Abstract
The motivation to engage in social behaviors is influenced by past experience and internal state, but also depends on the behavior of other animals. Across species, the oxytocin (Oxt) and vasopressin (Avp) systems have consistently been linked to the modulation of motivated social behaviors. However, how they interact with other systems, such as the mesolimbic dopamine system, remains understudied. Further, while the neurobiological mechanisms that regulate prosocial/cooperative behaviors have been extensively examined, far less is understood about competitive behaviors, particularly in females. In this chapter, we highlight the specific contributions of Oxt and Avp to several cooperative and competitive behaviors and discuss their relevance to the concept of social motivation across species, including humans. Further, we discuss the implications for neuropsychiatric diseases and suggest future areas of investigation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aggression
- Competitive behavior
- Cooperative behavior
- Dopamine
- Epigenetics
- Neuropsychiatric disorders
- Oxytocin receptor
- Pair bonding
- Social behavior network
- Social communication
- Social recognition memory
- Vasopressin 1a receptor
- Vasopressin 1b receptor
1 Overview
Motivation is a dominant construct in psychology, psychiatry, and neuroscience, as trying to understand why animals, including humans, do what they do is at the core of these disciplines. Although motivation can be defined in a variety of ways, a key component is that motivated behaviors are directed toward (approach) or away (avoidance) from a stimulus. Motivation also contains emotional elements with approach linked to positive hedonic valence and avoidance linked to negative valence. This review focuses on social motivation, which, like other forms of motivation, is influenced by past experience and an individual’s internal state. Social motivation is, however, intrinsically more dynamic and less predictable because the drive to approach or avoid another individual(s) depends in large measure on how that individual behaves.
Recent studies of the neurobiology of social behavior have often characterized social behavior as having a positive valence, described as prosocial or affiliative interactions (e.g.,pair bonding , maternal behavior), or as having a negative valence, described as negative social interactions (e.g., aggression, territoriality). Although such a dichotomy is convenient and can have descriptive value, a closer look at these behaviors suggests that social motivation is more complex. For example, while the formation of a pair bond in a species like prairie voles has positive behavioral elements, such as highly affiliative behaviors directed toward a partner, it is also associated with mate guarding, in which males display selective aggression toward other voles. Thus, in the context of a pair bond, simply ascribing positive valence to the affiliative behaviors and negative valence to aggression is an oversimplification. Further, all aggressive behaviors are not the same, nor are the effects on the players. The fact is, winning is rewarding (Martinez et al. 1995; Meisel and Joppa 1994), and there is even the possibility that losing can be rewarding as long as the defeat is not too severe (Gil et al. 2013). Therefore, assigning hedonic valence to social behaviors (e.g., aggressive behavior) or to mating strategies (e.g., pair bonding) must be done with great care, particularly when linking approach or avoidance with the neural mechanisms underlying motivation.
Historically, investigations into the neurobiology of motivation have primarily focused on the mesolimbic dopamine (DA) system where DA neurons were thought of as “reward” neurons. It has now been recognized that the role of the mesolimbic DA system in hedonic mechanisms is far more complex. Understanding social motivation requires us to expand our studies of the neural mechanisms of motivation beyond this system into the networks that control the expression of social behavior in response to social stimuli. Of the myriad of neurochemical signals that are known to be involved in the modulation of social behaviors, two neuropeptides, oxytocin (Oxt) and vasopressin (Avp), stand out as being critical across species. Because of the vast literature on the role of these two nine-amino acid neuropeptides, or nonapeptides, in regulating social behavior, this chapter will focus on mammalian social behavior and provide examples of the powerful contributions of these two neuropeptide systems to cooperative and competitive behaviors.
2 Origins and Mechanisms of Motivated Social Behaviors
When considering the evolutionary origins of motivated behaviors, most simply put, it all comes down to fitness. Animals engage in species-specific behaviors because over evolutionary time these behaviors were either selected for via natural or sexual selection, or occurred through some other mechanism of evolution. In the context of sexual behavior, males are described as “ardent” and females as “choosy,” which is reflected in their physiology. Males make a lot of sperm and often display behaviors that that will result in the fertilization of as many eggs as possible over their reproductive lifetime. Female mammals on the other hand typically have to invest in the gestation and the care of offspring, so they tend to be more selective about their mates. These differences in selective pressures result in vastly different behavioral displays between males and females. However, these behavioral differences are not limited to sexual behaviors. For instance, in mammals, biparental behavior is scarce, occurring in fewer than 6 % of rodent species and 5–10 % of all mammals (Kleiman 1977). Thus, a male that engages in parental care does so because the “cost” of not being paternal is too high; for instance, while reproductive success may be compromised, his proximity to the female increases the likelihood that he is the sire of the offspring. There are also sex differences in displays of cooperative and competitive behaviors, with females often displaying more cooperative behaviors across the lifetime and males displaying more competitive behaviors, particularly during the breeding season.
In nature, the diversity in cooperativity and competitiveness observed across animal species is striking. Some animals have social structures that are characterized by high levels of cooperativity, such as that observed in species that form long-term social bonds like pair bonds . In other species, high levels of competitive behaviors serve to establish and maintain social dominance relationships. Overlaid on the complexity of social life for a given species is a lack of stability, as social behaviors often change over the seasons and over the lifetime. Further, the different behavioral strategies employed by an animal have their particular costs and benefits. To explore these costs and benefits we can take a closer look at cooperative and competitive behaviors.
2.1 Cooperative and Competitive Behaviors
Cooperative behaviors, associated with affiliative behaviors, are thought to have evolved from reproductive and parental behaviors, in turn being permissive for the development of longer-term social bonds (Crews 1997). Competitive behaviors too are important in the formation of social bonds, as intraspecific interactions are universal and often governed by dominance relationships. Some evolutionary advantages to forming social bonds include localization of resources, lower predation due to group aggression , and increased reproductive opportunities (Alexander 1974). Social bonds have been extensively studied in primates and in some instances have been shown to increase evolutionary fitness (Silk 2007). In free-ranging baboons, females that have strong social bonds with one another live longer than those who have weaker social bonds (Silk et al. 2009). Even in humans social relationships can have profound effects on an individual’s health, including improved mood and a longer life (House et al. 1988; Rodriguez-Laso et al. 2007; Baumeister and Leary 1995).
