Abstract
Structural neuroplasticity in the adult brain is a process involving quantitative changes of the number and size of neurons and of their dendritic arborization, axon branching, spines, and synapses. These changes can occur in specific neural circuits as adaptive response to environmental challenges, exposure to stressors, tissue damage or degeneration. Converging studies point to evidence of structural plasticity in circuits operated by glutamate, GABA, dopamine, and serotonin neurotransmitters, in concert with neurotrophic factors such as Brain Derived Neurotrophic Factor (BDNF) or Insulin Growth Factor 1 (IGF1) and a series of modulators that include circulating hormones. Intriguingly, most of these endogenous agents trigger the activation of the PI3K/Akt/mTOR and ERK1/2 intracellular pathways that, in turn, lead to the production of growth-related structural changes, enhancing protein synthesis, metabolic enzyme functions, mitogenesis for energy, and new lipid-bilayer membrane apposition. The dopamine (DA) D3 receptor has been shown to play a specific role by inducing structural plasticity of the DAergic neurons of the nigrostriatal and mesocorticolimbic circuit, where they are expressed in rodents and humans, via activation of the mTORC1 and ERK1/2 pathways. These effects are BDNF-dependent and require the recruitment of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors to allow the structural changes. Since in mood disorders, depression and anhedonia have been proposed to be associated with impaired neuroplasticity and reduced DAergic tone in brain circuits connecting prefrontal cortex, ventral striatum, amygdala, and ventral mesencephalon, activation of D3 receptors could provide a therapeutic benefit. Sustained improvements of mood and anhedonia were observed in subjects with an unsatisfactory response to serotonin uptake inhibitors (SSRI) when treated with D3-preferential D2/D3 agonists such as pramipexole and ropinirole. The recent evidence that downstream mTOR pathway activation in human mesencephalic DA neurons is also produced by ketamine, probably the most effective antidepressant currently used in subjects with treatment-resistant depression, further supports the rationale for a D3 receptor activation in mood disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction: Cellular and Molecular Mechanisms of Neuronal Structural Plasticity
Neuronal structural plasticity is a critical feature of adult mammalian brain and not only a prerogative of the developing brain. It consists of changes of density of macro-processes such as neuronal soma, axons, and dendritic arborizations, as well as of micro-processes such as synapses and dendritic spines within a given circuit or brain nuclei in response to stressors, damage, or long-term functional adaptation (Koleske 2013; Duman et al. 2016).
During brain development, the neuronal dendrites branch and form spines, the latter being the target of synapses coming from other neurons, the cornerstone of neuronal communication, turning over dynamically to fulfill an ontogenetic program. By contrast, in the adult brain, most dendrite branches and spines are tendentially stable over long spans of time, turning over mostly functionally in response to activation or hyperactivation coming from specific circuits, or due to local factors e.g., mediators of inflammation, circulating stress hormones, interaction with glial cells and failure of intracellular organelles such as mitochondria. Convergent findings indicate that stability of dendritic arborization and synaptic spines has a key role in the functioning of the adult brain, loss of stability, and structural deficits being associated with psychiatric or neurodegenerative disorders (Koleske 2013; Duman et al. 2016), while reactive structural neuroplasticity aimed to adaptively normalize those circuits was proposed to be triggered by the re-engagement of neurodevelopmental programs (Castrén and Rantamäki 2010). Recent findings have provided better understanding of the molecular mechanisms that underlie the adaptive structural plasticity implicated in the stabilization of dendritic arborization, pointing to major players such as the intracellular pathways activated by Brain Derived Neurotrophic Factor (BDNF) and Insulin Growth Factor 1 (IGF1), by the level of activity of NMDA-mediated glutamate neurotransmission, of circulating glucocorticoids, the local expression of adhesion molecules, new protein synthesis and the integrity of energy-producing machinery associated with the mitochondria (Mattson et al. 2008; Liston and Gan 2011; Koleske 2013, Duman et al. 2016; Castrén and Monteggia 2021).
Here we will review the available evidence supporting the specific role for Dopamine D3 receptors (D3R) in producing and maintaining structural plasticity of the nigrostriatal and mesolimbic DAergic circuits in the adult brain, of potential relevance for mood disorders and, in particular, for treatment-resistant depression (Collo and Merlo Pich 2018).
