Abstract
When rats engage in playful interactions, they emit appetitive 50-kHz ultrasonic vocalizations (USVs). We investigated the role of 50-kHz USVs in the playful behavior of both juvenile and adult rats. A cohort of juvenile rats was surgically devocalized and allowed to interact with either devocalized or intact partners as juveniles and again as adults. A substantial decrease in playful motivation was seen for pairs of devocalized rats, as well as all intact rats housed with devocalized ones. In pairs in which at least one partner could vocalize, there was no difference in the number of playful interactions as compared to controls. Further investigation revealed that, within the playful episode itself, 50-kHz USVs are more likely to appear before a playful attack is launched than after, regardless of the attacking partner’s ability to vocalize, and when one partner is pinned on its back by another, it is the rat that is on top that is more likely to emit 50-kHz USVs. These findings suggest that, for juveniles, 50-kHz USVs may have a critical function in maintaining and facilitating playful motivation, but a more limited role in signaling playful actions. In adults, however, whatever the motivational role of such calling may be, the various kinds of USVs appear to serve critical communicatory functions. For instance, when pairs of adult males that are unfamiliar with one another encounter each other in a neutral arena, they play together, but if one partner is devocalized, there is a significantly higher likelihood that the interaction will escalate to become aggressive. While the relative roles of appetitive 50-kHz and aversive 22-kHz USVs in this context remain to be determined, our overall findings for play in both juveniles and adults suggest that 50-kHz USVs likely have multiple functions, with different functions being more prevalent at some ages and contexts than others.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
One of the most challenging forms of play is rough-and-tumble play or play-fighting, in which pairs of animals typically compete for some advantage over one another (Aldis 1975). This advantage, often involving contacting some target on the body (Aldis 1975; Pellis 1988), does not involve the unrestrained competition often seen in serious fighting (Blanchard and Blanchard 1994), but rather involves some measure of restraint that leads to play-fighting being reciprocal. Such reciprocity has been characterized by the 50:50 rule, whereby each player wins about 50 % of its playful encounters (Altmann 1962). Subsequent game theory models have shown that, as win–loss ratios deviate from 50:50, play-fights become progressively less stable. This is not to say that in some cases, the win–loss ratio cannot deviate from 50:50 (for review see Pellis et al. 2010), but what it does suggest is that play-fighting will not remain playful if one partner attempts to dominate the encounters completely. Indeed, some empirical studies have shown that, when individuals do attempt to dominate playful interactions completely, their potential play partners ostracize them, reducing their ability to engage in further interactions (e.g., Suomi 2005).
To maintain the reciprocity needed for play-fights to remain playful, animals have to follow rules of restraint (Pellis et al. 2010), which requires them to monitor both their own actions and those of their partner, to evaluate any potential transgressions of the rules. To be precise, this requires that, while engaged in play, animals need to assess whether an inappropriate action by a partner is a one-off act of exuberance or a deliberate bending of the rules. The possibility of deliberate transgressions becomes particularly likely as animals become sexually mature and increasingly use play-fighting as a tool for social assessment and manipulation (Palagi 2011). Indeed, comparative data suggest that social play is a more demanding activity than nonsocial play. Comparative analyses in primates have shown that the size of socioemotional brain systems increases in species that engage in more play-fighting, but not in species that engage in more nonsocial play (i.e., locomotor-rotational play, object play) (Graham 2011). One of the mechanisms thought to be involved in maintaining play-fighting, not only in primates but also in other species, is the use of play signals to negotiate interactions (Palagi et al. 2015). Rats not only engage in complex patterns of play-fighting, but they also use signals that can potentially serve the negotiating functions needed to maintain playfulness (Pellis and Pellis 2009).
2 Play Behavior in Rats
Rats engage in a variety of forms of play, including playing with inanimate objects, solitary locomotor-rotational play, and play-fighting (Hole and Einon 1984). However, even though locomotor-rotational play and play-fighting in rats can be very complex, object play is limited, and by far, it is play-fighting that occupies the majority of their time when they are playing (see Pellis and Pellis 2009 for a review). Not surprisingly, the rat has been an important model species for the study of the behavioral, developmental, and neurobiological mechanisms underlying mammalian play-fighting (e.g., Siviy and Panksepp 2011; Vanderschuren and Trezza 2014).
In rats, play-fighting involves attack and defense of the nape of the neck, which is then nuzzled with the snout when contacted (Pellis and Pellis 1987; Siviy and Panksepp 1987). Such dorsal contact by one partner is defended against by the recipient by using one of two major classes of defensive tactics: (1) evasion, in which the rat turns to look away from the oncoming attacker and swerves, leaps or runs away, and (2) facing defense, in which the rat turns to face the attacker and uses a variety of movements to block access to its nape. Facing defense, in turn, can involve two different classes of tactical maneuvers: (1) rotation around a vertical axis, usually the mid-body or pelvis, thus maintaining a prone position, and (2) rotation around the longitudinal axis, with either the whole body rotating so that the defender lays supine on its back or with only the forequarters rotating so that the defender still maintains contact on the ground with at least one hind foot (Himmler et al. 2013). If successfully executed, the defender can then launch counterattacks of its own, which, if successful, can lead to a role reversal as the original attacker defends itself (Kisko et al. 2015a). Moreover, the attacker may execute movements that facilitate successful counterattacks by the defender (Pellis et al. 2005), thus ensuring reciprocity. Regardless of the pattern of defense used, the repeated attacks and defense often lead to one rat lying on its back and its partner standing over it in a pinning configuration (Panksepp 1981).
Due to the repeated cycles of attack, defense, and counterattack, play-fighting in rats is thought to be more complex than that reported in many other species of rodents (Pellis and Pellis 1998) and as complex as that reported in many species of primates and carnivores (Pellis and Pellis 2009). Consequently, play-fighting in rats, like in many primates (Palagi 2011), involves complex cognitive assessments and the regulation of emotions (Pellis et al. 2014). To maintain such complex processes and thus allow playful interactions to proceed, rats likely depend on the use of play signals.
