Abstract
We provide in this chapter a brief overview of the present knowledge about social memory in laboratory rodents with a focus on mice and rats. We discuss in the first part the relevance of the processing of olfactory cues for social recognition in these animals and present information about the brain areas involved in the generation of a long-term social memory including cellular mechanisms thought to underlie memory consolidation. In the second part, we suggest that sensory modalities beyond olfaction may also be important in contributing to the long-term social memory trace including audition and taction (and vision). The exposure to stimuli activating the auditory system and taction is able to produce interference phenomena at defined time points during the consolidation of social memory. This ability of such—nonsocial—stimuli may provide a new approach to dissect the brain processes underlying the generation of the social memory trace in further studies.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Social memory
- Social discrimination
- Olfaction
- Auditory stimuli
- Behaviour
- Retroactive interference
- Memory consolidation
- Mice
- Rats
Most rodents are social animals, living substantial parts of their lives in societies in which they use complex ways to communicate with each other to form social bonds. The ability to recognize and distinguish between individuals is therefore vital for their lifestyle. Individual recognition is not only important for the formation of parent–offspring bonds but also forms the basis of territorial behaviour, identifying the individual or group, defending resources such as mates, food or nest sites and allowing the detection of intruders and the rejection of strange animals from a social group. Information from a range of senses can be used for discrimination familiar from unfamiliar conspecifics. For rodents, olfaction is their dominant sense and their social behaviour is heavily influenced by the chemosignals secreted by conspecifics. This chapter will focus on selected neurobiological aspects that provide the substrate to success in this complex behaviour, known as social recognition memory. In this context, the second part will be focused on the data obtained in interference studies that shed additional light on the importance of the different sensory modalities involved in social recognition in laboratory rodents.
1 Social Recognition Behaviours and Experimental Paradigms
1.1 Social Recognition Assessment
Social recognition can be assessed in the laboratory in tests that represent different variants of a basic design allowing the measure of familiarity recognition. Most of the studies published so far investigated social memory related to the storage of information about distinct qualities attributed to a specific individual, which allows the identification of this animal upon a subsequent encounter within a relevant time window. The sources for the chemosensory signals used by rodents primarily to identify conspecifics are body fluids as urine or secretions from skin, reproductive tract or specialized scent glands producing pheromones and other semiochemical compounds (Natynczuk and Macdonald 1994; Heiss et al. 2009). There is evidence that each individual has unique composition of its “smell”, often called “olfactory signature”. This olfactory signature is composed of volatile and nonvolatile compounds (Popik et al. 1991; Sawyer et al. 1984) which—after being detected in social encounters—are processed by two segregated neuronal pathways, the main and the accessory olfactory system (Noack et al. 2010), respectively. Several investigations in rats and mice proved the olfactory nature of the recognition cue, since lesions of the olfactory bulb and chemically induced anosmia impair their individual recognition (Popik et al. 1991; Noack et al. 2010).
1.2 Social Recognition Paradigms
Exploiting the spontaneous investigatory behaviour of the animals towards conspecifics including the innate drive to investigate unfamiliar over familiar items, several nonconditioned paradigms have been widely used to measure social recognition in rodents. Basically, all tests rely on the exposure of the subject under study (experimental subject) towards conspecific (stimulus animal) and the monitoring of the behaviour of the experimental subject. In using this principle of experimentation, animals can be tested repeatedly in social recognition memory, i.e. under different treatment conditions, which provides a high statistical power in data analysis and allows the detection of side effects that may affect the animal’s behaviour.
It has to be mentioned that for all paradigms, different versions exist in different laboratories using different exposure times, different inter-trial intervals and/or different stimulus animals. As shown in Table 1, social experiments with gerbils and hamsters have also been performed although not as extensively as with other taxa. In most of them, social scents including urine or secrets from the ventral gland were used instead of stimulus animals. Therefore, these studies should be better called “chemico-sensory” rather than “social”. As outlined below, presenting the odour alone omits the stimulation of other sensory modalities of the experimental subject that may play also an important role not only for the consolidation and durability of the memory trace, but also for the brain areas involved. Nevertheless, studies in gerbils and hamsters suggested that urine by itself is also used as odorant communication in rodents, as the scent marking behaviour corroborates. Scent marks deposited in the environment may communicate information on territory ownership, social, reproductive, health and nutritional status and enable recognition of individuals (Borelli et al. 2009). A good example for the behavioural relevance is the health assessment of conspecifics to minimize the potential exposure to parasites and to avoid contagion: rodents have the innate ability to discriminate between healthy and parasitized conspecifics. Such behaviour is referred as “sick conspecific avoidance” and can be evaluated using a social preference test (Boillat et al. 2015). A similar behaviour is observed in inbreeding avoidance, particularly evident across the mammalian taxa as inbreeding can also cause a reduction in fitness. It has been shown that the attraction of mice to the urinary odours of other mice is subject to a “parent-of-origin” effect which causes both males and females to prefer the odour of urine from mice of an unrelated strain to that of urine from mice of the same strain as their mothers (Isles et al. 2001).
An intensively studied model of social recognition refers to mate recognition in pair-bonding prairie voles in which long-term pair bonds between males and females are formed. Following mating, these animals display a well-characterized suite of behaviours including selective affiliation with the familiar partner and aggression towards unfamiliar conspecifics (Carter et al. 1995). The act of mating in conjunction with the exposure to the odour stimuli of the partner leads to a recognition memory for the partner as well as promoting the formation of a pair bond. Interestingly, these features of pair bonding resemble the imprinting. In the partner preference test, the experimental subject is paired with a sexually experienced male or female for 24 h and allowed to mate (learning). After a defined exposure interval, the second (memory) session offers experimental subjects a choice between two stimulus animals (the previously mated and an unfamiliar conspecific), and durations spent with each, measured as social proximity and immobile social contact, are used to calculate a preference score. Although—compared to rats and mice—little is known about general vole social recognition, the principle mechanisms described so far fit into the theoretical framework generated by rat and mouse studies. Indeed, the main and accessory olfactory bulbs are critical relay stations also to generate partner preference formation in prairie voles (Curtis et al. 2001) (Table 1).
