Abstract
Recent advances in inductively coupled plasma mass spectrometry (ICP-MS) as applied in mass cytometry, enabled its broad applicability to life science research. Mass cytometry enables the high-dimensional characterization of cellular systems by simultaneously measuring dozens of metal isotope reporter labeled antibodies bound to cell components. With the ability to simultaneously interrogate an unprecedented number of molecular components on a per cell basis, it offers the possibility to gain better understanding of single cell biology in heterogeneous samples. To upscale this single cell information to screening approaches by mass cytometry, a cell-based multiplexing technique, called mass-tag cellular barcoding (MCB), was developed. MCB enables the simultaneous analysis of multiple cell samples by using n metal ion tags to multiplex up to 2n samples. Different mass tag combinations are used to label individual cell samples with a unique mass barcode that allows multiple samples to be combined and immunostained together for a single analysis on the mass cytometer. Taken together, MCB enables increased sample throughput, reduces antibody consumption, and increases the overall data quality. In this chapter, we describe the MCB to array the samples in a 96-well format that allows for medium-scale profiling/screening experiments to be run on a standard mass cytometer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Mass cytometry is a recently developed technology platform that allows for high-content, multiparametric analysis of single cells in complex biological systems (Ornatsky et al. 2010). Using mass cytometry, one can simultaneously interrogate signaling components, cell cycle state, cell viability, DNA content, calcium flux, and many other cellular markers (Bendall et al. 2011). Coupled to simultaneous cell surface marker profiling, these unique single cell states can be put into the context of complex heterogeneous cell populations such as those encountered in human tissue samples. There are a wide range of possible applications for mass cytometry including drug screening, patient profiling, biomarker discovery, and time-course analysis to name just a few (Bodenmiller et al. 2012). Until recently, however, the number of samples that could be analyzed by mass cytometry was limited due to the protocols used for sample preparation and the mass cytometric measurement itself: Immune-staining over multiple samples was heterogeneous, the antibody reagent costs were high, and the fluidics systems on the mass cytometer allowed only a low sample throughput.
To address these issues, the multiplexing approach employed in fluorescent cell barcoding (FCB) (Krutzik and Nolan 2006; Krutzik et al. 2008) was adapted for use in mass cytometry in a method of cell multiplexing that we call mass-tag cellular barcoding (MCB) (Bodenmiller et al. 2012). MCB enables the simultaneous analysis of multiple samples in a single measurement (Bodenmiller et al. 2012). To accomplish this, individual cell samples are labeled with a unique combination of mass tags before being combined into a single sample. The pooled sample is stained with a single antibody mix and analyzed in one run on the mass cytometer. Measured cells are thereafter assigned to the corresponding source sample based on their unique “mass barcode” signatures (Bodenmiller et al. 2012). By mixing samples prior to staining, antibody consumption is typically reduced 30- to 50-fold. In addition, the overall data quality is increased through the identical processing of control and all samples of interest, which eliminates pipetting errors and staining variation and reduces artifacts due to instrument variations. Finally, the sample analysis throughput is greatly increased as the cleaning step of the fluidics system can be omitted. Taken together, MCB enables increased sample throughput, reduces antibody consumption, and increases the overall data quality (Bodenmiller et al. 2012), thereby enabling medium-scale profiling/screening experiments to be run on a standard mass cytometer. In this book chapter, we describe MCB procedures that can be used for any cell samples of interest.
2 Reagents
In MCB, cells are labeled with unique signatures or “barcodes”. This is accomplished by using mass tags—bifunctional chelating reagents that are able to strongly chelate specific metal ions and that can be covalently bound to a cell. For the MCB procedure described in this chapter, the molecule 1,4,7,10-tetraazacyclododecane-1,4,7-tris–acetic acid 10-maleimide ethylacetamide (maleimido-mono-amido-DOTA or mDOTA) is used as the bifunctional compound. The DOTA moiety readily chelates rare earth metal lanthanide (III) ions with a K d of ~10−16, and the maleimide moiety rapidly reacts covalently with cellular thiol groups as illustrated in Fig. 1 (Bodenmiller et al. 2012). Since the reactive bifunctional reagent is covalently attached to the cell, unreacted molecules can be readily removed by washing, the mass-tagged samples are stable over multiple sample processing steps, and the samples can be stored a week or more without cross contamination of tags between cells within the multiplexed sample.