3 Play Signals
Because the contact involved in play-fighting can be similar to that occurring in other social contexts, such as aggression and courtship, it has been hypothesized that animals can use play signals to inform their partners that the contact is playful (Fagen 1981). While play signals can be used to make amends if one animal is too rough in its actions (Aldis 1975), the traditional role of such play signals has been thought to be to inform a potential play partner that the imminent contact is playful (Bekoff 1975). Play signals can be produced in several sensory modalities, including olfactory (Wilson 1973) and auditory ones (Kipper and Todt 2002), but ones involving visual cues are the most widely reported (Palagi et al. 2015). Among canids and primates, facial gestures provide the richest source of signaling (Bekoff 1975; van Hoof 1967), but bodily movements and positions are also prevalent (Yanagi and Berman 2014). In rats, facial gestures are limited to basic ones exhibiting pleasure and revulsion (Berridge and Robinson 2003), and there is no evidence of olfactory signals being used in play (Hole and Einon 1984). Rats have a rich repertoire of jumps, twists, turns, and runs that are performed during playful interactions (Pellis and Pellis 1983), and these could potentially serve as play signals. Other rodents with complex playful wrestling, such as hamsters, do not have these bodily gyrations (Pellis and Pellis 1988), yet mice that do not engage in playful wrestling have a varied repertoire of jumps and rotations (van Oortmerssen 1971). Therefore, it is unlikely that all these bodily gestures function as play signals. Nonetheless, some of these jumps performed by rats are produced in contexts that are consistent with them being useful for facilitating play (Pellis and Pellis 1983); this suggests that some may serve as communicatory functions. More likely to function as play signals, however, are the rich diversity of ultrasonic vocalizations (USVs) that are emitted in a variety of prosocial contexts, including play-fighting (Burgdorf et al. 2008; Knutson et al. 1998; Wright et al. 2010).
4 Ultrasonic Vocalizations in Rats
Rats are able to emit sounds in the ultrasonic range, termed USVs. Typically, three main categories of USVs are distinguished, of which all serve distinct communicative functions as socioaffective signals (for a detailed overview, see Brudzynski 2013; Wöhr and Schwarting 2013). Infant rats emit 40-kHz USVs following separation from their mother and littermates. These 40-kHz USVs elicit maternal behaviors, most notably, search and retrieval behavior (Wöhr and Schwarting 2008). In juvenile and adult rats, two main USV types occur, with their occurrence strongly depending on the emotional valence of the situation. Low-frequency 22-kHz USVs occur in aversive situations and particularly high rates are observed during aggressive encounters of adult male rats (Lehman and Adams 1976; Lore et al. 1976; Sales 1972b; Sewell 1967). They likely reflect a negative affective state. In contrast, high-frequency 50-kHz USVs are observed in appetitive situations, most notably in juveniles both during rough-and-tumble play with peers (Burgdorf et al. 2008; Knutson et al. 1998; Wright et al. 2010) and when tickled by a human (Panksepp and Burgdorf 2000). However, some negative affective situations, such as resident–intruder tests, will also elicit 50-kHz USVs (Takahashi et al. 1983). In adulthood, 50-kHz USVs mainly occur during mating (Sales 1972a), but can also be seen in other rewarding situations, such as when given food (Burgdorf et al. 2000) and psychoactive drugs (Burgdorf et al. 2001, 2008). It is widely believed that they reflect a positive affective state. In the first study on 50-kHz USVs emitted during rough-and-tumble play, Knutson et al. (1998) showed that the emission of 50-kHz USVs is positively correlated with dorsal contacts during play and that 50-kHz USVs occur in anticipation of play. As described by Wöhr et al. (2015), they further found that rats exposed to a brief period of social isolation emitted more than twice as many 50-kHz USVs and that they played more vigorously than group-housed controls, possibly due to an increase in social motivation. In contrast, an aversive stimulus, such as a bright white light, led to a reduction in 50-kHz USV emission. In a subsequent study, Burgdorf et al. (2008) found that, of the many 50-kHz USV subtypes, the frequency-modulated (FM) 50-kHz USVs occur at particularly high rates during rough-and-tumble play. These subtypes are also greatly increased following a brief period of social isolation and are most closely associated with the occurrence of dorsal contacts during play, but are negatively correlated with pinning behavior. Interestingly, in rats selectively bred for low 50-kHz USV emission rates, rough-and-tumble play is altered and characterized by fewer dorsal contacts but more pinning behavior (Webber et al. 2012). Moreover, in rats selectively bred for low or high anxiety-related behavior, we found that highly anxious rats initiate less rough-and-tumble play and emit fewer 50-kHz USVs, possibly reflecting lack of positive affect (Lukas and Wöhr 2015). As the breeding lines differ in their hypothalamic vasopressin availability and vasopressin is strongly implicated in the regulation of social behavior, we further tested whether manipulating the vasopressin system alters the emission of 50-kHz USVs during rough-and-tumble play. While the administration of synthetic vasopressin did not alter rough-and-tumble play and the concomitant emission of 50-kHz USVs, blocking the central vasopressin system by means of a vasopressin 1a receptor antagonist resulted in lower levels of play behavior and fewer 50-kHz USVs (Lukas and Wöhr 2015). This indicates that the central vasopressin system is involved in the regulation of affiliative communication in rodents, which is of translational relevance because various findings repeatedly link alterations in the central vasopressin system to autism in humans. Recently, we further showed that rats exposed to valproic acid during pregnancy emit fewer 50-kHz USV during rough-and-tumble play (Raza et al. 2015). Exposure to valproic acid, which is a drug typically used to treat epilepsy and bipolar disorder, is one of the major environmental risk factors for developing autism in humans (Moore et al. 2000) and has been shown to induce autism-like phenotypes when administered to pregnant rats (Schneider and Przewłocki 2005).