The habituation–dishabituation paradigm was, and still is, one of the most widely used test to study social recognition. The experimental subject is exposed repeatedly to a given unfamiliar stimulus animal, these exposures are knows as habituation sessions and are separated by exposure intervals during which the experimental subject remains undisturbed in its cage. Throughout the time of each exposure, a trained investigator records the duration of the direct sniffing by the experimental subject towards the stimulus animal, mostly of its anogenital and perioral areas. The social investigative response declines upon the number of habituation sessions, since the familiarity towards the stimulus animal increases. In the last session, called dishabituation, the presentation of an unfamiliar stimulus animal is expected to reinstate the initial level of social investigation. Despite the popularity of this test, it presents some difficulties in data interpretation as repeated testing of the same animal can lead to nonspecific behavioural changes, such as sensitization to the testing procedure (Engelmann et al. 1995). Further, this test has only limited suitability to analyse the duration of the recognition memory performance.
Another and more direct way to assess social recognition is the use of the social discrimination paradigm (Engelmann et al. 1995, 2011). This paradigm evolved from the social recognition test (Thor and Holloway 1982) and consists of two sessions. During the first session, a given stimulus animal is introduced in the cage of the experimental subject, allowing the acquisition of its olfactory signature. In the subsequent session, separated by the desired exposure interval, two stimulus animals are introduced at the same time in the experimental subject’s cage, the familiar stimulus animal (presented in the first session) together with an unfamiliar one. Depending on the exposure interval chosen, different memories can be tested (from immediate-term memory lasting minutes to long-term memory lasting several days). The main difference between social recognition test (assessed in the habituation/dishabituation paradigm and in the original social recognition procedures) and social discrimination test is that the later measures the presence or absence of recognition categorically: during choice not only the previously encountered conspecific is presented (=social recognition test) but also—simultaneously—a novel, previously not encountered conspecific. Thus, the experimental subject is allowed to discriminate between both stimulus animals simultaneously in one session. This provides an internal control under identical experimental conditions and allows separating specific from nonspecific effects in pharmacological studies, thereby reducing the number of sessions for a given experimental series. By using different exposure intervals, the social discrimination test enables the investigation of the impact of manipulations on the different “stages” of memory. Moreover, social discrimination allows the emergence of social memory in animals that appeared to possess no social recognition when tested in the habituation/dishabituation test, thus showing a higher sensitivity in assessing this type of memory performance (Engelmann et al. 1995).
Using the social discrimination test, the performance of mice and rats has been investigated and revealed interesting findings: mice show a memory performance that lasts at least 24 h, whereas rats form short-term social recognition memory only (Table 1). A more detailed analysis in male rats revealed recognition memory to be extinct after ~45 min, whereas female rats, exposed to juveniles from both sexes, show a slightly, but significantly longer recognition lasting ~2 h (Dantzer et al. 1987; Engelmann et al. 1998). Although a great amount of studies confirm the absence of long-term memory in rats, it must be noted that some recently published studies suggested that male rats retain social long-term recognition memory for at least one week, attributing the discrepancies with the rest of studies to the different housing conditions (Shahar-Gold et al. 2013) (see 3.1.2 for a more detailed discussion).
Different modifications from the social discrimination test were described. Originally, the stimulus animals were allowed to move freely in the experimental subject’s cage, although there are some variants where they are confined in wired cups, frequently when a three-chambered apparatus is used, referred in the literature as social choice test, test for sociability or social novelty preference. Although this makes “preference” measurements easier, it limits the access of the experimental subjects to the nonvolatile fraction of the olfactory signature of the stimulus animal. However, in particular, rats need direct access to the conspecific’s body surface to show a proper social memory performance (Engelmann et al. 2011). Therefore, confining the stimulus animals in wired cups is of limited suitability for testing this taxon. Another modification is provided by the volatile fraction cage (Engelmann et al. 2011). Here, the juveniles are confined in two tubes separated by two fences from the experimental subject’s cage, preventing direct tactile contact. The tubes are connected to two fan units which provide an air stream towards the experimental subject’s cage, facilitating the access only to the volatile fraction of the olfactory signature of the stimulus animals. The volatile fraction cage is functional for studies aimed at discriminating the relevance of each fraction of the olfactory signature in order to establish the social recognition ability (Noack et al. 2010). Using this test, differences between mice and rats in the processing of the different fractions of the olfactory signatures of respective conspecifics have been confirmed. Mice recognize juvenile conspecifics on the basis of both, the volatile and nonvolatile components of their olfactory signatures. However, mice are also able to form long-term memory by just having access to the volatile fraction. Rats, in contrast, require access to the nonvolatile fraction of the olfactory signature, which is predominantly processed by the accessory olfactory bulb and results in short-term recognition memory only (Noack et al. 2010) (Table 1). Thus, the ability to form a long-term social recognition memory might be linked to the processing of the volatile fraction of the olfactory signature of the conspecific which does not play a significant role for social recognition in rats.
Interestingly, the wealth of data suggests that rats and mice differ concerning the persistence of social recognition memory under similar test conditions. In a seminatural environment, rats (i.e. Rattus norvegicus) show a quite similar social behavioural profile to mice (i.e. Mus musculus) (Eibl-Eibesfeldt 1950, 1952). Therefore, it remains remarkable that under the reported experimental conditions, long-term social memory can be measured in mice only. Recent studies suggest that the lack of being able to monitor long-term memory in rats might be linked not only to the fraction of the olfactory signature used to recognize a conspecific (Noack et al. 2010) but also to the isolation of the experimental subjects during the exposure interval between learning and retrieval (see 3.1.2). Although—considering the impact of interference by encountering conspecifics (see 3.2)—upon the first view contra-intuitive, testing this hypothesis might provide new insight in the social memory formation in rats. A detrimental impact of isolation on adult rats has been described in the context with brain plasticity (Stranahan et al. 2006). Further studies have to reveal the detailed effect of isolation on the performance in the social memory tests discussed here.
