Abstract
Recent advances in neuroimaging have offered a rich array of structural and functional markers to probe the organization of regional and large-scale brain networks. The current chapter provides a brief introduction into these techniques and overviews their contribution to the understanding of autism spectrum disorder (ASD), a neurodevelopmental condition associated with atypical social cognition, language function, and repetitive behaviors/interests. While it is generally recognized that ASD relates to structural and functional network anomalies, the extent and overall pattern of reported findings have been rather heterogeneous. Indeed, while several attempts have been made to label the main neuroimaging phenotype of ASD (e.g., ‘early brain overgrowth hypothesis’, ‘amygdala theory’, ‘disconnectivity hypothesis’), none of these frameworks has been without controversy. Methodological sources of inconsistent results may include differences in subject inclusion criteria, variability in image processing, and analysis methodology. However, inconsistencies may also relate to high heterogeneity across the autism spectrum itself. It, therefore, remains to be investigated whether a consistent imaging phenotype that adequately describes the entire autism spectrum can, in fact, be established. On the other hand, as previous findings clearly emphasize the value of neuroimaging in identifying atypical brain morphology, function, and connectivity, they ultimately support its high potential to identify biologically and clinically relevant endophenotypes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Autism spectrum disorder (ASD) encompasses a group of early-onset, lifelong, neurodevelopmental conditions currently diagnosed in >1 % of children (CDC 2014). Core behavioral impairments include atypical social cognition, language function, and sensory abnormalities, often together with narrow and repetitive behaviors and interests.
ASD is highly heterogeneous, with significant phenotypic variations across affected individuals. Individuals with ASD show an elevated risk for psychiatric comorbidities and neurological disorders. For example, around 30 % of children with ASD also meet criteria for attention-deficit/hyperactivity disorder (ADHD) (Simonoff et al. 2008); similarly, prevalent subgroups of individuals with ASD present with epileptic seizures (Bolton et al. 2011) or anxiety (van Steensel et al. 2011). Although comorbid conditions often co-occur (Simonoff et al. 2008), the extent to which they cluster in specific patterns is to date unknown. The etiology of ASD is complex and likely not uniform. To that end, an increasing catalog of genetic and developmental risk factors has been identified (De Rubeis and Buxbaum 2015). This remarkable neurobiological variability has challenged the development of novel diagnostic procedures and targeted therapies (Ecker et al. 2015).
Quantitative neuroimaging, particularly magnetic resonance imaging (MRI), offers a rich array of markers that describe the structural and functional organization of the human brain with increasing spatial resolution and validity (Fig. 1). These metrics promise to capture important anatomical aspects in both the typical and atypical brain, and have the potential to be translated into powerful tools that are able to stratify the affected individuals into biological subtypes. This chapter aims to overview the current state of neuroimaging data in ASD. Generally, findings have shown diverse brain anomalies in ASD, ranging from atypical brain growth patterns, morphological anomalies in both cortical and subcortical regions, and inter-regional structural as well as functional network alterations. However, a consistent, universally accepted imaging phenotype that adequately describes all facets of the disorder (and reliably characterizes all individuals with ASD) is lacking. While outlining major findings in ASD, we will specifically attempt to emphasize methodological sources contributing to the somewhat low reproducibility of findings in ASD.
