Abstract
Streptococcal adhesion, invasion, intracellular trafficking, dissemination, and persistence in eukaryotic cells have a variety of implications in the infection pathogenesis. While cell adhesion establishes the initial host contact, adhering bacteria exploit the host cell for their own benefit. Internalization into the host cell is an essential step for bacterial survival and subsequent dissemination and persistence, thus playing a key role in the course of infection. This chapter summarizes the current knowledge about the diverse mechanisms of streptococcal adhesion to and invasion into different eukaryotic cells and the impact on dissemination and persistence which is reflected by consequences for the pathogenesis of streptococcal infections.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Streptococci play an important role as clinically relevant pathogens worldwide. Streptococci cause approximately 700 million human infections each year, with estimated 500,000 deaths (Carapetis 2005). This clinical importance of streptococci was recognized as early as 1879 by Louis Pasteur when studying puerperal fever causing high mortality rates in pregnant women. Thus, streptococci have been recognized as one of the first microorganisms causing contagious disease. Historically, the classification of streptococci was based on Rebecca Lancefield’s scheme grouping streptococcal strains according to the different carbohydrate compositions of the cell wall. Nowadays, M- and T- typing is performed or the 5’-end variability of genes for the M protein, emm genes, are used for molecular typing. Streptococci consist of a heterogeneous genus that comprises important human pathogens like Streptococcus pyogenes, also referred to as Group A Streptococci (GAS), Streptococcus dysgalactiae subsp. equisimilis as a member of Group C Streptococci (GCS) and Group G Streptococci (GGS), S. agalactiae also known as Group B Streptococci (GBS), as well as S. pneumoniae (pneumococci). Today, streptococci are also often organized into six groupings (pyogenic, anginosus, mitis, mutans, salivarius, and bovis) based on their 16S rRNA gene sequence. Members of the pyogenic group, S. pyogenes and S. dysgalactiae subsp. equisimilis, are often associated with a range of diseases including pharyngitis, tonsillitis, impetigo, mastitis, and resulting sequelae like rheumatic fever or glomerulonephritis (Henningham et al. 2012a). Streptococci are also able to cause recurrent infections such as erysipel and tonsillitis. This phenomenon has been described as the carrier stage of streptococcal infection and it is believed that streptococci have a safe ecological niche within the human body, most properly residing intracellularly in eucaryotic cells, which allow them not only to persist but also to resist antibiotic treatment. Moreover, they can lead to severe invasive infections like bacteraemia, cellulites, and necrotizing fasciitis with high mortality due to streptococcal toxic shock syndrome (STSS). This demonstrates that streptococci are able to infect otherwise sterile deep tissue in the human host. For this purpose streptococci express a highly variable and extensive repertoire of virulence determinants that are differentially regulated and expressed in response to signals from the environment within the human host. In the past 15 years more and more evidence has become available which suggests that the long time considered extracellular pathogenic bacteria of the genus Streptococcus adhere to host cells, and subsequently invade these cells for their own benefit, namely escaping antibiotic treatment and the host immune system (for reviews see Cunningham 2000; Nitsche-Schmitz et al. 2007; Nobbs et al. 2009; Courtney et al. 2002). This chapter will mainly focus on Group A streptococci highlighting the repertoire of adhesins and invasins of streptococci, and their impact on the colonization and invasion of the human host the persistence of streptococci within the host.
2 Adhesion of GAS
The initial and essential step of streptococcal infectious colonization of the host depends on adhesion of the pathogen to the host tissue. To allow colonization of the site of infection, streptococci express an arsenal of surface bound components, named adhesins, which establish the contact to the host’s extracellular matrix (ECM) or directly to host cells. Since streptococci possess multiple adhesins, they are able to colonize various sites in the human body. Adhesion connects the bacteria firmly to the host cells and allows the pathogen to withstand host cleansing mechanisms like mechanical forces by excretion or peristalsis. Nevertheless, in some instances it might be an advantage for the bacteria to detach from a surface in case the growth conditions become unfavorable. Thus, adhesion can sometimes also be considered as being a dynamic process. Most host surfaces are covered by a protective epithelial or endothelial cell layer which is covered with extracellular matrix proteins like collagen, fibrinogen, laminin, vitronectin, or fibronectin (Cremer et al. 1998; Debelle and Tamburro 1999; Schvartz et al. 1999; Pankov and Yamada 2002; Dempfle and Mosesson 2003). Many streptococcal adhesins function by specifically binding to various compounds of the extracellular matrix. Since streptococci express multiple adhesins, it is most likely that different adhesins are expressed under the different environmental conditions encountered throughout an infection. Once adhesion has been established, streptococci can multiply extracellularly, establish colonization of the infection side, internalize into host cells, traffic intracellularly in the host cell and multiply within the cell, or escape from the host cell, or use the host cell as a Trojan horse for systemic spread of the infection.
2.1 Cell Wall-Anchored Adhesins
Adhesins can be attached in four different ways to the streptococcal surface (i) covalently linked through the C-terminus to the cell wall peptidoglycan through an LPxTz motif, (ii) tethered to the cell membrane through N-terminal modifications with lipid, (iii) retained on the surface by a yet unknown mechanism, or (iv) bound back to the cell surface through noncovalent interactions with cell surface compounds like polysaccharides or other proteins. Most adhesins belong to the family of cell wall-anchored proteins. A prerequisite for those proteins is a functioning membrane-associated transpeptidase, sortase A (Maraffini et al. 2006). Many streptococcal adhesins function by specifically recognizing and binding to the various components found in the ECM of the host. The ECM is the major support of cells and tissue and it is responsible for maintaining strength and elasticity of the body. Thus, it is ubiquitously present and frequently exposed under conditions such as trauma and injury, making the then exposed components an ideal prime target for streptococcal adhesion. In addition to the adhesins, other surface structures of streptococci like fimbriae, pili, lipoteichoic acid, and polysaccharides may also play an important role during the first steps of adhesion to the host cell.
2.1.1 Hyaluronic Acid Capsule and Lipoteichoic Acid
Many of the β-hemolytic streptococci are producing a polysaccharide capsule, consisting of hyaluronic acid (HA), a glycosaminoglycan that is a linear polymer of alternating monosaccharide units of N-acetylglucosamine and glucuronic acid. Streptococci show differently expressed HA capsules. While some strains are barely encapsulated when grown ex vivo on agar plates, others produce high amounts of the HA capsule that confers a mucoid appearance to the bacterial colonies that were grown on solid media (Wilson 1959). When compared to the host HA, the streptococcal HA is chemically very similar. The host HA is a widely distributed and abundant component of the host’s ECM. The chemical similarity might be the reason for the low immunogenicity of the streptococcal HA capsule in the infected patients. It is assumed that the HA capsule masks the bacteria and therefore provides protection against the host immune system. An epidemiological investigation demonstrated that encapsulated strains can often be isolated from severe invasive infections, whereas isolates from uncomplicated pharyngitis were only rarely of mucoid character. A total of 42 % of isolates from patients with acute rheumatic fever were found to be of the mucoid type (Johnson et al. 1992). In addition, the HA capsule can act as a nonprotein adhesin by binding to the hyaluronic acid receptor CD44 on keratinocytes (Schrager et al. 1998), and the HA capsule serves as a ligand for collagen IV (Dinkla et al. 2003a). These studies highlight that the HA capsule is not only a nonprotein adhesin, but can also be described as a virulence factor associated with invasive infections and their severe sequelae like acute rheumatic heart disease. Studies in the US concluded that outbreaks of acute rheumatic fever were associated with the spread of mainly M-type 18 highly encapsulated strains (Veasy et al. 2004; Kaplan et al. 1989). In addition, the contribution of the HA capsule for pharyngeal colonization was also demonstrated (Wessels et al. 1991). Most noteworthy, mucoid strains seem to help to breach epithelial cell layers for entering underlying sterile tissue in the process of dissemination in the host. Binding of the HA capsule to CD44 leads to cytoskeletal rearrangements in human epithelial cells (Schrager et al. 1998; Cywes et al. 2000) that cause disruption of intracellular junctions and allow the passage into deeper tissue. Streptococci therefore travel paracellularly through an epithelial cell barrier, such as keratinocytes, instead of an intracellular passage after internalization into the epithelial host cells (Cywes and Wessels 2001).
Evidence has been acquired demonstrating that the HA capsule is not always representing a nonprotein adhesin, since it was demonstrated that the HA capsule can also counteract internalization into human epithelial cells and keratinocytes (Jadoun et al. 2002; Darmstadt et al. 2000; Schrager et al. 1996). The observed decreased internalization is accompanied by an increase in tissue damage as observed in a mouse model (Schrager et al. 1996). The increased virulence was also attributed to an antiphagocytic effect of the HA capsule (Wessels et al. 1991, 1994; Wessels and Bronze 1994). The HA capsule was reported to decrease the association with PMNs, thereby counteracting phagocytosis (Dale et al. 1996).
Lipoteichoic acid (LTA) is composed of chain-like glycerol phosphate polymers that are covalently coupled to glycolipids of the plasma membrane and represent a component of the cell wall. LTA is thought to allow streptococci to come into first contact with the ECM or directly with the host cell. A two-step adhesion mechanism has been postulated. LTA has the role of a first-step adhesive component with low cell type selectivity (Beachey and Ofek 1976; Leon and Panos 1990; Courtney and Hasty 1991; Courtney et al. 1992), whereas the highly abundant extracellular ECM protein fibronectin functions as a ligand or “bridging-molecule” for LTA mediating the initial streptococcal cell adhesion (Simpson and Beachey 1983). For establishing a firm adhesion to host cells, a second step with high avidity and cell specificity is required (Courtney et al. 1992; Hasty et al. 1992). This second step has a crucial influence on the observed tissue tropism of streptococcal infection and the pathway of dissemination in the host. More importantly, interactions of the proposed second step were shown to promote internalization of the bacteria into eukaryotic cells (LaPenta et al. 1994, Greco et al. 1995). The involvement of LTA in adhesion and invasion during streptococcal pathogenesis was recently demonstrated. In a cell culture model consisting of human brain microvascular endothelial cells, Group B streptococci (GBS), the leading pathogen in neonatal meningitis, with an impaired anchoring of LTA in the cell wall due to an inactivated glycosyltransferase gene showed a decreased invasion capability. The mutant strain exhibited less virulence in the meningitis mouse model of infection (Doran et al. 2005). Although LTA promotes adhesion in a nonselective way, LTA adhesion might contribute to the onset of the subsequent infection pathway.