However, being social does have its cost, such as increased susceptibility to disease, parasites, or injury (Alexander 1974; Crews 1997). In order for animals to live in groups, they must be able to tolerate close proximity; thus, keeping levels of aggression in check becomes particularly important. It also requires a memory of the members of the social group, as this allows animals to identify familiar stimuli, which in turn is permissive for adaptive behavioral responses. While many mammalian species live in groups, some species show “shifts” in the nature of their social interactions depending on where they are in their breeding cycle. Some species display high levels of affiliative behaviors in the non-breeding season, while others increase their intraspecific aggression when resources are scarce (Anacker and Beery 2013).
Over the last several decades, investigation of the neural mechanisms underlying social behavior has focused primarily on “prosocial” behaviors, rather than competitive behaviors. The tremendous progress that has been made in understanding the neural mechanisms underlying phenomena such as maternal behavior and pair bonding has likely contributed to this imbalance. Unfortunately, investigation of more competitive behaviors, such as aggression, has been on the decline for a variety of reasons (see Blanchard et al. 2003). Within studies of competitive behavior, males have been the main experimental subjects, perhaps because of Darwin’s emphasis on male–male competition and female mate choice in the context of sexual selection (Darwin 1871). More recently, however, the importance of competitive behaviors in females has been recognized. Not only do female mammals compete for resources and mates to achieve reproductive benefits, but female competition is widespread in the animal kingdom (Rosvall 2011; Stockley and Bro-Jorgensen 2011; Huchard and Cowlishaw 2011). Females compete for resources such as food, nest sites, and protection using a variety of strategies including intergroup aggression, dominance relationships, and territoriality, as well as through the inhibition of the reproductive capacity of other females. In many primate species female aggression is associated with rank and ultimately reproductive goals (for review, see Stanyon and Bigoni 2014). As a result, investigation of female competitive behavior is essential to understanding social behavior and its translational implications.
In rodents , one reason that there are few data on female competitive behavior is that in commonly studied laboratory species, i.e., rats and mice, females display little or no competitive behavior (Blanchard and Blanchard 2003). This contrasts with what is observed in another laboratory rodent, whose utility as an experimental model continues to increase, Syrian hamsters (Mesoscricetus auratus). Female Syrian hamsters display a range of competitive strategies including the expression of high levels of spontaneous offensive aggression, the rapid formation of robust dominance relationships, and the ability to inhibit the reproductive capacity of other females (Albers et al. 2002; Huck et al. 1988). While there is very little known about the neural mechanisms controlling female offensive aggression in any mammalian species, studies in hamsters have provided a good deal of information about how gonadal hormones influence this form of aggression (Albers et al. 2002).
In males, high levels of aggressive behaviors, intermale and territorial in particular, are often at their peak with the onset of breeding. Other forms of aggression are closely linked to parental behaviors, thus allowing for the defense of young, mates, food, or territories. Seasonal shifts from high cooperativity/affiliative behaviors to competitive behaviors are mediated primarily by changes in gonadal steroids, which are often linked to changes in photoperiod, though numerous neurotransmitter/neuropeptides are also involved. In many mammalian species, androgen concentrations are very high during the breeding season, as they are needed to support reproductive behaviors as well as the physiology of the gonads. During the non-breeding season, seasonal breeders will often undergo a period of gonadal quiescence, whereby the testes shrink in size and levels of circulating androgens plummet. However, it should be noted that in some species, such as hamsters (also seen in birds), androgens, specifically dehydroepiandrosterone (DHEA), produced by the adrenal glands may help to support aggressive behavior during the non-breeding season by serving as a prohormone or neurosteroid for the brain when gonadally derived androgen levels are low (for review, see Soma et al. 2015).
2.2 Brain Areas that Regulate Cooperative and Competitive Behaviors
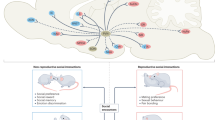
The social behavior neural network (SBNN) hypothesis by Newman (1999) proposes that a network composed of neural groups or “nodes” including, but not limited to, the extended amygdala, the bed nucleus of the stria terminalis (BNST), lateral septum (LS), periaqueductal gray (PAG), medial preoptic area (MPOA), ventromedial hypothalamus (VMH), and anterior hypothalamus (AH) controls social behavior. Each node within the SBNN meets several criteria: reciprocal connectivity, neurons with gonadal steroid hormone receptors, and having been identified as being important to more than one social behavior. The SBNN hypothesis has gained traction in the field in recent years (reviewed by Albers 2012, 2015; Crews 1997; Goodson and Kingsbury 2013). It represents a more nuanced and complicated approach to the understanding of social behavior, as it takes the regulation of these behaviors beyond the examination of just a single neuroanatomical area and supposes that the output of the network is an emergent property. The identification of SBNN for different species is an important next step in understanding the complexity of behavior. While previous approaches have been more simplistic, examining specific neural anatomical areas, single neurotransmitter/neuropeptides, and behaviors, the foundation has now been laid for impactful studies focused on how social behavior emerges from complex neural networks. A large number of different types of motivated social behaviors are thought to be controlled by the SBNN, including offensive and defensive aggression , social recognition memory , parental behavior, and social communication. Importantly, Oxt/Avp and their receptors are found throughout the SBNN and are ideally suited to regulate the expression of social behavior because of their plasticity in response to factors that influence social behavior (Fig. 1) (reviewed in Kelly and Goodson 2014; Goodson and Kingsbury 2013; Albers 2012, 2015; Caldwell et al. 2008a; Adkins-Regan 2009; Bosch and Neumann 2012).