Neuroimaging approaches previously applied to study ASD. a Markers of regional morphology: surface-based cortical thickness and cortical folding indices; b Neuroimaging-based structural networks have been derived mainly from diffusion MRI tractography and structural covariance analysis. Diffusion MRI tractography approximates plausible white matter pathways by following the directionality of water diffusion; covariance analysis infers inter-regional networks through the analysis of across-subjects correlations in morphological quantities. c Functional networks can be derived from inter-regional time series correlations
2 Probing Regional Morphology
Among the earliest and most extensively examined structural findings in ASD are those suggesting brain overgrowth. Assessing global brain volume based on MRI, Piven, and colleagues noted increased brain volumes in males with ASD relative to controls. These findings were complemented by cross-sectional assessments of head circumference (a proxy for overall brain growth) by Courchesne et al. (2001), who reported increased head size in up to 90 % of 2–4-year-old boys. As circumference was relatively normal in separate cohorts assessed shortly after birth and during adolescence, the authors suggested that ASD might relate to an atypical developmental trajectory characterized by early overgrowth, followed by normalization in later childhood and adolescence. In a follow-up study, the same group provided more direct evidence for this claim by using longitudinal analyses and pinpointed increases in head size to an interval between 1 and 14 months after birth (Courchesne et al. 2003). Notably, findings indicative of brain overgrowth were subsequently confirmed by several groups (Palmen et al. 2004). Nevertheless, a recent systematic review highlighted potential biases, when comparing head circumference measures in ASD relative to normative data (an approach chosen by some previous studies), in contrast to assessing locally recruited controls (Raznahan et al. 2013). Noteworthy, a recent longitudinal MRI study in newborns with ASD has demonstrated that increases in brain volume may co-occur with excessive cerebrospinal fluid (CSF), particularly in the frontal lobes, from 6 months onward (Shen et al. 2013). On the one hand, these findings suggest that CSF concentrations may confound findings based on global measures of head size. On the other hand, they point to the potential role of CSF circulation in the pathogenesis of ASD and may impact neurogenesis and neuronal migration patterns, eventually contributing to atypical cortical morphology in the disorder. Despite the support for brain overgrowth in ASD, remaining inconsistencies emphasize the need for future studies to prospectively enroll both individuals with ASD and controls, ideally from fetal stages onward to clarify trajectories of early brain development. To identify possible mechanisms that relate to overgrowth in subgroups with ASD, these studies would provide not only head circumference metrics, but also regionally specific measures of brain morphology and connectivity (see below), and are complemented by genetic assessments and monitoring of environmental factors.
Several quantitative neuroimaging analysis approaches have been brought forward to provide a more precise window into the morphology of individual brain regions. Common techniques include volumetry (the manual or automated tracing of a structure of interest to estimate its volume), voxel-based morphometry (VBM, an automated technique that statistically compares spatially normalized estimates of gray matter across subjects), together with surface-based measures of cortical thickness and surface area (the two components of gray matter volume), and gyrification as well as subcortical shape analysis. Using such regionally specific approaches, several studies have suggested gray matter increases in ASD compared to controls, particularly in frontal and temporal lobes in both children (Raznahan et al. 2010) and adults (Ecker et al. 2012). Given the putative functional roles of these areas, fronto-temporal anomalies might be compatible with marked impairments in social cognition and with atypical language often seen in autism (Rojas et al. 2006). Several assessments have indeed suggested a potential link between brain structure and atypical behavior in ASD. For example, fronto-temporal structural changes have been shown to correlate with abnormal sociocognitive and language function (Lai et al. 2014). Moreover, structural imaging studies in healthy populations have reported associations between the structure of similar regions known to play an important role in social cognition, such as the temporoparietal junction (TPJ) and superior temporal sulcus, and autistic traits both cross-sectionally (von dem Hagen et al. 2011) and longitudinally (Wallace et al. 2012)
Despite the frequency of fronto-temporal anomalies being reported in ASD at the group level, they may not be present in all affected individuals. A VBM study in adults and adolescents, for example, has suggested rather diffuse ASD-specific gray matter increases in all but the frontal lobes (Piven et al. 1996). Similarly, a study in children has reported temporal and parietal cortical thickening, while frontal and occipital regions appeared morphologically unaffected (Hardan et al. 2006). The difficulty in synthesizing a consistent pattern of findings is further increased by several studies reporting rather less and not more gray matter in ASD, both in children (Hardan et al. 2006) and in adult samples (Wallace et al. 2010). This high degree of divergence was also revealed in previous meta-analyses, suggesting a rather complex pattern of ASD-related regional anomalies with only modest convergence across studies (Duerden et al. 2012).
