Abstract
Epigenetic mechanisms have been linked to memory formation under physiological and pathological conditions. Therapeutic strategies that target the epigenetic machinery have been successfully used in preclinical studies investigating cognitive phenotypes linked to neuropsychiatric and neurodegenerative diseases. This chapter will specifically discuss the role of histone modification in the adult brain with a focus on learning and memory processes in the healthy and diseased brain. Data on dynamic changes in histone modification during memory processes as well as the most current knowledge on the corresponding enzymatic machinery in the adult brain will be summarized and discussed in the context of potential therapeutic opportunities to treat brain diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Epigenetic therapies
- Histone methylation
- Learning
- Memory
- Neurodegenerative diseases
- Neuroepigenetics
- Neuropsychiatric diseases
1 Introduction: Neuro-epigenetics and Histone Modifications
The tight control of gene expression programs is essential for cellular function. In turn, deregulated gene expression has been implicated with the pathogenesis of various diseases. In addition to the activity of transcription factors, epigenetic mechanisms are key processes that control gene expression at a systems level (Allis et al. 2007a). The term epigenetics has been originally introduced by Conrad Waddington to describe heritable changes of a phenotype that do not depend on altered DNA sequence (Waddington 1953). In addition to DNA methylation and the action of noncoding RNAs, a central epigenetic process is the modifications of histone proteins. The four core histones (H) build the nucleosome around which 147 bp of DNA is wrapped. The histone tails are subjected to posttranslational modifications such as acetylation, methylation, phosphorylation, ubiquitination, sumoylation, ADP-ribosylation, etc. (Vaquero et al. 2003) which are mediated by the counteracting activities of enzymes that add or remove histone modifications and are thus called “writers” and “erasers.” For example, histone acetylation is mediated by histone acetyltransferases (HAT, writers) and histone deacetylases (HDAC, erasers). The activity of such enzymes is believed to give rise to a combinatorial pattern of chromatin modifications, the so-called histone code (Strahl and Allis 2000). Such histone modifications are recognized by proteins that subsequently affect cellular processes such as gene expression and are referred to as chromatin “readers” (Fischer 2014a). The field of neuro-epigenetics investigates the abovementioned epigenetic processes in the context of neuronal plasticity, memory function, and brain diseases (Fischer 2014a; Day and Sweatt 2011; Jakovcevski et al. 2013b).
Various cellular mechanisms have been linked to memory function, and tightly controlled dynamic changes in gene expression are one requirement to enable the consolidation of long-term memories (Flexner et al. 1962, 1963; Davis 1984; Igaz et al. 2002; Frey and Morris 1998; Moncada et al. 2011; Fischer 2014a). In addition, deregulated gene expression is seen in brain diseases linked to memory impairment (Lu et al. 2004; Liang et al. 2010; Ginsberg et al. 2010; Twine et al. 2011; Blalock et al. 2011; Kim et al. 2012a; Arefin et al. 2012; Caldeira et al. 2013; Mills et al. 2013). Understanding the mechanisms that orchestrate gene expression programs in brain cells is thus of utmost importance, and epigenetic processes have emerged as key players that enable synaptic plasticity and memory formation.
A number of excellent review articles have discussed the role of epigenetic processes such as DNA methylation and noncoding RNAs in synaptic plasticity, memory function, and brain diseases (Day and Sweatt 2010; Fiore et al. 2011; Fischer 2014a; Jakovcevski and Akbarian 2012; Campbell and Wood 2019). This chapter will focus on recent findings on the role of histone modifications in memory formation and cognitive diseases.
1.1 Histone Modifications and Memory Consolidation in Health and Disease
First evidence that histone acetylation might play a role in memory consolidation stems already from 1979. Using C14-labeled acetate, Schmitt and Matthies could show that acetylation of histones is altered when rats are subjected to a learning paradigm that leads to the formation of long-term memories (Schmitt and Matthies 1979). Only at the beginning of this century, such studies were followed up, and a number of labs could demonstrate that memory formation in rodents is correlated to increased activity of HATs (Swank and Sweatt 2001) and changes in histone acetylation that were measured by semiquantitative immunoblot analysis using antibodies specific to acetylated lysine residues of histones (Levenson et al. 2004; Fischer et al. 2007; Bousiges et al. 2010). These methods are able to detect changes in bulk levels of histone acetylation but do not allow for any deeper insight to genes regulated by changes in histone acetylation. Such issues were circumvented when researchers started to perform chromatin immunoprecipitation (ChIP) followed by either qPCR or later next-generation sequencing approaches (ChIP-seq) to study histone acetylation at the single gene or genome-wide level (Peleg et al. 2010). An important consideration in this context is the complexity of cell types in the brain. Technological advances now also allow the analysis of histone modifications via ChIP-seq at the single-cell level, but these methods have not been applied yet to the neurosciences (Grosselin et al. 2019). Most researchers nowadays perform cell type-specific analysis of histone acetylation via ChIP-seq using FACS-sorted nuclei that distinguish at least between neuronal and non-neuronal cells (Shulha et al. 2012; Benito et al. 2015; Halder et al. 2016), which is of utmost importance for the analysis of brain diseases characterized by neuronal cell death in combination with inflammatory processes.
Thus, there is now substantial evidence confirming the initial observation by Schmitt and Matthies that changes in histone acetylation but also other histone modifications are observed in response to learning stimuli or during the pathogenesis of brain diseases. Moreover, there is now convincing evidence from the analysis of mutant mice or other model systems that these epigenetic processes play a key role in cognition. In the following we will discuss these data in detail.
1.2 Histone Acetylation
As discussed above, early findings reported altered histone acetylation in response to memory training, and in most cases, these changes were linked to active gene expression, hence an increase of histone marks linked to active gene expression such as histone acetylation at various lysine residues of H3 and H4 (Levenson et al. 2004; Alarcon et al. 2004; Fischer et al. 2007; Miller et al. 2008; Federman et al. 2009; Peleg et al. 2010; Dagnas and Mons 2013; Fischer 2014a). These data led to the hypothesis that inhibition of HDAC proteins might increase memory formation, which was confirmed in multiple studies (Alarcon et al. 2004; Levenson et al. 2004; Wood et al. 2005; Fischer et al. 2007; Fischer 2014b). As a consequence HDAC inhibitors were tested in models for cognitive diseases such as Alzheimer’s disease but also other cognitive diseases, and indeed administration of HDAC inhibitors was found to ameliorate memory impairments and cognitive phenotypes in numerous studies (Fischer 2014a, b). Some of the HDAC inhibitors such as vorinostat are already used in patients for other indications, and it should be mentioned that oral administration of vorinostat could ameliorate memory impairment and deregulated transcriptome plasticity in various mouse models for age-associated memory impairment and Alzheimer’s disease (Kilgore et al. 2010; Benito et al. 2015). Thus, this drug is currently tested in Alzheimer’s disease patients (https://clinicaltrials.gov/ct2/show/NCT03056495).
Histone acetylation is generally linked to active gene expression, and thus initially not much attention was paid to potential difference of, for example, learning-induced increases in H3K9ac, H4K14ac, or the H4 acetylation sites H4K5, H4K8, H4K12, and H4K12, and often histone acetylation was assayed using pan-antibodies that would detect all of the abovementioned H3 and H4 acetylation sites. While this is still a valid approach, there is also evidence that H3 and H4 acetylation sites might serve specific cellular functions. For example, the onset of cognitive decline during aging was linked to deregulated acetylation of H4K12 (H4K12ac). While memory training induced a transient increase in various histone acetylation marks in the hippocampus of young and aged mice, H4K12ac did not respond anymore to such a stimulus in old animals. When ChIP-Seq was combined with transcriptome analysis, the data revealed that H4K12ac is critical for the initiation of a learning-induced gene expression program via a mechanism that involved transcriptional elongation (Peleg et al. 2010). These data are interesting since the chromatin reader proteins BRD2 and BRD4, which belong to the BET (Bromodomain Extraterminal) subfamily of chromatin readers, were found to specifically recognize H4K12ac. Similar to HDAC inhibitors, the BRD2/BRD4 inhibitor JQ1 was found to improve memory function in wild-type mice and in a mouse model for Alzheimer’s disease (Benito et al. 2017).
All of these data are summarized in a number of excellent review articles (Fischer 2014a, b; Deussing and Jakovcevski 2013) and inspire the interested to better understand the enzymatic machinery that controls histone acetylation.
The mammalian genome encodes for at least 18 HATs that are subdivided into the GNAT (Gcn5 N-acetyltransferases) family, the MYST (MOZ, Ybf2/Sas3, Sas2, TIP60) family, the p300/CBP family, the SCR family nuclear receptor coactivators, and the several other HATs that cannot be grouped into a certain family (Lee and Workman 2007; Allis et al. 2007a). Most of these proteins are expressed in the mouse and human brain (Table 1). The best-studied HATs in the context of mammalian brain function belong to the p300/CBP family. Multiple studies demonstrated a role for CBP/KAT3A in memory consolidation (Alarcon et al. 2004; Korzus et al. 2004; Wood et al. 2005, 2006; Chen et al. 2010; Barrett et al. 2011). It should be mentioned at this point that memory function is routinely analyzed in rodents via a number of well-established memory tests that assay different cognitive domains such as spatial reference memory or associative fear learning. Loss of CBP/KAT3A does not affect all types of memory, and specifically spatial reference learning in the hippocampus-dependent Morris water maze is only medley affected in mutant mice (Josselyn 2005). In line with these observations, experiments in which CBP/KAT3A agonists were administered to mice during spatial reference learning did not improve the consolidation of memories that was measured 48 h after training but improved the consolidation of remote memories when analyzed up to 16 days after the training (Chatterjee et al. 2013). The role of CBP/KAT3A in neuronal plasticity has been linked to its potential to regulate the expression of specific genes via the modulation of histone acetylation. For example, its effect on memory consolidation was linked its ability to induce the expression of nuclear receptor 4a1 (Nr4a1) (Vecsey et al. 2007; McNulty et al. 2012). However, detailed genome-wide analysis of transcriptional networks associated with CBP function in the adult brain is sparse, and gene array approaches thus far linked CBP function to calcium signaling, transcription and synaptic plasticity (Chen et al. 2010), and deregulated gene expression in response to environmental enrichment training (Lopez-Atalaya et al. 2011). Evidence for a role of P300/KAT3B in memory function stems for work showing that genetic reduction of P300/KAT3B activity in the mouse brain impairs memory consolidation (Viosca et al. 2010; Oliveira et al. 2007, 2011; Lipinski et al. 2019). Moreover, CBP/KAT3A and P300/KAT3B are genetically linked to Rubinstein-Taybi syndrome, an autosomal dominantly inherited form of mental retardation that affects 1 in 125,000 individuals (Petrij et al. 1995; Oike et al. 1999). It is therefore important to note that administration of an HDAC inhibitor was able to ameliorate the phenotypes in KAT3A/CBP mutant mice (Alarcon et al. 2004). KAT3A/CBP has also been linked to Alzheimer’s disease. For example, it was found that phospho-CBP levels are downregulated in the 3xAD mice and that viral-mediated overexpression of CBP was able to ameliorate deficits in phospho-CBP levels and memory function (Caccamo et al. 2010, CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer’s disease). Moreover, deletion of presenilins 1 and 2 in mice affected CBP-mediated gene expression (Saura et al. 2004). There is also evidence PCAF/KAT2B is required for memory formation (Maurice et al. 2008; Duclot et al. 2010; Wei et al. 2012; Mitchnick et al. 2016). PCAF has also been linked to AD pathogenesis. One study found that mice which lack PCAF (KAT2B) are resistant to the detrimental effects of amyloid beta peptides that are directly injected into the lateral ventricles of mice (Duclot et al. 2010). This data is somewhat in conflict with the fact that the same PCAF mutant mice were found to show impaired memory function (Maurice et al. 2008), and it remains to be seen if reducing PCAF levels would have similar effects in more established models for amyloid pathology. Nevertheless, such findings remind us that the role of histone and protein acetylation in AD is likely very complex and should not be exclusively reduced to the hypothesis that inhibition of HDACs and activation of HATs would ameliorate cognitive impairment.