Oxytocin and vasopressin signaling in brain areas important to social motivation. Oxytocin (Oxtr) and vasopressin receptors (Avprs) are found throughout the structures of the social behavior neural network (SBNN) and the mesocorticolimbic dopamine (DA) system. Their localization in these nuclei is critical for oxytocin’s and vasopressin’s modulation of socially motivated behaviors and may serve as the functional connection between the SBNN and DA systems, particularly by their action in the lateral septum (LS) and extended amygdala, including the bed nucleus of the stria terminalis (BNST). Other abbreviations: AH anterior hypothalamus; BLA basolateral amygdala; HIPP hippocampus; MeA medial amygdala; NAcc nucleus accumbens; OB olfactory bulb; PAG periaqueductal gray; PFC prefrontal cortex; POA preoptic area; VP ventral pallidum; VTA ventral tegmental area; VMH ventromedial hypothalamus
Motivated behaviors also arise from a network of reciprocally connected brain regions that determine the salience of stimuli, assign motivational value, and initiate appropriate action (reviewed by Love 2014). The ventral tegmental area (VTA) is a key region in this network in that VTA neurons producing DA project to a large number of cortical and limbic structures, forming the foundation underlying the motivational circuitry. This network plays a critical role in social as well as nonsocial behavior and appears to provide an alerting signal for unexpected stimuli. Within this network, there are distinct groups of DA neurons that determine motivational value, being excited by appetitive stimuli and inhibited by aversive stimuli. Other groups of DA neurons appear to encode motivational salience, but not valence, in that they are excited by the intensity of the stimulus, regardless of whether it is appetitive or aversive (reviewed by Love 2014). See chapters by Bissonette and Roesch, Robinson et al., Redish et al., and Salamone et al. for detailed discussions of these issues.
While the SBNN and the mesolimbic DA system are distinct from one another, they are thought to dynamically interact and support decision making in the context of motivated social behaviors (O’Connell and Hofmann 2011a, b). O’Connell and Hofmann (2011b) have proposed that these two systems should be considered as part of a larger social decision-making network (SDM) that is relatively conserved across species. Across these two systems, there are two neuroanatomical areas, or nodes, of overlap—the LS and the extended amygdala—including the BNST. These areas provide the functional connection between the two systems by acting as relays, providing the SBNN with information from the motivational network about the salience of a social stimulus in turn allowing for an appropriate behavioral response. With these concepts in mind, in the following sections, we will discuss the possibility that Oxt and Avp provide critical links between specific elements within the SBNN and the motivational network that contribute to the motivational forces driving social behaviors.
3 Neuroendocrine Modulation of Social Behaviors
The first hormones implicated in the regulation of social behaviors were the gonadal steroids (Berthold 1849), since changes in social behaviors are observed following gonadectomy. While the gonadal steroids are important, so too are the evolutionarily ancient Oxt and Avp neuropeptide systems, as well as their non-mammalian homologues. Oxt and Avp are both primarily synthesized in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus. Their genes sit in opposite transcriptional direction on the chromosome as the result of the duplication of an ancestral vasotocin gene (Acher and Chauvet 1995; Acher et al. 1995) and they are synthesized as part of a larger precursor preprohormone (Hara et al. 1990). Since they are so structurally similar, Oxt and Avp are considered “sister” hormones, though their actions both peripherally and centrally can differ significantly from one another. Interestingly, across mammalian species, the roles of Oxt and Avp with regard to cooperative and competitive behaviors tend to be fairly conserved (Caldwell et al. 2008a; Caldwell and Young 3rd 2006; Lee et al. 2009a; Adkins-Regan 2009; Neumann 2008; Veenema and Neumann 2008; Carter et al. 2008; Albers 2012, 2015).
3.1 The Oxytocin System
Oxytocin literally meaning “sharp childbirth” is known for its peripheral actions on the regulation of uterine contraction as well as the facilitation of milk ejection (Dale 1906; Ott and Scott 1910). In rats, Oxt is synthesized in larger, magnocellular neurons, of the PVN and SON that project to the posterior pituitary and mediate the aforementioned actions. However, it is Oxt that is synthesized in the smaller, parvocellular neurons of the PVN that project centrally and mediate many of the central actions of Oxt. It should be noted, however, that this compartmentalization of function by magnocellular versus parvocellular neurons is not found in all species. For example, in Syrian hamsters, magnocellular neurons do not exclusively project to the posterior pituitary and seem to project centrally (Ferris et al. 1992b). Also, in mice and several vole species, there are reports of parvocellular Oxt neurons outside of the PVN (Castel and Morris 1988; Jirikowski et al. 1990; Wang et al. 1996) as well as reports of Oxt collaterals from the SON/PVN to the nucleus accumbens (NAcc) (Ross et al. 2009a).
Thus far, only a single Oxt receptor (Oxtr) has been identified, and it is thought to be the primary mechanism for the transduction of the Oxt signal (Kimura et al. 1992; Kubota et al. 1996); however, see the section titled “Signaling by Oxytocin and Vasopressin in the Brain.” The Oxtr is a member of the seven-transmembrane G-protein-coupled receptor family and signals through Gαq/11 GTP-binding proteins and Gβλ (Ku et al. 1995; Gimpl and Fahrenholz 2001; Zingg and Laporte 2003), which results in the hydrolysis of phosphatidylinositol. The structure and sequence of the Oxtr is similar to the Avp receptors (Gimpl and Fahrenholz 2001). In rats and mice, the Oxtr is most often visualized with receptor autoradiography through the use of a potent and specific 125I-labeled antagonist (Kremarik et al. 1993; Veinante and Freund-Mercier 1997). The Oxtr is observed in several areas of the brain, including the hippocampal formation, LS, central amygdala (CeA), olfactory tubercle, NAcc shell, dorsal caudate–putamen, BNST, medial amygdala (MeA), and VMH (Kremarik et al. 1993; Veinante and Freund-Mercier 1997; Insel et al. 1991), but there are seasonal as well as species- and sex-specific differences.
3.2 The Vasopressin System
Avp is named for its involvement in the constriction of blood vessels, but is also important to salt and water balance. The peripheral actions of Avp are primarily mediated by the magnocellular neurons of the PVN and SON, which result in Avp release from the posterior pituitary. Centrally, Avp is more widely expressed than Oxt, and its distribution can vary substantially between species. Avp immunoreactive (ir) cell bodies are consistently found in several hypothalamic nuclei including the suprachiasmatic nucleus (SCN), PVN, SON as well as in groups of accessary nuclei (Sofroniew 1983). Outside of the hypothalamus, Avp-ir neuronal cell bodies can be observed in the BNST and MeA in most rodent species examined to date (Sofroniew 1985). Interestingly, in Syrian hamsters, neuronal cell bodies containing Avp are absent in the BNST and MeA (Albers et al. 1991). Projections from Avp-producing neurons form a dense vasopressinergic network throughout the brain (Buijs et al. 1983, 1987; De Vries and Buijs 1983; Sawchenko and Swanson 1982).