It has frequently been suggested that inconsistent findings might have arisen as a function of age of the included cohorts. In other words, individuals with ASD may show a different pattern of changes relative to typical controls when tested at different neurodevelopmental stages and throughout aging. This hypothesis would be compatible with findings suggestive of altered developmental and age-related trajectories in ASD. Indeed, several studies have suggested divergent age effects on brain morphology changes in ASD relative to controls (Raznahan et al. 2010; Wallace et al. 2010; Duerden et al. 2012). Studying a cross-sectional sample of adolescents and young adults with ASD and controls, for example, Wallace and colleagues observed more rapid age-related cortical thinning in temporal and parietal regions compared to controls (Wallace et al. 2010). In two separate longitudinal studies, one including individuals aged 3–39 years (Zielinski et al. 2014) and the other including individuals aged 14–24 years (Wallace et al. 2015), researchers have also demonstrated accelerated thinning in ASD in adolescence. Overall, these data indicate that a comprehensive understanding of ASD may likely require accounting for its impact on dynamic brain changes from childhood into adulthood, in which complex morphological anomalies may vary over time throughout the life span.
A further example of heterogeneity of findings in morphological investigations of ASD comes from the study of the amygdala, a structure with a key role in emotional processing. A careful review of the initial inconsistent volumetric findings of amygdala suggested that age of the sample examined may affect the results (Haar et al. 2014). This notion was supported by the reports of increased amygdala volume in toddlers and preschoolers which were related to later measures of joint attention (Mosconi et al. 2009). Beyond age, other potential sources of variability on volumetric findings of the amygdala, such as the role of comorbidity with anxiety disorders or alexithymia (Bird et al. 2010), have been suggested but have not been fully clarified.
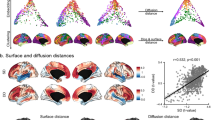
A generally limiting factor of reproducibility of findings relates to low statistical power resulting from relatively small samples studied, a condition often imposed by high costs and challenges associated with recruitment and scanning of individuals with ASD. Recently, the Autism Brain Imaging Data Exchange (ABIDE) initiative made a multicenter repository containing neuroimaging data from 539 individuals with ASD and 573 controls, together with standardized behavioral phenotypic information, freely available (Di Martino et al. 2014). Based on data from three independent ABIDE sites, Valk and colleagues performed a surface-based cortical thickness analysis and assessed overall patterns of findings as well as across-site reproducibility (Valk et al. 2015). Assessing data from more than 200 male participants that passed quality control and surface correction procedures, the authors observed cortical thickness increases in ASD relative to controls, particularly in medial and lateral prefrontal cortices, when pooling data across all imaging sites (Fig. 2a). While the authors reported a generally consistent direction of effects across each of the analysis sites, they emphasized variability in effects sizes across the sites (that may be due to variability in scanning parameters and inclusion criteria), together with increased effects in children compared to adults (Valk et al. 2015).
Recent findings in ASD based on the autism brain imaging data exchange (ABIDE) repository. a Cortical thickness increases in 107 ASD relative to 113 controls, based on the three sites that included children and adult data in both groups. Findings were consistent across all sites studied and measurable in children as well as adults, yet of variable effect size. b Structural covariance alterations in ASD, showing largely decreased covariance in ASD between medial/lateral prefrontal seed regions and posterior midline targets corresponding to the larger precuneus area. Similar to the thickness findings (panel A), findings were consistent across the three sites but of variable effect size. c Functional connectivity alterations showing primarily decreased connectivity between medial frontal and posterior midline regions in ASD relative to controls. Adapted from Valk et al. (2015) and Di Martino et al. (2014) with permission
In addition to methodological factors, diverse findings in human studies may be driven by intrinsic variability across the autism spectrum, which should be more directly addressed for a better understanding of the disorder. At the level of histopathology, several studies have pointed toward a rather complex substrate with multiple etiologies. A postmortem study by Bailey and colleagues, for instance, reported increased cortical thickness and/or increased neuronal numbers in the frontal cortex in 3/6 of cases (Bailey et al. 1998). Another study observed laminar rearrangement together with a poorly defined gray and white matter interface in some specimens (Avino and Hutsler 2010). Such cortical interface blurring is generally considered a common sign of atypical migration and aberrant organization, emerging in prenatal developmental stages. These findings may suggest an increased frequency of processes resembling known malformations of cortical development (MCD, generally considered to be a common cause of epilepsy) in ASD. Notably, several MCDs have indeed been shown to frequently co-occur with ASD, ranging from disruptions of cell proliferation, such as tuberous sclerosis complex, to those occurring during migration and cortical organization, such as polymicrogyria and schizencephaly. Their co-occurrence with ASD underlines that specific, possibly genetically mediated, developmental disruptions may result in variable neuropsychiatric and neurological phenotypes, emphasizing etiological commonalities across different clinicopathological entities.