Studies from our laboratory used an unbiased RNA-sequencing approach to compare the expression of the 18 HATs in the hippocampal CA1 region of adult mice, a region that is intimately linked to memory formation (Stilling et al. 2014) (Table 1). These data revealed that GCN5/KAT2A and TIP60/KAT5 are the highest expressed HATs in this brain region, at least at the mRNA level. Consequently, deletion of GCN5/KAT2A from excitatory forebrain neurons of the adult brain leads to severely impaired spatial reference and associative memory consolidation, as well as impaired hippocampal long-term potentiation (LTP), that is considered to be a molecular correlate of memory formation (Stilling et al. 2014). Loss of GCN5/KAT2A also had a profound impact on hippocampal gene expression that was linked to the acetylation of histone 4 at lysine 12 and histone 3 at lysine 14 and 18 as well as the acetylation of the p65, a co-regulation of the transcription factor NFkappaB (Stilling et al. 2014). Similarly, unpublished data from our laboratory suggest that deletion of the other highly expressed HAT in the hippocampus, namely, KAT5/TIP60, leads to severe memory impairment and a massive deregulation of neuronal gene expression when deleted from excitatory forebrain neurons. In line with these data, pharmacological inhibition of KAT5A/TIP60 impaired remote memory retrieval, a finding that was linked to the role of KAT5A/TIP60 in the acetylation of the histone variant H2A.Z (Narkaj et al. 2018). KAT5A7TIP60 has also been implicated with Alzheimer’s disease. To this end it was shown that increased TIP60 activity can rescue amyloid beta-induced neurotoxicity (Pirooznia et al. 2012) and axonal transport deficits in a Drosophila model (Johnson et al. 2013). In line with the data that KMTs do not exclusively act on histones, loss of TIP60 activity in drosophila causes reduced microtubule acetylation (Sarthi and Elefant 2011). Nevertheless, the protective effect of increasing TIP60 activity is – at least in part – also linked to the expression of genes that regulate apoptotic cell death (Pirooznia et al. 2012) and axonal transport (Johnson et al. 2013). A potential role for TIP60 in Alzheimer’s disease has been furthermore suggested even earlier since it was found that TIP60 regulates gene expression in a complex with the amyloid precursor protein intracellular domain (AICD) (Cao et al. 2004; Müller et al. 2013). Moreover TIP60 plays a critical role in orchestrating the cellular response to DNA damage (Kaidi and Jackson 2013) which is emerging as another critical player in AD pathogenesis (Mao and Reddy 2011; Herrup et al. 2013).
Comparatively little is known about the role of the other HATs in the adult brain and memory function. HAT1/KAT1 expression increases in mice treated with antidepressants, but the functional consequences are not understood (Kocki et al. 2018). These data are however in line with the observation that neuronal histone modifications are altered in depression (Sun et al. 2013). Myst4/KAT6B has (Merson et al. 2006) been linked to adult neurogenesis, a process that has been linked to cognitive function (Ernst and Frisén 2015), while MYST1/KAT8 has been linked to Alzheimer’s disease by a recent GWAS study (Marioni et al. 2018). RNAi-mediated knockdown of NCOA1/KAT13A in the hippocampal CA1 region of mice impairs spatial reference memory and LTP which was accompanied by reduced synapse density (Bian et al. 2018). The other HATs have not been linked to memory function yet, although mutations in TAF1/KAT4 are linked to X-linked dystonia-parkinsonism (Bragg et al. 2019). It is important to mention that HATs act as part of larger protein complexes and proteins with HAT activity are also part of the RNA-pol II complex (Lee and Workman 2007). This is of particular interest since acetyl-CoA is the main donor of acetyl groups essential for histone acetylation. It was recently shown that acetyl-CoA synthase 2 (ACSS2) is the main enzyme that provides acetyl-CoA to histone acetylation in the adult brain and that reduced ACSS2 levels lead to memory impairment (Mews et al. 2017). This is in line with previous data suggesting that age-associated memory decline is linked to reduced hippocampal citrate levels (Peleg et al. 2010), a main donor for acetyl-CoA synthesis.
The activity of HATs is counteracted by the histone deacetylases (HDAC) that are grouped into the zinc-dependent HDACs and the non-zinc-dependent sirtuins. A number of excellent reviews discuss the role of sirtuins and HDACs in various cellular systems (Haigis and Sinclair 2010; Donmez 2013; Fischer et al. 2010; Fischer 2014b). Here we will specifically focus on the role of the zinc-dependent HDACs in memory function. In line with the data that have generally shown that loss of HAT activity in linked to memory impairment, it was found that pharmacological inhibition of HDACs enhances the consolidation of memories in rodents (Levenson et al. 2004; Fischer et al. 2007; Stefanko et al. 2009; Federman et al. 2009; Benito et al. 2015; Agís-Balboa et al. 2017).
The 11 mammalian zinc-dependent HDACs belong to an ancient protein family and require a Zn2+ ion as cofactor (Gregoretti et al. 2004; de Ruijter et al. 2003). Under naïve conditions, all HDAC genes are expressed within the adult rodent brain (Broide et al. 2007). Mice that lack HDAC1 or overexpress it in all neurons from early developmental stages show no impairments in contextual fear learning or spatial memory formation (Guan et al. 2009), suggesting that HDAC1 has no obvious role at least in the abovementioned types of memory function. HDAC1 was however found to be essential for fear extinction learning, a process that is important for the treatment of neuropsychiatric diseases associated with aversive behaviors as they occur in post-traumatic stress disorder (Bahari-Javan et al. 2012). Here, the induction of immediate early genes was suppressed via HDAC1-mediated deacetylation of H3K9 and subsequent H3K9 tri-methylation (Bahari-Javan et al. 2012). Although HDAC1 and HDAC2 are close homologues that derived from gene duplications, their roles in memory function differ. Overexpression of HDAC2 in neurons impaired contextual fear learning and spatial memory formation in mice, while deletion of HDAC2 in neurons from early developmental stages improved memory function and synaptic plasticity (Guan et al. 2009). Notably, enhanced learning behavior in HDAC2 knockout mice correlated with increased hippocampal H4K12 acetylation, while H3K14 acetylation was not affected. Although the authors measured bulk changes, these data are interesting taking into account that genome-wide analysis of chromatin in the aging hippocampus suggested a key role for H4K12 acetylation in age-associated memory impairment (Peleg et al. 2010). Later studies suggest that the memory enhancing effect of HDAC2 reduction is linked to the activity of the transcription factor SP1 (Yamakawa et al. 2017). On the structural level, loss of HDAC2 increased the number of synapses (Guan et al. 2009) which is in line with a role of HDAC2 – but also HDAC1 – in synapse formation during development (Akhtar et al. 2009). Loss of HDAC2 was also found to improve fear extinction learning (Morris et al. 2013) which is opposite to the function of HDAC1 (Bahari-Javan et al. 2012). HDAC2 was found to bind promoter regions of genes linked to memory formation, but the precise mechanisms by which HDAC2 acts as a memory repressor are not well understood (Guan et al. 2009). One study showed that HDAC2 is essential for the survival of adult born neurons in the dentate gyrus (Jawerka et al. 2010). Since adult neurogenesis has been linked to memory function, it is clear that the role of HDAC2 in the adult brain awaits a more detailed analysis. An important next step would be to understand the role of HDAC1 and 2 in a cell type-specific manner. A recent study suggests that HDAC2 is produced in many neuronal cell types and in oligodendrocytes but not in astro- or microglia (Yao et al. 2013), a view that is however not undisputed since recent data point to an important role of HDAC1 and 2 in glia cells (Wendeln et al. 2018). HDAC1 has been also linked to neuropsychiatric diseases (Jakovcevski et al. 2013a). For example, a number of studies have demonstrated a role for HDAC1 in schizophrenia. Thus, HDAC1 was upregulated in postmortem brain tissue of schizophrenia patients (Dong et al. 2007; Sharma et al. 2008; Bahari-Javan et al. 2017). In a recent study, our laboratory was able to show that HDAC1 is increased specifically in schizophrenia patients that encountered early life stress (Bahari-Javan et al. 2017). Interestingly, this upregulation of HDAC1 was linked to DNA methylation-dependent regulation of the HPA axis and was also prominent in corresponding human blood samples (Bahari-Javan et al. 2017), suggesting the possibility that HDAC1 might serve as a biomarker for stratified therapy of patients with an unfavorable course of the disease (Bahari-Javan et al. 2017). Similar to the data available for HDAC2, mice that lack HDAC3 in the adult hippocampus show enhanced memory function (McQuown et al. 2011) which is mechanistically linked to the regulation of the Nr4a gene (Kwapis et al. 2019). The function of HDAC8 in adult brain has not been addressed in detail, but a recent study found that an HDAC inhibitor with some selectivity toward HDAC8 improves memory function in rats (Yang et al. 2013). In conclusion, it appears that the class I HDACs act as molecular inhibitors of memory formation and are thus potential therapeutic targets to ameliorate memory impairment.
As for the class II HDACs (Table 1), HDAC4 is known to shuttle between the cytoplasm and the nucleus of cultured hippocampal neurons in response to calcium signaling and CamKII activity (Chawla et al. 2003; Backs et al. 2006). In a C. elegans model, deletion of HDAC4 gene increases long-term memory for thermosensation in a CamKII-dependent manner (Wang et al. 2011). Specific expression of mammalian HDAC4 in the nucleus was able to revert this phenotype suggesting that nuclear export of HDAC4 is a critical process for memory formation. In line with this data, cytoplasmatic expression of HDAC4 increased memory formation in wild-type worms (Wang et al. 2011), suggesting that during learning HDAC4 regulates counteracting molecular processes in the nucleus and the cytoplasm. In line with these data, another study demonstrated that overexpression of HDAC4 impairs long-term memory in a Drosophila model (Fitzsimons et al. 2013). In contrast to such findings, a recent study suggests that HDAC4 is essential for memory function in mammalian systems. Mice that lack HDAC4 in the adult forebrain exhibit impaired hippocampus-dependent memory formation and plasticity (Kim et al. 2012b). This data is in line with findings showing that haploinsufficiency of HDAC4 is linked to mental retardation in humans (Williams et al. 2010). Another study confirmed that lack of HDAC4 in the adult brain results in impaired memory function and synaptic plasticity in mice and could identify synaptic plasticity genes that are regulated by nuclear HDAC4 (Sando et al. 2012). There is also evidence that HDAC4 controls genes essential for adult neurogenesis (Saha et al. 2019) that contribute to the development of psychiatric phenotypes in response to stressful events (Maddox et al. 2018).
Regarding HDAC5, there is a substantial amount of evidence implicating this HDAC with the mechanisms linked to drug abuse (Smith and Kenny 2018). Loss of HDAC5 in the nucleus accumbens renders mice hypersensitive to chronic cocaine (Renthal et al. 2007) and regulates cocaine-conditioned behaviors (Taniguchi et al. 2017), while 2-month-old mice that lack HDAC5 from the adult forebrain show no changes in hippocampus-dependent memory formation (Kim et al. 2012b). A role of HDAC5 in memory formation may not only be brain-region but also age-related. To this end 10-month-old mice that lack HDAC5 show hippocampus-dependent memory disturbances (Agis-Balboa et al. 2013).