Although it is well described that olfaction is the most important sense for rodents to enable social recognition, it is not the only one. Ultrasonic vocalizations have been reported in several rodent species, and the capability to hear and emit these calls has been intensively studied in laboratory mice and rats. Ultrasonic vocalization in pups is thought to modulate mother–offspring interaction during early postnatal days as they decreased as pups grow up. Adult mice and rats instead emit ultrasounds in different social contexts, with species differences being evident. 50–70 kHz vocalizations appeared to be closely linked to facilitate mating and coordinate sexual arousal and are highly present during social investigation/interaction. A recent study showed that vocalizations may contain signatures of individuality and kinship helping to avoid inbreeding, and introduced the possibility to use ultrasonic vocalizations as an index of social memory in female mice. It is of note that this behaviour, monitored as a decreased number of calls emitted by the female during the second encounter with the familiar female stimulus animal, vanished with an exposure interval of 60 min (Moles et al. 2007), allowing to test short-term memory only. The recording and possibly spectrographic analysis of the ultrasonic calls in mice is progressively gaining relevance in the context with social memory testing and could have great impact to bring new information on motivational aspects underlying social behaviour and subjective states related to social interaction.
2 Morphological Substrate and Mechanisms Underlying Social Memory
The ability to recognize, use and behave according to socially relevant information requires a neuronal system that not only processes the information of the perceived social cues but also links it to emotion, motivation and adaptive behaviour. The ability to generate these associations is essential for triggering what we call “memory”. Present research aimed at analysing the brain areas involved in social memory focused on those areas which can easily linked to processing of olfactory cues. As outlined above, in rodents, the olfactory system is the most important sensory system to form social memories. The initial processing of conspecific social cues takes place in the olfactory bulb, a well-described structure, ideal to study the involvement of the different neural substrates from the initial sensory detection through to limbic and higher cortical processing areas, which modulate complex social behavioural responses such as recognition memory. This type of memory is strongly modulated by neurotransmitter systems which act on the transduction and encoding of social information, which at the same time can be modulated by stress and social experiences (van der Kooij and Sandi 2012). There are numerous reviews describing in particular the relevance of vasopressin and oxytocin signalling in social recognition memory, which we highly recommend for further reading about this issue (Neumann and Landgraf 2012; Ferguson et al. 2002; Hammock 2015; Wacker and Ludwig 2012).
2.1 Selected Brain Areas Involved in Social Recognition
In this section, social memory formation will be presented and discussed on the basis of the present knowledge about the brain areas involved. Further, more details will be given on how this initial encounter has led to the formation of a long-term social memory enabling subsequent recognition of the previous encountered social stimulus.
2.1.1 Olfactory Bulb
The origin of segregating volatile odour and pheromone detection in the context with social encounters in rodents, is based on different sensory neurons localized either in the main olfactory epithelium or the vomeronasal organ (the latter is predominantly sensitive to nonvolatile molecules such as pheromones) that provide input to the olfactory bulb. The rodent olfactory bulb in turn is considered as the origin of the two distinct olfactory pathways: the main olfactory pathway and the accessory olfactory pathway, which are thought to transmit differential information about volatile and nonvolatile olfactory stimuli, respectively (Martinez-Marcos 2009). From the olfactory bulb, projections reach secondary and tertiary areas such as the cortex or limbic brain areas including the hypothalamus (Fig. 1). The olfactory bulb is essential for social recognition memory as it provides the first level of processing the olfactory information used to build social memories.
Main brain circuit processing olfactory information linked to social recognition memory in the rodent brain. Nonvolatile stimuli are processed mainly by the vomeronasal organ (VNO) which projects to the accessory olfactory bulb (AOB) transmitting the information to higher limbic and cortical areas essential to form social recognition memory. Volatile stimuli, instead, are mainly processed by the main olfactory epithelium (MOE) that projects to the main olfactory bulb (MOB) and sends information to the primary olfactory cortices from where they will be transferred to tertiary projection areas, including the amygdala and the hippocampus
2.1.2 Medial Amygdala
Different inputs mainly originating in the vomeronasal organ converge in the medial amygdala (MeA), which seems to act as a major site for the integration of accessory and main olfactory pathways. Efferences from the MeA signal back to the accessory olfactory bulb, thereby likely controlling the impact of the nonvolatile fraction of the conspecific’s “olfactory signature” on approach-avoidance behaviour (Fig. 1). Using the social discrimination test, the MeA had been proven to be essential in processing the nonvolatile fraction of the olfactory signature since its blockage immediately before the memory session, but not the learning session, impaired social recognition memory in mice (Noack et al. 2015). Studies in hamsters showed an activation of the anterior MeA in response to both conspecific chemosensory stimuli (Meredith and Westberry 2004). In addition, this area is the site of action of different steroids and neuropeptides, therefore being sensitive to hormonal states and able to strongly modulate social recognition memory through neuropeptides such as oxytocin and vasopressin.
2.1.3 Entorhinal and Perirhinal Cortex
The entorhinal cortex functions as the gateway to the hippocampal formation, because its output, through the perforant pathway, is the major cortical source of input to the hippocampus. Furthermore, together with the subiculum, it also receives the major output from the hippocampus (Witter et al. 1989). The lateral entorhinal cortex is a component of the olfactory cortex, receiving inputs from both the main olfactory system and piriform cortex, and it also provides feedback to these areas, thereby possibly modulating their functions and the olfactory acuity for familiar odours. In addition to the wiring to the hippocampus (Fig. 1), the entorhinal cortex also receives inputs from the perirhinal cortex, amygdala, thalamus, hypothalamus and other modulatory areas. This suggests the entorhinal cortex as a brain area with an integrative function linked to the generation of olfactory cued social memory. Indeed in rodents, lesions of the entorhinal cortex resulted in deficits of short-term odour memory (Kaut and Bunsey 2001). An area closely linked to the entorhinal cortex is the perirhinal cortex, which surrounds the hippocampal formation and receives incoming sensory information from the olfactory cortices. The perirhinal cortex contributes to recognition memories that require long-term storage of conjunctive feature representations, such as the olfactory signature of a conspecific of mice and/or rats (Feinberg et al. 2012). Perirhinal cortex-lesioned animals demonstrate greater levels of impairment as the degree of feature ambiguity increases, together with impairments in distinguishing simultaneously presented stimuli. This suggests that this area might mediate the perceptual disambiguation of overlapping stimulus representations, in addition to support the generation of recognition memory. Individual recognition by male hamsters in the context of the Coolidge effect (i.e. ability to distinguish a novel from a familiar female) was found to be disrupted by lesions of the perirhinal and entorhinal cortices (Petrulis and Eichenbaum 2003). Additionally, neurons in the entorhinal cortex of hamsters were reported to be responsive to individual social odours (Petrulis et al. 2005), supporting its role in social recognition.
