Abstract
Humans, like other animals, are fundamentally motivated to pursue rewarding outcomes and avoid aversive ones. Anxiety disorders are conceptualized, defined, and treated based on heightened sensitivity to perceived aversive outcomes, including imminent threats as well as those that are uncertain yet could occur in the future. Avoidance is the central strategy used to mitigate anticipated aversive outcomes – often at the cost of sacrificing potential rewards and hindering people from obtaining desired outcomes. It is for these reasons that people are often motivated to seek treatment. In this chapter, we consider whether and how anhedonia – the loss of interest in pursuing and/or reduced responsiveness to rewarding outcomes – may serve as a barrier to recovering from clinically impairing anxiety. Increasingly recognized as a prominent symptom in many individuals with elevated anxiety, anhedonia is not explicitly considered within prevailing theoretical models or treatment approaches of anxiety. Our goal, therefore, is to review what is known about anhedonia within the anxiety disorders and then integrate this knowledge into a functional perspective to consider how anhedonia could maintain anxiety and limit treatment response. Our overarching thesis is that anhedonia disrupts the key processes that are central to supporting anxiety recovery. We end this chapter by considering how explicitly targeting anhedonia in treatment can optimize outcomes for anxiety disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Anxiety disorders are defined based on excessive and persistent fear, anxiety, and/or avoidance of perceived threats – whether imminent (e.g., being criticized after sharing one’s opinion) or in the future (e.g., losing one’s job). They are classified into discrete categories based on the core source of threat and include social anxiety disorder (SAD; fear of embarrassment or negative evaluation in social/performance situations), panic disorder (PD; fear of having a panic attack and its consequences), and generalized anxiety disorder (GAD; worry about uncertain future threats). Responses to perceived threat originate from a defensive motivational system that operates to protect the organism from danger. The perception of threat activates heightened expectancies about the likelihood and cost of aversive outcomes. Inflated threat expectancies induce subjective distress (e.g., anxiety, fear), defensive physiological states (e.g., increased heart rate), and avoidance behaviors intended to mitigate perceived danger. Avoidance behaviors provide temporary relief from anxiety; however, they can maintain exaggerated threat responses over the long-term because the individual fails to learn the situation poses less danger than predicted. Cognitive behavioral models posit that recovery from anxiety disorders is predicated on individuals repeatedly confronting avoided threat-relevant cues or contexts and learning that the threat stimulus is no longer associated with aversive outcomes (Craske et al. 2008). This is the central premise underlying empirically supported exposure-based therapies for anxiety; however, only half of patients achieve a clinically significant response from these first-line treatments (Loerinc et al. 2015). We consider whether and how anhedonia may account in part for incomplete recovery that afflicts a sizeable proportion of the anxiety disorder population.
2 Anhedonia in Anxiety: Early Observations to Current Empirical Status
Initial signs pointing to the presence of anhedonia in anxiety disorders originated from the tripartite model of emotional disorders (Clark and Watson 1991). Anhedonia, a clinical symptom defined by diminished interest in pursuing and/or response to pleasurable or meaningful activities, was initially hypothesized to distinguish depression from anxiety disorders. Early tests of this framework suggested SAD was the exception to the rule – demonstrating associations with anhedonia comparable to those observed for depression (Brown et al. 1998). Accumulating evidence has since established a link between anxiety and anhedonia across various samples (e.g., clinical and non-clinical) and methods of assessment. Much of this evidence comes from surveys measuring temperament or personality (e.g., behavioral activation system; positive emotionality), positive affect (PA; i.e., the frequency and intensity of experiencing positive valence emotions), or clinical symptoms of anhedonia (i.e., pleasure related to specific activities). Among the anxiety disorders, SAD shows the most reliable associations with anhedonia – in studies ranging from surveys of trait-like positive emotionality (Naragon-Gainey et al. 2009) to daily PA (Kashdan and Steger 2006), and confirmed through meta-analysis (Kashdan 2007). Although depression symptoms frequently co-occur with anxiety, this does not fully explain the link between social anxiety and low PA (Kashdan 2007). Depression comorbidity, however, is more common in the subgroup of individuals with SAD characterized by low positive emotionality (Tung and Brown 2020). The link between self-reported anhedonia/low PA and GAD is less well-established – some studies observe an association (e.g., Prenoveau et al. 2010), whereas others do not (Brown et al. 1998; see review by Seager et al. 2019). Anhedonia is not associated with PD in the absence of co-occurring depression (Brown et al. 1998; Prenoveau et al. 2010).
Beyond subjective experiences of diminished interest or pleasure, anhedonia can be understood and studied as a set of dynamic and interactive components unfolding along the temporal stream of reward processing. Neuroscience-informed models of anhedonia (Der-Avakian and Markou 2012) generally agree that reward processing includes: (1) reward valuation – the process of predicting the magnitude, likelihood, time horizon, and effort required to obtain a reward; (2) reward responsiveness – hedonic experiences during anticipation and receipt of rewards; and (3) reward learning – i.e., the process of integrating information about expected vs. actual reward outcomes, which informs future expectancies of reward acquisition and behavior. Evidence supporting the link between anhedonia and deficits in each of these component processes comes primarily from research in depression (see chapter “Anhedonia in Depression and Bipolar Disorder NB” of this Book). Here, we summarize the empirical literature within anxiety disorder and high symptom (analogue) samples specifically.