Mass-tag cellular barcoding. Cells are covalently labeled with the bifunctional compound mDOTA. This compound can be loaded with lanthanide (III) isotope ions and reacts covalently with cellular thiol groups through the maleimide moiety. Reproduced from Bodenmiller et al. (2012)
Using binary combinations of n metal ion tags it is possible to multiplex up to 2n samples. By using seven or nine metal tags one can multiplex 96 or 384 samples, respectively (Bodenmiller et al. 2012). For mass cytometry, the lanthanide series of transition metal elements are used as they are not normally present in biological samples, and many different stable isotopes are available at high purity. In addition, their uniform +3 oxidation state gives great flexibility in choosing metal isotopes without the need of using different chelating chemistries. Although all rare-earth metals are amenable to MCB, highly pure isotopes at the high end of the lanthanide series (e.g., Yb171, Yb172, Yb174, Yb175, Yb176) are preferred as their use avoids false positive signals resulting from the isotope oxides (M + 16) that can result from oxidation of an isotope in the plasma (Ornatsky et al. 2010; Tanner et al. 2013). Since lanthanide metal isotopes are used in a combinatorial fashion to barcode large number of samples, individual mass barcodes will differ in the number of metals within a unique combination. In order to maintain the same ratio of maleimide groups to thiol groups across the barcoding plate, the amount of mDOTA per well is equalized across the plate by addition of mDOTA-conjugated metal isotopes that are not detected by mass cytometer (i.e., that do not interfere with the measurement channels) but that have similar analytical properties (e.g., Ga (III)). Metals used for this purpose must have a low mass so that their signals are excluded from the mass range used for detection and a +3 oxidation state such that they are efficiently chelated by mDOTA.
3 Preparing mDOTA-Lanthanide (III) DMSO Stocks for CyTOF® Barcoding
3.1 Materials
-
mDOTA (Macrocyclics, #B272)
-
Lanthanide (III) metal isotopes as chloride salts (DVS sciences)
-
Gallium (III) as chloride salt (Sigma-Aldrich, #427128)
-
20 mM acetate buffer, pH 5.5
-
Dimethyl sulfoxide (DMSO, Sigma-Aldrich, # D2438).
3.2 Method
The metal (III)-mDOTA complex should be prepared at 2:1 molar ratio of mDOTA:metal, in order to reduce or eliminate unchelated metal in the barcoding reagent.
-
1.
Weigh out 2 mg of solid mDOTA into a 1.5 mL microcentrifuge tube. Spin to the bottom of the tube for about 5 s.
-
2.
Dissolve the metal (III) salt stock solution in 20 mM acetate buffer, pH 5.5 to a concentration of 25 mM.
-
3.
Dissolve the solid mDOTA at 41.5 mg/mL (to 50 mM final concentration) in 20 mM acetate buffer pH 5.5 containing 25 mM metal (III) salt.
-
4.
Solid may not dissolve immediately. Vortex to dissolve it completely.
-
5.
Snap freeze by placing in liquid nitrogen for 10–20 s.
-
6.
Pierce the tube cap with a small hole, and transfer tube quickly to a lyophilization bell jar.
-
7.
Lyophilize overnight or as necessary depending on the total volume.
Note: Make sure sample does not thaw before putting on vacuum—this will lead to boiling.
Note: When removing the sample from the lyophilizer, the dried pellet may fly out of the microcentrifuge tube due to static electricity and/or the air rushing in when venting the lyophilization jar. One solution is to poke a hole in the tube cap with a wide-gauge needle.
-
8.
Spin tube to collect all powder at the bottom of the microcentrifuge tube.
-
9.
Dissolve the white pellet in DMSO, aliquot, and freeze for long-term storage at −20 °C. We typically use 10 mM stocks. Store at −20 °C.
4 Titrating Barcoding Reagents
Barcoding is well suited for analysis of both cultured immortalized cells and primary cell samples. The reaction between the maleimide group of the barcoding reagent and the cellular thiol groups is essentially complete in 15 min at room temperature. The optimal concentration of barcoding reagent depends on the total number of thiol groups in the sample, which is a function of cell number and cell volume. We recommend that similar cell numbers (or better, a similar number of thiol groups) be used in each well for the barcoding experiments. The concentration of each mass-tag barcoding reagent must be titrated for the experimentally desired number of target cells. MCB is typically used in experiments where cells have been crosslinked (fixed) and permeabilized for analysis of intracellular antigens such as protein phosphorylation sites (Bodenmiller et al. 2012). This workflow is preferable because the thiol-reactive reagents used find many more targets inside the cell as compared to the cell surface.