2 Defective Neuronal Structural Plasticity in Brain of Patients with Mood Disorders
Defective structural plasticity in circuits of frontal cortex, hippocampus, amygdala, and ventral mesencephalon has been consistently described in patients with mood disorders (Drevets et al. 2008) and in rodents after chronic stress (Christoffel et al. 2011). This reduced neuroplasticity is paralleled by reduced activity of the mTOR pathway, whose phosphorylation cascade controls cell survival and growth (Jernigan et al. 2011). Reversal of the reduced structural plasticity observed in dendrites produced by stress and depression was described in cortico-limbic circuits of rodents and humans exposed to clinically-effective electroconvulsive therapy (Chen et al. 2009; Dukart et al. 2014) and pharmacological treatments with ketamine or, to lesser extent, serotonin uptake inhibitors (SSRI) (Duman et al. 2016; Bessa et al. 2009). The antidepressant actions of these treatments involve the increase of BDNF levels and the activation of the BDNF-TrkB signaling that activate the main neurotrophic pathways in neurons, leading to enhanced structural plasticity at synaptic and dendritic levels, indicating that defective BDNF/TrkB could be a critical mechanism in determining the impaired structural plasticity in major depressive disorder (Duman et al. 2016; Castrén and Monteggia 2021). D3R appears to be a player in this process, being controlled by BDNF-TrkB signaling as part of the DA sensitization mechanisms (Guillin et al. 2001) and, in turn, acting as a trigger for the release of BDNF from the DAergic neurons (Yang et al. 2020). This reciprocal interaction was further explored in Chap. 4 “D3R Mediates Structural Plasticity in DAergic Neurons Engaging Neurotrophic Pathways”.
3 Dopamine D3 Receptors (D3R): Biological and Pharmacological Profile
The two most common dopaminergic receptors in mesolimbic and nigrostriatal circuits are the D3R and the D2R, the latter consisting of the “short” and the “long” subtypes. Even if they share a large sequence homology and several signaling pathways, they show differences in their action and regulation. The expression patterns of D2R and D3R partially differ in the mammalian brain, with differences between rodents and primates (Gurevich and Joyce 1999, Gurevich, chapter in the present book). At cellular level, D2R subtype and D3R are expressed either presynaptically as autoreceptors in DAergic neurons in the ventral mesencephalon, e.g., in substantia nigra (Diaz et al. 2000), or postsynaptically in neurons with various phenotypes present in the terminal regions of the DAergic projections, e.g., GABAergic neurons in striatum and putamen, and in substantia nigra (Gurevich and Joyce 1999, Gurevich, chapter in the present book) or glutamatergic neurons of prefrontal cortex layer 5 (Clarkson et al. 2017). Interestingly, when present in the same brain regions, as in prefrontal cortex of rodents, functional segregation of D1R, D2R, and D3R can be observed, affecting different circuits (Clarkson et al. 2017). In addition, pharmacological antagonism of either D2R or D3R in frontal cortex disrupts and promotes cognitive function, respectively (Watson et al. 2012). Further functional differences between D2R and D3R were observed in mice whose genes were knockout (KO). The endophenotype of D2R KO mice displayed behavioral hypoactivity, insensitivity to the D2R/D3R agonist quinpirole, and low extracellular DA levels in the striatum (Baik et al. 1995). Conversely, D3R KO mice showed the opposite phenotype, displaying behavioral hyperactivity, responded to quinpirole response and spontaneous high extracellular DA level in the striatum (Accili et al. 1996; Koeltzow et al. 1998). Finally, D3R were observed in glial cells of striatum, cortex, and substantia nigra (Miyazaki et al. 2004; Zhang et al. 2014), where they contribute to local inflammation (Montoya et al. 2019).