2.1 Reward Valuation
Reward valuation begins with the individual determining (1) reward probability, namely the value of a reinforcer according to its magnitude, valence, and likelihood, (2) the delay until the reinforcer will be delivered (immediate vs. future), and (3) the effort required to obtain the reward (i.e., perceived costs of physical or cognitive effort required). This information is integrated into a net value signal that informs decisions to pursue the reinforcer and motivation to perform actions required to obtain the reinforcer. A limited body of research links the presence of anxiety with reduced reward probability estimates. Blair et al. (2017) reported a significantly reduced likelihood of predicting future positive events in those with GAD relative to those with SAD and healthy controls. SAD and control participants did not differ, suggesting intact reward probability for those with SAD; however, future probability estimates were made in relation to both social and non-social events, which may have diluted effects in SAD. In support of this hypothesis, another study found individuals with SAD underestimated the likelihood and overestimated the aversiveness of positive social outcomes (Gilboa-Schechtman et al. 2000), suggesting reward probability may be diminished and negatively biased in response to future social events specifically (cf. generalized reward expectancy deficits in GAD). Beyond disorder-specific anxiety, evidence suggests that general anticipatory anxiety may also impact hedonic expectancy. In a non-clinical sample, different patterns of neural activation were observed under experimentally induced high vs. low anticipatory anxiety states (threat of shock) in mesolimbic regions that code for the subjective value of expected positive vs. negative valence outcomes (Engelmann et al. 2015). Specifically, when participants selected among outcomes that involved varying magnitudes of possible (but not certain) monetary gains vs. losses, heightened threat expectancies appeared to shift neural valuation from potential positive to aversive outcomes during decisions that could lead to either.
Research findings on the preference of reward timing (delay) in anxiety are mixed. Some studies found that higher self-reported social anxiety (Rounds et al. 2007) or intolerance of uncertainty (Luhmann et al. 2011) was associated with more preference for immediate versus delayed rewards, whereas others failed to find a relationship (Jenks and Lawyer 2015; Steinglass et al. 2017), and still others found trait anxiety was linked to more preference for delayed rewards (Steinglass et al. 2017). To the best of our knowledge, no studies have examined effort for rewards in the context of anxiety. Evidence from depression samples suggests, however, that anhedonia is related to less effort in pursuit of rewards (Treadway et al. 2012). In summary, although studies on reward valuation in anxiety are few and results are mixed across samples, findings generally point to alterations in reward valuation processes in the presence of anxiety.
2.2 Reward Responsiveness
After an incentive value has been assigned, anticipatory processes orient the individual and mobilize resources toward obtaining desired outcomes. Responsiveness to reward can be characterized as the processes evoked from (1) cues signaling a future positive reinforcer (anticipation); (2) initial presentation of a positive reinforcer (initial responsiveness); (3) changes in the incentive value of a reinforcer over time as that reinforcer is experienced (satiation). Neuroimaging work consistently demonstrates alterations in mesolimbic networks involved in reward processing in anxiety disorders (e.g., Richey et al. 2017). Specifically, there is a body of evidence showing hypoactivation in the ventral striatum in anticipation of reward. The majority of the research involved SAD samples using social reward (e.g., Cremers et al. 2015; Richey et al. 2017), but similar patterns of findings have been reported using monetary rewards in the consumption phase in GAD (e.g., Kessel et al. 2015) and the anticipation phase in PD (e.g., Held-Poschardt et al. 2018). Of note, hypoactivation in SAD may be specific to social versus monetary rewards (Richey et al. 2017) and is not observed in adolescents with SAD who instead show heightened neural response to reward incentives (Guyer et al. 2012; see Sect. 3.1). Data from daily diary studies (e.g., Kashdan and Steger 2006) show that people with elevated social anxiety report less pleasure from social and non-social positive everyday experiences; however, following positive events perceived as particularly intense, individuals with elevated (cf. low) social anxiety appear to experience greater psychological benefits (e.g., reduced anxiety; Doorley et al. 2021). Collectively, research suggests that aberrant neural patterns involved in reward motivation and generally blunted responsivity are present in those with anxiety. It remains unclear, however, whether anxious arousal/threat sensitivity or anhedonia is the mechanism underlying altered reward responsiveness.
2.3 Reward Learning
Actual reward outcomes are compared against anticipated rewards (reward prediction error), which guides learning about the likelihood of obtaining future rewards and the actions required to do so (probabilistic and reinforcement learning). Individuals with anxiety disorders make more errors than healthy controls on reinforcement-based decision-making tasks (e.g., choosing between two objects associated with different levels of reward or punishment; DeVido et al. 2009), which might suggest difficulty incorporating recent learning. For example, people with GAD made significantly more errors in the later (but not early) blocks of a reinforcement-based decision task compared to healthy controls (White et al. 2017). However, several studies failed to find an association between alterations in reward learning and anxiety disorders. For example, performance on a signal-detection task that rewarded one response option more frequently than the other (probabilistic reward task; Pizzagalli et al. 2005) revealed that individuals diagnosed with GAD showed intact reward learning compared to healthy controls (Morris and Rottenberg 2015). Using a similar task, people with major depressive disorder, SAD, and healthy controls did not differ on probabilistic reward learning performance (Reilly et al. 2020). However, self-reported anhedonia symptoms across diagnoses were associated with impaired reward learning, whereas anxious arousal and general distress symptoms were not. Therefore, poor reward learning may be a consequence of anhedonia rather than anxiety-related symptoms per se. Because some studies used tasks that involved the potential for either punishment or reward on a given trial (cf. reward only), discrepancies observed across studies may also reflect the interactive effect of stress in the presence of reward on learning.
3 Vulnerability and Amplifying Factors
3.1 Etiological Origins
Anxiety disorders appear to be characterized by deficits within each reward processing phase, at least in part driven by anhedonia rather than anxious arousal/threat sensitivity. Each of these processes is dynamically influenced by genes, temperament, culture, and social learning histories that shape currently held beliefs (e.g., perceived success of obtaining positive outcomes), values (e.g., outcome importance), and goals (e.g., balance of rewards to punishments; see review by Kujawa et al. 2020). To briefly expand, genetic susceptibility to heightened reactivity to both the positive and negative effects of environment possibly elevates the risk for anhedonic processes (Belsky 2013). Likewise, prior learning histories involving failed reward acquisition also contribute to the onset and maintenance of altered reward processing (e.g., Richey et al. 2019). Additionally, certain temperaments such as behavioral inhibition and activation (see review by Katz et al. 2020) and personality factors like neuroticism are associated with blunted reward processing (e.g., Bondy et al. 2021). That traits assumed to be parts of punishment/aversion systems are associated with blunted reward processing (in addition to those arising from the appetitive/approach system) could suggest a possible bimodal or interactive pathway to anhedonia. Beyond general approach-avoidance tendencies, individual and situation-specific reward-cost goals play a role in each phase of reward processing (e.g., Gable and Impett 2012).