2.1.4 Hippocampus
The activation of immediate early genes has been used to study the involvement of specific brain regions in social recognition memory formation after an initial social encounter mimicking the learning session in a social memory test. Male mice (Ferguson et al. 2001; Richter et al. 2005; Engelmann 2009; Samuelsen and Meredith 2011) showed increased c-Fos synthesis in a number of brain regions including the MeA, the medial preoptic area and the piriform cortex, whereas the number of c-Fos-positive cells in the dorsal hippocampal areas was not significantly affected. Although lesions studies in rats tend to confirm a lack of hippocampal involvement in short-term social recognition memory (Bannerman et al. 2001; Squires et al. 2006), permanent hippocampal lesion in mice impaired social recognition memory for a juvenile 30 min after the first exposure without affecting immediate social recognition (Kogan et al. 2000). Recently, the hippocampal area CA2 has been suggested to be critical for this impairment (Hitti and Siegelbaum 2014), since inactivation of CA2 pyramidal cells or lesion in this region impairs social recognition memory without impacting other forms of hippocampus-dependent memory.
The discrepancy between the findings on c-Fos activation in the dorsal hippocampus of mice after the social recognition test and the effect of hippocampal lesions, challenges the interpretation of data from immediate early-gene activation in the context of memory formation and highlights the need to do more accurate quantifications. It has been demonstrated that distinct parts of the hippocampus are involved in different behaviours. This functional dissociation is supported by its anatomical connectivity and gene expression; therefore, a more detailed look at c-Fos synthesis by analysing each subarea might help to clarify its involvement. In addition, it is likely that specific brain regions are only temporarily involved in acquisition, consolidation and/or retrieval encoding, as this time-dependent contribution has been demonstrated for the hippocampus (Kogan et al. 2000).
Different results obtained from hippocampal lesions in rats and mice suggest that the involvement of this brain area in social recognition memory seems to differ between the taxa. Similarly as observed in mice, studies performed on Degus also reported deficits in social recognition caused by hippocampal lesions (Uekita and Okanoya 2011). However, no impairment was observed in hippocampal-lesioned male hamsters tested for the Coolidge effect (Petrulis and Eichenbaum 2003).
To sum up, multiple brain areas downstream of the olfactory bulb and piriform cortex are involved in the processing of the information about the perceived olfactory cues, including the corticomedial amygdala, entorhinal cortex, perirhinal cortex and the hippocampus. Most of these areas are critical for declarative memory such as recognition memory. However, the specific function including the nature of their contribution to recognition memory is not completely understood. Hence, further studies are necessary to reveal which role plays each of these areas in the complex process of social memory formation and, thus, to clarify the inconsistencies in the data available.
2.2 Selected Cellular Mechanisms Activated During the Consolidation of Social Memories
One of the hallmarks of recognition memory is that newly learned information is sensitive to disruption after acquisition. This labile state after learning suggests that a period of consolidation occurs, which may last for hours or even days, before the memory may be called “stable”. It is well known that long-term, but not short-term, social memory requires consolidation and critically depends upon hippocampal functioning in mice (Squires et al. 2006; Kogan et al. 2000). Different studies investigated the molecular mechanisms underlying memory consolidation by producing irreversible lesions in the hippocampus or using drugs which interfere with protein synthesis. These studies revealed two distinct stages of protein synthesis being important for consolidation of olfactory recognition memory in mice: a short-term lasting stage, starting immediately after training and lasting for ~3 h, and a longer lasting stage, starting ~6 h after acquisition and lasting for ~12 h. The consolidation of the memory trace may have reached a reliable stability ~18 h after learning (Richter et al. 2005; Kogan et al. 2000; Wanisch et al. 2008). Analysis of immediate early-genes expression was used to investigate the brain areas involved in each of these stages in mice. The first stage coincided with an increase in the number of c-Fos immunoreactive cells in brain areas associated predominantly with the accessory olfactory bulb, such as the medial preoptic area and the medial nucleus of the amygdala, but also in the main olfactory bulb and piriform cortex (Richter et al. 2005). The relevance of the olfactory bulb was further supported, since the application of anisomycin (considered to act primarily as protein synthesis inhibitor blocking translation of the mRNA to the amino acid sequence) in this area, immediately and 6 h after the learning session, impaired social long-term memory formation in mice (Pena et al. 2014). During the second stage, the function of which depends upon the integrity of the first stage, proteins other than c-Fos are likely to be synthesized, and this process seems to be essential for olfactory engram formation, probably by enhancing intercellular communication (Richter et al. 2005). Although an increased c-fos transcription was not observed in the hippocampus, injection of anisomycin into mice dorsal hippocampus 3 h after the learning session also impaired long-term social recognition memory (Pena et al. 2014). This implies a distinct participation and provides additional information about the molecular basis of the social memory consolidation as tested in social recognition paradigm.
3 Conditions that Influence Social Memory Recognition
In the natural environment, learning episodes do not occur singly but are confronted with other, similar processes. Thus, memory formation takes place while other, potentially competitive, episodes induced by interfering conditions, and also requiring processing in the same areas of the central nervous system, are likely to happen at the same time. These interfering conditions can prevent the acquisition of information and/or impair or interrupt its consolidation. Usually, the laboratory conditions under which the memory tests take place try to avoid interference phenomena by isolating the animals to be tested in separated and undisturbed rooms. Such controlled and established conditions allow studying the effects of additional sensory modalities relevant for social long-term memory formation. Moreover, studies employing interfering conditions may help to better understand the mechanisms underlying the consolidation of the social memory trace. We will subsequently list some conditions that have been described to interfere with social recognition memory as tested in the laboratory.
3.1 Interference by Husbandry and Experimental Procedures
The disruption of cognitive function by stress-inducing elements from both housing and husbandry systems, as well as by experimental procedures, can additionally have potentially serious implications in the subject’s welfare and consequently altering their performance in memory tests (van der Kooij and Sandi 2012; Mendl 1999). Thus, the careful consideration of these conditions and procedures is essential in order to avoid spurious results.