3.1 D3R Role in Post-Synaptic Non-dopaminergic Neurons and Glia
D2R and D3R being members of the 7-transmembrane domain receptors display different coupling with the subunits of the G protein-coupled receptor (GPCR): DA D3R engages the βγ subunits, while D2R uses the Gαo subunits (Beom et al. 2004). Moreover, desensitization of D2R is associated with phosphorylation mediated by the G protein-coupled receptor kinase (GRK) and by interaction with β-arrestin for internalization, while D3R undergoes protein kinase C (PKC)-mediated phosphorylation, internalization and degradation, or translocation into membrane hydrophobic domains (Cussac et al. 1999; Kim et al. 2001; Beom et al. 2004). Indeed, in GABAergic medium spiny neurons of striatum, the activation of both D2R and D3R increases phosphorylation of the MAPK/ERK pathways, while D3R appears to be able to drive the selective activation of the Akt-mTOR signaling pathway produced by D2R/D3R agonists, since the latter was completely blocked by pretreatment with S-33084, a highly selective D3R antagonist (Salles et al. 2013). During development D3R begins its expression at the early embryological stages in neuronal precursors and immature oligodendrocytes (Bongarzone et al. 1998). In astrocytes of the adult mouse midbrain/striatum Montoya et al. (2019), showed that exposures to DA or to the D3-preferential DA agonist PD128907 were able to increase inducible Nitric Oxide Synthase (iNOS) to a similar extent to a systemic LPS administration, generating a pro-inflammatory-like response and increasing the expression of Glial Fibrillary Astrocytic Protein (GFAP). These effects were not observed in glial cells of D3R-KO mice, suggesting a possible permissive role of D3R neurotransmission in neuroinflammation. Intriguingly, LPS toxin exposures were shown to reduce the expression of D3R, suggesting a negative feedback possibly aimed to attenuate the local contribution of DA-dependent signals of inflammation.
3.2 D3R Role in Presynaptic DAergic Neurons
In DAergic neurons of substantia nigra (SN) and ventral tegmental area (VTA), both D2R-short and D3R are expressed in neuronal soma and functionally defined as “autoreceptors” (Diaz et al. 2000; Ford 2014). The role of “autoreceptors” has been seen as associated to negative feedback control of synaptic activities, in this case control of neuron firing rate and control of neurotransmitter release, mostly via modulatory effect on Ca++ efflux and/or via direct interaction with the Dopamine Transporter (DAT). Dopamine or D3R-preferential D2R/D3R agonists such as quinpirole, ropinirole, or pramipexole by binding to presynaptic D3R reduce DA uptake by interacting with DAT functions (Joyce et al. 2004). Interestingly, D3R requires functional D2R autoreceptors to exert its control on DA release (Zapata and Shippenberg 2005). Similar effects were observed when indirect DA agonists, such as amphetamine or cocaine, were tested on mesencephalic DAergic neurons. Cocaine increased extracellular DA by blocking DAT, an effect potentiated by the blockade of D3R using the selective antagonist SB-277011-A, but not by the selective D2R antagonist L-741,626 (McGinnis et al. 2016). Recent in vivo microdialysis studies support the evidence that D3R activation increases DA release from rat substantia nigra/VTA (Rodríguez-Sánchez et al. 2019). Previously, Schwarz et al. (2004) reported that the selective D3R antagonist SB-277011-A was able to potentiate pharmaco-Magnetic Resonance Imaging (MRI) response to amphetamine challenges in the ventral mesencephalon of rats, affecting the brain functional connectivity and suggesting a presynaptic effect. Another series of studies has recently profiled the activation of other D3R-mediated intracellular pathways: they are reviewed in the following paragraph.
4 D3R Mediates Structural Plasticity in DAergic Neurons Engaging Neurotrophic Pathways
4.1 Studies in Mesencephalic DA Neurons of Rodents
Based on the evidence collected by Diaz et al. (2000) on the localization of D3R in mesencephalic DAergic neurons, Du et al. (2005) studied the effects of D3R activation by incubating rodent primary mesencephalic culture containing astrocytes with D3R-preferential DA agonists, such as pramipexole and ropinirole, showing neurotrophic-like effects and increase in number of DAergic neurons. Similar results were obtained in mouse primary mesencephalic cultures that were maintained under conditions to prevent astrocytes growth using D3R-preferential D2R/D3R agonist such as 7-OH-DPAT and quinpirole at low doses, whose effects were blocked by selective D3R antagonist SB-277112-A (Collo et al. 2008). These data supported the tenet a neuronal-mediated D3R-dependent effect. Similar increases of dendritic arborization and