There are some differences in reward processing along development worth noting. Specifically, SAD in adolescents is associated with greater reward sensitivity (e.g., Guyer et al. 2012), whereas SAD in adults is associated with blunted reward reactivity (e.g., Richey et al. 2017). This may reflect the consequences of repeated failed attempts to obtain rewards throughout adolescence into adulthood (Richey et al. 2019) or may be suggestive of an initial pattern of hypersensitivity that diminishes over time from over-activation. Relatedly, anxiety disorders are characterized by heightened chronic stress and stress reactivity, which may consequently increase anhedonia (Pizzagalli 2014), consistent with the robust body of evidence showing anxiety disorders precede depression (e.g., Batterham et al. 2013). Finally, avoidance of situations due to anxiety limits exposure to positive experiences and reinforcers, possibly increasing both anxiety and hedonic atrophy (e.g., Winer et al. 2017).
3.2 Cognitive and Regulatory Anhedonia Amplifiers
Reward processing is also influenced by (1) cognitive processes that prioritize salient cues (attentional bias), alter the meaning of attended to information (interpretation bias), determine which information is encoded in memory and later retrieved (memory bias), and impact ability to envision personal future events (episodic future thinking), as well as (2) regulatory processes serving to up- or downregulate a given experience (e.g., emotion suppression vs. expression, dampening vs. amplifying). Together, these processes can shape how individuals experience positive emotions and events. Individuals with both clinical and non-clinical levels of anxiety have been shown to allocate attention away from rewards relative to threats (Winer and Salem 2016), interpret positive events negatively (Alden et al. 2008), recall positive memories as less positive (Glazier and Alden 2019), and rely on emotion regulation strategies to suppress the experience or expression of positive emotions (Eisner et al. 2009) – all of which may interfere with reward processing. For example, (a) diminished attentional allocation for reward cues may reduce their salience and influence reward valuation processes (Winer and Salem 2016); (b) negative interpretations of positive outcomes (e.g., Alden et al. 2008) could influence the predicted magnitude and valence of potential rewards and responsiveness to rewards by decreasing hedonic experiences and heightening aversive experiences; (c) diminished memory for positive valence events could reduce reward learning (e.g., outcomes were remembered as less positive than they were; Glazier and Alden 2019) which could in turn bias reinforcement learning and future reward valuation processes; and (d) reduced ability to imagine future positive outcomes and their hedonic impact (for a review see Miloyan et al. 2014) could decrease expected reward prediction. Emotion regulation strategies can change the intensity of affective experience (Sheppes et al. 2014). Therefore, positive emotion suppression (i.e., inhibiting outward expression; e.g., Kashdan and Breen 2008) and/or dampening of positive experiences (i.e., minimizing; Eisner et al. 2009) may lead to diminished responsiveness to reward cues and could interfere with reward learning and future valuation. Whether directly modifying cognitive and regulatory processes could augment reward processing in anxiety disorder samples is an open question for future research.
4 A Functional Account of How Anhedonia Could Impede Recovery from Anxiety
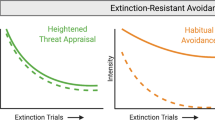
Overcoming excessive, chronic, and impairing anxiety is hard work. Current evidence-based therapy approaches require one to repeatedly confront and remain in the presence of (rather than avoid) perceived threat while tolerating associated aversive internal experiences in the service of facilitating new learning. Anhedonia is proposed to be a key disruptor of these processes and therefore a barrier to recovery. See Fig. 1.
4.1 Sacrificing Rewards Due to Costly Avoidance
The decision to engage with typically avoided threat-relevant contexts to achieve desired outcomes is determined by the valuation of potential rewards alongside threat-relevant costs. If anticipated rewards are perceived to be small, unlikely, temporally distant (delayed), and/or too effortful (costly), the balance will be tipped in favor of continued anxiety-related avoidance. Because anxiety disorders are characterized by blunted reward valuation, under-valued rewards may be sacrificed in favor of avoidance. Consistent with this perspective, individuals with elevated anxiety (and those with anxiety disorders; Pittig et al. 2021) fail to reduce threat-induced avoidance behavior that competes with potential reward acquisition (e.g., money, social approval; see review by Pittig et al. 2020). Reward-induced reduction of avoidance does not appear to attenuate fear responses but does facilitate fear extinction learning once the aversive outcome is no longer present (e.g., Pittig 2019), suggesting that intact reward sensitivity may encourage people to tolerate aversive experiences (Craske et al. 2008). Whether failure to reduce threat-related avoidance behavior reflects a lower sensitivity for competing rewards, a higher sensitivity to aversive outcomes, or some combination remains unknown. However, to the extent that anhedonia diminishes one’s sensitivity to rewards, it would be expected that anxious patients with co-occurring anhedonia would be especially likely to sacrifice potential rewards in service of continued avoidance of perceived threats. Those functional consequences could be reflected in avoidance of naturally occurring reward opportunities when threat is present, failure to seek out or initiate treatment, and/or reduced engagement in treatment activities that involve confronting threat-relevant contexts. Research is needed to test those predictions.
4.2 Consequences of Diminished Responsiveness
Diminished anticipatory and/or consummatory reward responsiveness may disrupt processes occurring before or during therapeutic exposures to threat that require the individual to initiate approach toward and remain engaged with threat-relevant contexts until new learning occurs. Reduced reward anticipation may lead the individual to devote more resources toward averting potential aversive outcomes (e.g., engaging in safety behaviors) rather than garnering positive ones, which may limit their success in achieving desired outcomes (Taylor and Alden 2011). Diminished responsiveness to rewarding outcomes during or after an exposure may bias outcome appraisals (i.e., reduced reward prediction error) and reduce the likelihood (through blunted reinforcement learning and subsequent valuation) that the individual will engage with similar contexts again in the future. Additionally, anhedonia may interrupt positively valenced subjective experiences following the omission of expected negative outcomes. Violation of negative (threat) expectancies is theorized as the core mechanism that facilitates response to exposure-based therapies (Craske et al. 2008). Relief experienced following the omission or reduction of an anticipated aversive outcome can be subjectively pleasurable and relies on the same mesolimbic circuit involved in reward processing (Leknes et al. 2011). Anhedonia may therefore blunt hedonic responses to either the presence of rewards and/or the absence of threats, both of which would be expected to perpetuate future avoidance behavior and prevent new threat-inconsistent learning.