4.1 Materials
-
Live cells
-
PBS (Sigma-Aldrich, #P4417-100TAB), at 4 °C
-
Doubly distilled water (ddH2O)
-
Cell Staining Media (CSM): PBS, pH 7.4. with 0.5 % bovine serum albumin and 0.02 % sodium azide, at 4 °C
-
1.6 % paraformaldehyde (PFA): dilute 16 % PFA (Electron Microscopy Sciences, #15710) 1:10 in PBS
-
Ice-cold methanol, HPLC grade (Sigma-Aldrich, #646377-1L)
-
FACS tubes (BD Falcon, #352008)
-
Filter-cap FACS tubes (BD Falcon, #352235)
-
Inject-F 1-mL syringes (Fisher, #S7510-1)
-
Iridium metallointercalator working solution: PBS, pH 7.4, with 1.6 % PFA and 0.02 % iridium metallointercalator (DVS Sciences, #Inter-1X-natIr).
4.2 Method
4.2.1 Preparation of Formaldehyde Crosslinked and Permeabilized Cells
-
1.
Bring cells into suspension and add 1.6 % PFA; incubate for 10 min at room temperature.
-
2.
Centrifuge cells at 600 × g for 5 min.
-
3.
Remove supernatant completely.
-
4.
Vortex vigorously to resuspend all cells, add ice-cold methanol to a concentration of ~1 × 106 cells/mL, incubate for 10 min at 4 °C to permeabilize cells.
Note: Optional pause point. Cells can be stored long-term in methanol at −80 °C.
-
5.
Take six FACS tubes per dilution series for each barcoding reagent to be titrated.
-
6.
Add 3 mL CSM and 1 mL cell suspension (1 × 106 cells) to each tube.
-
7.
Centrifuge at 600 × g for 5 min at 4 °C.
-
8.
Remove supernatant.
-
9.
Resuspend cell pellet in 3 mL PBS and centrifuge at 600 × g for 5 min at 4 °C.
-
10.
Remove supernatant and resuspend cell pellet in 1 mL PBS.
4.2.2 Titration of Barcoding Reagents
Prepare a six-step dilution series of each barcoding reagent; dilutions are made in DMSO.
-
1.
Dilute the 10 mM stock barcoding reagent to 500 μM with DMSO (i.e., 5 μL 10 mM stock barcoding reagent plus 95 μL DMSO).
-
2.
Use the 500 μM barcoding reagent to prepare a titration series according the following scheme:

-
3.
Add 4 μL barcoding reagent from each tube of the titration series to 1 mL cell suspension in PBS and vortex vigorously.
Final concentration of barcoding reagent [nM] | 1000 | 200 | 40 | 8 | 1.6 | 0.32 |
Note: DMSO tends to crystallize when pipetted into ice-cold (aqueous) solutions; therefore, it is critical to vortex immediately after pipetting the barcoding reagent into the cell suspension.
-
4.
Incubate for 30 min at room temperature. Keep cells in suspension by vortexing intermittently.
-
5.
Add 3 mL CSM and centrifuge at 600 × g for 5 min at 4 °C.
-
6.
Remove supernatant.
-
7.
Repeat washing step twice.
-
8.
Remove supernatant.
-
9.
Remove supernatant and add appropriate volume of Ir-metallointercalator working solution (1.6 % PFA in PBS with 1:5000 metallointercalator) to each tube.
-
10.
Use 1 mL of 1:5000 metallointercalator for ~1–2 × 106 cells.
-
11.
Incubate for 20 min at room temperature.
Note: Optional pause point. Cells can be stored in metallointercalator working solution overnight at 4 °C.
-
12.
Add 3 mL CSM and centrifuge at 600 × g for 5 min at 4 °C.
-
13.
Remove supernatant.
-
14.
Repeat washing step once.
-
15.
Add 3 mL PBS and centrifuge at 600 × g for 5 min at 4 °C.
-
16.
Remove supernatant.
-
17.
Add 3 mL ddH2O and centrifuge at 600 × g for 5 min at 4 °C.
-
18.
Remove supernatant.
-
19.