soma size were also observed with the indirect DA agonist amphetamine, producing effects that involved the activation of MAPK/ERK pathways via presynaptic D3R, probably due to the increased extracellular DA produced by amphetamine (Collo et al. 2008). These effects were in keeping with the dendritic outgrowth observed postmortem in VTA of rats repeatedly exposed to amphetamine (Mueller et al. 2006). Few years later, using both in vitro and in vivo studies on mice, Collo et al. (2012) show that exposure to cocaine, another indirect DA agonist, produced D3R-dependent increases of structural plasticity in mesencephalic DAergic neurons. These effects were seen in vitro on primary cultures of DAergic neurons from mouse embryo, where cocaine-induced increase of dendritic arborization and soma size were antagonized by the non-selective D2R/D3R antagonist sulpiride and by the selective D3R antagonists SB-277011-A and S-33084. These effects of cocaine were mediated by the activation of the ERK1/2 and Akt-mTOR pathways, since preincubation with selective phosphorylation blockers completely inhibited structural plasticity induced by cocaine. Moreover, when primary cultures of mesencephalic DA neurons from D3R KO mice were challenged with cocaine, no change in dendritic arborization was observed and no activation of ERK1/2 and Akt pathway phosphorylation was observed. These observations were corroborated in vivo by morphometric assessment of mesencephalic dopaminergic neurons of P1 newborns exposed to cocaine from E12.5 to E16.5. The experiments were performed in wild-type and D3R KO mice. Cocaine increased the soma area of wild-type but not of D3R KO mice, supporting the translational value of primary culture (Collo et al. 2012). Other in vivo studies support structural plasticity effects of D3R signaling: van Kampen and Eckman (2006) evaluated rats exposed to 6-OHDA acute neurotoxic damage of nigrostriatal DAergic pathways after a chronic treatment with D3-preferential DA agonist 7-OH-DPAT. They observed a significant induction of cell proliferation in the substantia nigra pars compacta with a time-dependent adoption of DAergic phenotype. Retrograde tracing revealed a restoration of striatal innervation from mesencephalic DAergic neurons and persistent recovery of locomotor function, a demonstration of induction of structural plasticity in vivo.
5 Studies in Human DA Neurons Differentiated from Inducible Pluripotent Stem Cells (iPSC)
The relatively recent observation that inducible Pluripotent Stem Cells (iPSC) from human donors can be differentiated in DAergic neurons (Kriks et al. 2011; Fedele et al. 2017) (Fig. 1a) has open the possibility to study their pharmacological phenotype in vitro (Fig. 1b). The changes of dendritic arborization and soma size induced by D3R-preferential agonists were studied in human iPSC-derived DAergic neurons (Collo et al. 2018) following a procedure schematically showed in Fig. 1b. An example of the application of this procedure is shown in Fig. 1c where the structural plasticity effects of pramipexole were quantified, resulting in a dose-dependent increases of maximal dendritic length, number of primary dendrites and soma size. Similar effects were also observed with ropinirole and antagonized by the selective D3R antagonists SB-277011-A and S-33084 (Collo et al. 2018). Visualization of phosphorylated p70S6 kinase indicated the recruitment of the mTOR pathway, a critical mediator of cell growth and structural plasticity. Phosphorylation of p70S6 kinase and structural plasticity induced by ropinirole and pramipexole were blocked by the kinase inhibitors LY294002 and by rapamycin, an mTORC1 inhibitor, confirming the involvement of the mTOR pathway. Since Ras-ERK and PI3K-mTOR pathways are also constitutive elements of the BDNF-TrkB signaling, different modalities of BDNF-TrkB pathway disruption previously used in rat telencephalic neurons (i.e., immunoneutralization of BDNF, inhibition of TrkB receptor and blockade of MEK-ERK signaling) (Jourdi et al. 2009) were applied to human DA neurons exposed to ropinirole, all procedures blocked D3R-dependent structural plasticity. These effects are consistent with the regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways previously described in the rat telencephalic neurons (Kumar et al. 2005). These data also indicate that active BDNF-TrkB signaling is necessary for D3R-dependent structural plasticity in human DA neurons. Interestingly, the behavioral relevance of reciprocal crosstalk between these two crucial pathways in DA neurons was demonstrated in rats with a unilateral nigrostriatal lesion of DA projections, showing structural recovery of axonal innervation and novel dendritic spine formation (Razgado-Hernandez et al. 2015).