Variable histopathological findings motivate the use of novel imaging biomarkers in ASD. Surface-based approaches most frequently applied to measure cortical thickness also provide a range of meaningful indices relating to cortical surface geometry (Hong et al. 2015), such as the folding complexity, sulcal depth, surface area, and geodesic distance mapping. Several recent studies have reported alterations in these features (Nordahl et al. 2007) in ASD, enriching the possibilities to perform an integrative neuroimaging phenotyping of the disorder. In a series of studies, Ecker and colleagues compared the spatial distribution of alterations in cortical thickness, volume, and surface area when comparing ASD to controls and reported relatively non-overlapping morphological anomalies across these feature dimensions (Ecker et al. 2013). Moreover, they have shown that patients can be accurately discriminated from controls based on supervised pattern learners that took advantage of these surface-based markers (Ecker et al. 2010). However, replicating these findings and demonstrating their discriminability from a plethora of other neurodevelopmental disorders (akin to differential diagnoses made in the clinic setting) is required before their potential clinical utility can be truly evaluated. It is conceivable that different morphological indices probe complementary facets of cortical architecture, myelination, connectivity, and development. Cortical thickness, for example, is assumed to reflect combinations of neuronal density, dendritic arborization, and myelin (Huttenlocher et al. 1982). Conversely, folding anomalies may indicate disrupted mechanical properties of the cortex, possibly secondary to disruptions in underlying white matter connectivity (Nordahl et al. 2007). Lastly, surface area changes may be driven specifically by radial unit progenitor cells that divide at the ventricular surface, thereby generating more proliferation units that ultimately lead to increased numbers of cortical columns (Ecker et al. 2015). Notably, a previous postmortem study of Casanova and colleagues suggested a smaller width but increased number of so-called mini-columns, neuronal assemblies centered on radially oriented pyramidal neurons, in ASD (Casanova et al. 2002).
3 Disturbances in Inter-regional Networks
Complex patterns of structural alterations in ASD may be reflective of reconfigurations of large-scale brain networks, a key determinant of functional, behavioral, and clinical outcomes. Recent advances in imaging acquisition and analysis techniques offer several opportunities to assess inter-regional structural and functional brain networks.
To study brain structural networks in the living human brain, the most well-established approaches are diffusion MRI tractography and structural MRI covariance analysis. Diffusion MRI data allows for the estimation of directionality and magnitude of water diffusion at each imaging voxel. As diffusivity is influenced by membrane permeability, myelination, and fiber packing (Concha et al. 2010), diffusion markers can serve as proxies of fiber microstructure and architecture. The most widely used parameters are fractional anisotropy (FA), estimating the deviation of water diffusion from random displacement, and mean diffusion (MD), a marker of bulk diffusion. Via tractographic techniques (Mori et al. 1999), it is possible to reconstruct plausible fiber tracts running through the white matter. While challenged close to gray mater regions, such as the neocortex and subcortical nuclei, where multiple fiber populations may intersect or merge (Jones et al. 2012), tractography within deep white matter achieves good correspondence with anatomically plausible tracts. Tractography of several deep bundles has furthermore been directly cross-validated using several different paradigms, for example, with functional connectivity analysis in humans (Johansen-Berg et al. 2005) and using manganese tracing in pigs (Knosche et al. 2015).
Structural networks can also be derived from covariance of MRI-derived morphology across subjects, such as cortical thickness or gray matter density (Lerch et al. 2006). In contrast to diffusion tractography, this framework is not tailored to an approximation of the course of anatomical connections between regions and only shows partial correspondence to diffusion-derived structural connectivity. On the other hand, high correlations in structural markers have been shown to relate to manifestations of persistent functional-trophic cross-talk, maturational interchange, together with common developmental and pathological influences (Alexander-Bloch et al. 2013). Compared to the diffusion anomalies, covariance analysis offers a direct seeding from cortical gray matter in a high-resolution space that only suffers from limited geometric distortions. It, thus, represents a meaningful complementary statistical approach to network mapping. Moreover, given the ubiquity of T1-weighted images in almost every clinical and research protocol, covariance analysis represents a pragmatic and cost-effective approach for network mapping in large populations.