Resuspend cells at ~1 × 106 cells/mL with ddH2O.
-
20.
Filter through filter-cap FACS tube, and transfer to 1 mL syringe.
-
21.
Acquire sample data on CyTOF®.
Note: Always begin the measurements with the LOWEST CONCENTRATION and proceed from lowest concentration to highest. If the signal becomes too high, the instrument can be damaged. Do not continue if the signal in a sample is >32 k ion counts per cell event.
4.2.3 Data Analysis
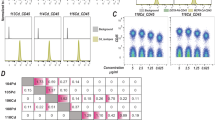
Analyze acquired data, and determine the optimal concentration for making the barcoding plates, taking into account signal intensity and separation between cells positive and negative for barcoding reagent as shown in Fig. 2.
5 Barcoding Plate Layout
Barcoding is performed by adding barcoding reagents in a combinatorial, binary fashion to each individual cell sample of interest. For convenience, a barcoding plate is made in a 96-well plate containing different combinations of barcoding reagents in each well. The pattern of binary combinations of the barcoding reagents distributed over the plate is called the barcoding key. The number of combinations depends on the number of different barcoding reagents (i.e., different metal isotopes used for making barcoding reagents). Additionally, in order to address the metal content variability across the plate, and avoid potential signal shifts, the metal content between wells and therefore samples must be equalized. Thus, in parallel to addition of a given barcoding reagent to each well (i.e., Lu175), the metal content is balanced by the addition of same amount of equalization reagent to wells not containing the given barcoding reagent. Importantly, the principle of the protocol remains the same regardless of a barcoding key used for making the barcoding plates. Multiple barcoding plates can be prepared at the same time by preparing one “master” barcoding plate containing barcoding reagents at high concentration, which can be diluted into individual barcoding plates. In the following section, we describe the generation of a 96-well barcoding plates similar to the setup described in Bodenmiller et al. (2012).
5.1 Materials
-
Thermo-fast 96 Detection Plate (Thermo, #AB1400-L)
-
Aluminum sealing film (Axygen, #PCR-AS-200)
-
DMSO (Sigma-Aldrich, # D2438)
-
Barcoding reagents
-
Equalization reagent
5.2 Method
5.2.1 Preparation of the Master Plate
The master plate will contain the mixed barcoding reagents at a 10-fold higher concentration than the concentrations needed for individual barcoding plates (Table 1). To generate the master plate, 10 μL of barcoding reagent working solution is pipetted into appropriate wells, and the reagent for equalization is pipetted in the wells not containing that barcoding reagent (Fig. 3). Equalization reagent is used in the same volume (10 μL) and in the same concentration as the barcoding reagent. This means that when seven barcoding reagents are used, the total volume in each well will be 100 μL: 70 μL barcoding reagent plus equalization reagent and 30 μL DMSO. Working solutions of the barcoding reagents and equalizing reagents are prepared by diluting the stock solutions (10 mM) to the concentrations needed for the master plate preparation.
Layout of barcoding matrix used to encode 96 samples. Seven unique lanthanide isotopes were used to generate 128 combinations, enough to barcode each sample in 96-well plate. The concentrations of each of the seven lanthanide isotopes and that of the equalizing reagent are shown as they are distributed on the 96-well plate
Note: In order to minimize pipetting errors, the barcoding key layout was designed to be pipetted using a multi-channel pipette. We advise that individual reagents are first pipetted in “patterns” suitable for the multi-channel pipette and at higher volume than it is required in “intermediate” 96-well plates, so that they can be easily transferred to the master plate using the multi-channel pipette.
5.2.2 Preparation of Barcoding Plates from Master Plate
Note : Use a multi-channel pipette for preparing the barcoding plates to minimize pipetting errors.
-
1.
Pipette 90 μL DMSO into each well of nine 96-well plates.
-
2.
Transfer 10 μL of barcoding reagent from the master plate to the appropriate wells in nine barcoding plates.
-
3.
Mix gently by pipetting up and down.
-
4.
Dilute the remaining 10 μL of barcoding reagent in the master plate by adding 90 μL of DMSO.
-
5.
Seal barcoding plates with aluminum foil and store at −20 °C until used.