Human iPSC-derived dopaminergic neurons methodological approach to study neuroactive compounds. (a) Schematic representation of the differentiation procedure used to obtain in vitro human DAergic neurons. Neuronal precursors derived from iPSCs are exposed to successive mix of growth factors and small molecules aimed to develop the A9-like DAergic phenotype, according to Kriks et al. (2011) and Fedele et al. (2017). KSR, knockout serum replacement; LDN, LDN193189;SB, SB431542; Shh, Shh C25II; FGF8, fibroblast growth factor 8; Purm, purmorphamine; CHIR, CHIR99021; BDNF, brain-derived neurotrophic factor; AA, ascorbic acid; cAMP, dibutyryl cAMP; TGFβ, transforming growth factor type β3; GDNF, glial cell line-derived neurotrophic factor. (b) Cartoon of the procedure used to test iPSC-derived human DA neurons with various pharmacological agents, performing activation and inhibition studies with a variety of concentrations of agonists and antagonists as described in Collo et al. (2018). The outcomes were imaging or biochemical parameters, aimed to characterize structural plasticity on dendrites and soma profiles and the molecular pathways involved in these responses. (c) Histograms representing the quantification of the morphological changes of dendrites and soma produced by pramipexole dose-dependent D3R activation as described in Collo et al. (2018)
6 Effects on DA Neuron Structural Plasticity Produced by the Activation of D3R Could Be Beneficial in Patients with Treatment-Resistant Depression
Dysfunction of dopaminergic neurotransmission within the mesolimbic and nigrostriatal systems, where D3R are expressed, may contribute to anhedonia, loss of motivation, and psychomotor retardation in severe depressive disorders that partially respond to treatment, and targeting D3R has been considered a possible therapeutic approach (Leggio et al. 2013). Preclinical studies showed association between low levels of BDNF in ventral mesencephalon with anhedonia, a core symptom of major depressive disorder (Der-Avakian et al. 2014). Low levels of TrkB expression were observed in postmortem striatum of patients with mood disorders (Reinhart et al. 2015). Indeed, increased BDNF signaling was recognized as a necessary step for the antidepressant effects of ketamine (Autry et al. 2011) and, partially, of SSRI (Bessa et al. 2009), leading to the concept of normalizing defective structural plasticity and dendritic arborization stability through a BDNF-TrkB orchestrated intracellular growth pathways activation (Castrén and Rantamäki 2010; Castrén and Monteggia 2021). Hence, the engagement of BDNF-TrkB signaling in mediating structural plasticity in DA neurons driven by D3R-preferential D2R/D3R agonists, such as 7-OH-DPAT, ropinirole or pramipexole, may be seen as a common feature involving D3R-mediated neurotransmission to address the problem of treatment-resistant depression. Accordingly, cariprazine, a D2R/D3R partial agonist with a 10-fold preferential affinity to D3R, improved symptoms in subjects with major depressive disorder that were poorly responsive to standard-of-care (Durgam et al. 2016). The intrinsic activity of cariprazine at D3R (Emax 70%) is comparable to that of aripiprazole, another D3R-preferential D2R/D3R partial agonist, that was approved for adjunctive treatment of major depressive disorder (Berman et al. 2007). Antidepressant effects of pramipexole were described in preclinical studies (Breuer et al. 2009), as well in clinical studies in Parkinson’s patients with diagnosis of depression (Barone et al. 2010) and as add-on to SSRI in individuals with treatment-resistant depression (Fawcett et al. 2016; Tundo et al. 2022).
7 D3R Signaling Is Involved in Structural Plasticity Produced by the Antidepressant Ketamine on Human and Mice Mesencephalic DAergic Neurons
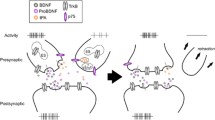
Ketamine is probably the most effective rapid-acting antidepressant for patients suffering from treatment-resistant depression (Schwartz et al. 2016; Zanos and Gould 2018; Collo and Merlo Pich 2018). Structural remodeling of prefrontal, hippocampal neurons involving dendritic arbors and spines has been proposed as a key neurobiological mechanism underlying antidepressant properties of ketamine (Duman et al. 2016; Zanos and Gould 2018). Ketamine-induced increase of dendritic arborization and soma size was also observed in mouse mesencephalic primary cultures and human iPSC-derived DAergic neurons (Cavalleri et al. 2018). These authors showed that the critical molecular mechanisms involved downstream activation of AMPA receptors which in turn trigger mTOR pathway-dependent structural plasticity via BDNF-TrkB activation. Both structural plasticity and neurotrophic pathway activation were blocked by MEK inhibitor PD98059, by PI3K inhibitor LY294002, and by rapamycin, an mTOR signaling inhibitor. The effects of ketamine were abolished by AMPA receptor antagonists and were mimicked by the AMPA positive allosteric modulator CX614, as shown also in telencephalic neurons (Li et al. 2010; Duman et al. 2016). Inhibition of BDNF-TrkB signaling achieved with various modalities prevented the induction of structural plasticity produced by ketamine. Intriguingly, ketamine effects on mesencephalic DAergic neurons required functional D3R, since its effects were abolished by pretreatment with selective D3R antagonists and were absent in D3R KO mice DAergic neurons (Cavalleri et al. 2018). These data are in line with the results of behavioral experiments in rodents using Forced Swim Test to assess depressive-like behavior, showing that the combined administration of sub-effective doses of ketamine and pramipexole exerted antidepressant-like effects compared with each drug alone (Li et al. 2015). The cartoon in Fig. 2 describes a working hypothesis about the molecular and cellular mechanisms involved in the interaction between ketamine and D3R-preferential DA agonist in producing beneficial effects in patients with treatment-resistant depression.