Avp receptors can be divided into two classes: Avp1 and Avp2 receptors (Avpr1 and Avpr2, respectively), both of which are seven-transmembrane G-protein-coupled receptors that are similar in structure to the Oxtr. There are two subtypes of the Avpr1: the Avpr1a and the Avpr1b. Peripherally, the Avpr1a mediates the effects of Avp on vasoconstriction and can be found in the liver, kidney, platelets, and smooth muscle (Ostrowski et al. 1992; Watters et al. 1998). Centrally, the Avpr1a is found in a variety of brain nuclei (Johnson et al. 1995; Tribollet et al. 1997; Ostrowski et al. 1994; Szot et al. 1994). The Avpr1a is modulated by gonadal hormones and photoperiod in some brain regions, but not others (Johnson et al. 1995; Young et al. 2000; Caldwell and Albers 2004b; Caldwell et al. 2008b). The Avpr1b was originally described in the anterior pituitary, where it is prominent on the corticotrophs; however, it can also be found in the brain (Antoni 1984; Lolait et al. 1995). In rats, there is a lack of consensus about the central distribution of the Avpr1b, with some groups reporting Avpr1b in the olfactory bulb, piriform cortical layer II, LS, cerebral cortex, hippocampus, PVN, SCN, cerebellum, and red nucleus (Lolait et al. 1995; Saito et al. 1995; Vaccari et al. 1998; Hernando et al. 2001; Stemmelin et al. 2005). However, a later study by Young and colleagues, which used more stringent conditions for in situ hybridization histochemistry (ISHH), determined that the Avpr1b in rats and mice is more discretely localized with prominent expression in hippocampal field CA2 pyramidal neurons (Young et al. 2006). The Avpr2 is found in the periphery and is primarily expressed in the kidney; it has not been localized to the brain. Its role in the kidney is to transduce the antidiuretic effects of Avp within the renal collecting ducts (Bankir 2001).
3.3 Signaling by Oxytocin and Vasopressin in the Brain
Neuropeptides can act in a highly localized manner, similar to classic neurotransmitter release at the synapse. However, neuropeptides can also be released in a much more diffuse manner, potentially impacting large numbers of neurons at multiple sites (Engelmann et al. 2000; Landgraf and Neumann 2004; Ludwig 1998; Ludwig and Leng 2006). This diversity of action has long been recognized, although the role of these different types of signaling mechanisms in the control of social behavior is not well understood. Neuropeptides such as Oxt and Avp are usually packaged in large dense-core vesicles (LDCV) that can be found in all areas of neurons including the presynaptic terminal (Jakab et al. 1991; Buijs and Swaab 1979; van Leeuwen et al. 1978). Because of the broad distribution of LDCV throughout the cell, neuropeptides can act locally at the synapse or much more broadly when released from non-synaptic regions (e.g., dendrites) in what is called volume transmission. Many factors affect the profiles of neuropeptide release such as the size of the neurons from which they are released, the spread of peptide after release, and the timing and intensity of its degradation by peptidases. While the spatial and temporal profiles of peptide release via volume transmission are not well understood (Leng and Ludwig 2008), estimates suggest that they may travel as far as 4–5 mm from their site of release (Engelmann et al. 2000). Magnocellular neurons in the hypothalamus represent the largest pool of nonapeptides in the brain, and there is evidence that they are activated by a variety of stimuli related to social behavior (Delville et al. 2000). As a result, it seems likely that volume transmission of nonapeptides from these neurons plays an important role in regulating social behavior by acting on nonapeptide receptors throughout the SBNN .
Another consideration is that neuropeptides are commonly found in neurons that also produce small molecular weight “classical neurotransmitters” (e.g., amino acids) (for review, see van den Pol 2012; Albers 2015), thus allowing for their co-release. In most cases, classical neurotransmitters are packaged in small synaptic vesicles (SSVs) in presynaptic terminals. The exocytosis of both SSVs and LDCVs at synapses is Ca2+ dependent. Because SSVs are usually in closer proximity to the membrane than LDCV, less activity is required for classic neurotransmitter release than for neuropeptide release. Therefore, synaptic release of neuropeptides is thought to lag behind that of neurotransmitter release and to require more electrical activity. The functional significance of synaptic co-release of classical neurotransmitters and neuropeptides is not known, but dynamic interactions of these signals will likely be important in understanding neuropeptide regulation of behavior (Bamshad et al. 1996). In summary, the ways that Oxt and Avp contribute to neurochemical signaling within the brain are varied and complex. As a result, researchers are left to untangle very intricate pharmacological interactions when they consider the effects of these neuropeptides on behavior.
In addition to the diversity seen in the ways that Oxt and Avp can signal, Oxt and Avp also have a high degree of similarity in their structure as well as in the structure of their canonical receptors (Maybauer et al. 2008; Gimpl and Fahrenholz 2001; Manning et al. 2012; Song et al. 2014b). Due to these structural similarities, there is a substantial amount of cross talk between these systems (Schorscher-Petcu et al. 2010; Sala et al. 2011), with Oxt and Avp having similar affinities for the Oxtr, Avpr1a, and Avpr1b in rats and mice (Manning et al. 2008, 2012). For example, both Oxt and Avp can induce communicative behavior in hamsters when injected into the AH by activating Avpr1a rather than the Oxtr (Song et al. 2014b). Similarly, both Oxt and Avp can enhance social recognition and social reward by activating the Oxtr and not the Avpr1a (Song et al. 2014a, b, 2015; Ragnauth et al. 2004). These data indicate that the effects of Oxt and Avp on different social behaviors can result from their activation of the Avpr1a, the Oxtr, or both.
Oxt and Avp receptors are distributed within structures of the mesolimbic DA system, providing a means by which these systems may interact. These areas include the amygdala, the hippocampus, the VTA, the prefrontal cortex (PFC), the NAcc, and the ventral pallidum (VP) (Vaccari et al. 1998; Baskerville and Douglas 2010; Curtis et al. 2008). It is also important to remember that there is a great deal of interspecies and interindividual variability in the distribution of Oxt and Avp receptors and that these differences play a major role in producing differences in the expression of social behavior.