3.1.1 Transportation and Context
It is well established that husbandry procedures can disrupt social memory and induce behavioural changes (Burman and Mendl 2000). As social experiments are often run in rooms specially installed for behavioural testing, but separated from the animal facility, the study of the effects of animal transportation is highly relevant. Transportation immediately before the learning session did not affect the recognition performance measured 24 h later in mice (Engelmann et al. 2011). Moreover, studies in rats showed that transportation of the experimental subject rat 6 h after a 2-h learning session did not impair long-term social recognition memory; however, it did when the transportation was made 0.5 h after the learning session (Moura et al. 2011). Therefore, the timing of transportation seems to be a critical factor that researchers must consider when designing their social recognition memory experiments.
Linked to the transportation, a change of context is often also present. Most behavioural tests take place in different contexts from the one the subjects are familiarized with; thus, a possible interference effect induced by an unfamiliar context was also studied (Zheng et al. 2013; Burman and Mendl 2002). In rats and voles, the exposure to different contextual cues failed to impair the recognition of conspecifics odours, although it interfered with the ability to distinguish between the stimulus animals for some individuals. Thus, the process of learning social identity was robust on familiar territory and comparably variable when social scents were absent. There are no studies available that systematically investigated the possible impact of the testing context on social memory in other taxa.
3.1.2 Isolation
Solitary housing/isolation is a potent stressor for social species, whose effect has been widely studied also in mice and rats. In both taxa, extended isolation leads to the modification of different physiological parameters coinciding with alterations in behaviour including aggression, mating and anxiety-like behaviour. Even more severe consequences on the brain morphology and local gene expression can be found in animals which have been reared in isolation, including a reduction on medial prefrontal cortex volume and changes in the regulation of gene expression. Although the mechanisms by which isolation affects these parameters may be of high interest, we will focus subsequently on the effects of isolation on social recognition in the course of the acute experiment only.
Different studies demonstrated that chronic and acute social isolation disrupts long-term, but not short-term, social memory in mice. Short-term social memory was intact in rats after one week of isolation, suggesting a robust performance unaffected by housing conditions. However, recent studies using the habituation/dishabituation test observed that long-term social isolation during adolescence strongly influenced subsequent social behaviour both in mice and rats (Zhao et al. 2009). This implies that not only acute isolation may have an impact on the behavioural performance but also long-lasting isolation episodes that may have occurred previously in particular during rearing.
Interestingly, several attempts were made to increase the duration of social memory in rats tested in the social recognition test. Most of them involved changing the length of the learning session(s) and altering the housing conditions. Two successive five-minutes learning sessions lead to the presence of social recognition memory in rats 2 h later (Dantzer et al. 1987), although long-term memory was not observed even when the learning session was prolonged up to 0.5 h (Sekiguchi et al. 1991). Group-housed juvenile female rats were tested to discriminate between an unfamiliar social odour and an odour from a cage-mate, under different isolation conditions. Only the rats with relatively short isolation periods prior testing (1 h and 48 h) recognized the odour from the cage-mate, but not the rats isolated for 96 h (Burman and Mendl 2006). These results suggest rats to be able to show longer lasting memories for conspecifics and their odours with significantly longer “learning session” and short isolation periods. Another study reported that social recognition memory in rats may last at least 24 h after 2 h or longer exposure to the conspecific during the learning session, showing for the first time that male rats exhibit long-term social recognition memory (Moura et al. 2010). A subsequent study also showing long-term memory formation in rats was recently published (Shahar-Gold et al. 2013), reporting rapid and profound, but reversible, effects of housing conditions on social recognition memory in adult rats when the learning and the memory sessions took place in a neutral arena. Interestingly, their methodology differed from the other studies published by several parameters; (i) between learning and memory sessions, the experimentator returned the experimental subjects to the home cages and housed them together with their home cage-mates. (ii) The experimentator used a learning session in which the stimulus animals had unrestricted access to the stimulus animal. (iii) However, during the memory session, the stimulus animals were confined to transparent and slotted plastic corrals. As mentioned above, the use of these corrals makes it more difficult for the experimental subject to gain access to the nonvolatile fraction of the olfactory signature which is—according to other studies (Noack et al. 2010)—necessary for rats to recognize the previously encountered conspecific. Interestingly, mice cannot recognize a previously encountered conspecific when during learning both the volatile and nonvolatile fraction of the olfactory signature were available (unrestricted, freely moving access) and during the memory session the experimental subjects have access to the volatile fraction of the olfactory signature of the (familiar) stimulus animal only (Noack et al. 2010). Thus, the manipulations used in the above-mentioned rat study (Shahar-Gold et al. 2013) do not easily explain the detection of long-term social memory in rats which differed from most of the previously published work, in which—moreover—the experimental subjects remained isolated between the learning and memory sessions, in order to avoid interference phenomena (see below). Nonetheless, it is important to note that all the referred studies, using indirect exposures and testing interference phenomena, were performed with animals isolated between the sessions. Besides, in all cases unfamiliar conspecifics were used as interference stimuli. Ongoing studies in our laboratory will further test the impact of social isolation on social recognition memory.
Taken together, the rapid and specific impairment of social recognition memory consolidation largely described in mice and rats suggests that molecular processes in the neuronal network underlying the consolidation of social memory are sensitive to manipulations by ongoing social activity. And probably this sensitivity is the cause for (at least some of) the discrepancies in this field due to the lack of standardized methods and analysis.