Schematic representation of the working hypothesis of molecular mechanisms involved in mediating the interaction between ketamine and D3R-preferential DA antagonists in producing structural plasticity-mediated changes in DAergic neurons suggested to improve depression in treatment-resistant depression. (a) This inset represents the hypothetical key steps mediating ketamine action on DAergic neurons via NMDA receptors expressed on GABAergic interneurons, as occurring in telencephalic neurons and summarized in Duman et al. (2016). (b) This inset represents an alternative hypothesis of ketamine action by a direct interaction on NMDA receptors expressed on DAergic neurons, as originally proposed for cortical neurons by Miller et al. (2016). Note that both proposed mechanisms upregulate synaptic AMPA receptors. (c) In this inset the exposure with D3R-preferential DA agonists, such as pramipexole or ropinirole (Collo et al. 2018), is highlighted as a way to drive a possible interaction with the ketamine-triggered intracellular pathways of relevance for structural changes, as proposed by Collo and Merlo Pich (2018)
8 Conclusions
The present review summarized the findings supporting a role for D3R activation through pharmacological agents such as pramipexole or, indirectly, ketamine to increase structural plasticity in human DA neurons via recruitment of BDNF-TrkB and the activation of the MAPK/ERK and mTOR signaling pathways. Given the evidence of disrupted stability and reduced plasticity of dendritic arborization in several brain circuits in mood disorders, the structural effects produced by pharmacological activation of D3R can be seen as a reasonable treatment approach for a combination treatment, not implemented yet in the clinics. D3R activation could, therefore, contribute to the enhancement of structural plasticity necessary to improve depression, providing a reasonable interpretation of the clinical effects observed with pramipexole or ropinirole as add-on treatment in patients with treatment-resistant depression.
References
Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S (1996) A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci U S A 93(5):1945–1949
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al (2011) NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature 475:91–95
Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A et al (1995) Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377(6548):424–428
Barone P, Poewe W, Albrecht S, Debieuvre C, Massey D, Rascol O et al (2010) Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 9:573–580
Beom SR, Cheong D, Torres G, Caron MG, Kim KM (2004) Comparative studies of molecular mechanisms of dopamine D2 and D3 receptors for the activation of extracellular signal-regulated kinase. J Biol Chem 279:28304–28314
Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK et al (2007) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 68:843–853
Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA et al (2009) The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14:764–777
Bongarzone ER, Howard SG, Schonmann V, Campagnoni AT (1998) Identification of the dopamine D3 receptor in oligodendrocyte precursors: potential role in regulating differentiation and myelin formation. J Neurosci 18(14):5344–5353
Breuer ME, Groenink L, Oosting RS, Buerger E, Korte M, Ferger B et al (2009) Antidepressant effects of pramipexole, a dopamine D3/D2 receptor agonist, and 7-OH-DPAT, a dopamine D3 receptor agonist, in olfactory bulbectomized rats. Eur J Pharmacol 616:134–140
Castrén E, Monteggia LM (2021) Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry 90(2):128–136
Castrén E, Rantamäki T (2010) The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70:289–297
Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, Collo G (2018) Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry 23(4):812–823
Chen F, Madsen TM, Wegener G, Nyengaard JR (2009) Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus. Eur Neuropsychopharmacol 19:329–338
Christoffel DJ, Golden SA, Russo SJ (2011) Structural and synaptic plasticity in stress-related disorders. Rev Neurosci 22:535–549
Clarkson RL, Liptak AT, Gee SM, Sohal VS, Bender KJ (2017) D3 receptors regulate excitability in a unique class of prefrontal pyramidal cells. J Neurosci 37(24):5846–5860
Collo G, Zanetti S, Missale C, Spano PF (2008) Dopamine D3 receptor-preferring agonists increase dendrite arborization of mesencephalic dopaminergic neurons via extracellular signal-regulated kinase phosphorylation. Eur J Neurosci 28:1231–1240