Recent work in primates has shed some light on the complexity of these systems in a broader evolutionary context, as there are some significant evolutionary changes in the Oxt and Avp systems. As discussed above, in rats and mice, the Oxtr and Avprs are relatively non-selective for Oxt and Avp; however, in humans, the OXTR has significant selectivity for Oxt over Avp. The functional significance of these differences in receptor selectivity is not known but is likely to be important. Aside from cross talk, there are also sequence differences in Oxt across primate species. Specifically, work by Lee et al. (2011) challenged the idea that Oxt is an invariant nine-amino acid sequence by determining that some New World primates have an amino acid substitution in the 8th position, a leucine rather than a proline. The implications of these findings are still being explored (Cavanaugh et al. 2014), but this coupled with variations in the Avpr1a gene suggests that there has been, and perhaps continues to be, an evolutionary shift in these systems and reinforces the importance of studying these systems together rather than in isolation (for review, see Ragen and Bales 2013). These findings are likely to impact work in preclinical models and may help to resolve some of the conflicting findings in the literature between primates and other mammalian species.
3.4 Epigenetics
The advent of epigenetics has also challenged our understanding of how these systems are regulated and potentially how they regulate other systems. Epigenetics refers to changes in gene transcription that are not due to changes in nucleotide sequence, but rather are “above” the genome. Epigenetic mechanisms include histone modification and gene methylation, both of which alter the ability of the transcriptional machinery to access promoter regions. While much of this work originally centered on Oxt and maternal behavior in animal models, it has now been expanded to human studies.
Elegant work from Dr. Michael Meaney’s laboratory found that in rats, the quality of mother–infant interactions affected DNA methylation and histone acetylation patterns in the offspring (e.g., Champagne et al. 2001, 2004; Fish et al. 2004; Francis et al. 1999; Weaver et al. 2004). During the first postpartum week, low licking-and-grooming (LG) dams have reduced Oxtr binding in the MPOA compared to high LG dams (Champagne et al. 2001; Francis et al. 2000). Further, microinjection of an Oxtr antagonist reduces licking and grooming in high LG dams, with essentially no effect in low LG dams (Champagne et al. 2001). For more complete reviews of this work, see (Champagne 2008; Bridges 2015).
More recently, work in humans suggests that changes in the methylation of the OXTR are associated with a variety of diseases/disorders, including anorexia nervosa (Kim et al. 2014), the perception of fear and anxiety (Puglia et al. 2015; Ziegler et al. 2015), psychopathy (Dadds et al. 2014), and autism (Gregory et al. 2009). However, a direct causal link between methylation patterns and behavior has yet to be made; however, given the animal literature, it seems likely that this is an important means by which early life experience may be able to directly impact behavior.
4 Oxytocin/Vasopressin and Cooperative and Competitive Behaviors: Social Memory, Social Interactions, and Aggression
Within mammals, social structures can vary widely. Take for instance naked mole rats and their large insect-like colony structure, prairie voles and their pair bonding that results in a lifetime social dyad, or more solitary species such as Syrian hamsters. With this diversity, it might be assumed that there are vast differences in the types of behaviors that these species are capable of displaying and in the neurotransmitters and/or brain areas that are important to their regulation. This, however, does not seem to be the case. While the details may differ, there are a limited number of social behaviors common to most species and they include the ability to remember others of the same species (social memory ) as well as the social behaviors that determine the nature of their relationships with conspecifics (e.g., affiliation/dominance). Furthermore, the roles of Oxt and Avp in the modulation of social behaviors have been evolutionarily conserved across species. For example, aggression is modulated by social experience-induced changes in the expression of Avpr1a in the hypothalamus in both male prairie voles and hamsters.
Since 90 % of mammals are not biparental, it is common for them to live in large social groups and display both cooperativity and competitiveness, depending on the context. Even in species that are more solitary and highly competitive, individuals have the capacity for social recognition, social communication, as well as the potential to form stable long-standing social relationships. There are also sex differences as well as environmental effects, such as photoperiod, which can cause shifts in social behaviors from the breeding to the non-breading season. In this section, we will explore the role of Oxt and Avp in selected forms of cooperative and competitive behavior in these differing and complex social contexts.
4.1 Social Recognition Memory
Displays of social behaviors often depend on whether the interaction is with an individual that is familiar or unknown. Thus, the ability to recognize individuals and remember them, i.e., social recognition memory, plays an important role in the decision to approach or avoid. There are considerable data that both Oxt and Avp contribute to social recognition memory. Oxt is thought to facilitate social memory by altering the processing of socially salient olfactory information (for review, see Lee et al. 2009a; Gabor et al. 2012; Wacker and Ludwig 2012). In males, Oxt infused into the olfactory bulb (OB), lateral ventricles, and MPOA facilitates social recognition memory (Dluzen et al. 1998; Benelli et al. 1995; Popik and Van Ree 1991). Some of the particulars of the circuit have been worked out with the assistance of genetic knockouts of the Oxt system, with Oxt knockout (Oxtr −/−) mice and forebrain-specific Oxtr knockout (Oxtr FB/FB, where CRE recombinase is driven by a CaMKIIα promoter) mice having deficits in social recognition memory (Lee et al. 2008a, b; Takayanagi et al. 2005; Macbeth et al. 2009; Ferguson et al. 2000; Hattori et al. 2015). Based on this work, a four-gene micronet involving Oxt, the Oxtr, estrogen receptor α, and estrogen receptor β has been proposed as being critical to the regulation of social recognition memory in both males and females (Choleris et al. 2003). In particular, estrogen-dependent Oxt signaling in the MeA appears to be key for normal social recognition memory. Infusion of Oxt into the MeA of Oxt −/− mice can rescue deficits in social recognition memory (Ferguson et al. 2001), and infusion of an Oxtr antisense DNA or an Oxtr antagonist into control mice can block social recognition memory (Choleris et al. 2007; Ferguson et al. 2001). Female Oxt −/− mice also have a disrupted Bruce effect (Bruce 1959), whereby they terminate their pregnancy when exposed to their mate, which suggests that they do not remember him (Wersinger et al. 2008).
Recent work suggests that Oxt may also play a role in human social recognition. Generally speaking, exogenous Oxt enhances the memory for faces (Savaskan et al. 2008; Guastella et al. 2008; Rimmele et al. 2009). Further, a common single-nucleotide polymorphism (SNP) (rs237887) in the OXTR is moderately associated with face recognition memory in families from the UK and Finland that have a child with an autism spectrum disorder (ASD) (Skuse et al. 2014). Taken together, these data suggest that Oxt and the Oxtr have a conserved role in the modulation of social memory across species.