4.3 Lessons from Positive Emotion Science
Positive affect is the subjective emotional experience that occurs in response to anticipating and/or receiving rewards. Although not synonymous with anhedonia, diminished PA represents its primary subjective experience. Research from non-clinical samples demonstrates that positive emotions support many of the processes believed to optimize responses to acute threat and promote new, non-threat learning – the key drivers of successful response to exposure-based treatments for anxiety. Individual differences in positive emotions as well as experimentally inducing positive emotions relative to neutral or negative emotions (e.g., sadness): (1) downregulates the physiological sequelae of threat reactivity, including speeding cardiovascular recovery following exposure to impending threat (Fredrickson et al. 2000); (2) facilitates tolerance of aversive experiences (de Wied and Verbaten 2001); (3) promotes adaptive coping strategies (e.g., positive reappraisal) in stressful situations (Tugade and Fredrickson 2004; and (4) increases awareness and assimilation of new information including widening attentional scope, increasing cognitive flexibility, and promoting openness to new information and patterns of information processing (see review by Fredrickson 2013). PA has also been shown to facilitate mechanisms that support learning and memory, including enhancing encoding, rehearsal, and retrieval (see review by Zbozinek and Craske 2017a). Finally, positive emotions can facilitate the initiation and maintenance of new behavioral intentions. Experimental studies in non-clinical samples reveal that PA experienced during a given activity induces approach motivation and effort for that activity (see review by Van Cappellen et al. 2018). This effect may even translate to supporting targeted longer-term behavior change (e.g., Cohn and Fredrickson 2010). To the extent that anhedonia robs individuals of positive emotional experiences, it would be expected to perpetuate heightened threat reactivity, reduce tolerability of aversive experiences, inhibit assimilation of new, threat-inconsistent information, and interfere with enduring behavior change directed toward threat-opposing actions.
4.4 Anhedonia and Threat Reactivity in Analogue and Clinical Samples
Studies using laboratory fear conditioning and extinction paradigms in healthy samples – an experimental analogue of exposure therapy – suggest positive emotions may inhibit the return of fear following extinction training (Zbozinek and Craske 2017b; Zbozinek et al. 2015). In a cross-sectional study, higher PA (but not negative affect) before and after extinction was associated with less return of fear during reacquisition as measured by skin conductance arousal and fear expectancy (Zbozinek and Craske 2017b). Experimental evidence shows positive mood induction prior to extinction training can decrease the subsequent negative valence of conditioned aversive stimuli and lessen the return of fear during reinstatement 1 week later (Zbozinek et al. 2015). A cross-sectional study of young adults found anhedonia (but not general distress or fears) was associated with increased activity in threat-related neural circuitry (e.g., amygdala, anterior insula) in response to an extinguished threat stimulus (Young et al. 2021). Those findings suggest a persistence of inflated threat reactivity when danger is no longer present – converging with prior studies linking positive emotions and extinction learning in healthy subjects. In a sample of adults with SAD and those without (Taylor et al. 2020b), higher PA significantly predicted lower anticipatory anxiety and less anxiety-related behavior (markers of diminished threat reactivity) in response to a social stressor, even beyond level of negative affect. In summary, emerging evidence from fear conditioning paradigms and anxiety disorder samples supports an association between anhedonia (including diminished PA) and both inflated threat reactivity and impaired non-threat learning. Although these studies cannot speak to the mechanism underlying the anhedonia-threat reactivity link (e.g., cognitive flexibility, openness to new information, fear tolerance), they suggest anhedonia may perpetuate anxiety and impede extinction learning.
5 Treatment Implications
5.1 Anhedonia as a Predictor of Treatment Response
To the extent that anhedonia perpetuates avoidance in the face of rewards, exaggerates threat reactivity, inhibits threat-inconsistent behavior change, and/or interferes new learning, it would be expected to predict response to contemporary exposure-based treatments for anxiety. Several studies support this prediction. In a sample of patients with PD or GAD receiving exposure-based cognitive behavioral therapy (CBT), higher pre-treatment levels of trait positive emotionality predicted superior treatment response (i.e., greater reduction in anxiety symptoms and fewer symptoms post-treatment), even when accounting for baseline depression, neuroticism, or disorder-specific symptom severity (Taylor et al. 2017a). Responder status was greater in participants who scored above the normative sample mean on positive emotionality vs. those who scored below (71% vs. 40%). Similarly, higher trait levels of self-reported reward responsiveness in youths (ages 7–17) completing CBT for anxiety predicted lower post-treatment anxiety and depression symptoms, improved functioning, and responder status (Norris et al. 2021). Younger (but not older) youth with higher reward sensitivity completed more exposure exercises, suggesting a greater willingness to confront threat-related contexts as part of treatment. One study, however, did not find evidence that baseline levels of PA predicted response to CBT or acceptance and commitment therapy for SAD (Sewart et al. 2019).
Initial evidence suggests neural markers of reward processing predict exposure therapy success. In a sample of youths (ages 9–14) receiving CBT for an anxiety disorder, treatment responders displayed greater pre-treatment striatal activation (encompassing the bilateral subgenual anterior cingulate cortex extending into the nucleus accumbens) to monetary rewards vs. losses relative to non-responders (Sequeira et al. 2021). In a sample of adults diagnosed with spider phobia, superior response to exposure therapy was predicted by higher neural activation in the ventrolateral prefrontal cortex during reward anticipation – a region involved in attentional allocation toward reward cues and goal-directed behavior that is positively associated with reward sensitivity (Papalini et al. 2019). Yet, hypothesized activation differences in mesolimbic brain regions involved in reward anticipation and outcome processing were not predictive of outcome. In contrast to the hypothesis that anhedonia predicts worse treatment outcomes, one study in adults receiving CBT for anxiety revealed that better treatment response was predicted by blunted reward responsiveness as measured using the reward positivity (RewP) event-related potential component (Burkhouse et al. 2016). Some evidence therefore suggests that anhedonia may interfere with treatment response, yet other studies did not find it to be predictive of response or to predict better response. Given the heterogeneity of samples, assessments, and treatment approaches, more work is needed to reconcile those outcomes. It also suggests that personalized approaches to treatment are likely needed based on an idiographic understanding of anhedonia across its different component processes.