5 High-Frequency 50-kHz USV as Play Signals?
The close relationship between the play behavior and the emission of 50-kHz USVs suggests that 50-kHz USVs might serve a communicative function as play signals. If 50-kHz USVs are being used as traditional play signals, signifying “I want to play with you” (Bekoff 1975), then the most important characteristic would be that they occur most frequently preceding playful attacks. In a recent study (Himmler et al. 2014), we provided support for such use of 50-kHz USVs in juvenile rats. We showed that there were significantly more 50-kHz USVs emitted preceding playful contact compared to when rats cease contact. We also showed that, consistent with other studies (Lukas and Wöhr 2015), 50-kHz USVs occur more often in males than in females during play-fighting. This sex difference may be associated with the play of males tending to be rougher (Pellis et al. 1997). Rougher play poses a bigger threat in escalating to serious aggression and so may be more reliant on play signals to avoid such escalation (Palagi et al. 2015). Furthermore, because of the variety of 50-kHz USVs, we also explored whether particular 50-kHz USV subtypes are associated with the onset of specific defense tactics. In the Himmler et al. (2014) study, the most frequently emitted 50-kHz USV subtype was the trill, but this subtype was not significantly associated with any specific defensive action. Short calls, although less frequent, mainly occurred when the defender used an evasive tactic. These findings, especially those showing the high frequency of calling preceding contact, provide compelling evidence supporting the traditional function of play signals, that of advertising imminent contact of one partner by another (Bekoff 1975). In this study, however, both rats could vocalize, so any particular call could not be empirically attributed to either partner. Therefore, it cannot be certain whether the rat launching the attack was in fact the one vocalizing prior to making contact.
A procedure to overcome this dilemma is surgical devocalization, which has been previously used to study the communicative function of USVs in adult rats (Lehman and Adams 1976; Takahashi et al. 1983; Takeuchi and Kawashima 1986; Thomas et al. 1983). Therefore, using pairs of juvenile rats in which one partner was vocal and the other devocalized, we examined which partner, prior to a playful attack, was vocalizing (Kisko et al. 2015a). It was predicted that, when a devocalized rat attacks a vocal partner, there should be very few, if any, 50-kHz USVs being emitted prior to that attack. However, this was not the case, and, in fact, the number of 50-kHz USVs emitted prior to an attack when the devocalized partner attacked was comparable to the number of 50-kHz USVs emitted when a vocal rat was attacking. That is, the same pattern (Fig. 1) that was found whether both partners could vocalize (Himmler et al. 2014) or only one could do so (Kisko et al. 2015a). Moreover, we found no difference in the subtypes of 50-kHz USV emitted, irrespective of which partner was attacking (Kisko et al. 2015a). These findings suggest that the rats are not only using 50-kHz USVs to announce an attack but also to solicit playful contact from a partner.
Percentage (mean and SEM) of 50-kHz USVs emitted immediately before playful contact and immediately following the termination of contact. More 50-kHz USVs are emitted prior to contact whether the attacker is the one able to vocalize or not (*p < 0.05; the control pair is from Himmler et al. 2014; the graph is a combined data set from Himmler et al. 2014 and Kisko et al. 2015a)
Tickling juvenile rats by a human experimenter elicits high rates of 50-kHz USVs (Panksepp and Burgdorf 2000); this action is thought to mimic rough-and-tumble play between two rats. In particular, when tickled, rats roll over onto their backs, thus adopting a configuration similar to that of the pinning present in play-fighting. This suggests that rats produce many calls while on their backs. If this were the case, it would seem reasonable that many, if not the majority of 50-kHz USVs emitted during play-fights, should be emitted by the rat that is being pinned.
Contrary to expectation, data analyzed from our pairs of rats in which one partner was devocalized (Kisko et al. 2015a) revealed that more 50-kHz USVs occurred when the vocal rat was pinning the devocalized rat than when the devocalized rat was pinning the vocal rat (Fig. 2). However, given that the rate of pinning by devocalized rats was low, data based on six pairs of rats, even though significant, should be considered preliminary. If substantiated by further studies, these observations would suggest that it is not the tickling of the belly itself that elicits 50-kHz USVs during play-fighting, but rather, it is the rat on top—the “tickler”—that receives the most enjoyment, thus emitting more 50-kHz USVs. Some level of resistance by the partner being attacked seems to be critical in motivating playful attacks (Pellis and McKenna 1995), so that initiating, soliciting, and gaining contacts together ensure rewarding tactile experiences during play. The presence of 50-kHz USVs in all these phases of play-fighting provides support for the hypothesis that 50-kHz USVs express the rats’ positive affective state and so function to maintain the animals’ playful motivation.
In further support of the hypothesis that juvenile rats are using 50-kHz USVs to keep the mood playful and in doing so maintain playful interactions, we found that pairs of devocalized rats had a reduced frequency of playful interactions (Kisko et al. 2015a). When compared to pairs of vocal rats, devocalized pairs had almost 50 % fewer play-fights (Fig. 3). This suggests that 50-kHz USVs are being used to promote and maintain a playful mood and, in their absence, the rats are not nearly as motivated to engage in play. It is possible that this playful mood is linked to dopamine. Studies have shown that play-fighting is associated with the release of dopamine in the nucleus accumbens (Trezza et al. 2010) and that activation of the mesolimbic dopamine system induces the production of 50-kHz USVs (Burgdorf et al. 2001, 2007). Using the playback paradigm, we found that hearing 50-kHz USVs results in increased neuronal activity (Sadananda et al. 2008) and dopamine release (Willuhn et al. 2014) in the nucleus accumbens. This suggests that the release of dopamine in the nucleus accumbens is linked to both the production and the perception of 50-kHz USVs, possibly indicating that dopamine release in the nucleus accumbens functions as a translator of a motivational acoustic signal into a prosocial action. Such a perception-and-action loop is particularly relevant for appetitive social and reciprocal communicatory signals, with 50-kHz USVs reflecting a positive affective state in the sender and evoking a similar affective state in the receiver, thus promoting positive social interactions.