In ASD, both approaches have generally suggested large-scale structural network breakdowns, contributing to the underconnectivity theory of autism. Several diffusion MRI studies, for example, have reported alterations in multiple fiber bundles in ASC (Fletcher et al. 2010; Barnea-Goraly et al. 2004; Sundaram et al. 2008). A recent analysis observed convergent decreases in FA, gray matter volume, and functional integration of the TPJ area in adults with ASD, and suggested an association between diffusion alterations and decreased emotionality (Mueller et al. 2013). Diffusion alterations have recently been confirmed by a systematic meta-analysis of 14 studies; in that report, consistent abnormalities were highlighted especially in pathways mediating frontal connectivity, such as in superior longitudinal and uncinate fasciculi as well as the corpus callosum (Aoki et al. 2013). Interestingly, a recent study showed a similar structural compromise in children with ASD and their unaffected siblings, suggesting a possible genetic basis for white matter anomalies in these conditions (Barnea-Goraly et al. 2010). Complementing diffusion MRI work, covariance analyses in ASD were also suggestive of compromised inter-regional structural integration (Bernhardt et al. 2014). A majority of covariance studies reported reduced network-level embedding of regions primarily involved in social cognition and affective processes, including the TPJ area (Bernhardt et al. 2014) and medial and lateral prefrontal cortices (Valk et al. 2015). In the latter study, the authors observed consistently decreased covariance between medial prefrontal and midline parietal regions across three independent imaging sites, indicating that structural segregation of these networks may be a reproducible finding in ASD (Fig. 2b) (Valk et al. 2015).
In the functional domain, several groups have studied inter-regional functional MRI time series, particularly during task-free conditions. Advantages of such resting-state over task-related paradigms include the possibility to examine multiple cortical areas in one relatively short session and relatively little demands on individuals with a reduced ability to perform tasks. Resting-state networks have been shown to be highly reproducible across subjects and are thought to correspond closely to systems engaging in specific tasks (Smith et al. 2009). Comparative (Mantini et al. 2011) and modeling (Honey et al. 2007) studies have furthermore suggested that anatomical pathways, in part, determine functional connections. In ASD, atypicalities in functional coupling of several large-scale networks have been reported, with the majority of findings supporting underconnectivity (Geschwind and Levitt 2007; Gotts et al. 2012), particularly of long-range inter-regional functional associations. Yet, several studies have emphasized that differential head motion may contribute to observing connectivity differences (Power et al. 2012), a finding important to consider given that many studies include children and the elevated comorbidity of ADHD with ASD. Moreover, systematic analyses and multi-method simulations have suggested a sizable impact of analytical choices on group differences reported in functional imaging studies, supporting efforts to establish consistent analytical routines, transparent reporting practices, work on openly accessible data, and more rigorous attempts to cross-validate functional connectivity measures (Muller et al. 2011). Based on ABIDE data, for example, the multi-site analysis by Di Martino and colleagues reconciled seemingly inconsistent findings of under- and overconnectivity in ASD. Evidence of both mechanisms were present but varies as a function of the circuits involved. Underconnectivity encompassed cortico-cortical connections and was predominant; yet, overconnectivity was also present and largely characteristic of subcortico-cortical circuits (Fig. 2c) (Di Martino et al. 2014).
Task-free functional studies suggesting connectivity differences have also been complemented by results based on alternative analytical paradigms and neuroimaging modalities. Task-based studies, for example, have suggested underconnectivity during social cognition tasks in ASD (Kana et al. 2009). On the other hand, a recent magnetoencephalography study suggested that connectivity alterations in ASD relative to controls may vary as a function of the frequency band. Moreover, findings may be different depending on the inclusion of frontal nodes in the analysis (Kitzbichler et al. 2015).