Studies examining anhedonia as a predictor of pharmacotherapy response for anxiety are sparse and were conducted in combined anxiety and depressive disorder samples – often collapsing outcomes across psychosocial and pharmacological interventions. Results are mixed and generally consistent with findings observed for prediction of exposure-based psychotherapy outcomes; for example, higher pre-treatment positive affect predicted superior response in adolescents receiving CBT, selective serotonin reuptake inhibitors (SSRIs), or their combination (Forbes et al. 2012), whereas reduced reward responsiveness (RewP) in adults predicted a greater reduction in depressive (but not anxiety) symptoms following SSRIs (Burkhouse et al. 2018). It remains to be established, however, whether anhedonia predicts recovery to pharmacotherapy for anxiety disorders specifically.
5.2 Anhedonia as a Treatment Target to Improve Outcomes for Anxiety
Extant literature suggests directly targeting anhedonia may improve response to traditional exposure-based therapies for anxiety. Emerging behavioral treatments focused on transdiagnostic deficits in PA and reward processing across the anxiety and mood disorders have shown promise in improving positive emotions to levels beyond that which is typically achieved with established negative valence treatments (Craske et al. 2019; Taylor et al. 2017b) as well as strengthening functional connectivity in reward processing brain regions (Kryza-Lacombe et al. 2021; see chapter “Psychological Treatments for Anhedonia” of this Book for a review of anhedonia-targeted interventions). These interventions use cognitive and behavioral strategies to increase exposure and responsiveness to rewarding experiences, for example, attending to positive aspects of events (including events perceived to be neutral or negative), savoring, gratitude, and engaging in kind or generous acts. Pharmacotherapies that engage neural systems believed to underlie anhedonia (e.g., enhancing dopamine signaling) have also shown promise in improving neural responsiveness to reward (in depression; Admon et al. 2017) and enhancing fear extinction learning (Esser et al. 2021). It remains to be established, however, whether and how these psychosocial or pharmacological treatments improve response to exposure-based therapies for anxiety disorders specifically. It is also unknown which reward processing components are most critical to target in facilitating treatment response, and which specific treatment activities are most efficacious in targeting those deficits. For example, some strategies may enhance reward outcome expectancies (e.g., visualizing one’s best possible future; Taylor et al. 2017b, or episodic future thinking; Hallford et al. 2020), whereas others may potentiate reward responsiveness and valuation (e.g., reminiscing about positive memories; Speer et al. 2014). Determining the optimal timing and dose of anhedonia-targeted interventions in the context of anxiety recovery will also be important (e.g., directly within the context of exposure exercises vs. sequencing treatments). Aside from facilitating anxiety reduction in therapy, targeting anhedonia may improve other outcomes governed by the positive valence system (e.g., social functioning; Taylor et al. 2020a; psychological well-being; Das et al. 2020) that show modest response to first-line approaches (Hofmann et al. 2014).
6 Concluding Remarks
Once considered to be a symptom that distinguished the anxiety disorders from depression, anhedonia is now recognized as a prominent feature of many individuals meeting diagnostic criteria for a principal anxiety disorder. Evidence across neural, behavioral, and self-report units of analysis points to deficits in multiple domains of reward processing, including valuation, responsiveness, and learning. This literature, however, is relatively nascent. Some reward processing domains (e.g., effort) have not been examined in anxiety disorder samples, and few attempts have been made to untangle the relative influence of anhedonia vs. anxious arousal/threat sensitivity to observed reward processing deficits. It remains an open question, therefore, to what extent such deficits are the result of low reward sensitivity, heightened threat sensitivity, or both. This issue is especially relevant for anxiety disorders wherein reward processing in real life often occurs against the backdrop of heightened sensitivity to aversive outcomes. Given heterogeneity within and across the anxiety disorders, it is likely that anhedonia varies considerably across individuals and may characterize a meaningful subtype within the overarching class of anxiety disorders (e.g., Tung and Brown 2020). Although this chapter focused on anhedonia as a maintenance factor in anxiety, it is also possible that anhedonia presages anxiety onset – a question ripe for empirical inquiry. To the extent that it is present, anhedonia may prevent anxious individuals from achieving optimal outcomes through several mechanisms, including perpetuating costly avoidance, elevating threat reactivity, diminishing tolerance of aversive experiences, and impairing new learning. Directly targeting anhedonia in anxiety disorder treatment may therefore boost response to first-line exposure-based treatments. Exactly when and how best to do so remains an important unanswered question. We hope this chapter will encourage researchers to address this and other related questions.