Number of playful attacks (mean and SEM) initiated by pairs of intact rats reared with other intact rats (control pairs), by pairs of devocalized rats (devocalized pairs), and by pairs of intact rats reared with devocalized partners (intact pairs) in 10-min trials. Both the devocalized rats and the intact cage mates of devocalized rats exhibit a reduced motivation to engage in play (*p < 0.05)
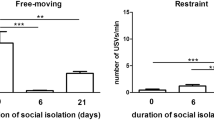
Interestingly, the playful mood can be reinstated to the typical control levels, seen in pairs of vocal rats, by pairing a devocalized rat with an unfamiliar vocal partner (Kisko et al. 2015b). This provides further support for the motivational role of 50-kHz USVs. The motivational role of 50-kHz USVs, however, may have a critical learning period. We observed that, in juveniles, the overall playful motivation was not only decreased in pairs of devocalized rats but was also significantly decreased in pairs of vocal rats that were housed with devocalized cage mates (Fig. 3). Juvenile cage mates often engage in playful interactions together, and it is possible that, in this critical learning period for juveniles, a vocal rat playing with a devocalized cage mate may not receive the necessary feedback from hearing 50-kHz USVs to learn about their contextual uses. That is not to say that the calls themselves are learned, but rather, that the proper context for their use in some situations could be learned through play (see, for further evidence, Wöhr et al. 2015). The animals in our study were housed in quads of two devocalized and two intact rats, so one would think that when the vocal cage mates played together it would be sufficient for them to learn the contextual cues, but this does not appear to be the case. In support of this critical learning period for juveniles, we have recently shown that prolonged social isolation in the four weeks after weaning, the juvenile period when play-fighting is most frequent results in a lack of appropriate behavioral responses toward 50-kHz USVs (Seffer et al. 2015). Specifically, while group-housed controls displayed social approach behavior in response to 50-kHz USVs, a response that is even more prominent in rats isolated for 24 h, rats exposed to long-term, post-weaning, social isolation did not display social approach behavior. Furthermore, these rats even showed some signs of social avoidance. In contrast, no social deficits were seen in rats given comparable levels of long-term social isolation following the juvenile period. Juvenile rats socially isolated for 24 h have an increased motivation to engage in playful interactions (Himmler et al. 2013); however, this increase can be curtailed by placing them with less playful partner. For example, a partner treated with scopolamine, a cholinergic antagonist, will explore the enclosure in which it is placed, but will not initiate playful attacks or respond to a playful attack (Pellis and McKenna 1995). Such a partner elicits playful attacks initially, but prolonged exposure to such a partner leads to reduced playful motivation in the un-drugged animal, as evidenced by a decrease in initiating playful attacks (Pellis and McKenna 1995). Furthermore, social play generally occurs only when a rat is free from physiological and social stress (Siviy et al. 2006). The decreased motivation to play that is seen in the devocalized cage mates could, in turn, negatively impact the playful motivation of the vocal cage mates. As a result, if a lack of playful motivation is consistent and prolonged, as would be the case for the vocal cage mates of the devocalized rats, the vocal rats may become depressed or stressed and thus much less motivated to play.
As well as regulating playful mood, 50-kHz USVs may also serve other important communicatory functions. For rats, pinning and being pinned during play-fighting appears to be highly rewarding and is thus a substantial component within their playful repertoire (Panksepp 1981). In a study by Siviy and Panksepp (1987), it was found that deafened rats pinned less, suggesting that not being able to hear 50-kHz USVs decreases the desire for close bodily contact in playful situations. Similarly, it was hypothesized that devocalized pairs would also show a reduction in playful pinning defenses, but the opposite turned out to be true (Kisko et al. 2015a). Pairs of devocalized rats had a higher frequency and preference for contact-promoting playful defenses than the intact control pairs. One hypothesis to explain these results could be that the 50-kHz USVs are acting as contact calls to help localize the partner within the play arena. Being nocturnal, the majority of playful interactions in rats take place in the dark, and so, being able to signal their location to their partner in a non-visual manner would be beneficial. If this were so, this could explain why devocalized pairs prefer to stay in close contact, in that it would avoid spending long amounts of time searching for one another in a test arena. Therefore, we predicted that, if a vocal rat were paired with a devocalized partner, the devocalized rat would adopt the typical playful defense tactics seen in control rat pairs, since calls from the vocal rat would provide the means to locate that partner. That is, by being able to hear their play partner’s 50-kHz USVs and adopting the more typical tactics of defense, the devocalized rats would return to the pinning frequencies present in control pairs. However, even when paired with a vocal partner, the devocalized animals still appeared to prefer to use contact-promoting defense tactics significantly more often than evasive defense tactics. This suggests that the change in defensive actions by the devocalized rats is not to compensate for the absence of 50-kHz USVs. Therefore, at least within the confines of the test arena used in this study, the results do not support the contact call hypothesis.
Moreover, when given the choice of being presented in the same test arena, vocal rats are no more attractive as a play partner than are silent ones (Kisko et al. 2015a). Indeed, even when confronted with unfamiliar animals, the rats were just as likely to launch playful attacks on devocalized partners as they were on vocal ones (Kisko et al. 2015b). That is to say, among juveniles, there is little evidence that rats use 50-kHz USVs as traditionally conceived play signals (Bekoff 1975; Palagi et al. 2015)—they appear to be unnecessary for both initiating playful contact and in soliciting playful contact. That for juvenile rats 50-kHz USVs do not appear to provide rewarding social incentives (Willey and Spear 2012) is consistent with these findings (although see below). Rather, the role of 50-USVs seems more closely tied to regulating playful motivation and possibly in promoting the development of prosocial neural systems.