2.1.2 Fimbrious Structures Like Pili
Examination of negatively stained streptococci reveals in some groups filamentous structures like fibrils and pili or fimbriae. Fibrils have been detected especially in oral streptococci with a peritrich or specifically localized structure on the cell wall. These structures have been related to growth and survival of oral streptococci since all fresh isolates express fibrils (Handley et al. 1984, 1987).
For many years, bacteriologists had evidence that Gram-negative bacteria adhere to host cells and pili, also sometimes referred to as fimbriae, serving as the primary colonization factor in the adhesion process. Pili are long hair-like extensions of the cell surface and can best be made visible by negative-staining of the bacteria and by electron microscopic observation. Although pili in Gram-positive bacteria were described first in 1968 in Corynebacteria and in the 1990s in streptococci (Yanagawa et al. 1968; Yanagawa and Honda 1976; Wu and Fives-Taylor 1999), they were more or less neglected in research in the years to follow. Just recently, pili in all three pathogenic streptococcal species causing invasive disease, S. pyogenes, S. agalactiae, and S. pneumonia, have been described (Mora et al. 2005; Lauer et al. 2005; Rosini et al. 2006; Barocchi et al. 2006). Since then, pili have come into the research focus and the newly detected pili in streptococci have been considered as most promising candidates for vaccine development against streptococci (Gianfoldoni et al. 2007).
In Gram-negative pathogens, pili consist of multiple subunits which are noncovalently attached to each other and the adhesin molecule is located exclusively at the tip of the pili. There are remarkable differences in the pili assembly in Gram-negative and Gram-positive bacteria. Whereas in Gram-negative bacteria the Sec-dependent secretion and chaperones are involved in the formation of the pili subunits (backbone) and the tip adhesin subunit (the most common assembly way is the chaperone/usher pathway) (Saulino et al. 2000), in Gram-positive bacteria pili subunits are covalently linked to each other and pili are covalently attached to the peptidoglycan. As in Gram-negative bacteria, the Sec-dependent machinery is also involved, but the polymerization of the subunit is controlled by an enzyme with homologies to sortases, called sortase A, which sorts and attaches proteins covalently to the peptidoglycan. For covalent linkage to the peptidoglycan, proteins exhibit a specific motif, the cell wall sorting signal (CWSS), in the C-terminus of the protein. The main characteristic is the LPxTG motif, a sequence that is conserved in all surface proteins anchored by sortase A. The LPxTG motive is followed by a hydrophobic membrane-spanning domain and a positively charged tail which is needed for the sortase A catalyzed anchoring with the peptidoglycan. For anchoring a surface protein to the cell wall, sortase A cleaves the LPxTG motif between threonine (T) and glycine (G), and the threonine residue of the cleaved peptide is covalently linked to the amino group of the cross-bridge within the peptidoglycan (Schneewind et al. 1993; Ton-That et al. 2004; Telford et al. 2006; Scott and Zähner 2006; Mandlik et al. 2007a). During the polymerization process, self-generated intramolecular isopeptide bonds are formed which give additional stabilization to the thin pilus structure and allow to withstand the tensile forces during the first steps in adhesion to the host cells. Remarkably, similar isopeptide bonds have been found in other surface displayed adhesins (Kang et al. 2007). In addition, genes encoding transpeptidases of the sortase C subfamily have been found functioning in polymerization of the subunits (Proft and Baker 2009).
For all streptococci it was found that the genes encoding for pilus proteins are located on pathogenicity islands (PI) and clustered at the same genetic locus in an operon. Sortase genes are in close proximity to the pili genes, implying that they might also belong to the operon. Up to now, nine PIs have been identified in GAS. Remarkably, the genes in GAS occur in the fibronectin binding, collagen binding, T-antigen region of the chromosome (FCT-region). Interestingly, this locus actually encodes the Lancefield T-antigen which has been used for serotype typing (Falugi et al. 2008). Mora et al. (2005) first identified that the T-antigen of serotyping actually represents the shaft of the pilus in a set of four GAS strains of different serotypes. Immune negative-staining with an antibody against the T-antigen revealed staining alongside the entire shaft of the pilus, but no labeling was detected at the pilus tip. The tip region was only stained by an antibody against the presumed adhesin. Thus, the pilus shaft represents the T-antigen used by R. Lancefield for serotyping. Additionally, a third component was identified in Gram-positive pili which is added at intervals into the shaft region of the pilus, the AP1 (ancillary protein 1) component, representing a collagen binding protein moiety (Podbielski et al. 1999). This observation confirms that pili are involved in the adhesion process especially with components of the ECM. Since pili were detected in streptococci, more evidence has been accumulating that pili are involved in the adhesion process, and in fact pili might actually mediate the first contact with the host cell. Subsequently, it was demonstrated that pili are involved in the adhesion process to a broad range of host epithelial cells including cells from the nasopharynx, tonsils, lung, cervix, and intestine (Abbot et al. 2007; Crotty et al. 2010). Remarkably, for GBS it was found that despite being involved in the adhesion process, pili in GBS are responsible for a paracellular passage through an epithelial cell barrier as well as for promoting invasion into brain microvascular endothelial cells (Maisey et al. 2007; Pezzicoli et al. 2008). Recent pili research has highlighted that pilus protein components of invasive streptococci have amino acid sequences similar to those of the members of the microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) which have been shown to interact with the ECM proteins like fibronectin, fibrinogen, collagen, laminin, and vitronectin (Patti and Höök 1994). Pili have also been shown to be responsible for tissue tropism during infections. Besides the long stretched extended pili, the so-called minor pilins have been identified in Corynebacterium by mutation analyses of pili. For these minor pilins, it has been demonstrated that they are displayed on the cell surface. Recombinant protein, when coated onto inert latex beads, clearly exhibited binding only to pharyngeal cells, but no binding resulted to cells of lung or laryngeal origin when tested in a cell culturing model (Mandlik et al. 2007b). In addition, the role of minor pilins in streptococcal tissue tropism has also been elucidated. These minor pilins mediate adhesion of GAS to human tonsil epithelium and primary keratinocytes, the prime colonization targets of GAS (Abbott et al. 2007).
From these observations, a putative model of cell adhesion through pili has been deduced (Telford et al. 2006). The first contact with the ECM of the host or directly with the host cell surface is mediated by the extended pili with the presumed adhesin (AC2-protein) on the tips of the pili. This interaction might be reversible to allow streptococci to find the specific tissue (cell tropism). Once the first contact has been established, the Ap1-protein can interact with collagen to attach the bacterium more firmly to the host cell. Due to this interaction, the bacterium comes closer to the host cell surface (in Gram-negative bacteria it has been shown that after the first attachment pili are retracted to get the bacteria closer to the cell surface) and cell surface-anchored adhesins can establish the intimate contact with the host cell and subsequently colonize the cell or tissue. The intimate contact favors three different functions which are needed to progress with the infection: (i) the proximity of the bacterial cell wall with the host cell membrane allows further ligand–receptor interactions, (ii) the close contact favors the efficient delivery of virulence factors as for example for the type three secretion system, TTSS, in Gram-negative bacteria, and (iii) the intimate adherence between streptococci and host cell membrane is the prerequisite for an successful invasion of the host.
2.1.3 M Proteins
M proteins were the first reported adhesins of GAS (Ellen and Gibbons 1972, 1974) and represent multifunctional virulence factors and adhesins of the streptococcal surface. Although structurally closely related, M proteins represent a heterogeneous group of adhesins with respect to their ligand molecules or target cells (Berkower et al. 1999). Some M proteins exhibit very distinct binding properties, whereas the interaction with glycosaminoglycanes represents a more common adherence mechanism displayed by several M protein serotypes. These interactions are mediated via the conserved C-terminal part of M protein (Frick et al. 2003). M proteins are hypervariable in the N-terminal region of the protein and today more than 200 emm-serotypes can be distinguished which differ in their adhesion capabilities (Beall et al. 1996). M proteins consist of four repeat regions with the A repeats region being hypervariable and the B repeat regions being semivariable. Structural-based studies revealed that M proteins are entirely α-helical coiled-coil dimers and can be detected on the bacterial surface as hair-like projections in ultra-thin sections. The N-terminal region is further stabilized by antiparallel interactions, which is thought to result in bacterial aggregation (Frick et al. 2000; Oemcke et al. 2010). The hypervariable regions of M proteins bind to a wide range of different human proteins including plasminogen, IgA, IgG, factor H, and C4b-binding protein (C4BP) which inhibit complement activation (Andre et al. 2006, McArthur and Walker 2006). The B repeats can bind to fibrinogen, human serum albumin, and IgG. For the ability to bind fibrinogen, it was shown that structural irregularities and instabilities in the coiled-coil structure of M1 protein facilitate binding to fibrinogen (McNamara et al. 2008). In addition, serotype M6 protein can bind to the membrane-bound cofactor protein CD46 on keratinocytes. The C-terminal region of M6 protein as well as the short consensus domains 3 and 4 of CD46 was shown to be crucial for M6/CD46-mediated keratinocyte attachment (Okada et al. 1995; Giannakis et al. 2002). The direct involvement of M proteins in cellular adhesion has been demonstrated for several M protein serotypes. For example, for M1 protein, it was shown that the ECM protein fibronectin is necessary for efficient adhesion to epithelial HeLa cells. As the cellular receptor for fibronectin α5β1 integrins have been identified. An M1 protein mutant showed significant reduced adhesion which could be blocked by specific M1 protein antibodies (Cue et al. 2000, 2001). Similar results were observed for the M24 serotype protein in which the serotype M24 protein mediated adhesion to HEp-2 cells, whereas the M24 protein deficient mutant was unable to bind to HEp-2 cells but was able to adhere to buccal cells demonstrating the cell type specificity (cell tropism) of the M24 protein. The mutant studies also revealed that another adhesin or adhesins other than M24 protein must be displayed on the bacterial surface (Courtney et al. 1994). Using the cell culture approach, other M proteins have been tested for their involvement in adhesion to epithelial cells. For example, for the M6 protein it was shown that M6 protein does not contribute to the adhesion to buccal and tonsillar epithelial cells. In these studies, again the involvement of fibronectin in the adhesion process was demonstrated (Caparon et al. 1991). The M3 protein serves as an important adhesin for soluble type I and type IV collagen as well as to native collagen matrices in the host. The N-terminal variable region, though specific for the M3 protein, was identified as the collagen binding region. This explains why all other M proteins lack the collagen binding properties of the M3 protein. Besides direct collagen binding, M3 protein is responsible for aggregation of soluble collagen, resulting in large aggregates of streptococci that are involved in the colonization process. Due to this aggregate formation streptococci can better resist phagocytosis and antibiotic treatment (Dinkla et al. 2003b). S. pyogenes expresses only one collagen binding protein, Cpa, which was identified in a serotype M49 isolate and was shown to mediate attachment to immobilized type I collagen (Podbielski et al. 1999). Nevertheless, the potential role of collagen binding by serotype M3 protein in the adhesion to host tissue has to be demonstrated. That collagen can play a role in cell adhesion can be deduced from investigations on GGS. In strains of this group, the M-like protein FOG is expressed which has a high affinity for collagen I and allows GGS to bind to human foreskin fibroblasts (Nitsche et al. 2006). Since it is known that two distinct collagen-like proteins, termed SclA/Scl1 and SclB/Scl2, can bind to pharyngeal and fibroblast cells reacting with the α2-domain of α2β1-integrin, and thereby triggering cellular signaling in lung fibroblasts, the conclusion could be draw that GAS employs collagen binding integrins to adhere to host cells (Humtsoe et al. 2005).