Avp also appears to be important for social memory. Androgen-dependent Avp projections from the MeA and BNST to the LS, all parts of the SBNN , are important for individual recognition (De Vries et al. 1984; Mayes et al. 1988; Bluthe et al. 1990, 1993). Microinjections of Avp into the LS of control or AVP-deficient Brattleboro rats facilitates social memory, whereas microinjections of Avpr1a antagonists or infusions of antisense Avpr1a oligonucleotides into the LS of control rats impairs social recognition memory (Engelmann and Landgraf 1994; Landgraf et al. 1995). The use of Avpr1a −/− and Avpr1b −/− mice has also provided some insight into the contributions of Avp to social memory. However, in the NIMH line of Avpr1a −/− mice, the findings have been mixed (Hu et al. 2003), with one group reporting that males have impaired social recognition that can be rescued by the overexpression of Avpr1a in the LS (Bielsky et al. 2003, 2005; Bielsky and Young 2004) and another group reporting no deficits in social recognition, but rather in olfaction (Wersinger et al. 2007b). While the reason for the discrepancy remains unknown, it is obvious from previous reports that Avpr1a in the LS is important for normal social recognition memory. Interestingly, Avpr1b −/− mice have mild impairments in social recognition memory (Wersinger et al. 2002). Further, lesions and genetic silencing of the CA2 region of the hippocampus, where the Avpr1b is prominently expressed, also results in impaired social recognition memory (Stevenson and Caldwell 2012; Hitti and Siegelbaum 2014).
While the aforementioned data provide strong support for a role of Avp in social recognition, how or if Avp modulates social recognition has really only been studied in a small number of species. Further, the data are limited by the fact that the vast majority of studies have only examined social recognition for very short intervals (<2 h). However, there are some studies that suggest that Avp may be important for long-term social recognition. Specifically, injection of an Avpr1a antagonist into the LS can block the formation of a mating-induced pair bond in male prairie voles, while injection of Avp into the LS institutes a pair bond in the absence of mating (Liu et al. 2001). Since social recognition is a necessary part of pair bonding and pair bonds can last a lifetime, these data suggest that Avp signaling via the Avpr1a can induce long-term changes in social recognition. There are also data to suggest that Avpr1a antagonists administered into the MeA can alter maternal memories in rats. Normally, after a ten-day separation from their pups, mothers display full maternal behavior within about 12 h of re-exposure. However, in peripartum mothers, in which an Avpr1a antagonist is infused into the MeA, the latency to display full maternal behavior does not occur for approximately 60 h; the antagonist had no effect on the initial expression of maternal behavior on the day of parturition (Nephew and Bridges 2008).
More recently, we have found that male Syrian hamsters can recognize social odors of other male Syrian hamsters for 24 h. Injection of Oxt or Avp intracerebroventricular (i.c.v) extends their social memory to 48 h. Interestingly, these effects are mediated by the Oxtr and not the Avpr1a (Song et al. 2015). In the broader context of animal behavior, it will also be important to determine whether Avp mediates some of the more complex forms of social recognition found by rodents such as kin recognition and the true recognition of specific individuals (e.g., Mateo and Johnston 2003; Johnston and Peng 2008; Petrulis 2009).
4.2 Cooperative Behavior
Although cooperative behavior is certainly not limited to species that pair bond, pair bonding species do provide an important model system in which to investigate the neurobiology of cooperation. That said, it remains important to recognize that these bonds are formed by mating and are limited to the cooperation of a male and a female. Pair bonding, formed by mating, represents one form of cooperative behavior. Pair bonds are somewhat unique among mammals in that they are only seen in 3–5 % of mammalian species (Kleiman 1977). Defined as a preference for contact with a familiar sexual partner, selective aggression toward unfamiliar conspecifics, biparental care, socially regulated reproduction, and incest avoidance (Carter et al. 1995; Carter and Getz 1993), pair bonding can be found in titi monkeys (Callicebus cupreus), marmosets (Callithrix penicillata and Callithrix jacchus jacchus), California mice (Peromyscus californicus), and prairie voles (Microtus ochrogaster). Humans too can have strong selective bonds between mates, but also show cooperative behaviors in many other contexts as well. The extent to which human pair bonds and other forms of human cooperative behavior are regulated by Oxt and Avp remains to be determined.
Our understanding of the mechanisms by which Oxt and Avp contribute to pair bonding comes primarily from work in prairie voles. Prairie voles live in extended family groups and are considered a socially monogamous species (Carter et al. 1995) (social monogamy is distinct from sexual monogamy as most individuals have extra-pair copulations). The formation of a pair bond is experimentally tested in the laboratory using a partner preference test (Williams et al. 1992), whereby the preference of a male or female for an animal in which they have previously cohabitated, i.e., the partner, versus a “stranger,” is assessed. If the experimental subject spends twice as much time with the “partner” animal, then it is said to have formed a pair bond with that individual (Insel and Hulihan 1995; Carter and Getz 1993; Williams et al. 1994; Carter et al. 1995).
Due to the diversity in social structures within the genus Microtus, comparative studies between vole species have provided significant insight into the neural regulation of social bonding. By comparing the neurochemistry of monogamous vole species, such as the prairie or pine vole (Microtus pinetorum), to non-monogamous voles, such as the montane (Microtus montanus) or meadow (Microtus pennsylvanicus) voles, scientists have explored how variations in Oxt and Avp neurochemistry between highly related species can result in significant differences in social behavior (Young et al. 2008, 2011; Adkins-Regan 2009). While there are differences in Oxt- and Avp-ir cells or their projections between species, most profound are the changes in the neuroanatomical distribution of the Oxtr and the Avpr1a. Relative to non-monogamous voles, monogamous voles have higher densities of Oxtr, as measured using Oxtr autoradiography and ISHH, in the NAcc, the PFC, and the BNST. In contrast, promiscuous voles have higher Oxtr density in the LS, VMH, and the cortical nucleus of the amygdala (Insel and Shapiro 1992; Young et al. 1996; Smeltzer et al. 2006). Evidence that the differences in the distribution of the Oxtr between species might be behaviorally meaningful comes primarily from pharmacological studies.