3.1.3 Anaesthesia
Some experimental manipulations involve for instance direct administration of substances in defined brain areas during the behavioural testing or briefly before or after a learning session. Therefore, the use of briefly acting anaesthetics is required to avoid stress that may interfere with the memory performance (see above). Moreover, a brief anaesthesia of the animal allows to monitor the successful treatment, rather than infusions in freely moving animals. The potency to interfere with memory varies among the different anaesthetics (Alkire and Gorski 2004), the dosage and the type of learning/memory task under study (Dutton et al. 2001). Among the different anaesthetics available, inhalation anaesthetics are particularly useful, and among them, isoflurane might be the substance of choice. Behavioural studies analysing hippocampus-dependent memory in animals exposed 24 h before the test for 15 min to 2.1 % isoflurane anaesthesia showed no impairment (Fidalgo et al. 2012). This is in line with observations that in adult mice a brief (~5 min), 1 % isoflurane anaesthesia immediately before the learning session failed to affect social recognition memory tested 24 h later (Engelmann et al. 2011). Nevertheless, due to the fact that different anaesthetics are used in the different laboratories, the different dose responses, the time point of administration (with respect to the experimental design) and the different learning tasks are employed; it is difficult to provide a general conclusion about the action of anaesthetics for social memory tests. However, it is important to note that also isoflurane anaesthesia might affect the outcome of social memory testing by directly affecting brain activity in an unintended manner: studies performed in mice hippocampal slices showed that high-dosage isoflurane anaesthesia (0.55 and 0.74 mM) blocked synaptic plasticity in the mouse hippocampus and impaired hippocampal long-term potentiation in a dose-dependent manner (Haseneder et al. 2009), anticipating severe effects on hippocampus-dependent memories such as social recognition memory under these extremely high doses. Therefore, a brief screening is suggested to monitor such effects in the defined experimental setup.
3.2 Interference Depends Upon the Nature of Stimuli and the Timing of Their Presentation
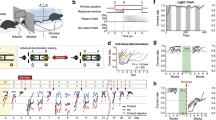
The nature of the interference stimuli and the timing of their presentation during consolidation determine retroactive interference for social recognition memory. Rats showed a short-term memory impairment by retroactive interference due to the exposure to another stimulus animal during the interval between the original learning and memory sessions in the social recognition test (Thor and Holloway 1982; Dantzer et al. 1987). Studies with mice showed that retroactive interference of social memory occurs only during the first 18 h after the original learning session (Engelmann 2009), if memory was tested 24 h later. The first 18 h after learning is a period in which the synthesis of proteins that are obviously required for the consolidation of long-term social recognition memory takes place (Wanisch et al. 2008; Richter et al. 2005). Retroactive interference experiments were done with the social discrimination task using different stimuli activating distinct sensory modalities including taction, audition, olfaction and vision. These stimuli were presented (for 1 min) at different time points after learning during the consolidation process. Interestingly, social recognition memory was sensitive to all stimuli presented within the first hours (up to 6 h in most cases), but not 22 h after the learning session (Perna et al. 2015) (Fig. 2). The insensitivity to all stimuli 22 h after learning might be linked to underlying consolidation processes of the information coding for the originally encountered stimulus animal that seems to be completed at this time point (Richter et al. 2005; Engelmann 2009; Wanisch et al. 2008). These findings highlight the wide diversity of stimuli able to impair social memory formation in mice, which therefore are worth of being considered when designing social memory tests.
Scheme illustrating the time-dependent ability of selected stimuli to interfere with the consolidation of long-term social memory in mice. The green rectangles represent the periods during which social memory consolidation is sensitive to the protein synthesis blocker anisomycin. In addition, interference effects are shown induced by exposure to (i) an unrestricted encounter with an unfamiliar conspecific (pictogram: small mouse), (ii) an object (grey toy brick), a monomolecular odour (grey cloud) and a loud tone (black speaker) at selected time points. Please note that the stimuli used provide different combinations of stimulating sensory modalities including (olfactory, tactile, visual and auditory) and that at least 22 h after the learning session memory performance is insensitive to them. Pure pictograms: induction of interference with social memory when presented at the given time point after learning; pictogram with black cross: failed to induce interference with social memory. All experiments were performed using the social discrimination test with an exposure interval of 24 h
Recently, experiments testing the persistence of the described interference effects during the ongoing consolidation of social memory were performed in our laboratory (Fig. 3). The aim of these experiments was to reveal whether a potential transient retrograde amnesia induced by isoflurane was able to suppress the interference phenomena induced by defined stimuli presented during the consolidation of long-term social memory. Whether this effect was dependent upon the activated sensory modalities was also tested by presenting different stimuli that potentially induced interference with social memory. Among other, an object and an unfamiliar juvenile (interference stimuli) were presented 3 h after the learning session, followed by a brief (~3 min) 1 % isoflurane anaesthesia of the experimental subjects. Social recognition memory towards the familiar stimulus animal (Fig. 3a, juvenile 1) was tested 24 h after the learning session. Interestingly, the brief anaesthesia was able to block the interference caused by the object but failed to block the interference induced by an unfamiliar conspecific (Fig. 3b). These findings shed first light on the processing of the interference-inducing stimuli interacting with ongoing memory consolidation and its timing. In the case of the interference produced by the encounter of the conspecific, obviously the processing of cues acquired via different sensory modalities during the interference session makes the poly-modal representations to compete and thus more difficult to be blocked. In contrast, the potential interference induced by stimuli that activate fewer sensory modalities is sensitive to the central nervous effects produced by the brief isoflurane anaesthesia. It is of note that beyond the missing nonvolatile odours and active movement of the interference stimuli, the main difference between conspecific and object relies on the presence or absence of the generation of social ultrasonic sounds. Sound presentation itself was found to produce interference 6 h after learning (Perna et al. 2015). Although previous studies showed no retrograde amnesia caused by isoflurane as tested in the Pavlovian fear conditioning, we observed that the defined anaesthesia is able to interfere with the processing of nonsocial stimuli and, thus, protects the ongoing consolidation of social memory.