Taken together, the role of M protein-mediated adhesion varies obviously with the M-serotype and represents a concerted action of the different ligand binding properties and sites within the M protein. We are just beginning to understand parts of the complex cell adhesion interactions mediated by the M proteins with respect to ligand–receptor binding. Once this process is fully understood, one might gain an insight into the so far mostly unknown interactions in streptococcal cell tropism and the onset of the cell tropism on the development of the disease.
2.1.4 Fibronectin-Binding Proteins
Fibronectin is a large glycoprotein which exists both as a soluble protein in plasma and as a fibrillar polymer in the ECM. Fibronectin is composed as a dimer of two 250 kDa subunits which are C-terminal linked via disulfide bonds. Each subunit exhibits three distinct modules, the type I, II, and III modules. Fibronectin interacts with host cell surface exposed integrins of which the α5β1 integrin is the classical fibronectin-binding integrin. Integrin binding is mediated through the RGD sequence within the fibronectin subunits (Pankov and Yamada 2002). Fibronectin can be seen as one of the prime targets in streptococcal adhesion and subsequent internalization when exploring the ECM during first steps of adhesion. Fibronectin acts as a bridging molecule, connecting bacterial adhesins with integrins on the surface of eukaryotic cells. All streptococci express fibronectin-binding proteins, but the proteins show differences in the fibronectin-binding capacities. Some serotype strains bind soluble fibronectin with high affinities (in the nanomolar range), whereas other strains can only adhere to immobilized fibronectin for a successful adherence. S. pyogenes possesses at least 11 distinct fibronectin-binding adhesins including SfbI/F1, protein F2, serum opacity factor (SOF), FbaA, and several M proteins. Some of these are present in a large number of serotypes, such as SfbI protein or FBP54, whereas others like the M1 or M3 proteins are exclusively expressed by M1 or M3 serotype streptococci (Talay et al. 1992, 1994; Hanski and Caparon 1992; Sela et al. 1993; Natanson et al. 1995; Ozeri et al. 1996; Cue et al. 2000; Kreikemeyer et al. 2004a). The expression of these fibronectin-binding proteins is highly regulated in response to environmental signals. For example, SfbI expression is regulated by a superoxide signal. At high partial pressures of O2, expression of SfbI is increased while expression of M protein is upregulated in a CO2-rich environment, which suggests that SfbI protein is expressed at the prime target sites of S. pyogenes adhesion, namely in the O2-rich environment of the respiratory tract and skin, where it is required for host adhesion and colonization. Whereas in deeper infections protection against the immune system becomes more important, the increased partial pressure of CO2 upregulates M protein (Gibson et al. 1995; Gibson and Caparon 1996; Kreikemeyer et al. 2003, 2004b).
Among all fibronectin-binding adhesins of S. pyogenes, SfbI protein and its allelic variant F1 are studied most extensively. Identified in 1992, SfbI/F1 was shown to act as an adhesin on epithelial cells (Talay et al. 1992; Hanski and Caparon 1992). SfbI protein exhibits a modular architecture with an aromatic domain, rich in aromatic amino acids, ARO, at the N-terminus, a proline-rich repeat region (PRR) in the middle of the molecule and a fibronectin-binding repeat region (FnBR) at the C-terminus as major modules (Talay et al. 1994). SfbI protein is very variable in the number of repeats in the PRR and FnBR regions. One study revealed that 34 distinct alleles of SfbI were found in 54 S. pyogenes strains as a result of horizontal gene transfer (Towers et al. 2003). The ARO region showed a high degree of sequence variability, whereas deletion or duplication of repeat units in PRR and FnBR resulted in variable numbers of PRR (1–11 repeats) and FnBR (1–5 repeats). Thus, SfbI protein exhibits a high antigenic variation, which might also reflect possible variable functional capabilities.
Binding to fibronectin is mediated by two distinct domains (Sela et al. 1993; Ozeri et al. 1996) and proceeds in the following way: the C-terminal fibronectin-binding repeat region and the adjacent nonrepetitive domain termed spacer 2 or UR synergistically bind to two distinct regions on the fibronectin molecule: the N-terminal fibrin-binding fragment harboring fibronectin F1 modules 1–5, and the gelatine/collagen-binding fragment harboring F1 modules 6–9 and the two F2 modules. Due to this cooperative binding, the RGD region in fibronectin gets exposed and can bind to integrins (Talay et al. 2000).
The molecular interaction between SfbI and fibronectin is based on three-dimensional structural data. Three-dimensional structure data are available describing a bacterial fibronectin-binding fragment of S. dysgalactiae bound to its target (Schwarz-Linek et al. 2003, 2004a, b). Since SfbI shows high similarity in respect to structural composition, conclusion can be drawn on the binding mechanism for SfbI and fibronectin. That this can indeed be observed was recently shown by Marjenberg et al. (2011) by applying microcalorimetry to reveal cooperative binding of fibronectin fragments to arrays of binding in SfbI (Marjenberg et al. 2011). The so far existing structural model for fibronectin-binding proteins is comprehensively reviewed by Schwarz-Linek et al. (2004a, b). Briefly, SfbI and fibronectin bind to each other in an antiparallel fashion. The C-terminal FnBR in SfbI recognizes the N-terminal domain of fibronectin with high specificity and high affinity (in the nanomolar range) by forming a novel protein–protein interaction mechanism, termed tandem ß-zipper. According to the tandem ß-zipper model, FnBRs can bind multiple copies of fibronectin. For SfbI from a S. pyogenes strain, it was demonstrated that a single SfbI molecule can bind up to five fibronectin molecules. The observed high affinity is considerably important since high-affinity binding is a prerequisite for firm bacterial attachment, because adherent streptococci have to withstand shear forces occurring on the mucosal surfaces or during the internalization process itself.
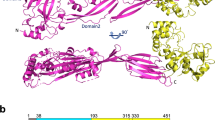
The C-terminal fibronectin-binding repeat region of SfbI was demonstrated to be sufficient to mediate adherence to epithelial cells (Talay et al. 2000). Several studies revealed that SfbI mediates attachment to epithelial cells of the oral mucosa and the lung, but also to endothelial cells (Rohde et al. 2003). Besides its potential to bind to cell exposed integrins, SfbI has the ability to recruit collagen via prebound fibronectin. This allows S. pyogenes to form aggregates and renders the organism capable of colonizing collagen matrices within the body (Dinkla et al. 2003a) as depicted in Fig. 1.
Protein F2 or PFBP are homologous but distinct fibronectin-binding proteins, found in most isolates of S. pyogenes lacking the sfbI/prtF1 gene (Jaffe et al. 1996; Rocha and Fischetti 1999; Kreikemeyer et al. 2004a). Protein F2 possesses two binding domains that interact with fibronectin. The most abundant fibronectin-binding protein found in all S. pyogenes isolates is FBP54. Although lacking a classical membrane anchor motif of Gram-positive surface proteins, it seems to be localized on the streptococcal surface by a distinct mechanism, thereby acting as an adhesin for buccal epithelial cells but not HEp2 cells (Courtney et al. 1996; Chhatwal 2002). In summary, this data suggests that distinct fibronectin-binding proteins target different cell types, and therefore might contribute to streptococcal cell tropism.
Streptococci can express two other fibronectin-binding proteins, Fba and FbaB. The fba gene was found only in five serotypes of S. pyogenes including serotype M1 and M49. An Fba mutant showed reduced adhesion to HEp2 cells, suggesting that this protein has adhesive properties. Interestingly, FbaB protein was only found in serotype M3/M18 S. pyogenes isolates and appears to be genetically most closely related to protein F2 (Terao et al. 2001, 2002). FbaB protein from serotype M3 S. pyogenes exhibits cell tropism since it mediates adhesion only to endothelial and not to epithelial cells (Amelung et al. 2011).