A commonly used measure of playful motivation is the frequency with which rats initiate playful contacts on the nape of their partner (Himmler et al. 2013). Such attacks are diminished when pairs of devocalized rats are tested together (Kisko et al. 2015a). Moreover, role reversals, in which the original defender launches a successful counterattack, forcing the original attacker to defend itself, are also reduced in such pairs (Kisko et al. 2015a). Given that the frequency of such counterattacks are decreased in tandem with initiating attacks (Pellis and Pellis 1990), the reduced frequency of role reversals is also likely to reflect a reduction in the motivation to play. That these reductions are, at least in part, due to an acute effect of the absence of 50-kHz USVs on playful motivation is suggested by the restoration of a high frequency of playful attacks when devocalized rats are tested with unfamiliar, vocal partners (Kisko et al. 2015b). However, that some of this effect is due to a more chronic influence of lack of exposure to normal levels of 50-kHz USVs over a prolonged period is shown by the finding that the vocal partners of devocalized cage mates also show a depressed level of initiating playful attacks (Fig. 3). In addition, the altered pattern of playful defense present in devocalized rats (Kisko et al. 2015a) is not ameliorated when playing with an unfamiliar, vocal partner (Kisko et al. 2015b), further suggesting deeper organizational changes in brain development due to a chronic lack of vocalizing. All our devocalized rats received their surgeries at around postnatal day 25, an age within a critical period for the development of several neurotransmitter and neuropeptide systems implicated in the regulation of social behavior (Trezza et al. 2010). The observation that the rats with sham surgeries did not display the same changes in play-fighting implicates the role of cutting laryngeal nerves, and the associated elimination of the ability to produce 50 or 22-kHz USVs, in these developmental disturbances.
Unlike the study by Wiley and Spear (2012), some playback studies have shown that 50-kHz USVs do appear to provide rewarding social incentives. For instance, Burgdorf et al. (2008) found that rats will nose-poke to elicit playback of 50-kHz USVs. Moreover, we showed that playback of 50-kHz USVs results in social approach behavior in the recipient (Wöhr and Schwarting 2007; Willuhn et al. 2014), and as described by Wöhr et al. (2015), this response is present in both juveniles and adults. However, as already mentioned, long-term, post-weaning social isolation results in a lack of social approach behavior in response to 50-kHz USVs (Seffer et al. 2015). These latter findings are consistent with the notion that the juvenile period is an important one for the development of the neural systems associated with 50-kHz USV production. Thus, given the possibility that the neural systems associated with the production of USVs and those associated with the regulation of social behavior overlap in their development, the changes in social play wrought by chronic devocalization in the early juvenile period that we have found (Kisko et al. 2015a, b) may not be coincidental. Such effects may be used as a vehicle for exploring how these neural systems may interact.
6 High-Frequency 50-kHz USVs as Appeasement Signals?
As noted above, for play-fighting to remain playful, the participants need to exercise some degree of reciprocity. Transgressions can lead to the partner escalating the encounter into serious aggression. Among juveniles, such escalation is rare, but not absent (Fagen 1981). It has been suggested that play signals can be used in such situations to de-escalate the encounter with the transgressor effectively using the signal to inform the partner that “it was only play” (Aldis 1975). That is, the signal can be used to appease the partner. In rats, play-fighting can also occasionally escalate into serious fighting, which can be unambiguously identified as when the rats stop attempting to nuzzle each others’ napes and instead switch to bite the partner’s lower flanks and rump (Pellis and Pellis 1987, 1990). If 50-kHz USVs are used as signals to de-escalate the risk of a playful encounter turning into aggression, then, in the absence of these calls, such escalation should be more likely. For none of our juvenile experimental animals were play-fights found to escalate into aggression—not when devocalized rats played together or when devocalized rats played with vocal partners (Kisko et al. 2015a). Even when tested with unfamiliar partners, so eliminating the possibility that rats with an established relationship can use other means to avoid escalation, there was no evidence that play-fights were more likely to escalate to aggression when one of the rats could not vocalize (Kisko et al. 2015b). The situation appears to be different when adult rats are involved.
In some species, adults also engage in play-fights, at which age it is likely to be used for social assessment and manipulation (Palagi 2011). Among adult male rats, dominance relationships can be negotiated with play-fights (Pellis and Pellis 2009). Within colonies of familiar rats, subordinate males will initiate and engage in a more gentle form of play with a dominant male. Furthermore, they will initiate less play with other subordinates, and when they do play together, it will be rougher. When unfamiliar adult rats encounter one another in a neutral arena, they can engage in a rough form of play-fighting which can lead to the establishment of a dominance relationship. When neither member of a pair adopts a submissive status, the encounter can escalate into serious fighting (reviewed in Pellis and Pellis 2009). It is hypothesized that, if 50-kHz USVs serve an important communicative function as appeasement signals, then this should become apparent when unfamiliar, adult males encounter one another in a neutral arena.
In pairs in which one play partner is devocalized, the risk of the interaction becoming aggressive is significantly higher than in pairs in which both rats can vocalize (Kisko et al. 2015b). In fact, in all pairs that included an unfamiliar devocalized partner, there were both agonistic displays, such as piloerection, lateral displays, and tail wiggles, and aggressive attacks, in which one partner directs bites at the flanks of the opponent. Such agonism was rare in the pairs in which both rats could vocalize, and their encounters never escalated to biting. This strongly indicates that, in potentially risky and ambiguous situations, adults may rely on 50-kHz USVs to modify each other’s behavior tactically in a way that is not essential among juveniles. These findings are thus consistent with ones that show that 50-kHz USVs are used as signals in agonistic encounters in adult rats.