In female prairie voles, central infusion of an Oxtr antagonist blocks the formation of the pair bond, but has no effect on sexual behavior, whereas central infusion of Oxt facilitates the pair bond in the absence of mating (Insel et al. 1995; Williams et al. 1994; Cho et al. 1999) and can decrease male-directed aggression (Bales and Carter 2003). In the aforementioned studies, the infusions were i.c.v.; however, manipulation of Oxtr signaling, using Oxtr antagonists and RNAi knockdown of the Oxtr within the NAcc, inhibits formation of a partner preference (Liu and Wang 2003; Young et al. 2001; Keebaugh et al. 2015), and overexpression of the Oxtr in the NAcc of adult female prairie voles accelerates the formation of partner preference (Ross et al. 2009b). However, overexpression of the Oxtr in the NAcc of non-monogamous meadow voles is not sufficient to promote pair bond formation (Ross et al. 2009b), which suggests that all of the required neurocircuitry is not in place for this species.
There are also differences in the distribution of the Avpr1a between vole species. Prairie voles have a higher density of Avpr1a, as measured using receptor autoradiography and ISHH, within the MeA, accessory olfactory bulb, diagonal band, thalamus, VP, and BNST compared to montane voles (Young et al. 1997; Insel et al. 1994). Montane voles, on the other hand, have a higher density of Avpr1a in the medial PFC and the LS (Smeltzer et al. 2006; Insel et al. 1994). These differences in Avpr1a distribution are thought to contribute to differences in social organization between monogamous and non-monogamous vole species. This hypothesis is supported by data in pine voles and meadow voles, which suggest similar social structure-specific distributions of Avpr1a between these species (Insel et al. 1994). Further support for this hypothesis comes from pharmacological manipulations of the Avpr1a in prairie voles. When an Avp antagonist is injected i.c.v. prior to mating, the formation of a partner preference is inhibited. Conversely, Avp infusion facilitates the formation of the partner preference (Winslow et al. 1993; Cho et al. 1999). Some of the more interesting data that support a role for the differential distribution of the Avpr1a in the formation of social bonds come from a study in which the prairie vole Avpr1a gene was overexpressed in the ventral forebrain of meadow voles, resulting in increases in the amount of time meadow voles spent huddled with their partners compared to controls (Lim et al. 2004).
4.3 Competitive Behavior
The most conspicuous form of competitive behavior is aggression . Offensive and defensive aggressions have been studied intensely and almost exclusively in male mammals. Further, neural circuits that overlap much of the SBNN have been proposed for each of these forms of aggression (Delville et al. 2000; Choi et al. 2005). Although frequently characterized as a negative social interaction, aggression plays a highly constructive role in the formation of social relationships. In the vast majority of mammals that do not form pair bonds, dominance relationships provide social bonds that serve many adaptive functions (e.g., resource distribution) and that ultimately reduce social conflict. Typically, dominance relationships are rapidly determined through aggressive interactions but are primarily maintained through social communication (e.g., scent marking, vocalization), thereby reducing the dangers associated with intense fighting (Albers et al. 2002; Fernald 2014).
4.3.1 Oxytocin and Competitive Behavior
Some of the earliest evidence that Oxt is involved in both aggression and social communication came from studies in squirrel monkeys. In male squirrel monkeys, with established dominant–subordinate relationships, i.c.v. administration of Oxt increases aggression in dominant males, while having no effect in subordinate males (Winslow and Insel 1991b). In contrast, Oxt stimulates scent marking in subordinate males but does not alter scent marking in dominant males (Winslow and Insel 1991b), thus demonstrating that social experience can determine the behavioral response to Oxt acting in the brain. Studies in rats suggest one mechanism that might contribute to the effects of social experience on the behavioral response to Oxt. Dominant male rats have significantly higher levels of Oxtr mRNA in the MeA 3 h after the social encounter that defined their relationship. Further, infusion of an Oxtr antagonist immediately after the establishment of subordinate status increases the duration of the dominant–subordinate relationship (Timmer et al. 2011). Social status also significantly impacts the circulating levels of serum Oxt in primates . In rhesus monkeys, dominant females have significantly higher levels of serum Oxt than subordinates (Michopoulos et al. 2011, 2012). The importance of social experience in determining the behavioral response to Oxt/Avp is a theme seen repeatedly in this chapter.
Historically, there have been little data supporting a role for Oxt in the regulation of intermale aggression in laboratory species of rodents . However, some recent work suggests that pharmacological treatment with Oxt may have antiaggressive effects in adult males. Work from Jaap Koolhaas’ laboratory has found that pharmacological enhancement of Oxt in rats, by intranasal treatment or i.c.v. infusion, reduces offensive aggression and promotes prosocial exploratory behaviors (Calcagnoli et al. 2013, 2014, 2015a). Furthermore, these inhibitory effects seem to be mediated by Oxt acting via the Oxtr in the CeA; however, it should be noted that blocking endogenous Oxt signaling in the CeA has no effect (Calcagnoli et al. 2015b). Thus, it is proposed that these findings should be considered in the context of pharmacological effects rather than being directly regulated by the local endogenous Oxt system (Calcagnoli et al. 2015b).
The role of Oxt in the neural regulation of female offensive aggression is sparse; as mentioned previously, females of the most commonly studied laboratory rodents, unlike many other mammalian species, rarely display aggressive behaviors outside of the peripartum period. However, there is evidence in female Syrian hamsters that Oxt can influence competitive behaviors. Specifically, microinjection of Oxt into the MPOA-AH reduces offensive aggression, and injection of an Oxtr antagonist increases offensive aggression directed toward a female intruder (Harmon et al. 2002a). Traditionally, studies of Oxt in female rodents have focused on maternal aggression. This is a unique, and transient, hormonal/physiological time in a female’s life and is characterized by high levels of nurturing behaviors directed toward pups and aggressive behaviors directed toward intruders. During the peripartum period, Oxt has anxiolytic effects (Bosch and Neumann 2008), specifically via its actions in the PVN and CeA (Blume et al. 2008; Jurek et al. 2012; Windle et al. 1997; Huber et al. 2005; Viviani et al. 2011; Knobloch et al. 2012). These decreases in anxiety permit females to attend to their pups. But even with lower levels of anxiety and strong bonds with her offspring, dams can display high levels of maternal aggression. Depending on the brain area and the behavioral state of the animal, Oxt and/or Oxtr antagonists can either increase or decrease maternal aggression (for review, please see Bosch 2013). For instance, in rats bred for low-anxiety rats, Oxt in the PVN increases maternal aggression (Bosch et al. 2005), but when microinjected into the BNST of Wistar rats decreases maternal aggression (Consiglio et al. 2005). In hamsters, injection of Oxt into the amygdala facilitates maternal aggression (Ferris et al. 1992a). So, while central Oxt is important to aggression in females, its effects are context and site specific. There are also numerous studies that support the assertion that developmental exposure to Oxt is important for the proper development of motivated social behaviors, including aggression , but those studies will not be reviewed in this chapter (recently reviewed in Miller and Caldwell 2015; Hammock 2015).