Effects of short-lasting anaesthesia administered immediately after the presentation of a potential interference stimuli, on mice long-term social recognition memory. a Schematic drawing showing the experimental protocol of the experiments: interference stimuli (toy brick, previously not encountered “interference” juvenile) were presented 3 h after learning, followed by a short-lasting 1 % isoflurane anaesthesia. Memory was tested 24 h after the learning session by simultaneous presentation of the previously encountered stimulus juvenile 1 and a novel stimulus juvenile 2 to the experimental subject. b Data obtained with the protocol shown in (a): the Investigation durations of the experimental subject towards the two stimulus juveniles measured during the memory session under the different treatment conditions is represented only. Exposure to isoflurane 3 h after the learning session failed to abolish the significantly reduced investigation duration of the (familiar) juvenile 1 and, thus, did not affect the intact long-term social recognition memory. Anaesthesia blocked the interference effect of the toy brick. No impact of the anaesthesia was observed on the interference effect produced by the “interference” juvenile: in both cases, investigation durations towards the juveniles 1 and 2 were statistically not significantly different. For the pictograms: see legend of Fig. 2. Means + SEM; n = 20. *** p < 0.01; paired student’s t test. Some of the data were obtained from Perna et al. (2015)
4 Conclusions
Social behaviour of rodents relies primarily on the emission and detection of olfactory cues to discriminate between familiar and strange conspecifics. Different methods were developed to study this behaviour including the underlying memory performance in the laboratory. In the course of these experiments, differences between distinct rodent taxa were observed, suggesting under different experimental conditions that some taxa show short-term social memory only (rats), whereas other taxa display long-term social recognition memory (mice, prairie voles). This type of memory is highly susceptible to disruption during the consolidation. Recent studies focused on the phenomena associated with the presentation of stimuli that potentially interfere with social memory at selected time points during its consolidation. The data of these studies revealed the sensitivity of social memory to disruption by different stimuli. It seems clear that the processing of the olfactory signature from a conspecific is superior for the ability to consolidate social memory in mice. However, all sensory modalities activated by the social encounter are likely to contribute to a complex neuronal representation that is required for long-term memory. The processing of the information obtained from the sensory modalities activated by the presentation of an interference stimulus might compete and interfere with the pattern completion necessary to remember the original conspecific. However, once consolidated, social recognition memory is more difficult to disrupt. We now know that the ability to transfer social recognition from short-term into long-term memory involves that odour cues being initially processed by the olfactory system and later distributed to primary, secondary and tertiary processing brain areas in mice. This knowledge provides the basis for the analysis of the impact of other sensory inputs to generate long-term social recognition memory. Indeed, the contribution of ultrasonic vocalization in mice during testing has been widely overlooked so far and provides an interesting substrate for more detailed studies of how the whole processing network encodes and organizes social memories. Understanding the neurophysiological basis of social recognition memory offers the access into the analysis of the development and—possibly also the treatment—of abnormal social disorders in humans.
References
Alkire MT, Gorski LA (2004) Relative amnesic potency of five inhalational anesthetics follows the Meyer–Overton rule. Anesthesiology 101(2):417–429
Bannerman DM, Lemaire M, Beggs S, Rawlins JN, Iversen SD (2001) Cytotoxic lesions of the hippocampus increase social investigation but do not impair social-recognition memory. Exp Brain Res 138(1):100–109
Boillat M, Challet L, Rossier D, Kan C, Carleton A, Rodriguez I (2015) The vomeronasal system mediates sick conspecific avoidance. Curr Biol 25(2):251–255
Borelli KG, Blanchard DC, Javier LK, Defensor EB, Brandao ML, Blanchard RJ (2009) Neural correlates of scent marking behavior in C57BL/6J mice: detection and recognition of a social stimulus. Neuroscience 162(4):914–923
Burman OH, Mendl M (2000) Short-term social memory in the laboratory rat: its susceptibility to disturbance. Appl Anim Behav Sci. 67(3):241–254
Burman OH, Mendl M (2002) Recognition of conspecific odors by laboratory rats (Rattus norvegicus) does not show context specificity. J Comp Psychol 116(3):247–252
Burman O, Mendl M (2006) Long-term social memory in the laboratory rat (Rattus norvegicus) Anim Welf 15(4):379–382
Carter CS, DeVries AC, Getz LL (1995) Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev 19(2):303–314
Curtis JT, Liu Y, Wang Z (2001) Lesions of the vomeronasal organ disrupt mating-induced pair bonding in female prairie voles (Microtus ochrogaster). Brain Res 901(1–2):167–174
Dantzer R, Bluthe RM, Koob GF, Le Moal M (1987) Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology 91(3):363–368
Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger EI 2nd (2001) The concentration of isoflurane required to suppress learning depends on the type of learning. Anesthesiology 94(3):514–519
Eibl-Eibesfeldt I (1950) Beiträge zur Biologie der Haus—und Ährenmaus nebst einigen Beobachtungen an anderen Nagern. Z Tierpsychol 7:558–587
Eibl-Eibesfeldt I (1952) Ethologische Unterschiede zwischen Hausratte und Wanderratte. Verh Deutsch Zool Ges Freiburg, pp 169–180
Engelmann M (2009) Competition between two memory traces for long-term recognition memory. Neurobiol Learn Mem 91(1):58–65
Engelmann M, Wotjak CT, Landgraf R (1995) Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav 58(2):315–321
Engelmann M, Ebner K, Wotjak CT, Landgraf R (1998) Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav Brain Res 90(1):89–94
Engelmann M, Hadicke J, Noack J (2011) Testing declarative memory in laboratory rats and mice using the nonconditioned social discrimination procedure. Nat Protoc 6(8):1152–1162
Feinberg LM, Allen TA, Ly D, Fortin NJ (2012) Recognition memory for social and non-social odors: differential effects of neurotoxic lesions to the hippocampus and perirhinal cortex. Neurobiol Learn Mem 97(1):7–16
Ferguson JN, Aldag JM, Insel TR, Young LJ (2001) Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21(20):8278–8285