In resident–intruder tests, in which an unfamiliar adult male is placed in the home cage of a resident male, the resident typically attacks the intruder (Blanchard and Blanchard 1994), and in such encounters, 50-kHz USVs are frequently emitted (Sales 1972b; Sewell 1967). Moreover, rats are even found to emit 50-kHz USVs when entering an area associated with the potential presence of an aggressor, with the number of 50-kHz USVs emitted by the intruder being positively correlated with the number of aggressive encounters it has experienced in this enclosure (Tornatzky et al. 1994, 1995). Importantly, devocalization (Takahashi et al. 1983; Thomas et al. 1983) and pharmacological (Vivian and Miczek 1993) studies have implicated the intruder as the source of the 50-kHz USVs. Together, these findings indicate that 50-kHz USVs are emitted as a signal of appeasement, thus reducing the likelihood of being attacked by the resident.
It should be noted that devocalization abolishes not only the ability to produce 50-kHz USVs, but also 22-kHz USVs, and there is evidence for 22-kHz USVs being used as an appeasement signal, but results are conflicting. For instance, it was reported that in the resident–intruder paradigm, aggressive behavior is rarely observed following the emission of 22-kHz USVs (Lehman and Adams 1976; Lore et al. 1976; Sales 1972b; Sewell 1967); yet devocalization experiments do not support the idea that 22-kHz USV emission modulates the aggressive behavior of the resident (Lehman and Adams 1976; Takeuchi and Kawashima 1986; Thomas et al. 1983). Thus, in the neutral test arena that we used (Kisko et al. 2015b), either 50-kHz USVs alone, 22-kHz USVs alone, or some combination of both may be used to diminish the likelihood of escalation from playful to serious fighting.
7 Conclusion
50-kHz USVs are emitted at a high frequency during play-fighting among juvenile rats (Burgdorf et al. 2008; Knutson et al. 1998; Wright et al. 2010). Examination of when these calls occur during play-fights shows that they are most likely to occur immediately prior to playful contact (Himmler et al. 2014), and this is true whether the attacker can vocalize or not (Kisko et al. 2015a). These findings suggest that the production of USVs is integral to play and that they may provide important communicatory functions. Such vocalizations may serve two kinds of communicatory functions that have been traditionally postulated for play signals (Bekoff 1975; Palagi et al. 2015): that of informing a potential recipient of a playful attack and that the imminent contact will be playful or that of a recipient soliciting such an attack from a nearby partner. However, our findings with devocalized rats indicate that play can occur in the absence of these presumed communicatory functions, at least among juveniles (Kisko et al. 2015a, b). More consistent with our data is the hypothesis advocated by Knutson et al. (1998) and supported by others that 50-kHz USVs are an expression of the positive affective state associated with play (Burgdorf et al. 2008). In this context, if these calls do serve a communicatory role, it is an indirect one, that of maintaining the playful mood of the producer and/or the receiver of the calls (Kisko et al. 2015a, b). From a developmental perspective, the production and perception of such calls may be important for the maturation of neural and behavioral systems that are associated with prosocial behavior. The case for a communicatory role of USVs is more compelling for adult males engaging in playful interactions with unfamiliar partners. In the absence of such calling, even when just one member of the pair cannot vocalize, there is a marked increase in the likelihood that play-fights escalate into aggression. Given the evidence from resident–intruder encounters (e.g., Lore et al. 1976; Sales 1972b), it seems highly likely that USVs, be they 50-kHz USVs, 22-kHz USVs, or both, are being used as appeasement signals to attenuate the risk of playful encounters escalating into aggression (Kisko et al. 2015b). Therefore, it seems that 50-kHz USVs may have multiple functions, and these may differ across different stages of development.
Further developmental studies are needed to understand the roles of maturation and learning in being able to emit 50-kHz USVs in contextually relevant ways during social interactions. Surgical devocalization provides a clear way to examine the role of such vocalizations and enables observers to identify, unambiguously, the rat that is vocalizing when only one member of a dyad is devocalized (Kisko et al. 2015a, b; Thomas et al. 1983). However, the technique is highly invasive and may have potential long-term side effects that have not yet been investigated. Therefore, other methods that do not require surgery to identify which member of the pair is calling would be helpful. One such technique, the use of multiple microphone arrays, has been successfully used to examine the emission of USVs during mating and other social interactions in mice (Neunuebel et al. 2015). However, play-fighting in rats is often very vigorous and fast-paced, with the rats alternating between wrestling and running. While worth trying, it seems unlikely that the multiple microphone array technique would be able to distinguish which rat is calling in all situations during play. Another new and potentially useful technique is one being used in songbirds, in which an ultraminiature backpack is used to record sound and acceleration in the bird carrying the pack (Anisimov et al. 2014). The small backpack, which weighs only 2.6 g, is harnessed on the bird’s back. Moreover, the weight of the backpack can be further decreased, to 1.4 g, if necessary. This backpack monitoring system may be an ideal way to record the vocalizations of individual rats or mice. Nonetheless, the utility of this technique in recording vocalizations from individual pair mates during play-fights needs to be evaluated, as the presence of the backpacks may inhibit the play or modify the play that is performed. After all, as already noted above, rolling over on to their backs is an important part of playful wrestling, and the presence of a backpack may hamper such behavior. Also, it is possible that the vigorous nature of play may dislodge the device or obstruct the recording abilities of the microphone. Irrespective of these concerns, the value of gaining information on the vocalizations emitted by individuals during social encounters, and do so while avoiding the potential side effects of surgical manipulation, is so great that these techniques should be tested empirically as tools for studying the USVs used during play-fights.