Another fibronectin-binding protein exposed on the bacterial surface is Protein H, a member of the M protein family. Protein H binds to the type III modules of fibronectin, unlike the so far described proteins that mainly interact with the type I or type II module containing domains of fibronectin. In addition, Protein H was shown to mediate streptococcal aggregation through a so called AHP sequence that also promoted adhesion to epithelial cells (Frick et al. 1995, 2000). M1 protein, another member of the M protein family, also binds to fibronectin and as in the case with SfbI, α5β1 integrins are the terminal receptor proteins on the cellular surface. M1 specific antibodies efficiently blocked adherence to epithelial HeLa cells. Moreover, an M1-deficient mutant showed reduced adherence indicating that M1 protein acts as an adhesin in serotype M1 S. pyogenes strains (Cue et al. 1998, 2000; Dombek et al. 1999).
2.2 Anchorless Adhesins
Most streptococcal surface proteins are attached through the C-termini via an LPxTz motif to the peptidoglycan of the cell wall. In recent years, a few proteins have been identified that bind to the cell surface without the LPxTz motif- the so called anchorless proteins. Noteworthy, these proteins also lack the N-terminal signal sequence. How these proteins are exported from the cytoplasm to the cell surface is not understood and remains an enigma as well as the attachment mechanism to the cell wall. Since the anchorless adhesins do not contain choline-binding repeats and can be removed from the bacterial surface with chaotropic agents, the interaction seems to be of hydrophobic nature, van der Waals forces, or of less-defined charges (Derbise et al. 2004). Anchorless adhesins are structurally and functionally very diverse and bind to different ligands. Most important is the fact that many anchorless adhesins display an enzymatic function mostly in the cytoplasm of the bacterium, especially in the glycolytic cycle. Triosephosphate isomerase, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 3-phosphoglycerate kinase, 3-phosphoglycerate mutase, and α-enolase are the last five enzymes in the glycolytic cycle and all have been found to be associated with the cell wall surface. Up to date, from these GAPDH and α-enolase are the best studied examples (Pancholi and Fischetti 1992, 1998; Kinnby et al. 2008). GAPDH can be detected in several species of streptococcus and targets multiple matrix proteins like fibronectin, fibrinogen, plasmin, and plasminogen. It is discussed that GAPDH contributes significantly to the colonization capabilities of GAS. Surprisingly, GAPDH seems also to be involved in interactions with the cytoskeletal proteins actin and myosin and with urokinase plasminogen activator receptor (Pancholi and Fischetti, 1992; Seifert et al. 2003; Jin et al. 2005). The above described anchorless adhesins have another feature in common when they function as a complex in the glycolytic cycle namely generation of ATP. Thus, the anchorless adhesins allow streptococci to produce extracellular ATP on the surface. It can be speculated that streptococci might use this ATP for host modulatory effects, since it is known that ATP is able to bind to P2X7 receptors on immune cells and can inhibit apoptosis. Streptococci seem to have evolved a system to manipulate the host immune cells for the benefit of a better progression of the infection after the first steps of adhesion to the host tissue (Yilmaz et al. 2008).
α-enolase serves as the major plasmin and plasminogen-binding protein of streptococci. After binding plasminogen, it can be converted into plasmin by plasminogen activators. Plasmin represents a very potent serine protease which degrades ECM matrix proteins to allow streptococci to gain access into deeper tissue. It is also discussed if plasmin is involved in facilitating bacterial dissemination through epithelial or endothelial barriers. Other members of anchorless adhesins are GtfG, SpeB, HtrA, Sib35, and FBP54. GtfG, SpeB, and Sib35 are secreted proteins which bind back to the cell wall surface (Hytonen et al. 2001; Kawabat et al. 2002). Other anchorless proteins like ADI, TF, and FBA were found by a post-proteomic approach. For ADI and TF, it was shown that both proteins elicited a protective immune response against intraperitoneal challenge with a serotype M1 isolate (Henningham et al. 2012b).
In summary, anchorless adhesins allow streptococci more flexibility compared to cell wall-linked adhesins since the anchorless proteins can be released and then bound back to the cell wall surface for better exploiting the environment by interacting with host molecules in a certain distance away from the bacterium. The question whether the glycolytic enzymes produce extracellular ATP or not and the function of extracellular ATP in the infection process has to be answered in further studies.
3 Invasion of Streptococci into Eukaryotic Cells
As outlined above, receptor recognition via ECM proteins as bridging molecules for adhesins is essential for a successful adherence to host tissue or cells. During the last two decades, it has become clear that after adhesion a subsequent process is triggered by the firmly attached streptococci, namely internalization into host cells. As mentioned, integrins play an important role in adhesion to the ECM proteins bound by streptococci and the integrin signaling pathways are then exploited by the bacteria to promote their own uptake by an outside–inside signal.
In 1994, LaPenta and colleagues first demonstrated in cell culture infection models that GAS can invade into nonphagocytic human epithelial cells at frequencies equal or greater than the classical intracellular pathogens such as Listeria or Salmonella (LaPenta et al. 1994). These experiments revealed that the long standing view of streptococci as being only extracellular human pathogenic bacteria has to be changed. These cell culture model studies were followed by the observation that excised tonsils from patients with recurrent tonsillitis contained viable streptococci (Oesterlund and Engstrand 1997; Oesterlund et al. 1997). Furthermore, it was demonstrated that besides GAS, other streptococci also invade nonphagocytic epithelial host cells such as Group B, Group C, and Group G streptococci, S. suis as well as S. pneumoniae and oral streptococci of the viridans group (Rubens et al. 1992; Talbot et al. 1996; Norton et al. 1999; Molinari and Chhatwal 1999; Haidan et al. 2000; Stinson et al. 2003).
Scanning electron microscopy (SEM) imaging revealed that streptococci use multiple morphological ways of invasion. This became evident when different serotypes with most probably different adhesins were tested for invasion. All invasion mechanism known so far of the classical intracellular bacteria could be detected. Some isolates exhibited the classical membrane-ruffling pattern (triggering mechanism) as described for Salmonella, whereas other isolates showed a well-defined zipper-like mechanism found in invading Listeria. Remarkably, a high number of streptococcal isolates expressing SfbI protein induced a third, so far unknown invasion pathway, with an easily detectable morphological feature, namely the formation of large invaginations during the internalization process, sometimes looking like a “hole” in the host cell membrane (Molinari et al. 2000). Interestingly, these different invasion patterns were also detected for Group C and Group G streptococci (Haidan et al. 2000) as well as for nonencapsulated strains of S. suis (Benga et al. 2004) and for S. aureus which also invades via α5ß1 integrins (Agerer et al. 2005).
These studies revealed that streptococci also possess invasins, like the classical intracellular pathogens Shigella, Listeria, or Salmonella. As for the classical intracellular bacteria, streptococcal invasins aid bacteria in their internalization process into nonphagocytic host cells like fibroblasts, keratinocytes, endothelial, and epithelial cells. Invasins are surface exposed and/or diffusible proteins that can promote (i) actin rearrangement of the host cytoskeleton that stimulates engulfment of the bacteria by membrane ruffles (Dombek et al. 1999), or (ii) interact with specific host cell receptors triggering signaling events which result in uptake of streptococci (Rezcallah et al. 2005; Ozeri et al. 1998), or (iii) co-opt host cell endocytotic pathways, caveolae, for their own internalization (Rohde et al. 2003).
The two streptococcal invasins are the streptococcal fibronectin-binding protein SfbI protein or F1 and the M proteins which are widely distributed in clinical isolates. Despite being only adhesins, these two proteins act also as invasins. SfbI/F1 protein, for example, is detectable in nearly 70–85 % of all clinical isolates and the M protein provides the basis of the Lancefield’s method of emm gene serotyping. As mentioned before, both proteins bind to fibronectin which acts as a bridging molecule for mediating adhesion to the host cell receptor. Another member of the invasin group of proteins is the surface exposed streptococcal dehydrogenase (SDH) which is involved in the invasion of M6 serotype streptococci. SDH triggers signaling events resulting in phosphorylation of several eukaryotic proteins (Pancholi and Fischetti 1997).
3.1 SfbI/F1 Invasion of Epithelial Cells
The work of Talay et al. (2000) explained the molecular mechanism of the binding properties of the distinct domains of SfbI protein with regard to fibronectin and dissected the adherence from the invasion process. The cooperative interaction, further explained by the tandem ß-zipper mechanism (Schwarz-Linek et al. 2003), subsequently resulted in exposure of the RGD region of the fibronectin molecule allowing to bind to α5ß1 integrins on the host cell surface. The interaction between the RGD region of fibronectin and integrins could be blocked by antibodies against the ß-subunit or with RGD peptides (Jadoun et al. 1998; Ozeri et al. 1998; Molinari et al. 2000). Ozeri et al. also demonstrated that the amount of bound fibronectin on the bacterial surface is responsible for the uptake efficiency into the host cell. Most properly, a certain threshold of bound fibronectin to integrins is needed to start the integrin signaling. According to the tandem ß-zipper mechanism, a single SfbI molecule is able to bind up to five fibronectin molecules. This means that the surface of SfbI-expressing streptococci is most properly covered by a high density of fibronectin. This allows streptococci to bind many integrins on the cell. A clustering of integrins underneath attached streptococci could be demonstrated by staining the ß-subunits of integrins or by a microscopic approach applying high-resolution field emission scanning electron microscopy (FESEM) to visualize integrin clustering directly on the host cell surface. Recombinant SfbI protein was coated onto 15 nm colloidal gold particles and the fate of the SfbI-gold particles on endothelial cells was observed. After 1 h, aggregates of SfbI-gold particles could be detected, meaning integrin clustering, and after 2 h SfbI-gold particle aggregates were taken up by invaginations into the cell comparable to the internalization of the SfbI-expressing S. pyogenes isolate (Rohde et al. 2003). This observation is in accordance with findings for other pathogens which also enter cells via integrins like Neisseria gonorrhoeae (van Putten et al. 1998), S. aureus (Fowler et al. 2000), and Yersiniae species (Isberg and Barnes 2001). Engagement and clustering of integrin receptors is the prerequisite for sufficient uptake and subsequent integrin signaling (Isberg and Leong 1990; Isberg 1991) (Fig. 2).