References
Admon R, Kaiser RH, Dillon DG et al (2017) Dopaminergic enhancement of striatal response to reward in major depression. Am J Psychiatry 174(4):378–386. https://doi.org/10.1176/appi.ajp.2016.16010111
Alden LE, Taylor CT, Mellings TM et al (2008) Social anxiety and the interpretation of positive social events. J Anxiety Disord 22(4):577–590. https://doi.org/10.1016/j.janxdis.2007.05.007
Batterham PJ, Griffiths KM, Barney LJ et al (2013) Predictors of generalized anxiety disorder stigma. Psychiatry Res 206(2–3):282–286. https://doi.org/10.1016/j.psychres.2012.11.018
Belsky J (2013) Differential susceptibility to environmental influences. Int J Child Care Educ Policy 7(2):15–31. https://doi.org/10.1007/2288-6729-7-2-15
Blair KS, Otero M, Teng C et al (2017) Reduced optimism and a heightened neural response to everyday worries are specific to generalized anxiety disorder, and not seen in social anxiety. Psychol Med 47(10):1806–1815. https://doi.org/10.1017/S0033291717000265
Bondy E, Baranger DAA, Balbona J et al (2021) Neuroticism and reward-related ventral striatum activity: probing vulnerability to stress-related depression. J Abnorm Psychol 130(3):223–235. https://doi.org/10.1037/abn0000618
Brown TA, Chorpita BF, Barlow DH (1998) Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol 107(2):179–192. https://doi.org/10.1037//0021-843x.107.2.179
Burkhouse KL, Kujawa A, Kennedy AE et al (2016) Neural reactivity to reward as a predictor of cognitive behavioral therapy response in anxiety and depression. Depress Anxiety 33(4):281–288. https://doi.org/10.1002/da.22482
Burkhouse KL, Gorka SM, Klumpp H et al (2018) Neural responsiveness to reward as an index of depressive symptom change following cognitive-behavioral therapy and selective serotonin reuptake inhibitor treatment. J Clin Psychiatry 79(4):17m11836. https://doi.org/10.4088/JCP.17m11836
Clark LA, Watson D (1991) Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol 100(3):316–336. https://doi.org/10.1037//0021-843x.100.3.316
Cohn MA, Fredrickson BL (2010) In search of durable positive psychology interventions: predictors and consequences of long-term positive behavior change. J Posit Psychol 5(5):355–366. https://doi.org/10.1080/17439760.2010.508883
Craske MG, Kircanski K, Zelikowsky M et al (2008) Optimizing inhibitory learning during exposure therapy. Behav Res Ther 46(1):5–27. https://doi.org/10.1016/j.brat.2007.10.003
Craske MG, Meuret AE, Ritz T et al (2019) Positive affect treatment for depression and anxiety: a randomized clinical trial for a core feature of anhedonia. J Consult Clin Psychol 87(5):457–471. https://doi.org/10.1037/ccp0000396
Cremers HR, Veer IM, Spinhoven P et al (2015) Neural sensitivity to social reward and punishment anticipation in social anxiety disorder. Front Behav Neurosci 8:439. https://doi.org/10.3389/fnbeh.2014.00439
Das A, Clerkin EM, Tolin DF et al (2020) Moving beyond the negative: contributions of positive and negative affect on quality of life in patients with generalized anxiety disorder. J Nerv Ment Dis 208(11):843–847. https://doi.org/10.1097/NMD.0000000000001228
de Wied M, Verbaten MN (2001) Affective pictures processing, attention, and pain tolerance. Pain 90(1–2):163–172. https://doi.org/10.1016/s0304-3959(00)00400-0
Der-Avakian A, Markou A (2012) The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35(1):68–77. https://doi.org/10.1016/j.tins.2011.11.005
DeVido J, Jones M, Geraci M et al (2009) Stimulus-reinforcement-based decision making and anxiety: impairment in generalized anxiety disorder (GAD) but not in generalized social phobia (GSP). Psychol Med 39:1153–1161. https://doi.org/10.1017/S003329170800487X
Doorley JD, Goodman FR, Disabato DJ et al (2021) The momentary benefits of positive events for individuals with elevated social anxiety. Emotion 21(3):595–606. https://doi.org/10.1037/emo0000725
Eisner LR, Johnson SL, Carver CS (2009) Positive affect regulation in anxiety disorders. J Anxiety Disord 23(5):645–649. https://doi.org/10.1016/j.janxdis.2009.02.001
Engelmann JB, Meyer F, Fehr E et al (2015) Anticipatory anxiety disrupts neural valuation during risky choice. J Neurosci 35(7):3085–3099. https://doi.org/10.1523/JNEUROSCI.2880-14.2015
Esser R, Korn CW, Ganzer F et al (2021) L-DOPA modulates activity in the vmPFC, nucleus accumbens, and VTA during threat extinction learning in humans. elife 10:e65280. https://doi.org/10.7554/eLife.65280
Forbes EE, Stepp SD, Dahl RE et al (2012) Real-world affect and social context as predictors of treatment response in child and adolescent depression and anxiety: an ecological momentary assessment study. J Child Adolesc Psychopharmacol 22(1):37–47. https://doi.org/10.1089/cap.2011.0085
Fredrickson BL (2013) Positive emotions broaden and build. In: Advances in experimental social psychology, vol 47. Academic Press, pp 1–53
Fredrickson BL, Mancuso RA, Branigan C et al (2000) The undoing effect of positive emotions. Motiv Emot 24(4):237–258. https://doi.org/10.1023/a:1010796329158
Gable SL, Impett EA (2012) Approach and avoidance motives and close relationships. Soc Personal Psychol Compass 6(1):95–108. https://doi.org/10.1111/j.1751-9004.2011.00405.x
Gilboa-Schechtman E, Franklin ME, Foa EB (2000) Anticipated reactions to social events: differences among individuals with generalized social phobia, obsessive compulsive disorder, and nonanxious controls. Cognit Ther Res 24(6):731–746. https://doi.org/10.1023/A:1005595513315
Glazier BL, Alden LE (2019) Social anxiety disorder and memory for positive feedback. J Abnorm Psychol 128(3):228–233. https://doi.org/10.1037/abn0000407
Guyer AE, Choate VR, Detloff A et al (2012) Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry 169:205–212. https://doi.org/10.1176/appi.ajp.2011.11010006
Hallford DJ, Farrell H, Lynch E (2020) Increasing anticipated and anticipatory pleasure through episodic thinking. Emotion. https://doi.org/10.1037/emo0000765