References
Aldis O (1975) Play fighting. Academic Press, New York, NY
Altmann SA (1962) Social behavior of anthropoid primates: analysis of recent concepts. In: Bliss EL (ed) Roots of behavior. Harper, New York, pp 277–285
Anisimov VN, Herbst JA, Abramchuk AN, Latanov AV, Hahnloser RH, Vyssotski AL (2014) Reconstruction of vocal interactions in a group of small songbirds. Nat Methods 11:1135–1137
Bekoff M (1975) The communication of play intention: are play signals functional? Semiotica 15:231–239
Berridge KC, Robinson TE (2003) Parsing reward. Trends Neurosci 26:507–513
Blanchard RJ, Blanchard DC (1994) Environmental targets and sensorimotor systems in aggression and defense. In: Cooper SJ, Hendrie CA (eds) Ethology and psychopharmacology. Wiley, New York, NY, pp 133–157
Brudzynski SM (2013) Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol 23:310–317
Burgdorf J, Knutson B, Panksepp J (2000) Anticipation of rewarding brain stimulation evokes ultrasonic vocalizations in rats. Behav Neurosci 97:320–327
Burgdorf J, Knutson B, Panksepp J, Ikemoto S (2001) Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci 115:940–944
Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J (2007) Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res 182:274–283
Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J (2008) Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol 122:357–367
Fagen R (1981) Animal play behavior. Oxford University Press, New York
Graham KL (2011) Coevolutionary relationship between striatum size and social play in nonhuman primates. Am J Primat 73:314–322
Himmler BT, Pellis VC, Pellis SM (2013) Peering into the dynamics of social interactions: measuring play fighting in rats. J Vis Exp 71:e4288
Himmler BT, Kisko TM, Euston DR, Kolb B, Pellis SM (2014) Are 50-kHz calls used as play signals in the playful interactions of rats? I. Evidence from the timing and context of their use. Behav Process 106:60–66
Hole GJ, Einon DF (1984) Play in rodents. In: Smith PK (ed) Play in animals and children. Blackwell, Oxford, pp 95–117
Kipper S, Todt D (2002) The use of vocal signals in the social play of Barbary macaques. Primates 43:3–17
Kisko TM, Euston DR, Pellis SM (2015a) Are 50-kHz calls used as play signals in the playful interactions of rats? III. The effects of devocalization on play with unfamiliar partners as juveniles and as adults. Behav Process 113:113–121
Kisko TM, Himmler BT, Himmler SM, Euston DR, Pellis SM (2015b) Are 50-kHz calls used as play signals in the playful interactions of rats? II. Evidence from the effects of devocalization. Behav Process 111:25–33
Knutson B, Burgdorf J, Panksepp J (1998) Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol 112:65–73
Lehman MN, Adams DB (1976) A statistical and motivational analysis of the social behaviors of the male laboratory rat. Behaviour, LXI:3–4
Lore R, Flannelly K, Farina P (1976) Ultrasounds produced by rats accompany decreases in intraspecific fighting. Aggress Behav 2:175–181
Lukas M, Wöhr M (2015) Endogenous vasopressin, innate anxiety, and the emission of pro-social 50-kHz ultrasonic vocalizations during social play behavior in juvenile rats. Psychoneuroendocrinology 56:35–44
Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T, Dean JCS (2000) A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet 37:489–497
Neunuebel JP, Taylor AL, Arthur BJ, Egnor SR (2015) Female mice ultrasonically interact with males during courtship displays. eLife 4:e06203
Palagi E (2011) Playing at every age: modalities and potential functions in non-human primates. In: Pellegrini AD (ed) Oxford handbook of the development of play. Oxford University Press, Oxford, pp 70–82
Palagi E, Burghardt GM, Smuts B, Cordoni G, Dall’Olio S, Fouts HN, Řeháková-Petrů M, Siviy SM, Pellis SM (2015). Rough-and-tumble play as a window on animal communication. Biol Rev, in press
Panksepp J (1981) The ontogeny of play in rats. Dev Psychobiol 14:327–332
Panksepp J, Burgdorf J (2000) 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav Brain Res 115:25–38
Pellis SM (1988) Agonistic versus amicable targets of attack and defense: consequences for the origin, function and descriptive classification of play-fighting. Aggress Behav 14:85–104
Pellis SM, McKenna MM (1995) What do rats find rewarding in play fighting? An analysis using drug-induced non-playful partners. Behav Brain Res 68:65–73
Pellis SM, Pellis VC (1983) Locomotor-rotational movements in the ontogeny and play of the laboratory rat (Rattus norvegicus). Dev Psychobiol 16:269–286
Pellis SM, Pellis VC (1987) Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat (Rattus norvegicus). Aggress Behav 13:227–242
Pellis SM, Pellis VC (1988) Play-fighting in the Syrian golden hamster (Mesocricetus auratus waterhouse), and its relationship to serious fighting during post-weaning development. Dev Psychobiol 21:323–337
Pellis SM, Pellis VC (1990) Differential rates of attack, defense and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol 23:215–231
Pellis SM, Pellis VC (1998) The play fighting of rats in comparative perspective: a schema for neurobehavioral analyses. Neurosci Biobehav Rev 23:87–101
Pellis SM, Pellis VC (2009) The playful brain: venturing to the limits of neuroscience. Oneworld Press, Oxford
Pellis SM, Pellis VC, Himmler BT (2014) How play makes for a more adaptable brain: a comparative and neural perspective. Am J Play 7:73–98
Pellis SM, Pellis VC, Foroud A (2005) Play fighting: aggression, affiliation and the development of nuanced social skills. In: Tremblay R, Hartup WW, Archer J (eds) Developmental origins of aggression. Guilford Press, New York, NY, pp 47–62
Pellis SM, Pellis VC, Reinhart CJ (2010) The evolution of social play. In: Worthman C, Plotsky P, Schechter D, Cummings C (eds) Formative experiences: the interaction of caregiving, culture, and developmental psychobiology. Cambridge University Press, Cambridge, pp 404–431