FESEM image of invading Group A streptococci via SfbI-mediated fibronectin binding, integrin clustering, and formation of invaginations in the host epithelial cell (left image). In a mutant strain lacking SfbI protein, streptococci invade via induction of cytoskeletal rearrangements, membrane ruffles (right image) giving evidence that a single strain has the potential to invade via two morphological distinct pathways depending on the protein repertoire expressed on the surface
In further studies, it was shown that formation of large invaginations is triggered by the SfbI-fibronectin-integrin signaling complex which is responsible for the aggregation of caveolae around adherent bacteria. Caveolae fuse with each other to form the invagination. Once inside the host cell, SfbI-expressing streptococci traffic in a new compartment called “caveosome”. The intriguing aspect of this type of streptococcal invasion is the fact that caveosomes do not fuse with lysosomes. By co-opting this caveolae-mediated cellular pathway, SfbI-carrying streptococci bypass the degradation machinery of the host cell, since no fusion with lysosomes is detectable (Rohde et al. 2003).
GGS exhibit GfbA, another adhesin and invasin, on their surface which binds equal amounts of fibronectin as SfbI and interacts with α5ß1 integrins. But GfbA-mediated invasion leads to the formation of membrane ruffles, i.e. rearrangement of the host cell cytoskeleton. Immunofluorescence studies demonstrated that GfbA-expressing streptococci follow the classical endocytic pathway with subsequent fusion with lysosomes to form phagolysosomes. By heterologous surface expression of GfbA in the nonpathogenic S. gordonii, it was demonstrated that GfbA alone is responsible for the morphologically distinct invasion mechanism compared to the SfbI-mediated caveolae dependent invasion mechanism. Sequencing of the GfbA gene demonstrated that the FnBR repeat region in the C-terminal part is very similar to SfbI. Only the N-terminal part, including the aromatic domain of GfbA (ARO) and SfbI, shows significant differences. Therefore, it was assumed that the ARO region might be responsible for the observed morphological differences in the invasion mechanism. To test this hypothesis, a GfbA protein was constructed without the aromatic domain, and the aromatic domain in SfbI was replaced by the aromatic domain of GfbA. FESEM studies revealed that GfbA with a deleted ARO region invades like SfbI-expressing strains, whereas the SfbI strain containing the ARO region of GfbA now induced membrane ruffles. By repeating the FESEM studies for integrin clustering, it was shown that only GfbA without the aromatic domain induced integrin clustering and signaling, whereas SfbI with the ARO region of GfbA was impaired in integrin clustering and signaling. Thus, for the first time a biological function for the ARO region in a fibronectin-binding protein was demonstrated (Rohde et al. 2011).
3.2 M Protein Invasion of Epithelial Cells
For M protein-mediated invasion, fibronectin, laminin, or plaminogen/plasmin (Colognato and Yurchenco 2000; Siemens et al. 2011) binding on the bacterial surface are the prerequisites. Serotypes M1, M3, M5, M6, M12, M18, and M49 (Berkower et al. 1999; Dombeck et al. 1999; Molinari et al. 2000; Nerlich et al. 2009) have been studied with respect to HEp-2 cell invasion and all serotypes were found to be invasive. Only the highly capsulated M18 serotype was found to be less invasive because of interference of the capsule with the initial adherence process to the cells (Hagman et al. 1999).
After binding to fibronectin and α5ß1-integrins, the serotype M1 promotes invasion of human lung epithelial cells. A M1 negative mutant had a significant reduced invasion capability. M1 protein can also bind to laminin with subsequent internalization in host cells (Cue et al. 1998). The mechanism of entrance was accompanied by cytoskeletal rearrangements and represented a zipper-like mechanism in HeLa cells as shown by scanning EM (Dombek et al. 1999). This observation is in contrast to the caveolae-mediated internalization of SfbI/F1 carrying strains, despite the fact that also M1 serotype strains bind fibronectin and subsequently α5ß1 integrins.
The zipper-like invasion was mediated by an early intimate contact of streptococci with host cell microvilli and invading streptococci were associated with polymerized actin at their site of entry. Identical observations of actin polymerization underneath the entry port were observed for a M5 serotype/A8 strain (Molinari et al. 2000) which does not express SfbI. M1 protein-mediated invasion subsequently leads to the fusion with lysosomes to form a phagolysosome.
4 Dissemination and Persistence
After a first episode of infection with streptococci, a small percentage of patients will experience recurrent tonsillitis or erysipelas episodes. These recurrent infections may be attributed to a bacterial subpopulation surviving beyond the first episode. There are two plausible reasons why the streptococci might persist: (i) by hiding in the matrix proteins of the human mucosa, especially serotype M3 and M18 by binding to collagen, (ii) or intracellularly by residing in an intracellular compartment or in the cytoplasm (Fig. 3).
For dissemination in the host, two possible ways are conceivable. Firstly, streptococci might invade certain cells and travel unrecognized in these cells through the body until reaching a safe niche in the body—Trojan horse theory. Secondly, after bacteraemia streptococci might be able to passage through the dense endothelial cell layer of the blood vessels to gain access into deeper tissue. For both ways, first experimental evidence has been obtained during recent years. Nevertheless, we are far away from understanding the entire multiple strategies of streptococci during dissemination in the body. For example, it was shown that GAS, which are resistant to killing in PMNs, may exploit the ability of the neutrophils to transmigrate through endothelial cell barrier of the vascular system for spread via the blood stream (Medina et al. 2003a, b). Surprisingly, streptococci were also found residing in macrophages when biopsies of patients with soft tissue infections were examined, supporting the Trojan horse theory (Thulin et al. 2006). These studies conclusively showed that host phagocytic cells, especially macrophages, were the reservoir for intracellular GAS during infection. Remarkably, the authors found evidence that localization of GAS varied and depended on the severity of tissue infection. Intracellular streptococci were predominantly found in noninflamed tissue with a low bacterial load, whereas in inflamed tissue, even after prolonged intravenous treatment with antibiotics, high loads of GAS were detected. It has been suggested that internalization could indeed promote the spread of GAS within the tissue. Macrophages might then play the role of the Trojan horse in the infected tissue. On the other hand, work by Cywes et al. (2000) described another type of streptococcal invasion. They demonstrated that binding to CD44 on a confluent layer of human keratinocytes via HA (capsule) of encapsulated strains induced cytoskeletal changes, membrane ruffling, and a disruption of cellular junctions after binding to the host cell lamellipodia. In contrast, nonencapsulated strains attached to host membrane regions devoid of lamellipodia. This type of invasion opens up the way for streptococci to gain access into deeper tissue layers. For Group B streptococci (GBS), an identical paracellular translocation through the epithelial barrier during onset of infection has been reported. Soriani et al. (2008) applied differentiated epithelial cells grown in cell culture transwell inserts for their studies. Translocation of bacteria occurred without measurable decrease in the transepithelial resistance and analysis of ultra-thin sections revealed an intimate association of GBS with the intercellular junctions (Soriani et al. 2008). Recently, a third mechanism has been found demonstrating that streptococci mediate an exocytosis process in which streptococci residing in a phagolysosome can trigger their own exocytosis process, with Rab27 being involved, to transmigrate through an endothelial barrier (Talay et al. personal communication).
For persistence in the host, only scant data are available. For example, S. dysgalactiae subsp. equisimilis (Group C or Group D) are relatively common in invasive infections, especially in older patients (Sylvetsky et al. 2002). This species (type stG485.0) was involved in several recurrent bacteremic sepsis episodes despite prolonged antibiotic treatment over years and using opsonizing antibodies against the isolate. The isolate was suspected to reside in an aortic aneurysm of the patient. With a cell culturing model, it was shown that the isolate possesses a very high invasiveness in endothelial HUVEC cells (Rohde et al. 2012) (Fig. 4).
That streptococci actually can survive for a long period of time was shown in batch cultures using sugar-limited Todd-Hewitt broth. Bacteria were still viable and culturable after 1 year (Wood et al. 2005). Other experiments extended the survival time to over 45 months. An overnight culture with an inoculation with 5 μl of the sedimented bacteria, grown in TBS, was sufficient to obtain fully viable streptococci. Even more surprising was the fact that FESEM imaging of the bacteria revealed no visible difference of 45-month-old streptococci to the overnight regrown bacteria; only the interior morphology of the bacteria was remarkably different (Rohde, unpublished). It is speculated that during persistence S. pyogenes might enter a kind of a quiescent state, due to conditions which do not support rapid bacterial growth, such as nutrition limitations. If a trauma from outside destroys areas of intracellular streptococci containing tissue bacteria, allowing access to new nutrition due to infiltration of immune cells etc., to the damaged tissue side, then bacteria can respond with a fulminant growth thereby causing a recurrent infection.
5 Conclusions
Adhesion of streptococci through the multiple adhesins and internalization into human epithelial and endothelial cells by invasins creates a safe sanctuary for streptococci protecting them against the host immune system and antibiotic treatment. Together, with the capability to penetrate cellular barrier for dissemination and hijacking immune cells like PMNs for their own spread in the body makes streptococci a very versatile and not easy to eradicate human pathogen. Since the work of Österlund and colleagues (Oesterlund and Engstrand 1997; Oesterlund et al. 1997), who provided first evidence for the existence of persisting intracellular streptococci, evidence has accumulated that streptococcal invasion into host cells is a severe therapeutic problem (Thulin et al. 2006; Rohde et al. 2012). The routinely used β-lactam antibiotics, penicillins, are not efficiently permeating into eukaryotic cells and fail to completely eradicate intracellular GAS. Recently, macrolides like erythromycin or derivatives thereof or an azalide, azithromycin were found to be more efficient in eradicating intercellular streptococci in vitro and may present an attractive therapeutic alternative for eradication of streptococci (Kaplan et al. 2006). Nevertheless, the use of macrolides faces another drawback since streptococci have developed a strong resistance against macrolides in some countries. The mostly in vitro obtained observations of streptococcal adhesion, internalization, trafficking inside the cell, dissemination, and persistence, taken together with in vivo studies on persistence of streptococci, should allow for a more effective approach for clinical management of Group A S. pyogenes carriers with the aim to eradicate bacteria at the onset of the infection.