HDAC6 is best known for its acetylation of tubulin or heat shock protein 90 (Govindarajan et al. 2013). Loss of HDAC6 has mild impact on memory function in wild-type mice but improves cognition in mouse models for Alzheimer’s disease-related protein aggregation (Govindarajan et al. 2013; Fan et al. 2018; Selenica et al. 2014) or Fragile X syndrome (Kozikowski et al. 2019). HDAC7 was found to be downregulated in response to memory training in mice, which was linked to the expression of genes essential for long-term memory consolidation (Jing et al. 2017). Little is known on the role of HDAc9 in the adult brain, but a recent study points to a role of an HDAC9-dependent circRNA in dementia (Lu et al. 2019). Moreover, HDAC9 has been linked to dendritic plasticity (Sugo et al. 2010) and is associated with stroke and schizophrenia (Lang et al. 2011; Markus et al. 2013). Almost nothing is known about the role of HDAC10 and 11 in the adult brain. In conclusion, several HATs and HDACs have emerged as key players in memory function. Future research will be essential to understand how these epigenetic enzymes are regulated and which gene expression pathways they control. Moreover, it is important to mention that this enzymatic machinery not only regulates the acetylation state of specific histone proteins but also affects non-histone proteins such as transcription factors but also proteins generally linked, for example, to metabolic processes (Choudhary et al. 2009).
1.3 Histone Methylation: H3K4
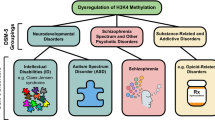
In addition to acetylation, another well-studied histone modification is methylation (Vaquero et al. 2003). Similar to histone acetylation, it is regulated by the counteracting activity of histone methyltransferases (HMTs) and histone demethylases (HMDs). However, in contrast to acetylation, the lysine residues of histones can be either mono-, di-, or tri-methylated which is catalyzed by specific enzymes. The functional consequences of histone methylation are thus also more complex and have been either linked to active euchromatin or the formation of facultative and constitutive heterochromatin that is associated with gene silencing. As such, there are more HMTs and HDMs than there are HATs and HDACs, and their general role has been discussed in a number of review articles (Shi 2007; Shi and Whetstine 2007; Badeaux and Shi 2013). In the context of memory formation, most data has been generated on the role of histone 3 methylation (H3K4me3) that is enriched around transcription start site (TSS) regions of actively transcribed and/or poised genes (Guenther et al. 2006), whereas histone 3 lysine 4 monomethylation (H3K4me1) is enriched at enhancers (Heintzman et al. 2009). H3K4 methylation is mediated by SET proteins. Set1 is the only H3K4 methylase in yeast (Roguev et al. 2001), whereas in Drosophila, three different SET proteins – Set1, trithorax (trx), and trithorax-related (trr) – are expressed (Mohan et al. 2011). Mammals, in turn, possess six SET-related H3K4 methyltransferases – Kmt2a (Mll1), Kmt2b (Mll2), Kmt2c (Mll3), Kmt2d (Mll4), Setd1a, and Setd1b – that have been linked to mono-, di-, and tri-methylation. These proteins are subdivided into three groups, based on their homology to each other and to the corresponding Drosophila homologues. Kmt2a/Kmt2b are related to trx, Kmt2c/Kmt2d to trr, and Setd1a/Setd1b to Set1 (Shilatifard 2012). The existence of several homologous histone-modifying enzymes mediating the same modifications raises the question of redundancy vs. specificity of their actions. This is especially true for brain regions such as the hippocampus, which is intimately linked to memory function and cognitive diseases, where all six H3K4-KMTs are strongly expressed (Kerimoglu et al. 2017 }. It is also important to note that mutations in any of the six H3K4-KMTs are linked to various rare diseases that include cognitive phenotypes and intellectual disability (Kleefstra et al. 2014).
In line with this, a number of studies implicated the regulation of H3K4me3 with memory function. For example, it was found that H3K4me3 correlates with the expression of glutamate receptors in the human brain (Stadler et al. 2005). Moreover, bulk levels of hippocampal H3K4me3 were found to increase in response to fear learning (Gupta et al. 2010), and mice that constitutively lack one allele of the H3K4-KMT KMT2A display impaired memory formation (Gupta et al. 2010). In line with this, conditional deletion of KMT2A from neurons of the adult brain impaired synaptic plasticity and working memory (Jakovcevski et al. 2015). Another study also demonstrated a role for the H3K4-specific HMT Mll2/KMT2B in memory function. Mice that lack KMT2B in the dorsal dentate gyrus of hippocampal region show memory impairment that is linked to deregulation of learning-relevant genes (Kerimoglu et al. 2013). Loss of KMT2B not only affected H3K4me3 at the promoter regions of learning-regulated genes but also reduced H3K9 acetylation, while H4K16 acetylation and H3K4me1 were unaffected at the same gene promoters (Kerimoglu et al. 2013). Such data further confirm the view that histone methylation and histone acetylation are tightly linked and also demonstrate the need to better understand the protein complexes that regulate chromatin plasticity in the adult brain. Especially the latter is in utmost importance since recent data compared the role of two closely related H3K4me3 KMTs – KMT2A and KMT2B – in excitatory neurons of the adult brain (Kerimoglu et al. 2017). These data show that loss of either enzyme leads to the impairment of hippocampus-dependent memories, but it was somewhat surprising to see that the gene expression pathways controlled by KMT2A and KMT2B in the hippocampus were entirely different. In fact, loss of neuronal KTM2A mainly affected gene expression pathways linked to neuronal identity and synaptic plasticity, while loss of KMT2B was linked to more general metabolic functions (Kerimoglu et al. 2013, 2017). Preliminary data from our lab suggest that similar observations are made for other KMTs that regulate H3K4me3. Mechanistically this phenomenon is presently not understood, but multiple explanations can be considered. Different KMTs could simply occupy different genomic regions, a hypothesis that is at present difficult to be tested since antibodies that allow the reliable analysis of KMTs in ChIP-seq experiments are necessary for this approach. Some evidence for this hypothesis stems however from the finding that in the neurons of KMT2A mutant mice, the deregulated genes contain different transcription factor binding sites when compared to KMT2B (Kerimoglu et al. 2017). KMTs may however also occupy similar genomic regions but might be regulated via different posttranslational modifications that are associated with distinct protein complexes. This could have different function outcomes. For example, KMT2A has been also linked to the regulation of higher-order chromatin structure and long-distance interactions (Bharadwaj et al. 2014). Mechanistic data on the other H3K4 HMTs in memory formation are comparatively rare. Loss of KMT2D in mice leads to phenotypes that partially recapitulate the Kabuki syndrome including hippocampus-dependent memory impairment, which can be rescued by the inhibition of HDAC inhibitors (Bjornsson et al. 2014) or a ketogenic diet (Benjamin et al. 2017). These data are highly interesting, since they suggest that defects in neural H3K4me3 can be attenuated by HDAC inhibitors that increase histone acetylation. While this is in line with other finding suggesting that also in neurons H3K4me3 is functionally coupled to histone acetylation (Kerimoglu et al. 2013, 2017), data from T-cells had suggested that genes which do not carry H3K4me3 cannot be regulated by HDAC inhibitors (Wang et al. 2009). Virtually nothing is known on the role of SETD1A in the adult brain, but it has been genetically linked to autism and schizophrenia (Singh et al. 2016; Takata et al. 2016). Also on the role of SET1B in the adult brain, there is so far little data available. Mutations in the corresponding genes are also linked to intellectual disability and autism (Labonne et al. 2016; Hiraide et al. 2018).
Four histone demethylases (KDMs) counteract the activity of the H3K4-KMTs. These are KDM5A (JARID1A), KDM5B (JARID1B), KDM5C (JARIC1C), and KDM5D (JARID1C). Little is known on the role of KDM5A and KDM5D. There is in vitro evidence that reducing the levels of KDM5B might increase neurogenesis in the subventricular zone of the adult brain (Zhou et al. 2016). Mutations in KDM5C have been linked to mental retardation (Jensen et al. 2005; Rujirabanjerd et al. 2012) and to short-term memory deficits in female humans (Simensen et al. 2012). A recent study analyzed mutant mice that lack one KDM5C allele either constitutively from early developmental stages and in addition tested mice that lack KDM5C in excitatory neurons of the adult brain (Scandaglia et al. 2017). While loss of KMD5C from developmental stages leads to memory impairment, this effect is less pronounced when KDM5C is deleted at adult stages. Similar findings are observed when H3K4me3-dependent gene expression was analyzed. The finding that deletion of KDM5C from the adult brain has no severe phenotype is interesting taking into account that reduced H3K4me3 has been linked to adult onset memory impairment and cognitive disease such as Alzheimer’s disease. These data suggest that inhibition of KMD5 proteins may ameliorate corresponding disease phenotypes. In line with this, a study available via bioRxiv shows that the phenotypes including memory impairment which are linked to the loss of KMT2A are ameliorated when these mutant mice are crossed with mice that lack KDM5C (Vallianatos et al. 2019). In conclusion, the current data point to a key role of H3K4 methylation in cognitive function and suggest that loss of H3K4 methylation in neurons is linked to memory impairment. In turn, drugs that increase H3K4 methylation could be suitable avenues for the treatment of cognitive diseases. Whether this could be achieved by the inhibition of HDAC or KDMs or both needs to be investigated. Also, clearly more research on the role of the H3K4 regulating enzymes in brain circuitries linked to memory function is needed.
1.4 Histone Methylation: H3K9
In addition to H3K4 methylation, there is a substantial amount of data on H3K9 methylation in the adult brain that is a mark for heterochromatin-mediating gene silencing. Similar to the situation described for H3K4 methylation, a number of different enzymes that are all expressed in the adult brain mediate H3K9 methylation. These are KMT1A (SUV39H), KMT1B (SUV39H2), KMT1C (G9A/EHMT2), KMT1D (GLP/EHMT1), KMT1E (SETDB1), and KMT8A (PRDM2).
While little is known on the role of H3K9 tri-methylase KMT1A in the adult brain, KMT1B was found to be upregulated in the hippocampus of rats in response to restraint stress, a procedure that affects memory function (Hunter et al. 2012). KMT1C and KMT1D have been linked to H3K9 mono- and di-methylation and act mainly as a complex. Pharmacological compounds affect in most cases both proteins. KMT1C and KMT1D were linked to intellectual disability and autism spectrum disorder (Koemans et al. 2018). Mice that lack KMT1C in the adult forebrain develop mental retardation-like phenotypes (Schaefer et al. 2009). However, loss of KMT1C in the nucleus accumbens was shown to increase cocaine-induced neuronal plasticity (Maze et al. 2010). In Drosophila, loss of KMT1C impaired memory formation (Kramer et al. 2011). Another study found that pharmacological inhibition of KMT1C/KMT1D in the entorhinal cortex facilitated memory function, while administration of the same inhibitor into the hippocampus resulted in impaired memory function in mice (Gupta-Agarwal et al. 2012). Another KMT1C/KMT1D inhibitor was found to enhance hippocampal LTP (Sharma et al. 2018), while yet another compound reduced anxiety when administered to adult animals, whereas administration during development increased anxiety when the animals were adult (Wang et al. 2018). Another recent study reported that pharmacological inhibition of KMT1C/KMT1D rescues memory impairment and synaptic function in a mouse model for amyloid deposition (Zheng et al. 2019). In sum, these data do not leave a clear picture on the role of KMT1C/KMT1D in the adult brain, but they clearly demonstrate an important role for these enzymes. One likely explanation to this could be the different roles of KMT1C/KMT1D in the developing and adult brain. Thus, timing of KMT1C/KMT1D manipulation appears to be essential for the interpretation of the resulting phenotypes.
Increased expression of KMT1E in the forebrain of mice reduced depressive-like behavior via a mechanism that involved regulation of NMDA receptor subunit 2B (Jiang et al. 2010). KMT1E is linked to H3K9-tri-methylation and was also found to be an essential regulator chromosomal conformation in mouse neurons of the adult brain (Bharadwaj et al. 2014; Jiang et al. 2017). KMT8A has been linked to mono-methylation of H3K9. It has been implicated with the detrimental effect of alcohol abuse (Barbier et al. 2017).