6 Validation of the MCB Master Plate
To validate the accuracy and robustness of the MCB method, it is essential to verify that the MCB method does not alter mass cytometry measurements or introduce artifacts. In addition, it is important to ensure that the pattern of the barcoding reagent distribution in the barcoding plate can be retrieved upon deconvolution of the data acquired on the mass cytometer. Typically, two types of cell populations differing in a component to be immunostained (e.g., stimulated vs. unstimulated cells) are stained and used to validate the MCB master plate. Two cell types are arranged in geometrical shapes across the 96-well plate to create a checkerboard or striped pattern. After 96-well multiplexing, mass cytometry analysis of a particular antigen abundance is performed. Deconvolution should yield the expected pattern of cell sample distribution on the 96-well plate (Fig. 4) (Bodenmiller et al. 2012). In addition, the data acquired for multiplexed cell samples should be compared to non-multiplexed samples to ensure similar behavior (Bodenmiller et al. 2012; Krutzik and Nolan 2006; Krutzik et al. 2008).
Checkerboard and striped patterns of cell sample distribution used for validation of MCB analysis. Reproduced from Bodenmiller et al. (2012)
6.1 Materials
-
20 × 106 crosslinked and permeabilized cells from stimulated and unstimulated samples
-
Metal-tagged antibodies (e.g., DVS Sciences or self-conjugated)
-
CSM at 4 °C
-
Iridium metallointercalator working solution (DVS Sciences, #Inter-1X-natIr)
-
ddH2O
-
PBS (Sigma Aldrich, #P4417-100TAB)
-
Cluster tubes (Corning, #4418)
-
FACS tubes (BD Falcon, #352008)
-
Filter cap FACS tubes (BD Falcon, #352235)
-
Inject-F 1 mL syringes (Fisher, #S7510-1).
6.2 Method
-
1.
Aliquot cells into the cluster tubes by pipetting 0.2 × 106 cells/tube (200 μL of 106 cell/mL suspension).
-
2.
Organize cell samples according to one of the patterns shown in Fig. 4.
-
3.
Add CSM to a volume of 1 mL; centrifuge at 600 × g for 5 min at 4 °C.
-
4.
Remove supernatant.
-
5.
Resuspend cells in 1 mL PBS and centrifuge at 600 × g for 5 min at 4 °C.
-
6.
Remove supernatant.
-
7.
Resuspend cells in 500 μL PBS.
-
8.
Add 5 μL of barcoding reagent from the barcoding plate and mix immediately by pipetting.
-
9.
Incubate for 30 min at room temperature.
-
10.
Add 500 μL CSM and centrifuge at 600 × g for 5 min at 4 °C.
-
11.
Remove supernatant.
-
12.
Repeat washing step twice.
-
13.
Mix all cells from the checkerboard or striped pattern into a single FACS tube.
-
14.
Perform immunostaining by resuspending the cell pellets in 300 μL of an appropriate antibody mix.
-
15.
Mix gently and incubate of 60 min at room temperature.
-
16.
Add CSM to a volume of 3 mL.
-
17.
Centrifuge at 600 × g for 5 min at 4 °C.
-
18.
Remove supernatant.
-
19.
Add 3 mL CSM and centrifuge at 600 × g for 5 min at 4 °C.
-
20.
Remove supernatant.
-
21.
Proceed with Ir-metallointercalation (1:5000) protocol and cell preparation for mass cytometric analysis (see above).
-
22.
Acquire sample data on CyTOF® (unlimited cell number, acquisition time of 1,200 s).
-
23.
Deconvolute data.
7 Data Deconvolution
An essential step for multiplexed mass cytometry is the ability to retrieve the information on each original individual sample based on their barcoded mass signatures. This is achieved by using Boolean gating of barcoded cells in biaxial plots of mDOTA-lanthanide (III) channels, to define cell populations containing defined barcoding signatures as presented in the Fig. 5 (Bodenmiller et al. 2012). Boolean algebraic operations (AND, NOT, and OR) are combined with standard cell population gating techniques to generate Boolean gates in flow cytometry (Brown 2003). By creating multiple combinations of operations, any gating structure can be implemented during analysis to define cell populations containing a particular barcoding signature and subsequently to assign cells to their original sample.