Held-Poschardt D, Sterzer P, Schlagenhauf F et al (2018) Reward and loss anticipation in panic disorder: an fMRI study. Psychiatry Res Neuroimaging 271:111–117. https://doi.org/10.1016/j.pscychresns.2017.11.005
Hofmann SG, Wu JQ, Boettcher H (2014) Effect of cognitive-behavioral therapy for anxiety disorders on quality of life: a meta-analysis. J Consult Clin Psychol 82(3):375–391. https://doi.org/10.1037/a0035491
Jenks CW, Lawyer SR (2015) Using delay discounting to understand impulsive choice in socially anxious individuals: failure to replicate. J Behav Ther Exp Psychiatry 46:198–201. https://doi.org/10.1016/j.jbtep.2014.10.010
Kashdan TB (2007) Social anxiety spectrum and diminished positive experiences: theoretical synthesis and meta-analysis. Clin Psychol Rev 27(3):348–365. https://doi.org/10.1016/j.cpr.2006.12.003
Kashdan TB, Breen WE (2008) Social anxiety and positive emotions: a prospective examination of a self-regulatory model with tendencies to suppress or express emotions as a moderating variable. Behav Ther 39(1):1–12. https://doi.org/10.1016/j.beth.2007.02.003
Kashdan TB, Steger MF (2006) Expanding the topography of social anxiety. An experience-sampling assessment of positive emotions, positive events, and emotion suppression. Psychol Sci 17(2):120–128. https://doi.org/10.1111/j.1467-9280.2006.01674.x
Katz BA, Matanky K, Aviram G et al (2020) Reinforcement sensitivity, depression and anxiety: a meta-analysis and meta-analytic structural equation model. Clin Psychol Rev 77:101842. https://doi.org/10.1016/j.cpr.2020.101842
Kessel EM, Kujawa A, Hajcak Proudfit G et al (2015) Neural reactivity to monetary rewards and losses differentiates social from generalized anxiety in children. J Child Psychol Psychiatry 56(7):792–800. https://doi.org/10.1111/jcpp.12355
Kryza-Lacombe M, Pearson N, Lyubomirsky S et al (2021) Changes in neural reward processing following amplification of positivity treatment for depression and anxiety: preliminary findings from a randomized waitlist controlled trial. Behav Res Ther 15(142):103860. https://doi.org/10.1016/j.brat.2021.103860
Kujawa A, Klein DN, Pegg S et al (2020) Developmental trajectories to reduced activation of positive valence systems: a review of biological and environmental contributions. Dev Cogn Neurosci 43:100791. https://doi.org/10.1016/j.dcn.2020.100791
Leknes S, Lee M, Berna C et al (2011) Relief as a reward: hedonic and neural responses to safety from pain. PLoS One 6(4):e17870. https://doi.org/10.1371/journal.pone.0017870
Loerinc AG, Meuret AE, Twohig MP et al (2015) Response rates for CBT for anxiety disorders: need for standardized criteria. Clin Psychol Rev 42:72–82. https://doi.org/10.1016/j.cpr.2015.08.004
Luhmann CC, Ishida K, Hajcak G (2011) Intolerance of uncertainty and decisions about delayed, probabilistic rewards. Behav Ther 42(3):378–386. https://doi.org/10.1016/j.beth.2010.09.002
Miloyan B, Pachana NA, Suddendorf T (2014) The future is here: a review of foresight systems in anxiety and depression. Cogn Emot 28(5):795–810. https://doi.org/10.1080/02699931.2013.863179
Morris BH, Rottenberg J (2015) Heightened reward learning under stress in generalized anxiety disorder: a predictor of depression resistance? J Abnorm Psychol 124(1):115–127. https://doi.org/10.1037/a0036934
Naragon-Gainey K, Watson D, Markon KE (2009) Differential relations of depression and social anxiety symptoms to the facets of extraversion/positive emotionality. J Abnorm Psychol 118(2):299–310. https://doi.org/10.1037/a0015637
Norris LA, Rabner JC, Mennies RJ et al (2021) Increased self-reported reward responsiveness predicts better response to cognitive behavioral therapy for youth with anxiety. J Anxiety Disord 80:102402. https://doi.org/10.1016/j.janxdis.2021.102402
Papalini S, Lange I, Bakker J et al (2019) The predictive value of neural reward processing on exposure therapy outcome: results from a randomized controlled trial. Prog Neuro-Psychopharmacol Biol Psychiatry 92:339–346. https://doi.org/10.1016/j.pnpbp.2019.02.002
Pittig A (2019) Incentive-based extinction of safety behaviors: positive outcomes competing with aversive outcomes trigger fear-opposite action to prevent protection from fear extinction. Behav Res Ther 121:103463. https://doi.org/10.1016/j.brat.2019.103463
Pittig A, Wong AHK, Glück VM et al (2020) Avoidance and its bi-directional relationship with conditioned fear: mechanisms, moderators, and clinical implications. Behav Res Ther 126:103550. https://doi.org/10.1016/j.brat.2020.103550
Pittig A, Boschet JM, Glück VM et al (2021) Elevated costly avoidance in anxiety disorders: patients show little downregulation of acquired avoidance in face of competing rewards for approach. Depress Anxiety 38(3):361–371. https://doi.org/10.1002/da.23119
Pizzagalli DA (2014) Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol 10:393–423. https://doi.org/10.1146/annurev-clinpsy-050212-185606
Pizzagalli DA, Jahn AL, O'Shea JP (2005) Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry 57(4):319–327. https://doi.org/10.1016/j.biopsych.2004.11.026
Prenoveau JM, Zinbarg RE, Craske MG et al (2010) Testing a hierarchical model of anxiety and depression in adolescents: a tri-level model. J Anxiety Disord 24(3):334–344. https://doi.org/10.1016/j.janxdis.2010.01.006
Reilly EE, Whitton AE, Pizzagalli DA et al (2020) Diagnostic and dimensional evaluation of implicit reward learning in social anxiety disorder and major depression. Depress Anxiety 37(12):1221–1230. https://doi.org/10.1002/da.23081
Richey JA, Ghane M, Valdespino A et al (2017) Spatiotemporal dissociation of brain activity underlying threat and reward in social anxiety disorder. Soc Cogn Affect Neurosci 12(1):81–94. https://doi.org/10.1093/scan/nsw149
Richey JA, Brewer JA, Sullivan-Toole H et al (2019) Sensitivity shift theory: a developmental model of positive affect and motivational deficits in social anxiety disorder. Clin Psychol Rev 72:101756. https://doi.org/10.1016/j.cpr.2019.101756