H3K9 methylation is reversed by the action of H3K9 KDMs that are however also known to affect other methylation sites such as H3K36. These are KDM2A (JHDM2A), KDM4A (JMJD2A), KDM4B (JMJD2B), KDM4C (JMJD2C), KDM4D (JMJD2D), and KDM4E (JMJD2E). KDM4A was found in a complex with HDAC1 and protein phosphatase 1 (PP1) which was required for memory formation (Koshibu et al. 2009). When KMT4B was deleted from adult forebrain neurons, the authors observed altered morphology of dendritic spines. Moreover mutant mice exhibited impaired working memory (Fujiwara et al. 2016). Another KMD that acts on H3K4 and K3K9 is KMD1 (LSD1). It was shown that pharmacological inhibition of LSD1 impairs memory function in mice (Neelamegam et al. 2012). In line with this data, recent data show that deletion of KDM1 from adult forebrain neurons in mice leads to neurodegeneration and memory impairment and induces gene expression changes linked to Alzheimer’s disease and frontotemporal dementia (Christopher et al. 2017).
In conclusion, the current data suggest that H3K4 methylation, as well as the activity of the corresponding HMTs and HDMs play a role in memory formation, are heavily linked to intellectual disability disorders and that targeting this machinery could a suitable approach for the treatment of cognitive diseases.
1.5 Histone Methylation: H3K27
Tri-methylation of H3K27 has been linked to facultative heterochromatin and gene silencing, while its mono- and di-methylation has been linked to active gene expression. H3K27me3 counteracts the role of H3K4me3, and both marks are often present at the same gene promoter which is then called bivalent promoter since they carry an active and repressive mark. Such genes are often induced by specific stimuli; hence they are repressed but can be induced rapidly by decreasing, for example, H3K27me3. Thus, H3K27me3 is associated with facultative heterochromatin. Interestingly, large portions of non-facultative heterochromatin are also marked by histone H3K9me3 and are thereby tethered to the nuclear lamina and associate with it in what is called lamina-associated domains (LADs) (van Steensel and Belmont 2017). Interestingly a recent publication elegantly showed that mechanical forces derived from tensile loading in human epithelial progenitor cells resulted in a switch from the constitutive heterochromatin marker H3K9me3 to the facultative H3K27me3 (Le et al. 2016). Deregulation of H3K27 methylation has been linked to neurodegenerative diseases. For example, changes in H3K27me3 a specific form of frontotemporal dementia is caused by mutations in the C9ORF72 genes that result in GGGGCC expanded repeats. It was found that mutant H3K27me3 level is increased in the mutant C9ORF72 gene and contributes to its downregulation (Belzil et al. 2013). Mutant C9ORF72 variants are also linked to translation leading to the generation of dipeptide repeat proteins (DRPs). A recent study showed that one of these DRPs, namely, proline-arginine (PR)-DRP, causes heterochromatin abnormalities linked to altered H3K27 methylation (Zheng et al. 2019). H3K27 methylation is mediated by the polycomb repression complex 2 (PRC2) with the essential subunits KMT6A (EZH2) and KMT6B (EZH1). KMT3F methylates H3K4 and H3K27. Neuronal deletion of KMT6A in mice from early developmental stages resulted in hippocampal memory impairment and reduced adult neurogenesis (Zhang et al. 2014). Another study found that in response to memory training, KMT6A regulated H3K27me3-dependent expression of the PTEN gene (Jarome et al. 2018). Ataxia-telangiectasia (A-T), also named Louis-Bar syndrome, is a rare progressive neurodegenerative disease that leads to cerebellar ataxia and caused by mutations of the ATM kinase. ATM was found to act on KMT6A, and the corresponding changes in H3K27 methylation have been linked to the disease progression (Li et al. 2013). Regarding KMT6B, it was shown that its expression is regulated by microRNA-132 (Johnstone et al. 2018), a microRNA intimately linked to memory formation (Fischer 2014a). The same study reports the microRNA-132-dependent downregulation of KMT6B in mice that were chronically exposed to antipsychotics (Johnstone et al. 2018). Consequently, knockdown of KMT6B in the prefrontal cortex affected motivational behavior. Another H3K27 KMT is KMT3F (NSD3) that can however also methylate H3K4. Nothing is known on the role of this enzyme in the adult brain. Among the H3K27 KDMs, KMD6A (UTX) and KDM6B (JMJD3) are rather specific to the demethylation of H3K27me2 and HeK27me3. KMD6A is encoded on the X chromosome, and there is indeed evidence for a sex-specific expression of KMD6A in neurons; more specifically KMD6A levels are higher in females (Xu et al. 2008). KMD6A has also been linked to the Kabuki syndrome, and deleting KDM6A from neurons from early developmental stages in mice results in impaired hippocampus-dependent memory formation and reduced LTP (Tang et al. 2017). Gene expression analysis revealed that KDM6A control genes linked to serotonergic signaling (Tang et al. 2017). Knockdown of KDM6B in hippocampal neurons impaired the stimulus-dependent expression of candidate genes (Wijayatunge et al. 2014). There is also evidence that KMD6B function is essential to keep neurogenesis genes in a poised state and thereby regulates neurogenesis in the developing and neurogenic niches of the adult brain (Park et al. 2014). Moreover, pharmacological inhibition of KMD6A in mice was shown to impair reinstatement of cocaine reward memory (Zhang et al. 2018). Nothing is known on the role of KDM7B on memory function in the adult brain.
In sum, although comparatively less studied than H3K4, the current data link H3K27 methylation and the corresponding enzymatic machinery to cognitive function and brain diseases.
1.6 Other Histone Methylation Sites
Little is known about the dynamic regulation of H3K36 methylation, a mark linked to complex regulation of gene expression depending on its mono-, di-, or tri-methylation, in the adult brain. This histone lysine residue can be methylated by KMT3A (SET2) that mediates H3K36me3, while KMT3B (NDS1) and KMT3C (SMYD2) mainly regulate H3K36 mono- and di-methylation. H3K36 demethylation is catalyzed by KDM2A (JHDM1A), KDM2B (JHDM1B), and KDM8 (JMJD5) that act on H3K36 mono- and di-methylation. Besides the observation that KDM2B has been genetically linked to intellectual disability and neuronal progenitor dysfunction leading to exencephaly (Fukuda et al. 2011; Labonne et al. 2016), nothing is known on the role of these enzymes in the adult brain. Demethylation of H3K36me3 is mediated by KDM4A–E that have been discussed above. Another histone modification is H3K79 methylation which has been linked to active gene expression but is also implicated with more complex regulations depending on the cellular context. Dynamic changes in H3K79 methylation are involved with neurodevelopmental processes (Büttner et al. 2010) and are reduced in FTLD/ALS patients that carry mutations in the C9ORF72 gene (Belzil et al. 2013). H3K79 mono-, di-, and tri-methylation is mediated by KMT4 (Dot1) that play a role in cortical development (Roidl et al. 2016). Demethylation of H3K79 has been linked to the activity of KDM2B that also acts on H3K36 and has been discussed above. H4K20 is so far the main methylation site on histone 4. H4K20me1 has been linked to active transcription, while H4K20me2 occurs during specific cellular processes such as cell cycle control. In contrast H4K20me3 is linked to repression of gene expression. Reduced H4K20me3 was observed in FTLD/ALS patients with the C9ORF72 mutation (Belzil et al. 2013) and in a mouse model for accelerated aging (Wang et al. 2010). H4K20 methylation is mediated by KMT5A (SET8) that mediated mono-methylation, while KMT5B (SUV4-20 h1) and KMT5C (SUV4-20H2) control di- and tri-methylation. While nothing is known on the role of KMT5A and C in the brain, KMT5B is associated with autism and developmental-disability biases (Stessman et al. 2017). A very interesting finding was that LSD1n is a H4K20 demethylase. LSD1n is a neuronal-specific splice variant of KMT1 (LSD1). Interestingly, while LSD1 affect H3K4 and H3K9, LSD1n de-methylates H4K20. Genetic ablation of LSD1n caused memory impairment and deregulation of neuronal gene expression (Wang et al. 2015).
1.7 Other Histone Modifications
Histones can also be reversibly ubiquitylated, which is probably the most severe form of a histone modification considering that a 76-amino-acid polypeptide is added to a histone tail. Histone ubiquitylation occurs mainly at lysine 119 of H2A (H2AK119ub) and lysine 120 of histone H2B (H2BK120ub). Both modifications occur as mono-ubiquitylation are thus not priming histones for degradation but serve signaling function that are diverse and have been linked to activation and repression of gene expression depending on the cellular context (Allis et al. 2007b). Early studies already observed increased H2A and H2B ubiquitylation in the aging mouse brain (Morimoto et al. 1993) which is in line with more recent data showing that elevated H2A ubiquitylation is a general marker for aging in a Drosophila model which is conserved in rodents, primates, and humans and that reduction of H2AK119ub in Drosophila increases life span (Yang et al. 2019). H2AK119ub not only increases in aging but also in neurodegenerative disease such as Huntington chorea (McFarland et al. 2013). Ubiquitination of histones is, for example, mediated by the E3 ubiquitin ligases RNF20 (BRE1A) and RNF40 (BRE1B) which have been linked to astrocyte differentiation (Liang et al. 2018). Histones can also be phosphorylated, and a prime example is the phosphorylation of the noncanonical histone gamma-H2AX that plays an important role in the DNA-damage response (Kinner et al. 2008). Interestingly, activity-induced DNA damage has been implicated with synaptic plasticity, memory consolidation, and neurodegenerative disease (Suberbielle et al. 2013; Madabhushi et al. 2015). Many other histone modifications have been reported (Vaquero et al. 2003), but their role in the adult brain remains to be studied. It has also been mentioned that in addition to the comparatively well-studied acetylation and methylation modifications of histone 3 and 4, proteome analysis suggests that more sites may exist, which still await to be studied in the brain (Brunner et al. 2012).
In addition to histone modifications, it is interesting to mention that under certain conditions, the canonical histones can be replaced by histone variants. A prime example is the replacement of H2A with H2A.Z that is considered represent a mechanisms for a transcriptional memory (Buschbeck and Hake 2017). Other variants are, for example, H3.3 or macroH2A. While in the brain H3 is constantly replaced by H3.3 along aging, so that older individuals contain almost exclusively to the replication-independent H3.3, the amount of H2A.Z was initially found to be rather constant (Piña and Suau 1987). Interestingly, it was shown that memory training in mice induced the eviction of H2A.Z at selected genes in the hippocampus and cortex of mice. Moreover H2A.Z levels were found to increase in the hippocampus of aging mice, and viral-mediated knockdown of H2A.Z improved memory function in mice (Zovkic et al. 2013; Stefanelli et al. 2018). Interestingly KAT5/TIP60 has been implicated with the deposition of H2A.Z, and more recent data suggest that inhibition of this process can improve memory consolidation in mice (Narkaj et al. 2018). While H3.3 has been linked to the general aging process (Bano et al. 2017), little is known on it and other histone variants in the adult brain, and also the role of reader proteins is only beginning to emerge.
2 Conclusion
Histone modifications are dynamically regulated during memory formation and in cognitive diseases. Histone acetylation appears to be a prerequisite for neuronal plasticity, and loss of the corresponding KMTs leads to memory impairments. In turn, the inhibition of class I HDAC has emerged as a suitable and promising approach to improve memory function in cognitive diseases. Histone methylation is also critical for proper neuronal plasticity, but the picture is more complex. Mutations in many of the enzymes machinery that control the various histone methylation events are linked to intellectual disability disorders. Best studied is the role of H3K4 and H3K9. Future research is needed to explore the full spectrum of histone modifications in the adult brain and understand how these modifications build a combinatorial code that controls transcriptome plasticity in neuronal circuitries.