Boolean gating. a Density dot biaxial plots of barcoded cells are shown with DNA content on the y axis and barcoding channel on the x axis. Cells positive and negative for a given channel are indicated. b Standard gating techniques are combined with Boolean algebraic operations (AND, NOT, OR) to generate Boolean gates and, subsequently, to assign to each cell sample a particular barcode signature and position on a barcoding plate
8 Barcoding and Intracellular Staining of Cell Samples
Here, we describe a straightforward procedure for combining MCB with immunostaining of cell samples. The protocol can easily adapt to lab-specific requirements. Important for a successful barcoding is the cell permeabilization and the removal of unbound barcoding reagent.
8.1 Materials
-
Crosslinked and permeabilized cells (see above)
-
PBS (Sigma Aldrich, #P4417-100TAB)
-
ddH2O
-
Cluster tubes (Corning, #4418)
-
FACS tubes (BD Falcon, #352008)
-
Filter cap FACS tubes (BD Falcon, #352235)
-
Inject-F 1 mL syringes (Fisher, #S7510-1)
-
CSM at 4 °C
-
Iridium metallointercalator working solution (DVS Sciences, #Inter-1X-natIr).
8.2 Method
-
1.
Use one cluster tube per sample; add 0.2–1 × 106 cells to each tube.
-
2.
Add CSM to 1 mL.
-
3.
Centrifuge at 600 × g for 5 min at 4 °C.
-
4.
Remove supernatant.
-
5.
Resuspend cell pellet in 1 mL PBS and centrifuge at 600 × g for 5 min at 4 °C.
-
6.
Remove supernatant.
-
7.
Resuspend cell pellet in 500 μL PBS.
-
8.
Add 5 μL of barcoding reagent and mix immediately by pipetting.
-
9.
Incubate for 30 min at room temperature. Keep cells in suspension by vortexing intermittently.
-
10.
Add 500 μL CSM and centrifuge at 600 × g for 5 min at 4 °C.
-
11.
Remove supernatant.
-
12.
Add 1 mL CSM and centrifuge at 600 × g for 5 min at 4 °C.
-
13.
Remove supernatant.
-
14.
Repeat washing step twice.
-
15.
Combine all cell samples into a single FACS tube.
-
16.
Centrifuge at 600 × g for 5 min at 4 °C.
-
17.
Perform immunostaining by resuspending the cell pellets in 300 μL of an appropriate antibody mix.
-
18.
Mix gently and incubate for 60 min at room temperature.
-
19.
Add CSM to 3 mL and centrifuge at 600 × g for 5 min at 4 °C.
-
20.
Remove supernatant.
-
21.
Add 3 mL CSM and centrifuge at 600 × g for 5 min at 4 °C.
-
22.
Remove supernatant.
-
23.
Proceed with Ir-metallointercalation (1:5000) protocol and cell preparation for mass cytometric analysis (see above).
-
24.
Acquire sample data on CyTOF® (unlimited cell number, acquisition time of 1,200 s).
-
25.
Deconvolute data.
References
Bendall SC, Simonds EF, Qiu P, Amir el-AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe’er D, Tanner SD, Nolan GP (2011) Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332:687–696
Bodenmiller B, Zunder ER, Finck R, Chen TJ, Savig ES, Bruggner RV, Simonds EF, Bendall SC, Sachs K, Krutzik PO, Nolan GP (2012) Multiplexed mass cytometry profiling of cellular states perturbed by small-molecule regulators. Nat Biotechnol 30:858–867
Brown FM (2003) Boolean reasoning: the logic of Boolean equations. Dover Publications, New York
Krutzik PO, Nolan GP (2006) Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat Methods 3:361–368
Krutzik PO, Crane JM, Clutter MR, Nolan GP (2008) High-content single-cell drug screening with phosphospecific flow cytometry. Nat Chem Biol 4:132–142
Ornatsky O, Bandura D, Baranov V, Nitz M, Winnik MA, Tanner S (2010) Highly multiparametric analysis by mass cytometry. J Immunol Methods 361:1–20
Tanner SD, Baranov VI, Ornatsky OI, Bandura DR, George TC (2013) An introduction to mass cytometry: fundamentals and applications. Cancer Immunol Immunother 62:955–965
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Zivanovic, N., Jacobs, A., Bodenmiller, B. (2013). A Practical Guide to Multiplexed Mass Cytometry. In: Fienberg, H., Nolan, G. (eds) High-Dimensional Single Cell Analysis. Current Topics in Microbiology and Immunology, vol 377. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2013_335
Download citation
DOI: https://doi.org/10.1007/82_2013_335
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54826-0
Online ISBN: 978-3-642-54827-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)