Collo G, Bono F, Cavalleri L, Plebani L, Merlo Pich E, Millan MJ et al (2012) Pre-synaptic dopamine D3 receptor mediates cocaine-induced structural plasticity in mesencephalic dopaminergic neurons via ERK and Akt pathways. J Neurochem 120:765–778
Collo G, Cavalleri L, Bono F, Mora C, Fedele S, Invernizzi RW, Gennarelli M, Piovani G, Kunath T, Millan MJ, Merlo Pich E, Spano P (2018) Ropinirole and pramipexole promote structural plasticity in human iPSC-derived dopaminergic neurons via BDNF and mTOR signaling. Neural Plast:4196961
Collo G, Merlo Pich E (2018) Ketamine enhances structural plasticity in human dopaminergic neurons: possible relevance for treatment-resistant depression. Neural Regen Res 13(4):645–646
Cussac D, Newman-Tancredi A, Pasteau V, Millan MJ (1999) Human dopamine D3 receptors mediate mitogen-activated proteinkinase activation via a phosphatidylinositol 3-kinase and an atypical protein kinase C-dependent mechanism. Mol Pharmacol 56:1025–1103
Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A (2014) Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry 76:542–549
Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P (2000) Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci 20(23):8677–8684
Drevets WC, Price JL, Furey ML (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118
Du F, Li R, Huang Y, Li X, Le W (2005) Dopamine D3 receptor-preferring agonists induce neurotrophic effects on mesencephalic dopamine neurons. Eur J Neurosci 22(10):2422–2430
Dukart J, Regen F, Kherif F, Colla M, Bajbouj M, Heuser I et al (2014) Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc Natl Acad Sci U S A 111:1156–1161
Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249
Durgam S, Earley W, Guo H, Li D, Németh G, Laszlovszky I et al (2016) Efficacy and safety of adjunctive cariprazine in inadequate responders to antidepressants: a randomized, double-blind, placebo-controlled study in adult patients with major depressive disorder. J Clin Psychiatry 77:371–378
Fawcett J, Rush AJ, Vukelich J, Diaz SH, Dunklee L, Romo P et al (2016) Clinical experience with high dose pramipexole in treatment resistant mood disorder patients. Am J Psychiatry 173:107–111
Fedele S, Collo G, Behr K, Bischofberger J, Müller S, Kunath T, Christensen K, Gündner AL, Graf M, Jagasia R, Taylor V (2017) Expansion of human midbrain floor plate progenitors from induced pluripotent stem cells increases dopaminergic neuron differentiation potential. Sci Rep 7(1):6036
Ford CP (2014) The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 282:13–22
Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P (2001) BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411(6833):86–89
Gurevich EV, Joyce JN (1999) Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology 20(1):60–80
Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA et al (2011) The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35:1774–1779
Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M (2009) Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci 29:8688–8697
Joyce JN, Woolsey C, Ryoo H, Borwege S, Hagner D (2004) Low dose pramipexole is neuroprotective in the MPTP mouse model of Parkinson's disease, and downregulates the dopamine transporter via the D3 receptor. BMC Biol 2:22
Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG (2001) Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem 276(40):37409–37414
Koeltzow TE, Xu M, Cooper DC, Hu XT, Tonegawa S, Wolf ME, White FJ (1998) Alterations in dopamine release but not dopamine autoreceptor function in dopamine D3 receptor mutant mice. J Neurosci 18(6):2231–2238
Koleske AJ (2013) Molecular mechanisms of dendrite stability. Nat Rev Neurosci 14(8):536–550
Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A et al (2011) Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480(7378):547–551
Kumar V, Zhang MX, Swank MV, Kunz J, Wu GY (2005) Regulation of dendritic morphogenesis by Ras-PI3K-AktmTOR and Ras-MAPK signaling pathways. J Neurosci 25(49):11288–11299
Leggio GM, Salomone S, Bucolo C, Platania C, Micale V, Caraci F et al (2013) Dopamine D(3) receptor as a new pharmacological target for the treatment of depression. Eur J Pharmacol 719:25–33
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964
Li Y, Zhu ZR, Ou BC, Wang YQ, Tan ZB, Deng CM, Gao YY et al (2015) Dopamine D2/D3 but not dopamine D1 receptors are involved in the rapid antidepressant-like effects of ketamine in the forced swim test. Behav Brain Res 279:100–105