References
Abbot EL, Smith WD, Siou GP, Chriboga C, Smith RJ, Wilson JA, Hirts BH, Kehoe MA (2007) Pili mediate specific adhesion of Streptococcus pyogenes to human tonsils and skin. Cell Microbiol 9:1822–1833
Agerer F, Lux S, Michel A, Rohde M, Ohlsen K, Hauck CR (2005) Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J Cell Sci 118:2189–2200
Amelung S, Nerlich A, Rohde M, Spellerberg B, Cole JN, Nizet V, Chhatwal GS, Talay SR. (2011) The FbaB-type fibronectin-binding protein of S. pyogenes promote specific invasion into endothelial cells. Cell Microbiol 13:1200–1211
Andre I, Persson J, Blom AM, Nilsson H, Drakenberg, T, Lindahl G, Linse S (2006) Streptococcal M protein: structural studies of the hypervariable regions, free and bound to human C4BP. Biochemistry 45:4559–4568
Barocchi MA, Ries J, Zogaj X, Hemsley C, Albiger B, Kanth A, Dahlberg S, Fernebro J, Moschioni M, Masignani V, Hultenby K, Taddei AR, Beiter K, Wartha F, von Euler A, Covacci A, Holden DW, Normark S, Rappuoli R, Henriques-Normark B (2006) A pneumococcal pilus influences virulence and host inflammatory responses. Proc Natl Acad Sci USA 103: 2857–2862
Beachey EH, Ofek I (1976) Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med 143:759–771
Beall B, Facklam R, Thompson T (1996) Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol 34:9533–958
Benga L, Goethe R, Rohde M, Valentin-Weigand P (2004) Non-encapsulated strains reveal novel insights in invasion and survival of Streptococcus suis in epithelial cells. Cell Microbiol 6:867–881
Berkower C, Ravins M, Moses AE, Hanski E (1999) Expression of different group A streptococcal M proteins in an isogenic background demonstrates diversity in adherence to and invasion of eukaryotic cells. Mol Microbiol 31:1463–1475
Caparon MG, Stephens S, Olsen A, Scott JR (1991) Role of M protein in adherence of group A streptococci. Infect Immun 59:1811–1817
Carapetis (2005) Acute rheumatic fever. Lancet 366:155-168
Chhatwal GS (2002) Anchorless adhesins and invasion of Gram-positive bacteria: a new class of virulence factors. Trends in Microbiol 10:205–208
Colognato H, Yurchenco PD (2000) Form and function: the laminin family of heterotrimers. Dev Dyn 218:213–234
Courtney HS, Hasty DL (1991) Aggregation of group A streptococci by human saliva and effect of saliva on streptococcal adherence to host cells. Infect Immun 59:1661–1666
Courtney HS, von Hunolstein C, Dale JB, Bronze MS, Beachey EH, Hasty DL (1992) Lipoteichoic acid and M protein: dual adhesins of group A streptococci. Microb Pathog 12:199–208
Courtney HS, Bronze M S, Dale JB, Hasty DL (1994) Analysis of the role of M24 protein in group A streptococcal adhesion and colonization by use of omega-interposon mutagenesis. Infect Immun 62:4868–4873
Courtney HS, Dale JB, Hasty DI (1996) Differential effects of the streptococcal fibronectin-binding protein, FBP54, on adhesion of group A streptococci to human buccal cells and HEp-2 tissue culture cells. Infect Immun 64:2415–2419
Courtney HS, Hasty DL, Dale JB (2002) Molecular mechanisms of adhesion, colonization, and invasion of group A streptococci. Ann Med 34:77–87
Cremer MA, Rosloniec EF, Kang AH (1998) The cartilage collagens: a review of their structure, organization, and role in the pathogenesis of experimental arthritis in animals and in human rheumatic disease. J Mol Med 76:275–288
Crotty Alexander LE, Maisey HC, Timmer AM, Rooijakkers SHM, Gallo RL, von Köckritz-Blickwede M, Nizet V. (2010) M1T1 group A streptococci pili promote epithelial colonization but diminish systemic virulence through neutrophil extracellular entrapment. J Mol Med 88:371–381
Cue D, Dombek PE, Lam H, Cleary PP (1998) Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect Immun 66:4593–4601
Cue D, Southern SO, Southern PJ, Prabhakar J, Lorelli W, Smallheer JM, Mousa SA, Cleary PP (2000) A nonpeptide integrin antagonist can inhibit epithelial cell ingestion of Streptococcus pyogenes by blocking formation of integrin alpha 5beta 1-fibronectin-M1 protein complexes. Proc Natl Acad Sci USA 97:2858–2863
Cue D, Lam H, Cleary PP (2001) Genetic dissection of the Streptococcus pyogenes M1 protein: regions involved in fibronectin-binding and intracellular invasion. Microb Pathog 31:231–242
Cunningham MW (2000) Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13:470–511
Cywes C, Wessels MR (2001) Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414:648–652
Cywes C, Stamenkovic I, Wessels MR (2000) CD44 as a receptor for colonization of the pharynx by group A Streptococcus. J Clin Invest 106:995–1002
Dale JB, Washburn RG, Marques MB, Wessels MR (1996) Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect Immun 64:1495–1501
Darmstadt GL, Mentele L, Podbielski A, Rubens CE (2000) Role of group A streptococcal virulence factors in adherence to keratinocytes. Infect Immun 68:1215–1221
Debelle L, Tamburro AM (1999) Elastin: molecular description and function. Int J Biochem Cell Biol 31:261–272
Dempfle CE, Mosesson MW (2003) Theme issue: Fibrinogen and fibrin - structure, function, interactions and clinical applications. Thromb Haemost 89:599–600
Derbise A, Song YP, Parikh S, Fischetti VA, Pancholi V (2004) Role of the C-terminal lysine residues of streptococcal surface enolase in Glu- and Lys-plasminogen-binding activities of group A streptococci. Infect Immun 72:94–105
Dinkla K, Rohde M, Jansen WTM, Kaplan EL, Chhatwal GS, Talay SR (2003a) Rheumatic fever-associated Streptococcus pyogenes isolates aggregate collagen. J Clin Invest 111:1905–1912
Dinkla K, Rohde M, Jansen WTM, Carapetis JR, Chhatwal GS, Talay SR (2003b) Streptococcus pyogenes recruits collagen via surface-bound fibronectin: a novel colonization and immune evasion mechanism. Mol Microbiol 47:861–869
Dombek PE, Cue D, Sedgewick J, Lam H, Ruschkowski S, Finlay BB, Cleary PP (1999) High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol Microbiol 31:859–870
Doran KS, Engelson EJ, Khosravi A, Maisey HC, Fedtke I, Equils O, Michelsen KS, Arditi, M, Peschel A, Nizet V (2005) Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J Clin Invest 115:2499–2507
Ellen RP, Gibbons RJ (1972) M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun 5:826–830
Ellen RP, Gibbons RJ (1974) Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun 9:85–91
Falugi F, Zingaretti C, Pinto V, Mariani M, Amodeo A, Manetti G, Capo S, Musser JM, Orefici G, Margarit I, Telford L, Grandi G, Mora M (2008) Sequence variation in group A Streptococcus pili and associatition of pilus backbone types with Lancefield T serotypes. J Infect Dis 198:1834–1841
Fowler T, Wann ER, Joh D, Johansson S, Foster TJ, Höök M (2000) Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur J Cell Biol 79:672–679
Frick IM, Crossin KL, Edelman GM, Björck L (1995) Protein H-a bacterial surface protein with affinity for both immunoglobulin and fibronectin type III domains. EMBO J 14:1674–1679
Frick IM, Mörgelin M, Björck L (2000) Virulent aggregates of Streptococus pyogenes are generated by hemophilic protein–protein interactions. Mol Microbiol 37:1232–1247
Frick IM, Schmidtchen A, Sjobring U (2003) Interactions between M proteins of Streptococcus pyogenes and glycosaminoglycans promote bacterial adhesion to host cells. Eur J Biochem 270:2303–2311
Gianfoldoni C, Censini S, Hilleringmann M, Moschioni M, Facciotti C, Pansegrau W, Masigani V, Covacci A, Rappuoli R, Barocchi MA, Rugiero P (2007) Streptococcus pneumoniae pilus subunits protect mice against lethal challange. Infect Immun 75:1059–1062
Giannakis E, Jokiranta TS, Ormsby RJ, Duthy TG, Male DA, Christiansen D, Fischetti VA, Bagley C, Loveland BE, Gordon DL (2002) Identification of the streptococcal M protein binding site on membrane cofactor protein (CD46). J Immunol 168:4585–4592
Gibson CM, Caparon MG (1996) Insertional inactivation of Streptococcus pyogenes sod suggests that prtF is regulated in response to a superoxide signal. J Bacteriol 178:4688–4695
Gibson CM, Fogg G, Okada N, Geist RT, Hanski E, Caparon MG (1995) Regulation of host cell recognition in Streptococcus pyogenes. Dev Biol Stand 85:137–144
Greco R, De Martino L, Donnarumma G, Conte MP, Seganti L, Valenti P (1995) Invasion of cultured human cells by Streptococcus pyogenes. Res Microbiol 146:551–560
Hagman MM, Dale JB, Stevens DL (1999) Comparison of adherence to and penetration of a human laryngeal epithelial cell line by group A streptococci of various M protein types. FEMS Immunol Med Microbiol 23:195–204
Haidan A, Talay SR, Rohde M, Sriprakash KS, Currie BJ, Chhatwal GS (2000) Pharyngeal carriage of group C and group G streptococci and acute rheumatic fever in an Aboriginal population. Lancet 356:1167–1169
Handley PS, Carter PL, Fielding J (1984) Streptococcus salivarius strains carry either fibrils or fimbriae on the cell surface. J Bacteriol 157:64–72
Handley PS, Harty DW, Wyatt JE, Brown CR, Doran JP, Gibbs AC (1987) A comparison of the adhesion, coaggregation and cell-surface hydrophobicity properties of fibrillar and fimbriae strains of Streptococcus salivarius. J Gen Microbiol 133:3207–3217