References
Agis-Balboa RC, Pavelka Z, Kerimoglu C, Fischer A (2013) Loss of HDAC5 impairs memory function: implications for Alzheimer’s disease. J Alzheimers Dis 33:35–44
Agís-Balboa RC, Pinhero P, Rebola N, Kerimoglu C, Benito E, Gertig M, Bahari-Javan S, Jain G, Burkhardt S, Delalle I, Jatzko A, Dettenhofer M, Zunszain PA, Schmitt A, Falkai P, Pape JC, Binder EB, Mulle C, Fischer A, Sananbenesi F (2017) Formin 2 links neuropsychiatric phenotypes at young age to an increased risk for dementia. EMBO J 36:2815–2828
Akhtar MW, Raingo J, Nelson ED, Montgomery RL, Olson EN, Kavalali ET, Monteggia LM (2009) Histone deacetylases 1 and 2 form a developmental switch that controls excitatory synapse maturation and function. J Neurosci 29(25):8288–8297
Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A (2004) Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42:947–959
Allis CD, Berger SL, Cote J, Dent S, Jenuwein T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y (2007a) New nomenclature for chromatin-modifying enzymes. Cell 131:633–636
Allis CD, Jenuwein T, Reinberg D, Caparros ML (2007b) Epigenetics. Cold Spring Habor Laboratory Press, Cold Spring Habor
Arefin AS, Mathieson L, Johnstone D, Berretta R, Moscato P (2012) Unveiling clusters of RNA transcript pairs associated with markers of Alzheimer’s disease progression. PLoS One 7:e45535
Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN (2006) CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 116:1853–1864
Badeaux AI, Shi Y (2013) Emerging roles for chromatin as a signal integration and storage platform. Nat Rev Mol Cell Biol 14:211–224
Bahari-Javan S, Maddalena A, Kerimoglu C, Wittnam J, Held T, Bähr M, Burkhardt S, Delalle I, Kügler S, Fischer A, Sananbenesi F (2012) HDAC1 Regulates Fear Extinction in Mice. J Neurosci 32:5062–5073
Bahari-Javan S, Varbanov H, Halder R, Benito E, Kaurani L, Burkhardt S, Anderson-Schmidt H, Anghelescu I, Budde M, Stilling RM, Costa J, Medina J, Dietrich DE, Folkerts H, Gade K, Heilbronner U, Koller M, Konrad C, Nussbeck SY, Scherk H, Spitze C, Stierl S, Stöckel J, Thiel J, Hagen M, Zimmermann J, Zitzelsberger A, Schulz A, Schmitt A, Delalle I, Falkai P, Schulze TG, Dityatev A, Sananbenesi F, Fischer A (2017) HDAC1 links early life stress to schizophrenia-like phenotypes. Proc Natl Acad Sci U S A 114:E4686–E4694
Bano D, Piazzesi A, Salomoni P, Nicotera P (2017) The histone variant H3.3 claims its place in the crowded scene of epigenetics. Aging (Albany NY) 9:602–614
Barbier E, Johnstone AL, Khomtchouk BB, Tapocik JD, Pitcairn C, Rehman F, Augier E, Borich A, Schank JR, Rienas CA, Van Booven DJ, Sun H, Nätt D, Wahlestedt C, Heilig M (2017) Dependence-induced increase of alcohol self-administration and compulsive drinking mediated by the histone methyltransferase PRDM2. Mol Psychiatry 22:1746–1758
Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, Lynch G, Greene RW, Wood MA (2011) Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology 36:1545–1556
Belzil VV, Bauer PO, Prudencio M, Gendron TF, Stetler CT, Yan IK, Pregent L, Daughrity L, Baker MC, Rademakers R, Boylan K, Patel TC, Dickson DW, Petrucelli L (2013) Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol 126:895–905
Benito E, Urbanke E, Barth J, Halder R, Capece V, Jain G, Burkhardt S, Navarro M, Schutz AL, Bonn S, Fischer A (2015) Reinstating transcriptome plasticity and memory function in mouse models for cognitive decline. J Clin Invest 125:3572–3584
Benito E, Ramachandran B, Schroeder H, Schmidt G, Urbanke H, Burkhardt S, Capece V, Dean C, Fischer A (2017) The BET/BRD inhibitor JQ1 improves brain plasticity in WT and APP mice. Transl Psychiatry 7:e1239
Benjamin JS, Pilarowski GO, Carosso GA, Zhang L, Huso DL, Goff LA, Vernon HJ, Hansen KD, Bjornsson HT (2017) A ketogenic diet rescues hippocampal memory defects in a mouse model of Kabuki syndrome. Proc Natl Acad Sci U S A 114:125–130
Bharadwaj R, Peter CJ, Jiang Y, Roussos P, Vogel-Ciernia A, Shen EY, Mitchell AC, Mao W, Whittle C, Dincer A, Jakovcevski M, Pothula V, Rasmussen TP, Giakoumaki SG, Bitsios P, Sherif A, Gardner PD, Ernst P, Ghose S, Sklar P, Haroutunian V, Tamminga C, Myers RH, Futai K, Wood MA, Akbarian S (2014) Conserved higher-order chromatin regulates NMDA receptor gene expression and cognition. Neuron 84:997–1008
Bian C, Huang Y, Zhu H, Zhao Y, Zhao J, Zhang J (2018) Steroid receptor coactivator-1 knockdown decreases synaptic plasticity and impairs spatial memory in the hippocampus of mice. Neuroscience 1:114–125
Bjornsson HT, Benjamin JS, Zhang L, Weissman J, Gerber EE, Chen YC, Vaurio RG, Potter MC, Hansen KD, Dietz HC (2014) Histone deacetylase inhibition rescues structural and functional brain deficits in a mouse model of Kabuki syndrome. Sci Transl Med 6:256ra135
Blalock EM, Buechel HM, Popovic J, Geddes JW, Landfield PW (2011) Microarray analyses of laser-captured hippocampus reveal distinct gray and white matter signatures associated with incipient Alzheimer's disease. J Chem Meuroanat 42:118–126
Bousiges O, Vasconcelos AP, Neidl R, Cosquer B, Herbeaux K, Panteleeva I, Loeffler JP, Cassel JC, Boutillier AL (2010) Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetyltransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus. Neuropsychopharmacology 35:2521–2537
Bragg DC, Sharma N, Ozelius LJ (2019) X-linked dystonia-parkinsonism: recent advances. Curr Opin Neurol 32(4):604–609
Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ (2007) Distribution of histone deacetylases 1-11 in the rat brain. J Mol Neurosci 31:47–58
Brunner AM, Tweedie-Cullen RY, Mansuy IM (2012) Epigenetic modifications of the neuroproteome. Proteomics 12:2404–2420
Buschbeck M, Hake SB (2017) Variants of core histones and their roles in cell fate decisions, development and cancer. Nat Rev Mol Cell Biol 18:299–314
Büttner N, Johnsen SA, Kügler S, Vogel T (2010) Af9/Mllt3 interferes with Tbr1 expression through epigenetic modification of histone H3K79 during development of the cerebral cortex. Proc Natl Acad Sci U S A 107:7042–7047
Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S (2010) CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 107:22687–22692
Caldeira GL, Ferreira IL, Rego AC (2013) Impaired transcription in Alzheimer's disease: key role in mitochondrial dysfunction and oxidative stress. J Alzheimers Dis 34:115–131
Campbell RR, Wood MA (2019) How the epigenome integrates information and reshapes the synapse. Nat Rev Neurosci 20:133–147
Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36:827–835
Chatterjee S, Mizar P, Cassel R, Neidl R, Selvi BR, Mohankrishna DV, Vedamurthy BM, Schneider A, Bousiges O, Mathis C, Cassel JC, Eswaramoorthy M, Kundu TK, Boutillier AL (2013) A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J Neurosci 33:10698–10712
Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H (2003) Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem 85:151–159
Chen G, Zou X, Watanabe H, van Deursen JM, Shen J (2010) CREB binding protein is required for both short-term and long-term memory formation. J Neurosci 30:13066–13070
Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325:834–840
Christopher MA, Myrick DA, Barwick BG, Engstrom AK, Porter-Stransky KA, Boss JM, Weinshenker D, Levey AI, Katz DJ (2017) LSD1 protects against hippocampal and cortical neurodegeneration. Nat Commun 8:805
Dagnas M, Mons N (2013) Region- and age-specific patterns of histone acetylation related to spatial and cued learning in the water maze. Hippocampus 23:581–591
Davis HS, Squire LR (1984) Protein synthesis and memory: a review. Psychol Bull 96:518–559
Day JJ, Sweatt JD (2010) DNA methylation and memory formation. Nat Neurosci 13:1319–1323
Day JJ, Sweatt JD (2011) Epigenetic mechanisms in cognition. Neuron 70:813–829
de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB (2003) Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370:737–749
Deussing JM, Jakovcevski M (2013) Histone modifications in major depressive disorder and related rodent models. Adv Exp Med Biol 978:169–183
Dong E, Guidotti A, Grayson DR, Costa E (2007) Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc Natl Acad Sci U S A 104:4676–4681
Donmez G (2013) Sirtuins as possible targets in neurodegenerative diseases. Curr Drug Targets 14:644–647
Duclot F, Meffre J, Jacquet C, Gongora C, Maurice T (2010) Mice knock out for the histone acetyltransferase p300/CREB binding protein-associated factor develop a resistance to amyloid toxicity. Neuroscience 167:850–863
Ernst A, Frisén J (2015) Adult neurogenesis in humans- common and unique traits in mammals. PLoS Biol 13:e1002045
Fan SJ, Huang FI, Liou JP, Yang CR (2018) The novel histone de acetylase 6 inhibitor, MPT0G211, ameliorates tau phosphorylation and cognitive deficits in an Alzheimer's disease model. Cell Death Dis 9:655
Federman N, Fustiñana MS, Romano A (2009) Histone acetylation is recruited in consolidation as a molecular feature of stronger memories. Learn Mem 16:600–606
Fiore R, Khudayberdiev S, Saba R, Schratt G (2011) MicroRNA function in the nervous system. Prog Mol Biol Transl Sci 102:47–100
Fischer A (2014a) Epigenetic memory: the Lamarckian brain. EMBO J 33:945–967
Fischer A (2014b) Targeting histone-modifications in Alzheimer's disease. What is the evidence that this is a promising therapeutic avenue? Neuropsychopharmacology 80:95–012
Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH (2007) Recovery of learning & memory after neuronal loss is associated with chromatin remodeling. Nature 447:178–182
Fischer A, Sananbenesi F, Mungenast A, Tsai LH (2010) Targeting the right HDAC(s) to treat cognitive diseases. Trends Pharmacol Sci 31:605–617
Fitzsimons HL, Schwartz S, Given FM, Scott MJ (2013) The histone deacetylase HDAC4 regulates long-term memory in Drosophila. PLoS One 8:e83903
Flexner JB, Flexner LB, Stellar E, De La Haba G, Roberts RB (1962) Inhibition of protein synthesis in brain and learning and memory following puromycin. J Neurochem 9:595–605
Flexner JB, Flexner LB, Stellar E (1963) Memory in mice as affected by intracerebral puromycin. Science 141:57–59
Frey U, Morris RG (1998) Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci 21:181–188
Fujiwara K, Fujita Y, Kasai A, Onaka Y, Hashimoto H, Okada H, Yamashita T (2016) Deletion of JMJD2B in neurons leads to defective spine maturation, hyperactive behavior and memory deficits in mouse. Transl Psychiatry 6:e766
Fukuda T, Tokunaga A, Sakamoto R, Yoshida N (2011) Fbxl10/Kdm2b deficiency accelerates neural progenitor cell death and leads to exencephaly. Mol Cell 46:614–624