Liston C, Gan WB (2011) Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A 108(38):16074–16079
Mattson MP, Gleichmann M, Cheng A (2008) Mitochondria in neuroplasticity and neurological disorders. Neuron 60(5):748–766
McGinnis MM, Siciliano CA, Jones SR (2016) Dopamine D3 autoreceptor inhibition enhances cocaine potency at the dopamine transporter. J Neurochem 138(6):821–829
Miller OH, Moran JT, Hall BJ (2016) Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: direct inhibition and disinhibition. Neuropharmacology 100:17–26
Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N (2004) Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res 1029(1):120–123
Montoya A, Elgueta D, Campos J, Chovar O, Falcón P, Matus S, Alfaro I, Bono MR, Pacheco R (2019) Dopamine receptor D3 signaling in astrocytes promotes neuroinflammation. J Neuroinflammation 16(1):258
Mueller D, Chapman CA, Stewart J (2006) Amphetamine induces dendritic growth in ventral tegmental area dopaminergic neurons in vivo via basic fibroblast growth factor. Neuroscience 137:727–735
Razgado-Hernandez LF, Espadas-Alvarez AJ, Reyna-Velazquez P, Sierra-Sanchez A, Anaya-Martinez V, Jimenez-Estrada I, Bannon MJ, Martinez-Fong D, Aceves-Ruiz J (2015) The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PLoS One 10(2):e0117391
Reinhart V, Bove SE, Volfson D, Lewis DA, Kleiman RJ, Lanz TA (2015) Evaluation of TrkB and BDNF transcripts in prefrontal cortex, hippocampus, and striatum from subjects with schizophrenia, bipolar disorder, and major depressive disorder. Neurobiol Dis 77:220–227
Rodríguez-Sánchez M, Escartín-Pérez RE, Leyva-Gómez G, Avalos-Fuentes JA, Paz-Bermúdez FJ, Loya-López SI, Aceves J, Erlij D, Cortés H, Florán B (2019) Blockade of intranigral and systemic D3 receptors stimulates motor activity in the rat promoting a reciprocal interaction among glutamate, dopamine, and GABA. Biomol Ther 9(10):511
Salles MJ, Hervé D, Rivet JM, Longueville S, Millan MJ, Girault JA, Mannoury la Cour C (2013) Transient and rapid activation of Akt/GSK-3β and mTORC1 signaling by D3 dopamine receptor stimulation in dorsal striatum and nucleus accumbens. J Neurochem 125(4):532–544
Schwarz A, Gozzi A, Reese T, Bertani S, Crestan V, Hagan J, Heidbreder C, Bifone A (2004) Selective dopamine D3 receptor antagonist SB-277011-A potentiates phMRI response to acute amphetamine challenge in the rat brain. Synapse 54:1–10
Schwartz J, Murrough JW, Iosifescu DV (2016) Ketamine for treatment-resistant depression: recent developments and clinical applications. Evid Based Ment Health 19(2):35–38
Tundo A, Betro’ S, Iomm MI, de Filippis R (2022) Efficacy and safety of 24-week pramipexole augmentation in patients with treatment resistant depression. A retrospective cohort study. Prog Neuropsychopharmacol Biol Psychiatry 112:110425
Van Kampen JM, Eckman CB (2006) Dopamine D3 receptor agonist delivery to a model of Parkinson's disease restores the nigrostriatal pathway and improves locomotor behavior. J Neurosci 26:7272–7280
Watson DJ, Loiseau F, Ingallinesi M, Millan MJ, Marsden CA, Fone KC (2012) Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacology 37:770–786
Yang P, Perlmutter JS, Benzinger T, Morris JC, Xu J (2020) Dopamine D3 receptor: a neglected participant in Parkinson disease pathogenesis and treatment? Ageing Res Rev 57:100994. https://doi.org/10.1016/j.arr.2019.100994
Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23(4):801–811
Zapata A, Shippenberg TS (2005) Lack of functional D2 receptors prevents the effects of the D3-preferring agonist (+)-PD 128907 on dialysate dopamine levels. Neuropharmacology 48(1):43–50
Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P et al (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34(36):11929–11947
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Merlo Pich, E., Cavalleri, L., Toma, C., Collo, G. (2022). Involvement of DA D3 Receptors in Structural Neuroplasticity of Selected Limbic Brain Circuits: Possible Role in Treatment-Resistant Depression. In: Boileau, I., Collo, G. (eds) Therapeutic Applications of Dopamine D3 Receptor Function. Current Topics in Behavioral Neurosciences, vol 60. Springer, Cham. https://doi.org/10.1007/7854_2022_348
Download citation
DOI: https://doi.org/10.1007/7854_2022_348
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23057-8
Online ISBN: 978-3-031-23058-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)