Pellis SM, Field EF, Smith LK, Pellis VC (1997) Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci Biobehav Rev 21:105–120
Raza S, Himmler BT, Himmler SM, Harker A, Kolb B, Pellis SM, Gibb R (2015) Effects of prenatal exposure to valproic acid on the development of juvenile-typical social play in rats. Behav Pharmacol 26:707–719
Sadananda M, Wöhr M, Schwarting RKW (2008) Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci Lett 435:17–23
Sales GD (1972a) Ultrasound and mating behaviour in rodents with some observations on other behavioural situations. J Zool 168:149–164
Sales GD (1972b) Ultrasound and aggressive behaviour in rats and other small mammals. Anim Behav 20:88–100
Schneider T, Przewłocki R (2005) Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30:80–89
Seffer D, Rippberger H, Schwarting RKW, Wöhr M (2015) Pro-social 50-kHz ultrasonic communication in rats: post-weaning but not post-adolescent social isolation leads to social impairments—phenotypic rescue by re-socialization. Front Behav Neurosci 9:102
Sewell GD (1967) Ultrasound in adult rodents. Nature 215:512
Siviy SM, Panksepp J (1987) Sensory modulation of juvenile play in rats. Dev Psychobiol 20:39–55
Siviy SM, Panksepp J (2011) In search of the neurobiological substrates for social playfulness in mammalian brains. Neurosci Biobehav Rev 35:1821–1830
Siviy SM, Harrison KA, McGregor LS (2006) Fear, risk assessment, and playfulness in the juvenile rat. Behav Neurosci 120:49–59
Suomi SJ (2005) Genetic and environmental factors influencing the expression of impulsive aggression and serotonergic functioning in rhesus monkeys. In: Tremblay RE, Hartup WW, Archer J (eds) Developmental origins of aggression. Guilford Press, New York, pp 63–82
Takahashi LK, Thomas DA, Barfield RJ (1983) Analysis of ultrasonic vocalizations emitted by residents during aggressive encounters among rats (Rattus norvegicus). J Comp Psychol 97:207–212
Takeuchi H, Kawashima S (1986) Ultrasonic vocalizations and aggressive behavior in male rats. Physiol Behav 38:545–550
Thomas DA, Takahashi LK, Barfield RJ (1983) Analysis of ultrasonic vocalizations emitted by intruders during aggressive encounters among rats (rattus norvegicus). J Comp Psychol 97:201–206
Tornatzky W, Miczek KA (1994) Behavioural and autonomic responses to intermittent social stress: differential protection by clonidine and metoprolol. Psychopharmacol 116:346–356
Tornatzky W, Miczek KA (1995) Alcohol, anxiolytics and social stress in rats. Psychopharmacol 121:135–144
Trezza V, Baarendse PJ, Vanderschuren LJ (2010) The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci 31:463–469
Vanderschuren LJ, Trezza V (2014) What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. Curr Top Behav Neurosci 16:189–212
van Hooff J (1967) The facial displays of the catarrhine monkeys and apes. In: Morris D (ed) Primate Ethology. Weidenfeld & Nicolson, London, pp 9–88
van Oortmerssen GA (1971) Biological significance, genetics, and evolutionary origin of variability in behavior within and between inbred strains of mice (Mus musculus). Behav 38:1–91
Vivian JA, Miczek KA (1993) Diazepam and gepirone selectively attenuate either 20–32 or 32–64 kHz ultrasonic vocalizations during aggressive encounters. Psychopharmacol (Berl.) 112:66–73
Webber ES, Harmon KM, Beckwith TJ, Peña S, Burgdorf J, Panksepp J, Cromwell HC (2012) Selective breeding for 50 kHz ultrasonic vocalization emission produces alterations in the ontogeny and regulation of rough-and-tumble play. Behav Brain Res 229:138–144
Willey AR, Spear LP (2012) Development of anticipatory 50 kHz USV production to a social stimuli in adolescent and adult male Sprague-Dawley rats. Behav Brain Res 226:613–618
Willuhn I, Tose A, Wanat MJ, Hart AS, Hollon NG, Phillips PE, Schwarting RKW, Wöhr M (2014) Phasic dopamine release in the nucleus accumbens in response to pro-social 50 kHz ultrasonic vocalizations in rats. J Neurosci 34:10616–10623
Wilson SC (1973) The development of social behaviour in the vole (Microtus agrestis). Zool J Linn Soc 52:45–62
Wöhr M, Schwarting RKW (2007) Ultrasonic communication in rats: can playback of 50-kHz calls induce approach behavior? PLoS ONE 2:e1365
Wöhr M, Schwarting RKW (2008) Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav Neurosci 122:310
Wöhr M, Schwarting RKW (2013) Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res 354:81–97
Wöhr M, Engelhardt AK, Seffer D, Sungur AÖ, Schwarting RKW (2015) Acoustic communication in rats: effects of social experiences on ultrasonic vocalizations as socio-affective signals. Curr Topics Behav Neurosci, in press
Wright JM, Gourdon JM, Clarke PB (2010) Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacol 211:1–13
Yanagi A, Berman CM (2014) Body signals during social play in free-ranging Rhesus macaques (Macaca mulatta): a systematic analysis. Am J Primatol 76:168–179
Acknowledgements
M.W. is supported by the Deutsche Forschungsgemeinschaft (DFG WO 1732/4-1). S.M.P. is supported by the National Sciences and Engineering Research Council of Canada (NSERC—4058).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kisko, T.M., Wöhr, M., Pellis, V.C., Pellis, S.M. (2015). From Play to Aggression: High-Frequency 50-kHz Ultrasonic Vocalizations as Play and Appeasement Signals in Rats. In: Wöhr, M., Krach, S. (eds) Social Behavior from Rodents to Humans. Current Topics in Behavioral Neurosciences, vol 30. Springer, Cham. https://doi.org/10.1007/7854_2015_432
Download citation
DOI: https://doi.org/10.1007/7854_2015_432
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47427-4
Online ISBN: 978-3-319-47429-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)