Ginsberg SD, Alldred MJ, Counts SE, Cataldo AM, Neve RL, Jiang Y, Wuu J, Chao MV, Mufson EJ, Nixon RA, Che S (2010) Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer’s disease progression. Biol Psychiatry 68:885–893
Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schlüter OM, Bradke F, Lu J, Fischer A (2013) Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol Med 5:52–63
Gregoretti IV, Lee YM, Goodson HV (2004) Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol 338:17–31
Grosselin K, Durand A, Marsolier J, Poitou A, Marangoni E, Nemati F, Dahmani A, Lameiras S, Reyal F, Frenoy O, Pousse Y, Reichen M, Woolfe A, Brenan C, Griffiths AD, Vallot C, Gérard A (2019) High-throughput single-cell ChIP-seq identifies heterogeneity of chromatin states in breast cancer. Nat Genet 51:1060–1066
Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH (2009) HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459:55–60
Guenther MG, Jenner RG, Chevalier B, Nakamura T, Croce CM, Canaani E, Young RA (2006) Global and Hox-specific roles for the MLL1 methyltransferase. Proc Natl Acad Sci U S A 102:8603–8608
Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD (2010) Histone methylation regulates memory formation. J Neurosci 30:3589–3599
Gupta-Agarwal S, Franklinm AV, Deramusm T, Wheelockm M, Davism RL, McMahonm LL, Lubin FD (2012) G9a/GLP histone lysine dimethyltransferase complex activity in the hippocampus and the entorhinal cortex is required for gene activation and silencing during memory consolidation. J Neurosci 32:5440–5453
Haigis MC, Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 5:253–295
Halder R, Hennion M, Vidal RO, Shomroni O, Rahman RU, Rajput A, Centeno TP, van Bebber F, Capece V, Vizcaino JC, Schuetz AL, Burkhardt S, Benito E, Sala MN, Javan SB, Haass C, Schmid B, Fischer A, Bonn S (2016) DNA methylation changes in plasticity genes accompany the formation and maintenance of memory. Nat Neurosci 19:102–110
Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, Ching KA, Antosiewicz-Bourget JE, Liu H, Zhang X, Green RD, Lobanenkov VV, Stewart R, Thomson JA, Crawford GE, Kellis M, Ren B (2009) Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459:108–112
Herrup K, Li J, Chen J (2013) The role of ATM and DNA damage in neurons: upstream and downstream connections. DNA Repair 12:600–604
Hiraide T, Nakashima M, Yamoto K, Fukuda T, Kato M, Ikeda H, Sugie Y, Aoto K, Kaname T, Nakabayashi K, Ogata T, Matsumoto N, Saitsu H (2018) De novo variants in SETD1B are associated with intellectual disability, epilepsy and autism. Hum Genet 137:95–104
Hunter RG, Murakami G, Dewell S, Seligsohn M, Baker ME, Datson NA, McEwen BS, Pfaff DW (2012) Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proc Natl Acad Sci U S A 109:17657–17662
Igaz LM, Vianna MR, Medina JH, Izquierdo I (2002) Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J Neurosci 22:6781–6791
Jakovcevski M, Akbarian S (2012) Epigenetic mechanisms in neurological disease. Nat Med 18:1194–1204
Jakovcevski M, Bharadwaj R, Straubhaar J, Gao G, Gavin DO, Jakovcevski I, Mitchell AC, Akbarian S (2013a) Prefrontal cortical dysfunction after overexpression of histone deacetylase 1. Biol Psychiatry 74:696–705
Jakovcevski M, Bharadwaj R, Straubhaar J, Gao G, Gavin DP, Jakovcevski I, Mitchell AC, Akbarian S (2013b) Prefrontal cortical dysfunction after overexpression of histone deacetylase 1. Biol Psychiatry 74:696–705
Jakovcevski M, Ruan H, Shen EY, Dincer A, Javidfar B, Ma Q, Peter CJ, Cheung I, Mitchell AC, Jiang Y, Lin CL, Pothula V, Stewart AF, Ernst P, Yao WD, Akbarian S, Impey S (2015) Neuronal Kmt2a/Mll1 histone methyltransferase is essential for prefrontal synaptic plasticity and working memory. J Neurosci 35:5097–5108
Jarome TJ, Perez GA, Hauser RM, Hatch KM, Lubin FD (2018) EZH2 methyltransferase activity controls Pten expression and mTOR signaling during fear memory reconsolidation. J Neurosci 38:7635–7648
Jawerka M, Colak D, Dimou L, Spiller C, Lagger S, Montgomery RL, Olson EN, Wurst W, Göttlicher M, Götz M (2010) The specific role of histone deacetylase 2 in adult neurogenesis. Neuron Glia Biol 14:1–15
Jensen LR, Amende M, Gurok U, Moser B, Gimmel V, Tzschach A, Janecke AR, Tariverdian G, Chelly J, Fryns JP, Van Esch H, Kleefstra T, Hamel B, Moraine C, Gecz J, Turner G, Reinhardt R, Kalscheuer VM, Ropers HH, Lenzner S (2005) Mutations in the JARID1C gene, which is involved in transcriptional regulation and chromatin remodeling, cause X-linked mental retardation. Am J Hum Genet 76:227–236
Jiang Y, Jakovcevski M, Bharadwaj R, Connor C, Schroeder FA, Lin CL, Straubhaar J, Martin G, Akbarian S (2010) Setdb1 histone methyltransferase regulates mood-related behaviors and expression of the NMDA receptor subunit NR2B. J Neurosci 30:7152–7267
Jiang Y, Loh YE, Rajarajan P, Hirayama T, Liao W, Kassim BS, Javidfar B, Hartley BJ, Kleofas L, Park RB, Labonte B, Ho SM, Chandrasekaran S, Do C, Ramirez BR, Peter CJ, Safaie BM, Morishita H, Roussos P, Nestler EJ, Schaefer A, Tycko B, Brennand KJ, Yagi T, Shen L, Akbarian S (2017) The methyltransferase SETDB1 regulates a large neuron-specific topological chromatin domain. Nat Genet 49:1239–1250
Jing X, Sui WH, Wang S, Xu XF, Yuan RR, Chen XR, Ma HX, Zhu YX, Sun JK, Yi F, Chen ZY, Wang Y (2017) HDAC7 ubiquitination by the E3 ligase CBX4 is involved in contextual fear conditioning memory formation. J Neurosci 37:3848–3863
Johnson AA, Sarthi J, Pirooznia SK, Reube W, Elefant F (2013) Increasing Tip60 HAT levels rescues axonal transport defects and associated behavioral phenotypes in a Drosophila Alzheimer’s disease model. J Neurosci 33:7535–7547
Johnstone AL, O'Reilly JJ, Patel AJ, Guo Z, Andrade NS, Magistri M, Nathanson L, Esanov R, Miller BH, Turecki G, Brothers SP, Zeier Z, Wahlestedt C (2018) EZH1 is an antipsychotic-sensitive epigenetic modulator of social and motivational behavior that is dysregulated in schizophrenia. Neurobiol Dis 119:149–158
Josselyn SA (2005) What’s right with my mouse model? New insights into the molecular and cellular basis of cognition from mouse models of Rubinstein-Taybi Syndrome. Learn Mem 12:80–83
Kaidi A, Jackson SP (2013) KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature 498:70–74
Kerimoglu C, Agis-Balboa RC, Kranz A, Stilling R, Bahari-Javan S, Benito-Garagorri E, Halder R, Burkhardt S, Stewart AF, Fischer A (2013) Histone-methyltransferase mll2 (kmt2b) is required for memory formation in mice. J Neurosci 33:3452–3464
Kerimoglu C, Sakib MS, Jain G, Benito E, Burkhardt S, Capece V, Kaurani L, Halder R, Agís-Balboa RC, Stilling R, Urbanke H, Kranz A, Stewart AF, Fischer A (2017) KMT2A and KMT2B mediate memory function by affecting distinct genomic regions. Cell Rep 20:538–548
Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G (2010) Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 35:870–880
Kim KH, Moon M, Yu SB, Mook-Jung I, Kim JI (2012a) RNA-Seq analysis of frontal cortex and cerebellum from 5XFAD mice at early stage of disease pathology. J Alzheimers Dis 29:793–808
Kim MS, Akhtar MW, Adachi M, Mahgoub M, Bassel-Duby R, Kavalali ET, Olson EN, Monteggia LM (2012b) An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J Neurosci 32:10879–10886
Kinner A, Wu W, Staudt C, Iliakis G (2008) Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res 36:5678–5694
Kleefstra T, Schenck A, Kramer JM, van Bokhoven H (2014) The genetics of cognitive epigenetics. Neuropharmacology 80:83–94
Kocki T, Urbańska EM, Kocki J, Kloc R, Kocka K, Olajossy M, Owe-Larsson B (2018) Prolonged therapy with antidepressants increases hippocampal level of kynurenic acid and expression of Kat1 and Kat2 genes. Pharmacol Rep 70:737–745
Koemans TS, Kleefstra T, Chubak MC, Stone MH, Reijnders MRF, de Munnik S, Willemsen MH, Fenckova M, Stumpel CTRM, Bok LA, Sifuentes B, Saenz M, Byerly KA, Baughn LB, Stegmann APA, Pfundt R, Zhou H, van Bokhoven H, Schenck A, Kramer JM (2018) Functional convergence of histone methyltransferases EHMT1 and KMT2C involved in intellectual disability and autism spectrum disorder. PLoS Genet 13(10):e1006864
Korzus E, Rosenfeld MG, Mayford M (2004) CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42:961–972
Koshibu K, Gräff J, Beullens M, Heitz FD, Berchtold D, Russig H, Farinelli M, Bollen M, Mansuy IM (2009) Protein phosphatase 1 regulates the histone code for long-term memory. J Neurosci 29:13079–13089
Kozikowski AP, Shen S, Pardo M, Tavares MT, Szarics D, Benoy V, Zimprich CA, Kutil Z, Zhang G, Bařinka C, Robers MB, Van Den Bosch L, Eubanks JH, Jope RS (2019) Brain penetrable histone deacetylase 6 inhibitor SW-100 ameliorates memory and learning impairments in a mouse model of fragile X syndrome. ACS Chem Nerosci 10:1679–1695
Kramer JM, Kochinke K, Oortveld MA, Marks H, Kramer D, de Jong EK, Asztalos Z, Westwood JT, Stunnenberg HG, Sokolowski MB, Keleman K, Zhou H, van Bokhoven H, Schenck A (2011) Epigenetic regulation of learning and memory by Drosophila EHMT/G9a. PLoS Biol 9:e1000569
Kwapis JL, Alaghband Y, López AJ, Long JM, Li X, Shu G, Bodinayake KK, Matheos DP, Rapp PR, Wood MA (2019) HDAC3-mediated repression of the Nr4a family contributes to age-related impairments in long-term memory. J Neurosci 39:4999–5009
Labonne JD, Lee KH, Iwase S, Kong IK, Diamond MP, Layman LC, Kim CH, Kim HG (2016) An atypical 12q24.31 microdeletion implicates six genes including a histone demethylase KDM2B and a histone methyltransferase SETD1B in syndromic intellectual disability. Hum Genet 135:757–771
Lang B, Alrahbeni TM, Clair DS, Blackwood DH, International Schizophrenia Consortium, McCaig CD, Shen S (2011) HDAC9 is implicated in schizophrenia and expressed specifically in post-mitotic neurons but not in adult neural stem cells. Am J Stem Cells 18:31–41
Le HQ, Ghatak S, Yeung CY, Tellkamp F, Günschmann C, Dieterich C, Yeroslaviz A, Habermann B, Pombo A, Niessen CM, Wickström SA (2016) Mechanical regulation of transcription controls polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol 18:864–875
Lee KK, Workman JL (2007) Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol 8:285–295
Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD (2004) Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279:40545–40559
Li J, Hart RP, Mallimo EM, Swerdel MR, Kusnecov AW, Herrup K (2013) EZH2-mediated H3K27 trimethylation mediates neurodegeneration in ataxia-telangiectasia. Nat Neurosci 16:1745–1753
Liang WS, Dunckley T, Beach TG, Grover A, Mastroeni D, Ramsey K, Caselli RJ, Kukull WA, McKeel D, Morris JC, Hulette CM, Schmechel D, Reiman EM, Rogers J, Stephan DA (2010) Neuronal gene expression in non-demented individuals with intermediate Alzheimer’s disease neuropathology. Neurobiol Aging 31:549–566