Hanski E, Caparon M (1992) Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus. Proc Natl Acad Sci USA 89:6172–6176
Hasty DL, Ofek I, Courtney HS, Doyle RJ (1992) Multiple adhesins of streptococci. Infect Immun 60:2147–2152
Henningham A, Barnett TC, Maamary PG, Walker MJ (2012a) Pathogenesis of group A infections. Discov Med 13:329–342
Henningham A, Chiarot E, Gillen CM, Cole JN, Rohde M, Fulde M, Ramachandran V, Cork AJ, Hartas J, Magor G, Djordjevic SP, Cordwell SJ, Kobe B, Sriprakash KS, Nizet V, Chhatwal GS, Margarit IY, Batzloff MR, Walker MJ (2012b) Conserved anchorless surface proteins as group A vaccine candidates. J Mol Med (Epub ahead)
Humtsoe JO, Kim JK, Xu,Y, Keene DR, Höök M, Lukomski S, Wary KK (2005) A streptococcal collagen-like protein interacts with the alpha2beta1 integrin and induces intracellular signaling. J Biol Chem 280:13848–13857
Hytonen J, Haataja S, Gerlach D, Podbielski A, Finne J (2001) The SpeB virulence factor of streptococcus pyogens, a multifuntional secreted and cell surface molecule with strepadhesin, laminin-binding and cysteine protease activity. Mol Microbiol 39:512–519
Isberg RR (1991) Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science 252:934–938
Isberg RR, Barnes P (2001) Subversion of integrins by enteropathogenic Yersinia. J Cell Sci 114:21–28
Isberg RR, Leong JM (1990). Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861–871
Jadoun J, Ozeri V, Burstein E, Skutelsky E, Hanski E, Sela S (1998) Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J Infect Dis 178:147–158
Jadoun J, Eyal O, Sela S (2002) Role of CsrR, hyaluronic acid, and SpeB in the internalization of Streptococcus pyogenes M type 3 strain by epithelial cells. Infect Immun 70:462–469
Jaffe J, Natanson-Yaron S, Caparon MG, Hanski E (1996) Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains. Mol Microbiol 21:373–384
Jin H, Song YP, Boel G, Kochar J, Pancholi V (2005) Group A streptococcal surface GAPDH, SDH, recognizes uPAR/CD87 as its receptor on the human pharyngeal cell and mediates adherence to host cells J Biol Chem 350:27–41
Johnson DR, Stevens DL, Kaplan EL (1992) Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J Infect Dis 166: 374–382
Kang HJ, Coulibaly F, Clow F, Proft T, Baker EN (2007) Stabilizing isopeptide bonds revealed in gram-positive bacterial pilus structure. Science 318:1625–1628
Kaplan EL, Johnson DR, Cleary PP (1989) Group A streptococcal serotypes isolated from patients and sibling contacts during the resurgence of rheumatic fever in the United States in the mid-1980s. J Infect Dis 159:101–103
Kaplan EL, Chhatwal GS, Rohde M (2006) Reduced ability of penicillin to eradicate ingested group A streptococci from epithelial cells: clinical and pathogenetic implication. Clin Infect Dis 43:1398–1406
Kawabat S, Tamura Y, Murakami J, Teroa Y, Nakagawa I, Hamada S (2002) A novel, anchorless streptococcal surface protein that binds to human immunoglobulins. Biochem Biophys Res Comm 296:1329–1333
Kinnby B, Booth NA, Svensater G (2008) Plasminogen binding by oral streptococci from dental plaque and inflammatory lesions. Microbiology 154:924–931
Kreikemeyer B, McIver KS, Podbielski A (2003) Virulence factor regulation and regulatory networks in Stretococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol 11:224–232
Kreikemeyer B, Oemcke S, Nakata M, Hoffrogge R, Podbielski A (2004a) Streptococcus pyogenes fibronectin-bindung protein F2: expression profile, binding characteristics, and impact on eukaryotic cell interactions. J Biol Chem 279:15850–15859
Kreikemeyer B, Klenk M, Podbielski A (2004b) The intracellular status of Streptococcus pyogenes; role of extracellular matrix-binding proteins and their regulation. Int J Med Micriobiol 294:177–188
LaPenta D, Rubens C, Chi E, Cleary PP (1994) Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci USA 91:12115–12119
Lauer P, Rinaudo CD, Soriani M, Margarit I, Maione D, Rosini R, Taddei AR, Mora M, Rappuoli R, Grandi G, Telford JL (2005) Genome analysis reveals pili in Group B Streptococcus. Science 309:105
Leon O, Panos C (1990) Streptococcus pyogenes clinical isolates and lipoteichoic acid. Infect Immun 58:3779–3787
Maisey HC, Hensler M, Nizet V, Doran KS (2007) Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J Bacteriol 189:1464–1467
Mandlik A, Swierczynski A, Das A., Ton-That H. (2007a) Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends in Microbiol 16:33–39
Mandlik A, Swierczynski A, Das A, Ton-That H (2007b) Corynebacterium diphtheria employs specific minor pilins to target human pharyngeal epithelial cells. Mol Microbiol 64:11–124
Maraffini LA, Dedent AC, Schneewind O (2006) Sortases and the art of anchoring proteins to the envelope of gram-positive bacteria. Microbiol Mol Biol Rev 70:192–221
Marjenberg ZR, Ellis IR, Hagan RM, Prabhakaran S, Höök M, Talay SR, Potts JR, Staunton D, Schwarz-Linek U (2011) Cooperative binding and activation of fibronectin by a bacterial surface protein. J Biol Chem 286:1884–1894
McArthur JD, Walker MJ (2006) Domains of group A streptococcal M protein that confer resistance to phagocytosis, opsonozation and protection: implications for vaccine development. Mol Microbiol 59:1–4
McNamara C, Zinkernagel AS, Machebeouf M, Cunningham M, Nizet V. (2008) Coiled-coil irregularities and instabilities in group A streptococcus M1 are required for virulence. Science 319: 1405–1408
Medina E, Rohde M, Chhatwal GS (2003a) Intracellular survival of Streptococcus pyogenes in polymorphonuclear cells results in increased bacterial virulence. Infect Immun 71:5376–5380
Medina E, Goldmann O, Toppel AW, Chhatwal GS (2003b) Survival of Streptococcus pyogenes within host phagocytic cells: a pathogenic mechanism for persistence and systemic invasion. J Infect Dis 187:597–603
Molinari G, Chhatwal GS (1999) Streptococcal invasion. Curr Opin Microbiol 2:56–61
Molinari G, Rohde M, Guzman CA, Chhatwal GS (2000) Two distinct pathways for the invasion of Streptococcus pyogenes in non-phagocytic cells. Cell Microbiol 2:145–154
Mora M, Bensi G, Capo S, Falugi F, Zingaretti C, Manetti AG, Maggi T, Taddei AR, Grandi G, Telford JL (2005) Group A streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc Natl Acad Sci USA 102:15641–15646
Natanson S, Sela S, Moses AE, Musser JM, Caparon MG, Hanski, E (1995) Distribution of fibronectin-binding proteins among group A streptococci of different M types. J Infect Dis 171:871–878
Nerlich A, Rohde M, Talay, SR, Genth H, Just I, Chhatwal GS (2009) Invasion of endothelial cells by tisuue-invasive M3 type Group A streptococci requires Src kinase and activation of Rac1 by a phosphatidylinositol 3-kinase-independent mechanism. J Biol Chem 284:20319–20328
Nitsche DP, Johansson HM, Frick IM, Mörgelin, M (2006) Streptococcal protein FOG, a novel matrix adhesin interacting with collagen I in vivo. J Biol Chem 281:1670–1679
Nitsche-Schmitz DP, Rohde M, Chhatwal GS (2007) Invasion mechanisms of gram-positive pathogenic cocci. Thromb Haemost 98:488–496
Nobbs AH, Lamont RJ, Jenkinson HF (2009) Streptococcus adherence and colonization. Microbiol Mol Biol Rev 73:407–450
Norton PM, Rolph C, Ward PN, Bentley RW, Leigh JA (1999) Epithelial invasion and cell lysis by virulent strains of Streptococcus suis is enhanced by the presence of suilysin. FEMS Immunol Med Microbiol 26:25–35
Oemcke S, Shannon O, Mörgelin M, Herwald H. (2010) Streptococcal M proteins and their role as virulence determninants. Clinica Chemica Acta 411:1172–1180
Oesterlund A, Engstrand L (1997a) An intracellular sanctuary for Streptococcus pyogenes in human tonsillar epithelium-studies of asymptomatic carriers and in vitro cultured biopsies. Acta Otolaryngol 117:883–888
Oesterlund A, Popa R, Nikkila T, Scheynius, A, Engstrand L (1997b) Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope 107:640–647
Okada N, Liszewski MK, Atkinson JP, Caparon M (1995) Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A Streptococcus. Biochem Biophys Res Commun 377:1128–1134
Ozeri V, Tovi A, Burstein I, Natanson-Yaron S, Caparon MG, Yamada KM, Akiyama SK, Vlodavsky I, Hanski E (1996) A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J 15:989–998
Ozeri V, Rosenshine I, Mosher DF, Fässler R, Hanski E (1998) Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol Microbiol 30:625–637
Pancholi V, Fischetti VA (1992) A major surface protein on group A streptococci is a glyceraldehydes-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med 176:415–426
Pancholi V, Fischetti VA (1997) Regulation of the phosphorylation of human pharyngeal cell proteins by group A streptococcal surface dehydrogenase: signal transduction between streptococci and pharyngeal cells. J Exp Med 186:1633–1643
Pancholi V, Fischetti VA (1998) alpha-enolase, a novel strong plasminogen-(ogen) binding protein on the surface of pathogenic streptococci. J Biol Chem 273:14503–14515
