hemagglutinin elicit cross-protective immunity against influenza. Acta Virol 56:169–176
Jegaskanda S, Job ER, Kramski M et al (2013) Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 190:1837–1848. doi:10.4049/jimmunol.1201574
Kanekiyo M, Wei CJ, Yassine HM et al (2013) Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. doi:10.1038/nature12202
Kashyap AK, Steel J, Oner AF et al (2008) Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc Natl Acad Sci USA 105:5986–5991
Kashyap AK, Steel J, Rubrum A et al (2010) Protection from the 2009 H1N1 pandemic influenza by an antibody from combinatorial survivor-based libraries. PLoS Pathog 6:e1000990. doi:10.1371/journal.ppat.1000990
Katz JM, Hancock K, Xu X (2011) Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther 9:669–683. doi:10.1586/eri.11.51
Krammer F, Palese P (2013) Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 3:521–530. doi:10.1016/j.coviro.2013.07.007
Krammer F, Palese P (2014) Universal influenza virus vaccines: need for clinical trials. Nat Immunol 15:3–5. doi:10.1038/ni.2761
Krammer F, Pica N, Hai R, Tan GS, Palese P (2012) Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J Virol 86:10302–10307. doi:10.1128/JVI.01336-12
Krammer F, Pica N, Hai R, Margine I, Palese P (2013) Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 87:6542–6550. doi:10.1128/JVI.00641-13
Krammer F, Hai R, Yondola M et al (2014a) Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J Virol 88:3432–3442. doi:10.1128/JVI.03004-13
Krammer F, Margine I, Hai R et al (2014b) H3 Stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J Virol 88:2340–2343. doi:10.1128/JVI.03183-13
Krause JC, Tsibane T, Tumpey TM, Huffman CJ, Basler CF, Crowe JE Jr (2011) A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. J Virol 85:10905–10908. doi:10.1128/JVI.00700-11
Lee PS, Yoshida R, Ekiert DC, Sakai N, Suzuki Y, Takada A, Wilson IA (2012) Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc Natl Acad Sci USA 109:17040–17045. doi:10.1073/pnas.1212371109
Li GM, Chiu C, Wrammert J et al (2012) Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci USA 109:9047–9052. doi:10.1073/pnas.1118979109
Li S, Nakaya HI, Kazmin DA, Oh JZ, Pulendran B (2013) Systems biological approaches to measure and understand vaccine immunity in humans. Semin Immunol 25:209–218. doi:S1044-5323(13)00032-8, 10.1016/j.smim.2013.05.003
Lu Y, Welsh JP, Swartz JR (2014) Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc Natl Acad Sci USA 111:125–130. doi:10.1073/pnas.1308701110
Margine I, Hai R, Albrecht RA et al (2013a) H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol 87:4728–4737. doi:10.1128/JVI.03509-12
Margine I, Krammer F, Hai R et al (2013b) Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J Virol 87:10435–10446. doi:10.1128/JVI.01715-13
McNeil S, Shinde V, Andrew M, et al (2014) Interim estimates of 2013/2014 influenza clinical severity and vaccine effectiveness in the prevention of laboratory-confirmed influenza-related hospitalisation. Euro Surveill Mar 6; 19(9). pii 20729.
Miller MS, Gardner TJ, Krammer F, Aguado LC, Tortorella D, Basler CF, Palese P (2013a) Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med 5:198ra107. doi:10.1126/scitranslmed.3006637
Miller MS, Tsibane T, Krammer F, Hai R, Rahmat S, Basler CF, Palese P (2013b) 1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans. J Infect Dis 207:98–105. doi:10.1093/infdis/jis652
Moody MA, Zhang R, Walter EB et al (2011) H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 6:e25797. doi:10.1371/journal.pone.0025797
Muthuri SG, Venkatesan S, Myles PR et al (2014) Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2(5):395–404. doi: 10.1016/52213-2600 (14) 70041-4
Nakamura G, Chai N, Park S et al (2013) An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe 14:93–103. doi:10.1016/j.chom.2013.06.004
Neumann G, Noda T, Kawaoka Y (2009) Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459:931–939. doi:10.1038/nature08157
Ohshima N, Iba Y, Kubota-Koketsu R, Asano Y, Okuno Y, Kurosawa Y (2011) Naturally occurring antibodies in humans can neutralize a variety of influenza virus strains, including H3, H1, H2, and H5. J Virol 85:11048–11057. doi:10.1128/JVI.05397-11
Okuno Y, Isegawa Y, Sasao F, Ueda S (1993) A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol 67:2552–2558
Okuno Y, Matsumoto K, Isegawa Y, Ueda S (1994) Protection against the mouse-adapted A/FM/1/47 strain of influenza A virus in mice by a monoclonal antibody with cross-neutralizing activity among H1 and H2 strains. J Virol 68:517–520
Osterholm MT, Kelley NS, Sommer A, Belongia EA (2012) Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12:36–44. doi:http://dx.doi.org/10.1016/S1473-3099(11)70295-X
Palese P, Wang TT (2011) Why do influenza virus subtypes die out? A hypothesis. MBio 2. doi:10.1128/mBio.00150-11
Pica N, Hai R, Krammer F et al (2012) Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci USA 109:2573–2578. doi:10.1073/pnas.1200039109
Polakova K, Russ G, Styk B (1978) Antigenic glycopolypeptides HA1 and HA2 of influenza virus haemagglutinin. I. Gel filtration in 6 M guanidine hydrochloride. Acta Virol 22:362–370
Renegar KB, Small PA Jr (1991) Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol 65:2146–2148
Russ G, Styk B, Polakova K (1978a) Antigenic glycopolypeptides HA1 and HA2 of influenza virus haemagglutinin. II. Reactivity with rabbit sera against intact virus and purified undissociated haemagglutinin. Acta Virol 22:371–382