Liang Q, Xia W, Li W, Jiao J (2018) RNF20 controls astrocytic differentiation through epigenetic regulation of STAT3 in the developing brain. Cell Death Differ 25:294–306
Lipinski M, Del Blanco B, Barco A (2019) CBP/p300 in brain development and plasticity: disentangling the KAT’s cradle. Curr Opin Neurobiol 8:1–8
Lopez-Atalaya JP, Ciccarelli A, Viosca J, Valor LM, Jimenez-Minchan M, Canals S, Giustetto M, Barco A (2011) CBP is required for environmental enrichment-induced neurogenesis and cognitive enhancement. EMBO J 30:4287–4298
Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429:883–891
Lu Y, Tan L, Wang X (2019) Circular HDAC9/microRNA-138/Sirtuin-1 pathway mediates synaptic and amyloid precursor protein processing deficits in Alzheimer’s disease. Neurosci Bull 18. https://doi.org/10.1007/s12264-019-00361-0
Madabhushi R, Gao F, Pfenning AR, Pan L, Yamakawa S, Seo J, Rueda R, Phan TX, Yamakawa H, Pao PC, Stott RT, Gjoneska E, Nott A, Cho S, Kellis M, Tsai LH (2015) Activity-induced DNA breaks govern the expression of neuronal early-response genes. Cell 161:1592–1605
Maddox SA, Kilaru V, Shin J, Jovanovic T, Almli LM, Dias B, Norrholm SD, Fani N, Michopoulos V, Ding Z, Conneely KN, Binder EB, Ressler KJ, Smith AK (2018) Estrogen-dependent association of HDAC4 with fear in female mice and women with PTSD. Mol Psychiatry 23:658–665
Mao P, Reddy PH (2011) Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: implications for early intervention and therapeutics. Biochim Biophys Acta 1812:1359–1370
Marioni RE, Harris SE, Zhang Q, McRae AF, Hagenaars SP, Hill WD, Davies G, Ritchie CW, Gale CR, Starr JM, Goate AM, Porteous DJ, Yang J, Evans KL, Deary IJ, Wray NR, Visscher PM (2018) GWAS on family history of Alzheimer’s disease. Transl Psychiatry 8:161
Markus HS, Mäkelä KM, Bevan S, Raitoharju E, Oksala N, Bis JC, O’Donnell C, Hainsworth A, Lehtimäki T (2013) Evidence HDAC9 genetic variant associated with ischemic stroke increases risk via promoting carotid atherosclerosis. Stroke 44:1220–1225
Maurice T, Duclot F, Meunier J, Naert G, Givalois L, Meffre J, Célérier A, Jacquet C, Copois V, Mechti N, Ozato K, Gongora C (2008) Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology 33:1584–1602
Maze I, Covington HE 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, Nestler EJ (2010) Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 327:213–216
McFarland KN, Das S, Sun TT, Leyfer D, Kim MO, Xia E, Sangrey GR, Kuhn A, Luthi-Carter R, Clark TW, Sadri-Vakili G, Cha JH (2013) Genome-wide increase in histone H2A ubiquitylation in a mouse model of Huntington’s disease. J Huntingtons Dis 2:263–277
McNulty SE, Barrett RM, Vogel-Ciernia A, Malvaez M, Hernandez N, Davatolhagh MF, Matheos DP, Schiffman A, Wood MA (2012) Differential roles for Nr4a1 and Nr4a2 in object location vs. object recognition long-term memory. Learn Mem 19:588–592
McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, Wood MA (2011) HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci 31:764–774
Merson TD, Dixon MP, Collin C, Rietze RL, Bartlett PF, Thomas T, Voss AK (2006) The transcriptional coactivator Querkopf controls adult neurogenesis. J Neurosci 26:11359–11370
Mews P, Donahuem G, Drakem AM, Luczakm V, Abelm T, Bergerm SL (2017) Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature 546(7658):381–386
Miller CA, Campbell SL, Sweatt JD (2008) DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem 89:599–603
Mills JD, Nalpathamkalam T, Jacobs HI, Janitz C, Merico D, Hu P, Janitz M (2013) RNA-Seq analysis of the parietal cortex in Alzheimer’s disease reveals alternatively spliced isoforms related to lipid metabolism. Neurosci Lett 1:90–95
Mitchnick KA, Creighton SD, Cloke JM, Wolter M, Zaika O, Christen B, Van Tiggelen M, Kalisch BE, Winters BD (2016) Dissociable roles for histone acetyltransferases p300 and PCAF in hippocampus and perirhinal cortex-mediated object memory. Genes Brain Behav 2016:6
Mohan M, Herz HM, Smith ER, Zhang Y, Jackson J, Washburn MP, Florens L, Eissenberg JC, Shilatifard A (2011) The COMPASS family of H3K4 methylases in Drosophila. Mol Cell Biol 31:4310–4318
Moncada D, Ballarini F, Martinez MC, Frey JU, Viola H (2011) Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc Natl Acad Sci U S A 108:12931–12936
Morimoto S, Komatsu S, Takahashi R, Matsuo M, Goto S (1993) Age-related change in the amount of ubiquitinated histones in the mouse brain. Arch Gerontol Geriatr 16:217–224
Morris MJ, Mahgoub M, Na ES, Pranav H, Monteggia LM (2013) Loss of histone deacetylase 2 improves working memory and accelerates extinction learning. J Neurosci 33:6401–6411
Müller T, Schrötter A, Loosse C, Pfeiffer K, Theiss C, Kauth M, Meyer HE, Marcus K (2013) A ternary complex consisting of AICD, FE65, and TIP60 down-regulates Stathmin1. Biochim Biophys Acta 1834:387–394
Narkaj K, Stefanelli G, Wahdan M, Azam AB, Ramzan F, Steininger CFDJ, Walters BJ, Zovkic IB (2018) Blocking H2A.Z incorporation via Tip60 inhibition promotes systems consolidation of fear memory in mice. eNeuro 5(5). pii: ENEURO.0378-18.2018
Neelamegam R, Ricq EL, Malvaez M, Patnaik D, Norton S, Carlin SM, Hill IT, Wood MA, Haggarty SJ, Hooker JM (2012) Brain-penetrant LSD1 inhibitors can block memory consolidation. ACS Chem Nerosci 3:120–128
Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K (1999) Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet 8:387–396
Oliveira AM, Wood MA, McDonough CB, Abel T (2007) Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn Mem 14:564–572
Oliveira AM, Estévez MA, Hawk JD, Grimes S, Brindle PK, Abel T (2011) Subregion-specific p300 conditional knock-out mice exhibit long-term memory impairments. Learn Mem 18:161–169
Park DH, Hong SJ, Salinas RD, Liu SJ, Sun SW, Sgualdino J, Testa G, Matzuk MM, Iwamori N, Lim DA (2014) Activation of neuronal gene expression by the JMJD3 demethylase is required for postnatal and adult brain neurogenesis. Cell Rep 8:1290–1299
Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Java S, Agis-Balboa RC, Cota P, Wittnam J, Gogul-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, KAng H, Farinelli L, Chen W, Fischer A (2010) Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328:753–756
Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, van Ommen GJ, Goodman RH, Peters DJ (1995) Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376:348–351
Piña B, Suau P (1987) Changes in histones H2A and H3 variant composition in differentiating and mature rat brain cortical neurons. Dev Biol 123:51–58
Pirooznia SK, Sarthi J, Johnson AA, Toth MS, Chiu K, Koduri S, Elefant F (2012) Tip60 HAT activity mediates APP induced lethality and apoptotic cell death in the CNS of a Drosophila Alzheimer’s disease model. PLoS One 7:e41776
Renthal W, Maze I, Krishnan V, Covington HE 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, TA MK, Bassel-Duby R, Olson EN, Nestler EJ (2007) Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 56:517–529
Roguev A, Schaft D, Shevchenko A, Pijnappel WW, Wilm M, Aasland R, Stewart AF (2001) The Saccharomyces cerevisiae Set1 complex includes an Ash2 homologue and methylates histone 3 lysine 4. EMBO J 20:7137–7143
Roidl D, Hellbach N, Bovio PP, Villarreal A, Heidrich S, Nestel S, Grüning BA, Boenisch U, Vogel T (2016) DOT1L activity promotes proliferation and protects cortical neural stem cells from activation of ATF4-DDIT3-mediated ER stress in vitro. Stem Cells 34:233–245
Rujirabanjerd S, Nelson J, Tarpey PS, Hackett A, Edkins S, Raymond FL, Schwartz CE, Turner G, Iwase S, Shi Y, Futreal PA, Stratton MR, Gecz J (2012) Identification and characterization of two novel JARID1C mutations: suggestion of an emerging genotype-phenotype correlation. Eur J Hum Genet 18:330–335
Saha P, Gupta RK, Sen T, Sen N (2019) Histone deacetylase 4 downregulation elicits post-traumatic psychiatric disorders through impairment of neurogenesis. J Neurotrauma. https://doi.org/10.1089/neu.2019.6373
Sando R 3rd, Gounko N, Pieraut S, Liao L, Yates J 3rd, Maximov A (2012) HDAC4 governs a transcriptional program essential for synaptic plasticity and memory. Cell 151:821–834
Sarthi J, Elefant F (2011) dTip60 HAT activity controls synaptic bouton expansion at the Drosophila neuromuscular junction. PLoS One 6:e26202
Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ 3rd, Kandel ER, Duff K, Kirkwood A, Shen J (2004) Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42:23–36
Scandaglia M, Lopez-Atalaya JP, Medrano-Fernandez A, Lopez-Cascales MT, Del Blanco B, Lipinski M, Benito E, Olivares R, Iwase S, Shi Y, Barco A (2017) Loss of Kdm5c causes spurious transcription and prevents the fine-tuning of activity-regulated enhancers in neurons. Cell Rep 21:47–59
Schaefer A, Sampath SC, Intrator A, Min A, Gertler TS, Surmeier DJ, Tarakhovsky A, Greengard P (2009) Control of cognition and adaptive behavior by the GLP/G9a epigenetic suppressor complex. Neuron 64:678–691
Schmitt M, Matthies H (1979) Biochemical studies on histones of the central nervous system. III. Incorporation of [14C]-acetate into the histones of different rat brain regions during a learning experiment. Acta Biol Med Ger 38:683–689
Selenica ML, Benner L, Housley SB, Manchec B, Lee DC, Nash KR, Kalin J, Bergman JA, Kozikowski A, Gordon MN, Morgan D (2014) Histone deacetylase 6 inhibition improves memory and reduces total tau levels in a mouse model of tau deposition. Alzheimers Res Ther 6. https://doi.org/10.1186/alzrt1124
Sharma RP, Grayson DR, Gavin DP (2008) Histone deacetylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: analysis of the National Brain Databank microarray collection. Schizophr Bull 98:111–117
Sharma M, Razali NB, Sajikumar S (2018) Inhibition of G9a/GLP complex promotes long-term potentiation and synaptic tagging/capture in hippocampal CA1 pyramidal neurons. Cereb Cortex 27:3161–3171
Shi Y (2007) Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet 8:829–833
Shi Y, Whetstine JR (2007) Dynamic regulation of histone lysine methylation by demethylases. Mol Cell 25:1–14
Shilatifard A (2012) The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu Rev Biochem 81:65–95
Shulha HP, Crisci JL, Reshetov D, Tushir JS, Cheung I, Bharadwaj R, Chou HJ, Houston IB, Peter CJ, Mitchell AC, Yao WD, Myers RH, Chen JF, Preuss TM, Rogaev EI, Jensen JD, Weng Z, Akbarian S (2012) Human-specific histone methylation signatures at transcription start sites in prefrontal neurons. PLoS Biol 10:e1001427