Rounds JS, Beck JG, Grant DM (2007) Is the delay discounting paradigm useful in understanding social anxiety? Behav Res Ther 45(4):729–735. https://doi.org/10.1016/j.brat.2006.06.007
Seager I, Mennin DS, Aldao A (2019) Positive emotion in generalized anxiety disorder. In: Gruber J (ed) The Oxford handbook of positive emotion and psychopathology. Oxford University Press, New York, pp 298–311
Sequeira SL, Silk JS, Ladouceur CD et al (2021) Association of neural reward circuitry function with response to psychotherapy in youths with anxiety disorders. Am J Psychiatry 178(4):343–351. https://doi.org/10.1176/aapi.ajp.2020.20010094
Sewart AR, Niles AN, Burklund LJ et al (2019) Examining positive and negative affect as outcomes and moderators of cognitive-behavioral therapy and acceptance and commitment therapy for social anxiety disorder. Behav Ther 50(6):1112–1124. https://doi.org/10.1016/j.beth.2019.07.001
Sheppes G, Scheibe S, Suri G et al (2014) Emotion regulation choice: a conceptual framework and supporting evidence. J Exp Psychol Gen 143(1):163–181. https://doi.org/10.1037/a0030831
Speer ME, Bhanji JP, Delgado MR (2014) Savoring the past: positive memories evoke value representations in the striatum. Neuron 84(4):847–856. https://doi.org/10.1016/j.neuron.2014.09.028
Steinglass JE, Lempert KM, Choo TH et al (2017) Temporal discounting across three psychiatric disorders: anorexia nervosa, obsessive compulsive disorder, and social anxiety disorder. Depress Anxiety 34(5):463–470. https://doi.org/10.1002/da.22586
Taylor CT, Alden LE (2011) To see ourselves as others see us: an experimental integration of the intra and interpersonal consequences of self-protection in social anxiety disorder. J Abnorm Psychol 120(1):129–141. https://doi.org/10.1037/a0022127
Taylor CT, Knapp SE, Bomyea JA et al (2017a) What good are positive emotions for treatment? Trait positive emotionality predicts response to cognitive behavioral therapy for anxiety. Behav Res Ther 93:6–12. https://doi.org/10.1016/j.brat.2017.03.006
Taylor CT, Lyubomirsky S, Stein MB (2017b) Upregulating the positive affect system in anxiety and depression: outcomes of a positive activity intervention. Depress Anxiety 34(3):267–280. https://doi.org/10.1002/da.22593
Taylor CT, Pearlstein SL, Stein MB (2020a) A tale of two systems: testing a positive and negative valence systems framework to understand social disconnection across anxiety and depressive disorders. J Affect Disord 266:207–214. https://doi.org/10.1016/j.jad.2020.01.041
Taylor CT, Tsai TC, Smith TR (2020b) Examining the link between positive affectivity and anxiety reactivity to social stress in individuals with and without social anxiety disorder. J Anxiety Disord 74:102264. https://doi.org/10.1016/j.janxdis.2020.102264
Treadway MT, Bossaller NA, Shelton RC et al (2012) Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol 121(3):553–558. https://doi.org/10.1037/a0028813
Tugade MM, Fredrickson BL (2004) Resilient individuals use positive emotions to bounce back from negative emotional experiences. J Pers Soc Psychol 86(2):320–333. https://doi.org/10.1037/0022-3514.86.2.320
Tung ES, Brown TA (2020) Distinct risk profiles in social anxiety disorder. Clin Psychol Sci 8(3):477–490. https://doi.org/10.1177/2167702620901536
Van Cappellen P, Rice EL, Catalino LI et al (2018) Positive affective processes underlie positive health behaviour change. Psychol Health 33(1):77–97. https://doi.org/10.1080/08870446.2017.1320798
White SF, Geraci M, Lewis E et al (2017) Prediction error representation in individuals with generalized anxiety disorder during passive avoidance. Am J Psychiatry 174(2):110–117. https://doi.org/10.1176/appi.ajp.2016.15111410
Winer ES, Salem T (2016) Reward devaluation: dot-probe meta-analytic evidence of avoidance of positive information in depressed persons. Psychol Bull 142(1):18–78. https://doi.org/10.1037/bul0000022
Winer ES, Bryant J, Bartoszek G et al (2017) Mapping the relationship between anxiety, anhedonia, and depression. J Affect Disord 221:289–296. https://doi.org/10.1016/j.jad.2017.06.006
Young KS, Bookheimer SY, Nusslock R et al (2021) Dysregulation of threat neurociruitry during fear extinction: the role of anhedonia. Neuropsychopharmacology. https://doi.org/10.1038/s41386-021-01003-8
Zbozinek TD, Craske MG (2017a) Positive affect predicts less reacquisition of fear: relevance for long-term outcomes of exposure therapy. Cogn Emot 31(4):712–725. https://doi.org/10.1080/02699931.2016.1142428
Zbozinek TD, Craske MG (2017b) The role of positive affect in enhancing extinction learning and exposure therapy for anxiety disorders. J Exp Psychopathol 8(1):1–52. https://doi.org/10.5127/jep.052615
Zbozinek TD, Holmes EA, Craske MG (2015) The effect of positive mood induction on reducing reinstatement fear: relevance for long term outcomes of exposure therapy. Behav Res Ther 71:65–75. https://doi.org/10.1016/j.brat.2015.05.016
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Dr. Taylor’s effort was partially supported by the National Institute of Mental Health (R33MH113769). Dr. Taylor declares that in the past 3 years he has been a paid consultant for Bionomics, and receives payment for editorial work for UpToDate and the journal, Depression and Anxiety. All other authors report no potential conflicts of interest.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Taylor, C.T., Hoffman, S.N., Khan, A.J. (2022). Anhedonia in Anxiety Disorders. In: Pizzagalli, D.A. (eds) Anhedonia: Preclinical, Translational, and Clinical Integration. Current Topics in Behavioral Neurosciences, vol 58. Springer, Cham. https://doi.org/10.1007/7854_2022_319
Download citation
DOI: https://doi.org/10.1007/7854_2022_319
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-09682-2
Online ISBN: 978-3-031-09683-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)