Pankov R, Yamada KM (2002) Fibronectin at a glance. J Cell Sci 115:3861–3863
Patti JM, Höök M (1994) Microbial adhesions recognizing extracellular matrix molecules. Curr Opin Cell Biol 6:752–758
Pezzicoli A, Santi I, Lauer P, Rosini R, Rinaudo D, Grandi, G, Telford JL, Soriani M (2008) Pilus backbone contributes to group B Streptococcus paracellular translocation through epithelial cells. Infect Immun 198:890–898
Podbielski A, Woischnik M, Leonard BA, Schmidt KH (1999) Characterization of nra, a global negative regulator gene in group A streptococci Mol Microbiol 31:1051–1064
Proft T, Baker EN (2009) Pili in gram-negative and gram-positive bacteria-structure, assembly and role in disease. Cell Mol Life Sci 66:613–635
Rezcallah M S, Hodges K, Gill DB, Atkinson JP, Wang B, Cleary PP (2005) Engagement of CD46 and alpha5beta1 integrin by group A streptococci is required for efficient invasion of epithelial cells. Cell Microbiol 7:645–653
Rocha CL, Fischetti VA (1999) Identification and characterization of a novel fibronectin-binding protein on the surface of group A streptococci. Infect Immun 67:2720–2728
Rohde M, Müller E, Chhatwal GS, Talay SR (2003) Host cell caveolae act as an entry-port for Group A streptococci. Cell Microbiol 5:323–342
Rohde M, Graham RM, Branitzki-Heinemann K, Borchers P, Preuss C, Schleicher I, Zähner D, Talay SR, Fulde M, Dinkla K, Chhatwal GS (2011) Differences in the aromatic domain of homologous streptococcal fibronectin-binding proteins trigger different cell invasion mechanisms and survival rates. Cell Microbiol 13:450–468
Rohde M, Talay SR, Rasmussen M (2012) Molecular mechanisms of Streptococcus dysgalactiae subsp. Equisimilis enabling intravascular persistence. Microbes Infect 14:329–334
Rosini R, Rinaudo CD, Soriani M, Lauer P, Mora M, Maione D, Taddei A, Santi I, Ghezzo C, Brettoni C, Buccato S, Margarit I, Grandi G, Telford JL. (2006) Identification of novel genomic islands coding for antigenic pilus-like structures in Streptococcus agalacticae. Mol Microbiol 61:126–141
Rubens, CE, Smith S, Hulse M, Chi EY, van Belle G (1992) Respiratory epithelial cell invasion by group B streptococci. Infect Immun 60:5157–5163
Saulino ET, Bullitt E, Hultgren SJ (2000) Snapshots of usher mediated protein secretion and ordered pilus assembly. Proc Natl Acad Sci USA 97:9240–9245
Schneewind O, Mihaylova-Petkov D, Model P (1993) Cell wall sorting signals in surface proteins of gram-positive bacteria EMBO J 12:4803–4811
Schrager HM, Rheinwald JG, Wessels MR (1996) Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Invest 98:1954–1958
Schrager HM, Alberti S, Cywes C, Dougherty GJ, Wessels MR (1998) Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J Clin Invest 101:1708–1716
Schvartz I, Seger D, Shaltiel S (1999) Vitronectin. Int J Biochem Cell Biol 31:539–544
Schwarz-Linek U, Werner JM, Pickford AR, Gurusiddappa S, Kim JH, Pilka ES, Briggs JA, Gough TS, Höök M, Campbell ID, Potts JR (2003) Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature 423:177–181
Schwarz-Linek U, Höök M, Potts JR (2004a) The molecular basis of fibronectin-mediated bacterial adherence to host cells Mol Microbiol 52:631–641
Schwarz-Linek U, Pilka ES, Pickford AR, Kim JH, Höök M, Campbell ID, Potts J R (2004b) High affinity streptococcal binding to human fibronectin requires specific recognition of sequential F1 modules. J Biol Chem 279:39017–39025
Scott JR, Zähner D (2006) Pili with strong attachments: Gram-positive bacteria do it differently Mol Microbiol 62:320–330
Seifert KN, McArthur WP, Bleiweis AS, Brady LJ (2003) Characterization of group B streptococcal glyceraldehydes-3-phosphate dehydrogenase: surface localization, enzymatic activity, and protein–protein interactions. Can J Microbiol 49:350–356
Sela S, Aviv A, Tovi A, Burstein I, Caparon MG, Hanski E (1993) Protein F: an adhesin of Streptococcus pyogenes binds fibronectin via two distinct domains. Mol Microbiol 10:1049–1055
Siemens N, Patenge N, Otto J, Fiedler T, Kreikemeyer B (2011) Streptococcus pyogenes M49 plasminogen/plasmin binding facilitates keratinocyte invasion via integrin–integrin-linked kinase (ILK) pathways and protects from macrophage killing. J Biol Chem 286:21612–21622
Simpson WA, Beachey EH (1983) Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun 39:275–279
Soriani M, Santi I, Taddei A, Rappuoli R, Grandi G, Telford JL (2008) Group B streptococci crosses human epithelial cells by a paracellular route. J Infect Dis 193:241–250
Stinson M W, Alder S, Kumar S (2003) Invasion and killing of human endothelial cells by viridans group streptococci. Infect Immun 71:2365–2372
Sylvestky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM (2002) Bacteremia due to beta-hemolytic Streptococcus group G: increasing incidence and clinical characteristics of patients. Am J Med 112:622–626
Talay SR, Valentin-Weigand P, Jerlstrom PG, Timmis KN, Chhatwal GS (1992) Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun 60: 3837–3844
Talay SR, Valentin-Weigand P, Timmis KN, Chhatwal GS (1994) Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol Microbiol 13:531–539
Talay SR, Zock A, Rohde M, Molinari G, Oggioni M, Pozzi G, Guzman CA, Chhatwal GS (2000) Co-operative binding of human fibronectin to SfbI protein triggers streptococcal invasion into respiratory epithelial cells. Cell Microbiol 2:521–535
Talbot UM, Paton AW, and Paton JC (1996). Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect Immun 64:3772–3777
Telford JT, Barocchi MA, Margarit I, Rappuoli R, Grandi G (2006) Pili in Gram-positive pathogens. Nat Rev Microbiol 4:509–519
Terao Y, Kawabata S, Kunitomo E, Murakami J, Nakagawa I, Hamada S (2001) Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol Microbiol 42:75–86
Terao Y, Kawabata S, Nakata M, Nakagawa I, Hamada S (2002) Molecular characterization of a novel fibronectin-binding protein of Streptococcus pyogenes strains isolated from toxic shock-like syndrome patients. J Biol Chem 277:47428–47435
Thulin P, Johansson L, Low DE, Gan BS, Kotb M, McGeer A, Norrby-Teglund A (2006) Viable Group A streptococci in macrophage during acut soft tissue infection. PLos Med 3:e53
Ton-That H, Marraffini LA, Schneewind O (2004) Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim Biophys Acta 1694:269–278
Towers RJ, Fagan PK, Talay SR, Currie BJ, Sriprakash KS, Walker MJ, Chhatwal GS (2003) Evolution of sfbI encoding streptococcal fibronectin-binding protein I: horizontal genetic transfer and gene mosaic structure. J Clin Microbiol 41:5398–5406
van Putten JP, Duensing, TD, and Cole RL (1998) Entry of OpaA+gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol Microbiol 29:369–379
Veasy LG, Tani LY, Daly JA, Korgenski K, Miner L, Bale J, Kaplan EL, Musser JM, Hill HR (2004) Temporal association of the appearance of mucoid strains of Streptococcus pyogenes with a continuing high incidence of rheumatic fever in Utah. Pediatrics 113, e168–172
Wessels MR, Bronze MS (1994) Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc Natl Acad Sci USA 91:12238–12242
Wessels MR, Moses AE, Goldberg JB, DiCesare TJ (1991) Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci USA 88:8317–8321
Wessels MR, Goldberg JB, Moses AE, DiCesare TJ (1994) Effects on virulence of mutations in a locus essential for hyaluronic acid capsule expression in group A streptococci. Infect Immun 62:433–441
Wilson AT (1959) The relative importance of the capsule and the M-antigen in determining colony form of group A streptococci. J Exp Med 109:257–270
Wood DN, Chaussee MA, Chaussee MS, Buttaro BA (2005) Persistence of Streptococcus pyogenes in stationary-phase cultures. J Bacteriol 187:3319–3328
Wu H, Fives-Taylor PM (1999) Identification of dipeptide repeats and cell wall sorting signal in the fimbriae-associated adhesion, Fap1, of Streptococcus parasanguis. Mol Microbiol 34:1070–1081
Yanagawa R, Honda E (1976) Presence of pili in species of human and animal parasites and pathogens of the genus corynebacterium. Infect Immun 13:1293–1295
Yanagawa R, Otsuki K Tokui T (1968) Electron microscopy of fine structure of Corynebacterium renale with special reference to pili. Jpn J Vet Res 16:31–37
Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, Lamont RJ, Ojcius DM (2008) ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol 10:863–875
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Rohde, M., Chhatwal, G.S. (2012). Adherence and Invasion of Streptococci to Eukaryotic Cells and their Role in Disease Pathogenesis. In: Chhatwal, G. (eds) Host-Pathogen Interactions in Streptococcal Diseases. Current Topics in Microbiology and Immunology, vol 368. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2012_281
Download citation
DOI: https://doi.org/10.1007/82_2012_281
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36339-9
Online ISBN: 978-3-642-36340-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)