Simensen RJ, Rogers RC, Collins JS, Abidi F, Schwartz CE, Stevenson RE (2012) Short-term memory deficits in carrier females with KDM5C mutations. Genet Couns 23:31–40
Singh T, Kurki MI, Curtis D, Purcell SM, Crooks L, McRae J, Suvisaari J, Chheda H, Blackwood D, Breen G, Pietiläinen O, Gerety SS, Ayub M, Blyth M, Cole T, Collier D, Coomber EL, Craddock N, Daly MJ, Danesh J, DiForti M, Foster A, Freimer NB, Geschwind D, Johnstone M, Joss S, Kirov G, Körkkö J, Kuismin O, Holmans P, Hultman CM, Iyegbe C, Lönnqvist J, Männikkö M, McCarroll SA, McGuffin P, McIntosh AM, McQuillin A, Moilanen JS, Moore C, Murray RM, Newbury-Ecob R, Ouwehand W, Paunio T, Prigmore E, Rees E, Roberts D, Sambrook J, Sklar P, St Clair D, Veijola J, Walters JT, Williams H, Swedish Schizophrenia Study; INTERVAL Study; DDD Study; UK10 K Consortium, Sullivan PF, Hurles ME, O’Donovan MC, Palotie A, Owen MJ, Barrett JC (2016) Rare loss-of-function variants in SETD1A are associated with schizophrenia and developmental disorders. Nat Neurosci 19:571–577
Smith AC, Kenny PJ (2018) HDAC5 regulates the formation of drug memories. Trends Mol Med 24:106–108
Stadler F, Kolb G, Rubusch L, Baker SP, Jones EG, Akbarian S (2005) Histone methylation at gene promoters is associated with developmental regulation and region-specific expression of ionotropic and metabotropic glutamate receptors in human brain. J Neurochem 94:324–336
Stefanelli G, Azam AB, Walters BJ, Brimble MA, Gettens CP, Bouchard-Cannon P, Cheng HM, Davidoff AM, Narkaj K, Day JJ, Kennedy AJ, Zovkic IB (2018) Learning and age-related changes in genome-wide H2A.Z binding in the mouse Hippocampus. Cell Rep 22:1124–1131
Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA (2009) Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A 106:9447–9452
Stessman HA, Xiong B, Coe BP, Wang T, Hoekzema K, Fenckova M, Kvarnung M, Gerdts JTS, Cosemans N, Vives L, Lin J, Turner TN, Santen G, Ruivenkamp C, Kriek M, van Haeringen A, Aten E, Friend K, Liebelt J, Barnett C, Haan E, Shaw M, Gecz J, Anderlid BM, Nordgren A, Lindstrand A, Schwartz C, Kooy RF, Vandeweyer G, Helsmoortel C, Romano C, Alberti A, Vinci M, Avola E, Giusto S, Courchesne E, Pramparo T, Pierce K, Nalabolu S, Amaral DG, Scheffer IE, Delatycki MB, Lockhart PJ, Hormozdiari F, Harich B, Castells-Nobau A, Xia K, Peeters H, Nordenskjöld M, Schenck A, Bernier RA, Eichler EE (2017) Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat Genet 49:515–526
Stilling R, Rönicke R, Benito-Garagorri E, Urbanke H, Capece V, Burckhard S, Bahari-Javan S, Barth J, Sananbenesi F, Schütz AL, Dyczkowski J, Martinez-Hernandez A, Kerimoglu C, Dent SR, Bonn S, Reymann KG, Fischer A (2014) K-Lysine acetyltransferase 2A regulates a hippocampal gene-expression network linked to memory formation. EMBO J 33:1912–1927
Strahl BD, Allis CD (2000) The language of covalent histone modifications. Nature 403:41–45
Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, Devidze N, Kreitzer AC, Mucke L (2013) Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat Neurosci 16:613–621
Sugo N, Oshiro H, Takemura M, Kobayashi T, Kohno Y, Uesaka N, Song WJ, Yamamoto N (2010) Nucleocytoplasmic translocation of HDAC9 regulates gene expression and dendritic growth in developing cortical neurons. Eur J Neurosci 31:152–1532
Sun H, Kennedy PJ, Nestler EJ (2013) Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology 38:124–137
Swank MW, Sweatt JD (2001) Increased histone acetyltransferase and lysine acetyltransferase activity and biphasic activation of the ERK/RSK cascade in insular cortex during novel taste learning. J Neurosci 21:3383–3391
Takata A, Ionita-Laza I, Gogos JA, Xu B, Karayiorgou M (2016) De novo synonymous mutations in regulatory elements contribute to the genetic etiology of autism and schizophrenia. Neuron 89:940–947
Tang GB, Zeng YQ, Liu PP, Mi TW, Zhang SF, Dai K, Tang QY, Yang L, Xu YJ, Yan HL, Du HZ, Teng ZQ, Zhou FQ, Liu CM (2017) The histone H3K27 demethylase UTX regulates synaptic plasticity and cognitive behaviors in mice. Front Mol Neurosci 10:267
Taniguchi M, Carreira MB, Cooper YA, Bobadilla AC, Heinsbroek JA, Koike N, Larson EB, Balmuth EA, Hughes BW, Penrod RD, Kumar J, Smith LN, Guzman D, Takahashi JS, Kim TK, Kalivas PW, Self DW, Lin Y, Cowan CW (2017) HDAC5 and its target gene, Npas4, function in the nucleus accumbens to regulate cocaine-conditioned behaviors. Neuron 96:130–144
Twine NA, Janitz K, Wilkins MR, Janitz M (2011) Whole transcriptome sequencing reveals gene expression and splicing differences in brain regions affected by Alzheimer’s disease. PLoS One 6:e16266
Vallianatos CN, Raines B, Porter RS, Wu MC, Garay PM, Collette KM, Seo YA, Dou Y, Keegan CE, Tronson N, Iwase S (2019) Amelioration of brain histone methylopathies by balancing a writer-eraser duo KMT2A-KDM5C. bioRxiv. doi: https://doi.org/10.1101/567917
van Steensel B, Belmont AS (2017) Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell 169(5):780–791
Vaquero A, Loyola A, Reinberg D (2003) The constantly changing face of chromatin. Sci Aging Knowledge Environ 14:RE4
Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA (2007) Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 27:6128–6140
Viosca J, Lopez-Atalaya JP, Olivares R, Eckner R, Barco A (2010) Syndromic features and mild cognitive impairment in mice with genetic reduction on p300 activity: differential contribution of p300 and CBP to Rubinstein-Taybi syndrome etiology. Neurobiol Dis 37:186–194
Waddington CH (1953) Epigenetics and evolution. Symp Soc Exp Biol 7:186–199
Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K (2009) Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138:1019–1031
Wang CM, Tsai SN, Yew TW, Kwan YW, Ngai SM (2010) Identification of histone methylation multiplicities patterns in the brain of senescence-accelerated prone mouse 8. Biogerontology 11:87–102
Wang WH, Cheng LC, Pan FY, Xue B, Wang DY, Chen Z, Li CJ (2011) Intracellular trafficking of histone deacetylase 4 regulates long-term memory formation. Anat Rec (Hoboken) 294:1025–1034
Wang J, Telese F, Tan Y, Li W, Jin C, He X, Basnet H, Ma Q, Merkurjev D, Zhu X, Liu Z, Zhang J, Ohgi K, Taylor H, White RR, Tazearslan C, Suh Y, Macfarlan TS, Pfaff SL, Rosenfeld MG (2015) LSD1n is an H4K20 demethylase regulating memory formation via transcriptional elongation control. Nat Neurosci 18:1256–1264
Wang DY, Kosowan J, Samsom J, Leung L, Zhang KL, Li YX, Xiong Y, Jin J, Petronis A, Oh G, Wong AHC (2018) Inhibition of the G9a/GLP histone methyltransferase complex modulates anxiety-related behavior in mice. Acta Pharmacol Sin 39:866–874
Wei W, Coelho CM, Li X, Marek R, Yan S, Anderson S, Meyers D, Mukherjee C, Sbardella G, Castellano S, Milite C, Rotili D, Mai A, Cole PA, Sah P, Kobor MS, Bredy TW (2012) p300/CBP-associated factor selectively regulates the extinction of conditioned fear. J Neurosci 32:11930–11941
Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G, Wagner J, Häsler LMWK, Skodras A, Blank T, Staszewski O, Datta M, Centeno TP, Capece VIM, Kerimoglu C, Staufenbiel M, Schultze JL, Beyer M, Prinz M, Jucker M, Fischer ANJ (2018) Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556(7701):332–338
Wijayatunge R, Chen LF, Cha YM, Zannas AS, Frank CL, West AE (2014) The histone lysine demethylase Kdm6b is required for activity-dependent preconditioning of hippocampal neuronal survival. Mol Cell Neurosci 61:187–200
Williams SR, Aldred MA, Der Kaloustian VM, Halal F, Gowans G, McLeod DR, Zondag S, Toriello HV, Magenis RE, Elsea SH (2010) Haploinsufficiency of HDAC4 causes brachydactyly mental retardation syndrome, with brachydactyly type E, developmental delays, and behavioral problems. Am J Hum Genet 87:219–228
Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T (2005) Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem 12:111–119
Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T (2006) A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem 13:609–617
Xu J, Deng X, Watkins R, Disteche CM (2008) Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J Neurosci 28:4521–4527
Yamakawa H, Cheng J, Penney J, Gao F, Rueda R, Wang J, Yamakawa S, Kritskiy O, Gjoneska E, Tsai LH (2017) The transcription factor Sp3 cooperates with HDAC2 to regulate synaptic function and plasticity in neurons. Cell Rep 20:1319–1334
Yang YC, Chen CN, Wu CI, Huang WJ, Kuo TY, Kuan MC, Tsai TH, Huang JS, Huang CY (2013) NBM-T-L-BMX-OS01, semisynthesized from osthole, is a novel inhibitor of histone deacetylase and enhances learning and memory in rats. Evid Based Complement Alternat Med 2013:514908. https://doi.org/10.1155/2013/514908
Yang L, Ma Z, Wang H, Niu K, Cao Y, Sun L, Geng Y, Yang B, Gao F, Chen Z, Wu Z, Li Q, Shen Y, Zhang X, Jiang H, Chen Y, Liu R, Liu N, Zhang Y (2019) Ubiquitylome study identifies increased histone 2A ubiquitylation as an evolutionarily conserved aging biomarker. Nat Commun 10(1):2191
Yao ZG, Zhang L, Huang L, Zhu H, Liu Y, Ma CM, Sheng SL, Qin C (2013) Regional and cell-type specific distribution of HDAC2 in the adult mouse brain. Brain Strct Funct 218:563–573
Zhang J, Ji F, Liu Y, Lei X, Li H, Ji G, Yuan Z, Jiao J (2014) Ezh2 regulates adult hippocampal neurogenesis and memory. J Neurosci 34:5184–5199
Zhang YX, Akumuo RC, España RA, Yan CX, Gao WJ, Li YC (2018) The histone demethylase KDM6B in the medial prefrontal cortex epigenetically regulates cocaine reward memory. Neuropharmacology 141:113–125
Zheng Y, Liu A, Wang ZJ, Cao Q, Wang W, Lin L, Ma K, Zhang F, Wei J, Matas E, Cheng J, Chen GJ, Wang X, Yan Z (2019) Inhibition of EHMT1/2 rescues synaptic and cognitive functions for Alzheimer’s disease. Brain 142:787–807
Zhou Q, Obana EA, Radomski KL, Sukumar G, Wynder C, Dalgard CL, Doughty ML (2016) Inhibition of the histone demethylase Kdm5b promotes neurogenesis and derepresses Reln (reelin) in neural stem cells from the adult subventricular zone of mice. Mol Biol Cell 27:627–639
Zovkic IB, Meadows JP, Kaas GA, Sweatt JD (2013) Interindividual variability in stress susceptibility: a role for epigenetic mechanisms in PTSD. Front Psych 4. https://doi.org/10.3389/fpsyt.2013.00060
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fischer, A. (2019). The Role of Dynamic Histone Modifications in Learning Behavior. In: Binder, E., Klengel, T. (eds) Behavioral Neurogenomics. Current Topics in Behavioral Neurosciences, vol 42. Springer, Cham. https://doi.org/10.1007/7854_2019_108
Download citation
DOI: https://doi.org/10.1007/7854_2019_108
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31264-0
Online ISBN: 978-3-030-31265-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)