Ferguson JN, Young LJ, Insel TR (2002) The neuroendocrine basis of social recognition. Front Neuroendocrinol 23(2):200–224
Fidalgo AR, Cibelli M, White JP, Nagy I, Wan Y, Ma D (2012) Isoflurane causes neocortical but not hippocampal-dependent memory impairment in mice. Acta Anaesthesiol Scand 56(8):1052–1057
Hammock EA (2015) Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology 40(1):24–42
Haseneder R, Kratzer S, von Meyer L, Eder M, Kochs E, Rammes G (2009) Isoflurane and sevoflurane dose-dependently impair hippocampal long-term potentiation. Eur J Pharmacol 623(1–3):47–51
Heiss E, Natchev N, Rabanser A, Weisgram J, Hilgers H (2009) Three types of cutaneous glands in the skin of the salamandrid Pleurodeles waltl. A histological and ultrastructural study. J Morphol 270(7):892–902
Hitti FL, Siegelbaum SA (2014) The hippocampal CA2 region is essential for social memory. Nature 508(7494):88–92
Isles AR, Baum MJ, Ma D, Keverne EB, Allen ND (2001) Urinary odour preferences in mice. Nature 409(6822):783–784
Kaut KP, Bunsey MD (2001) The effects of lesions to the rat hippocampus or rhinal cortex on olfactory and spatial memory: retrograde and anterograde findings. Cogn Affect Behav Neurosci 1(3):270–286
Kogan JH, Frankland PW, Silva AJ (2000) Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10(1):47–56
Martinez-Marcos A (2009) On the organization of olfactory and vomeronasal cortices. Prog Neurobiol 87(1):21–30
Mendl M (1999) Performing under pressure: stress and cognitive function. Appl Anim Behav Sci 65(3):221–244
Meredith M, Westberry JM (2004) Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci 24(25):5719–5725
Moles A, Costantini F, Garbugino L, Zanettini C, D’Amato FR (2007) Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav Brain Res 182(2):223–230
Moura PJ, Meirelles ST, Xavier GF (2010) Long-term social recognition memory in adult male rats: factor analysis of the social and non-social behaviors. Braz J Med Biol Res 43(7):663–676
Moura PJ, Venkitaramani DV, Tashev R, Lombroso PJ, Xavier GF (2011) Transport of animals between rooms: a little-noted aspect of laboratory procedure that may interfere with memory. Behav Process 88(1):12–19
Natynczuk SE, Macdonald DW (1994) Scent, sex, and the self-calibrating rat. J Chem Ecol 20(8):1843–1857
Neumann ID, Landgraf R (2012) Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 35(11):649–659
Noack J, Richter K, Laube G, Haghgoo HA, Veh RW, Engelmann M (2010) Different importance of the volatile and non-volatile fractions of an olfactory signature for individual social recognition in rats versus mice and short-term versus long-term memory. Neurobiol Learn Mem 94(4):568–575
Noack J, Murau R, Engelmann M (2015) Consequences of temporary inhibition of the medial amygdala on social recognition memory performance in mice. Front Neurosci 9:152
Pena RR, Pereira-Caixeta AR, Moraes MF, Pereira GS (2014) Anisomycin administered in the olfactory bulb and dorsal hippocampus impaired social recognition memory consolidation in different time-points. Brain Res Bull 109:151–157
Perna JC, Wotjak CT, Stork O, Engelmann M (2015) Timing of presentation and nature of stimuli determine retroactive interference with social recognition memory in mice. Physiol Behav 143:10–14
Petrulis A, Eichenbaum H (2003) The perirhinal-entorhinal cortex, but not the hippocampus, is critical for expression of individual recognition in the context of the Coolidge effect. Neuroscience 122(3):599–607
Petrulis A, Alvarez P, Eichenbaum H (2005) Neural correlates of social odor recognition and the representation of individual distinctive social odors within entorhinal cortex and ventral subiculum. Neuroscience 130(1):259–274
Popik P, Vetulani J, Bisaga A, van Ree JM (1991) Recognition cue in the rat’s social memory paradigm. J Basic Clin Physiol Pharmacol 2(4):315–327
Richter K, Wolf G, Engelmann M (2005a) Social recognition memory requires two stages of protein synthesis in mice. Learn Mem 12(4):407–413
Richter K, Wolf G, Engelmann M (2005b) Social recognition memory requires two stages of protein synthesis in mice. Learn Memory. 12(4):407–413
Samuelsen CL, Meredith M (2011) Oxytocin antagonist disrupts male mouse medial amygdala response to chemical-communication signals. Neuroscience 180:96–104
Sawyer TF, Hengehold AK, Perez WA (1984) Chemosensory and hormonal mediation of social memory in male rats. Behav Neurosci 98(5):908–913
Sekiguchi R, Wolterink G, van Ree JM (1991) Short duration of retroactive facilitation of social recognition in rats. Physiol Behav 50(6):1253–1256
Shahar-Gold H, Gur R, Wagner S (2013) Rapid and reversible impairments of short- and long-term social recognition memory are caused by acute isolation of adult rats via distinct mechanisms. PLoS ONE 8(5):e65085
Squires AS, Peddle R, Milway SJ, Harley CW (2006) Cytotoxic lesions of the hippocampus do not impair social recognition memory in socially housed rats. Neurobiol Learn Mem 85(1):95–101
Stranahan AM, Khalil D, Gould E (2006) Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci 9(4):526–533
Thor DH, Holloway WR (1982) Social memory of the male laboratory rat. J Comp Physiol Psychol 96(6):1000–1006
Uekita T, Okanoya K (2011) Hippocampus lesions induced deficits in social and spatial recognition in Octodon degus. Behav Brain Res 219(2):302–309
van der Kooij MA, Sandi C (2012) Social memories in rodents: methods, mechanisms and modulation by stress. Neurosci Biobehav Rev 36(7):1763–1772
Wacker DW, Ludwig M (2012) Vasopressin, oxytocin, and social odor recognition. Horm Behav 61(3):259–265
Wanisch K, Wotjak CT, Engelmann M (2008) Long-lasting second stage of recognition memory consolidation in mice. Behav Brain Res 186(2):191–196
Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH (1989) Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol 33(3):161–253
Zhao X, Sun L, Jia H, Meng Q, Wu S, Li N et al (2009) Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment-related gene expression in rats. Prog Neuropsychopharmacol Biol Psychiatry 33(7):1173–1177
Zheng DJ, Foley L, Rehman A, Ophir AG (2013) Social recognition is context dependent in single male prairie voles. Anim Behav 86(5):1085–1095
Acknowledgments
We thank Rita Murau for expert technical assistance. J.C.P was supported by stipends from the Leibniz Graduate School and the Medical Faculty Otto-von-Guericke-Universität Magdeburg.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Camats Perna, J., Engelmann, M. (2015). Recognizing Others: Rodent’s Social Memories. In: Wöhr, M., Krach, S. (eds) Social Behavior from Rodents to Humans. Current Topics in Behavioral Neurosciences, vol 30. Springer, Cham. https://doi.org/10.1007/7854_2015_413
Download citation
DOI: https://doi.org/10.1007/7854_2015_413
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47427-4
Online ISBN: 978-3-319-47429-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)