Methodological options for evaluating large-scale networks in ASD have recently been extended by the introduction of graph theory to neuroimaging (Bullmore and Sporns 2009). Systematic connectivity mapping among all pairs of regions (usually derived from parcellations) can be used to build a connectivity matrix, the substrate for graph-theoretical analyses. Brain graphs representing these matrices can be visualized, which facilitate an intuitive understanding of topological properties. Fundamental topological parameters include clustering coefficient, a measure of local network integration that also relates to network stability, and characteristic path length, a proxy for global network efficiency. Graph theory can also be used to calculate the centrality metrics that quantify the embedding of regions within the network; high centrality scores are a feature of hub regions relevant for the overall network architecture and communication.
Graph-theoretical analyses of healthy human brain networks have consistently demonstrated a small-world topology that is characterized by separate groups of densely clustered regions (modules) interconnected by short paths passing through core hub regions (such as the midline parietal cortex). Several neurological and neuropsychiatric conditions, including schizophrenia (Bassett et al. 2008), ADHD (Cao et al. 2013), and epilepsy (Bernhardt et al. 2011, 2015a), have recently been associated with topological disruptions based on this framework. While only relatively few graph-theoretical assessments have been published in the ASD literature to date, initial findings have shown decreased clustering, suggesting that overall connectomic alterations may be indicative of network reorganization toward a more random arrangement (Rudie et al. 2012). These findings have recently been confirmed (Itahashi et al. 2014); moreover, they have been complemented by data indicating shifts in the overall hub organization in ASD (Di Martino et al. 2013).
4 Summary and Outlook
Neuroimaging has greatly advanced our understanding of brain anomalies associated with ASD. As detailed above, the previous literature has revealed a rather complex pattern of structural and functional alterations in cohorts of individuals with ASD. Among structural MRI studies, a frequently reported finding has been gray matter increases in frontal and temporal regions that might be developmentally constrained and related to aberrant growth patterns in early age and possibly to malformation processes. Investigations of functional and structural network alterations have primarily suggested global underconnectivity, together with islands of focal connectivity increases. Initial graph-theoretical reports have suggested an association between ASD and topological randomization, together with shifts in the spatial distribution of network hubs. Nevertheless, findings at all levels have not been free from controversy, and ongoing as well as future efforts to increase homogeneity and transparency of subject inclusion criteria as well as analysis routines are highly recommended.
ASD is likely best understood as a developmentally dynamic disorder, motivating longitudinal studies that carefully assess brain development and aging-related change in large cohorts across the life span. The increasing use of high and ultra-high field MRI has the potential to achieve an adequate resolution to address layer-specific structural as well functional alterations and connectivity arrangements in vivo. This scale of analysis is likely needed to detect possibly an elusive pathological substate in specific ASD subgroups (e.g., those with subtle cortical malformations). Based on increasingly complex and high-dimensional neuroimaging datasets, machine-learning techniques offer an appropriate analytic framework to integrate data and to discover and evaluate imaging biomarkers of ASD. In other brain disorders, these techniques are able to discover latent subtypes within seemingly homogeneous cohorts (Bernhardt et al. 2015b). The high variability documented across prior studies of ASD is indicative of claims that this population is very likely composed of different biological subtypes. Variability across the spectrum, thus, needs to be explicitly addressed in research rather than ignored. In this light, cross-site collaborative efforts to aggregate and share large datasets, such as the ABIDE initiative, represent useful and urgent avenues to pursue in our efforts to better understand the neural underpinnings of the complex autism spectrum.