Russ G, Styk B, Polakova K (1978b) Radioimmunoassay of influenza A virus haemagglutinin. I. Preparation and properties of radioactive 125I-labelled bromelain-released haemagglutinin. Acta Virol 22:1–10
Sagawa H, Ohshima A, Kato I, Okuno Y, Isegawa Y (1996) The immunological activity of a deletion mutant of influenza virus haemagglutinin lacking the globular region. J Gen Virol 77 ( Pt 7):1483–1487
Samson M, Pizzorno A, Abed Y, Boivin G (2013) Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 98:174–185. doi:S0166-3542(13)00068-5, 10.1016/j.antiviral.2013.03.014
Schneemann A, Speir JA, Tan GS, Khayat R, Ekiert DC, Matsuoka Y, Wilson IA (2012) A virus-like particle that elicits cross-reactive antibodies to the conserved stem of influenza virus hemagglutinin. J Virol 86:11686–11697. doi:10.1128/JVI.01694-12
Seibert CW, Rahmat S, Krause JC et al (2013) Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs. J Virol 87:7793–7804. doi:10.1128/JVI.00979-13
Smirnov YA, Lipatov AS, Gitelman AK, Okuno Y, Van Beek R, Osterhaus AD, Claas EC (1999) An epitope shared by the hemagglutinins of H1, H2, H5, and H6 subtypes of influenza A virus. Acta Virol 43:237–244
Steel J, Lowen AC, Wang TT, et al (2010) Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 1. doi:10.1128/mBio.00018-10
Subbarao K, Matsuoka Y (2013) The prospects and challenges of universal vaccines for influenza. Trends Microbiol 21:350–358. doi:S0966-842X(13)00074-7, 10.1016/j.tim.2013.04.003
Sui J, Hwang WC, Perez S et al (2009) Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 16:265–273. doi:10.1038/nsmb.1566
Sui J, Sheehan J, Hwang WC et al (2011) Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza a antibodies. Clin Infect Dis 52:1003–1009. doi:10.1093/cid/cir121
Talaat KR, Luke CJ, Khurana S et al (2014) A live attenuated H5N1 vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis. doi:10.1093/infdis/jiu123
Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P (2012) A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol 86:6179–6188. doi:10.1128/JVI.00469-12
Terajima M, Cruz J, Co MD et al (2011) Complement-dependent lysis of influenza a virus-infected cells by broadly cross-reactive human monoclonal antibodies. J Virol 85:13463–13467. doi:10.1128/JVI.05193-11
Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K (2004) Influenza-associated hospitalizations in the United States. J Am Med Assoc 292:1333–1340. doi:10.1001/jama.292.11.1333
Thomson CA, Wang Y, Jackson LM et al (2012) Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front Immunol 3:87. doi:10.3389/fimmu.2012.00087
Throsby M, van den Brink E, Jongeneelen M et al (2008) Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS ONE 3:e3942
Tricco AC, Chit A, Soobiah C et al (2013) Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med 11:153. doi:10.1186/1741-7015-11-153
Wang TT, Tan GS, Hai R et al (2010a) Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Nat Acad Sci USA 107:18979–18984. doi:10.1073/pnas.1013387107
Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, Palese P (2010b) Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog 6:e1000796. doi:10.1371/journal.ppat.1000796
Wei CJ, Boyington JC, McTamney PM et al (2010) Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329:1060–1064. doi:10.1126/science.1192517
Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC (1988) Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333:426–431. doi:10.1038/333426a0
Whittle JR, Zhang R, Khurana S et al (2011) Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc Natl Acad Sci USA 108:14216–14221. doi:10.1073/pnas.1111497108
Whittle JR, Wheatley AK, Wu L et al (2014) Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy chain lineages. J Virol. doi:10.1128/JVI.03422-13
Wilson IA, Skehel JJ, Wiley DC (1981) Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature 289:366–373
Wrammert J, Smith K, Miller J et al (2008) Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671. doi:10.1038/nature06890
Wrammert J, Koutsonanos D, Li GM et al (2011) Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 208:181–193. doi:10.1084/jem.20101352
Xu R, Krause JC, McBride R, Paulson JC, Crowe JE Jr, Wilson IA (2013) A recurring motif for antibody recognition of the receptor-binding site of influenza hemagglutinin. Nat Struct Mol Biol 20:363–370. doi:10.1038/nsmb.2500
Yoshida R, Igarashi M, Ozaki H et al (2009) Cross-protective potential of a novel monoclonal antibody directed against antigenic site B of the hemagglutinin of influenza A viruses. PLoS Pathog 5:e1000350. doi:10.1371/journal.ppat.1000350
Zhu X, Guo YH, Jiang T et al (2013) A unique and conserved neutralization epitope in H5N1 influenza viruses identified by an antibody against the A/Goose/Guangdong/1/96 hemagglutinin. J Virol 87:12619–12635. doi:10.1128/JVI.01577-13
Acknowledgments
PP and FK are supported, in part, by NIH P01 AI097092, U19 AI109946, and HHSN272201400008C. JS is supported in part by HHSN272201400004C. We very much thank Caitlin Mullarkey for helpful discussions and editorial review.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Krammer, F., Palese, P., Steel, J. (2014). Advances in Universal Influenza Virus Vaccine Design and Antibody Mediated Therapies Based on Conserved Regions of the Hemagglutinin. In: Oldstone, M., Compans, R. (eds) Influenza Pathogenesis and Control - Volume II. Current Topics in Microbiology and Immunology, vol 386. Springer, Cham. https://doi.org/10.1007/82_2014_408
Download citation
DOI: https://doi.org/10.1007/82_2014_408
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11157-5
Online ISBN: 978-3-319-11158-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)