References
Alexander-Bloch A, Giedd JN, Bullmore E (2013) Imaging structural co-variance between human brain regions. Nat Rev Neurosci 14:322–336
Aoki Y, Abe O, Nippashi Y, Yamasue H (2013) Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Mol Autism 4:25
Avino TA, Hutsler JJ (2010) Abnormal cell patterning at the cortical gray-white matter boundary in autism spectrum disorders. Brain Res 1360:138–146
Bailey A, Luthert P, Dean A et al (1998) A clinicopathological study of autism. Brain 121(Pt 5):889–905
Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL (2004) White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry 55:323–326
Barnea-Goraly N, Lotspeich LJ, Reiss AL (2010) Similar white matter aberrations in children with autism and their unaffected siblings: a diffusion tensor imaging study using tract-based spatial statistics. Arch Gen Psychiatry 67:1052–1060
Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A (2008) Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28:9239–9248
Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N (2011) Graph-theoretical analysis reveals disrupted small-world organization of cortical networks in temporal lobe epilepsy. Cerebral Cortex 21:2147–57
Bernhardt BC, Valk SL, Silani G, Bird G, Frith U, Singer T (2014) Selective disruption of sociocognitive structural brain networks in autism and alexithymia. Cereb Cortex 24:3258–3267
Bernhardt BC, Bernasconi N, Hong S, Dery S, Bernasconi A (2015a) Subregional mesiotemporal network topology is altered in temporal lobe epilepsy. Cereb Cortex (in press)
Bernhardt BC, Hong SJ, Bernasconi A, Bernasconi N (2015b) Magnetic resonance imaging pattern learning in temporal lobe epilepsy: classification and prognostics. Ann Neurol 77(3):436–46
Bird G, Silani G, Brindley R, White S, Frith U, Singer T (2010) Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain J Neurol 133:1515–1525
Bolton PF, Carcani-Rathwell I, Hutton J, Goode S, Howlin P, Rutter M (2011) Epilepsy in autism: features and correlates. Br J Psychiatry 198:289–294
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198
Cao Q, Shu N, An L et al (2013) Probabilistic diffusion tractography and graph theory analysis reveal abnormal white matter structural connectivity networks in drug-naive boys with attention deficit/hyperactivity disorder. J Neurosci 33:10676–10687
Casanova MF, Buxhoeveden DP, Switala AE, Roy E (2002) Minicolumnar pathology in autism. Neurology 58:428–432
CDC (2014) Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and mortality weekly report Surveillance summaries 63:1–21
Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW (2010) In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci 30:996–1002
Courchesne E, Karns CM, Davis HR et al (2001) Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57:245–254
Courchesne E, Carper R, Akshoomoff N (2003) Evidence of brain overgrowth in the first year of life in autism. JAMA 290:337–344
De Rubeis S, Buxbaum JD (2015) Genetics and genomics of autism spectrum disorder: embracing complexity. Human molecular genetics 24(R1):R24–R31
Di Martino A, Zuo XN, Kelly C et al (2013) Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biological psychiatry
Di Martino A, Yan CG, Li Q et al (2014) The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry 19:659–667
Duerden EG, Mak-Fan KM, Taylor MJ, Roberts SW (2012) Regional differences in grey and white matter in children and adults with autism spectrum disorders: an activation likelihood estimate (ALE) meta-analysis. Autism Res 5:49–66
Ecker C, Marquand A, Mourao-Miranda J et al (2010) Describing the brain in autism in five dimensions–magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci 30:10612–10623
Ecker C, Suckling J, Deoni SC et al (2012) Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiatry 69:195–209
Ecker C, Ginestet C, Feng Y et al (2013) Brain surface anatomy in adults with autism: the relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiatry 70:59–70
Ecker C, Bookheimer SY, Murphy DG (2015) Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet neurology 14(11):1121–34
Fletcher PT, Whitaker RT, Tao R et al (2010) Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage 51:1117–1125
Geschwind DH, Levitt P (2007) Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 17:103–111
Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A (2012) Fractionation of social brain circuits in autism spectrum disorders. Brain 135:2711–2725
Haar S, Berman S, Behrmann M, Dinstein I (2014) Anatomical abnormalities in autism? Cereb Cortex
Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ (2006a) An MRI study of increased cortical thickness in autism. Am J Psychiatry 163:1290–1292
Hardan AY, Girgis RR, Lacerda AL et al (2006b) Magnetic resonance imaging study of the orbitofrontal cortex in autism. J Child Neurol 21:866–871
Honey CJ, Kotter R, Breakspear M, Sporns O (2007) Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA 104:10240–10245
Hong S-J, Bernhardt B, Schrader D, Caldairou B, Bernasconi N, Bernasconi A (2015) MRI-based lesion profiling of epileptogenic cortical malformations. In: Navab N, Hornegger J, Wells W, Frangi A (eds) Medical image computing and computer-assisted intervention—MICCAI 2015, Springer, Berlin, pp 501–509
Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H (1982) Synaptogenesis in human visual cortex–evidence for synapse elimination during normal development. Neurosci Lett 33:247–252
Itahashi T, Yamada T, Watanabe H et al (2014) Altered network topologies and hub organization in adults with autism: a resting-state fMRI study. PLoS ONE 9:e94115
Johansen-Berg H, Behrens TE, Sillery E et al (2005) Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex 15:31–39
Jones DK, Knosche TR, Turner R (2012) White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage 73:239–254
Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA (2009) Atypical frontal-posterior synchronization of theory of mind regions in autism during mental state attribution. Soc Neurosci 4:135–152
Kitzbichler MG, Khan S, Ganesan S et al (2015) Altered development and multifaceted band-specific abnormalities of resting state networks in autism. Biol Psychiatry 77:794–804
Knosche TR, Anwander A, Liptrot M, Dyrby TB (2015) Validation of tractography: comparison with manganese tracing. Hum Brain Mapp 36:4116–4134
Lai MC, Lombardo MV, Ecker C et al (2014) Neuroanatomy of individual differences in language in adult males with autism. Cereb Cortex
Lerch JP, Worsley K, Shaw WP et al (2006) Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. NeuroImage 31:993–1003
Mantini D, Gerits A, Nelissen K et al (2011) Default mode of brain function in monkeys. J Neurosci Official J Soci Neurosci 31:12954–12962
Mori S, Crain BJ, Chacko VP, van Zijl PC (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269
Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J (2009) Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry 66:509–516
Mueller S, Keeser D, Samson AC et al (2013) Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism: a multimodal MRI study. PLoS ONE 8:e67329
Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK (2011) Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex 21:2233–2243
Nordahl CW, Dierker D, Mostafavi I et al (2007) Cortical folding abnormalities in autism revealed by surface-based morphometry. J Neurosci 27:11725–11735
Palmen SJ, Hulshoff Pol HE, Kemner C et al (2004) Larger brains in medication naive high-functioning subjects with pervasive developmental disorder. J Autism Dev Disord 34:603–613
Piven J, Arndt S, Bailey J, Andreasen N (1996) Regional brain enlargement in autism: a magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry 35:530–536
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2152
Raznahan A, Toro R, Daly E et al (2010) Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex 20:1332–1340
Raznahan A, Wallace GL, Antezana L et al (2013) Compared to what? Early brain overgrowth in autism and the perils of population norms. Biol Psychiatry 74:563–575
Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR (2006) Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry 6:56
Rudie JD, Shehzad Z, Hernandez LM et al (2012) Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex 22:1025–1037
Shen MD, Nordahl CW, Young GS et al (2013) Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain 136:2825–2835
Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G (2008) Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 47:921–929
Smith SM, Fox PT, Miller KL et al (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045
Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC (2008) Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex 18:2659–2665
Valk SL, Di Martino A, Milham MP, Bernhardt BC (2015) Multicenter mapping of structural network alterations in autism. Hum Brain Mapp 36:2364–2373
van Steensel FJ, Bogels SM, Perrin S (2011) Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev 14:302–317
von dem Hagen EA, Nummenmaa L, Yu R, Engell AD, Ewbank MP, Calder AJ (2011) Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cereb Cortex 21:493–500
Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A (2010) Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain 133:3745–3754
Wallace GL, Shaw P, Lee NR et al (2012) Distinct cortical correlates of autistic versus antisocial traits in a longitudinal sample of typically developing youth. J Neurosci 32:4856–4860
Wallace GL, Eisenberg IW, Robustelli B et al (2015) Longitudinal cortical development during adolescence and young adulthood in autism spectrum disorder: increased cortical thinning but comparable surface area changes. J Am Acad Child Adolesc Psychiatry 54:464–469
Zielinski BA, Prigge MB, Nielsen JA et al (2014) Longitudinal changes in cortical thickness in autism and typical development. Brain 137:1799–1812
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bernhardt, B.C., Di Martino, A., Valk, S.L., Wallace, G.L. (2016). Neuroimaging-Based Phenotyping of the Autism Spectrum. In: Wöhr, M., Krach, S. (eds) Social Behavior from Rodents to Humans. Current Topics in Behavioral Neurosciences, vol 30. Springer, Cham. https://doi.org/10.1007/7854_2016_438
Download citation
DOI: https://doi.org/10.1007/7854_2016_438
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47427-4
Online ISBN: 978-3-319-47429-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)