4.3.2 Vasopressin and Competitive Behavior
Most of what we know about the role of nonapeptides in competitive behavior comes from studies of Avp and aggressive behavior. Further, much of the work has focused on the Avpr1a, as this was the first centrally identified receptor, and as such, there are a number of pharmacological tools available. However, the Avpr1b appears also to be important for displays of aggressive behavior. Because of the differences in the distribution of these two receptor subtypes, this section will be divided by subtype.
4.3.3 The Vasopressin 1a Receptor and Competitive Behavior
The first evidence for a role for Avp in aggression came from studies in male hamsters, in which the injection of Avpr1a antagonists into the AH significantly inhibited offensive aggression (Ferris and Potegal 1988; Potegal and Ferris 1990). Subsequent studies have replicated these results and shown that Avp injected into the AH stimulates offensive aggression (Caldwell and Albers 2004a; Ferris et al. 1997). However, the ability of Avp to stimulate aggression by its action in the AH appears to depend on an individual’s prior social experience. Avp injected into the AH increases aggression in male hamsters previously trained to fight other hamsters and in hamsters socially isolated for at least four weeks, but not in hamsters housed in social groups. The ability of social experience to enhance the response of the AH to Avp appears to be mediated by experience-dependent increases in the number of Avpr1a in the AH (Albers et al. 2006; Cooper et al. 2005). In summary, Avp has powerful effects on offensive aggression in male hamsters, but only if social experience has upregulated Avpr1a receptors in the AH.
Avp can also have potent effects on aggression in male prairie voles through a similar mechanism. Sexually naïve male voles are essentially non-aggressive, choosing to explore intruder males as opposed to attacking them (Winslow et al. 1993). Following mating-induced pair bonding , males display high levels of aggression toward conspecifics other than their mate and have increases in the density of Avpr1a receptors in the AH (Gobrogge et al. 2009). Further, overexpression of the Avpr1a in the AH using viral vector gene transfer increases aggression in non-pair-bonded males. Thus, in male hamsters and prairie voles, species with very different types of social organization, an individual’s social experience can modulate the number of Avpr1a in the AH and thereby regulate the intensity of aggression that is expressed.
Another hypothalamic region where Avp influences aggression is the ventrolateral hypothalamus (VLH). Avp injected into the VLH facilitates aggression in gonadally intact males and castrated males given testosterone but does not facilitate aggression in castrated controls (Delville et al. 1996). Avpr binding is also reduced in the VLH following castration and precastration levels of binding can be restored by testosterone. In contrast, it is not known whether testosterone influences the ability of Avp to alter aggression when injected into the AH. However, since castration reduces Avpr binding within this region (Delville et al. 1996), it seems possible that castration could reduce aggression stimulated by Avp. It should be noted that the relationship between gonadal hormones and aggression is complex; however, it is clear that testosterone does not simply induce aggression in males (for review, see Johnson et al. 1995; Young et al. 2000; Demas et al. 2007). In females, while there is evidence that gonadal hormones can affect Avpr1a binding within the VLH (Delville and Ferris 1995), the specific effects on aggression are unknown.
There are also extra-hypothalamic regions where aggression and Avp activity have been linked. In both male rats and mice selected for varying levels of aggression, a negative correlation has been observed between Avp fiber density in the LS and the amount of intermale aggression (Compaan et al. 1993; Everts et al. 1997). Interestingly, these differences in Avp and aggression are not related to differences in circulating levels of testosterone (Elkabir et al. 1990). The role of septal Avp in aggression has also been studied in male rats bred for low or high anxiety. Release of Avp into the LS is significantly lower in the much more aggressive low-anxiety rats than in the high-anxiety rats that exhibit lower levels of aggression. In addition, septal administration of Avp to the highly aggressive group and administration of the Avpr1a antagonist to the low aggressive group did not alter the levels of aggression expressed (Beiderbeck et al. 2007). The level of aggressiveness and the pattern of Avp expression in the LS and BNST are also correlated in mice. The monogamous California mice (Peromyscus californicus) have shorter attack latencies and increased Avp-ir in the BNST and LS compared to the polygamous, white-footed mice (Peromyscus leuopus) (Bester-Meredith et al. 1999). Interestingly, when California mice pups are cross-fostered to white-footed mice dams, they are less aggressive in adulthood than those reared by the same species, and they have less Avp-ir in the BNST and SON compared to controls (Bester-Meredith and Marler 2001). These data in mice, like those from hamsters, suggest that changes in the social environment are able to alter Avp neurocircuitry and the behavior driven by that circuitry. In addition, although relationships between aggressiveness and Avp expression and release within the LS have been found, Avp may not have any direct effects on male aggression by its actions in the LS.
Despite the powerful effects of Avp on aggression in the hypothalamus, not all central manipulations of Avp have been found to influence offensive aggression. Although i.c.v. injections of an Avpr1a antagonist increase the latency to the onset of aggression in highly aggressive California mice, i.c.v. injections of Avp or Avpr1a antagonists have no effect on aggression in white-footed mice (Bester-Meredith and Marler 2001). In non-pair-bonded male prairie voles with extensive experience with aggression, i.c.v. administration of an Avpr1a antagonist does not inhibit aggression (Winslow et al. 1993). There is other evidence from rats that i.c.v. administration of Avp does not alter the expression of aggression nor does deletion of the Avpr1a receptor in mice (Elkabir et al. 1990; Wersinger et al. 2007b). While it is clear that Avp can stimulate Aggression by its action in some brain sites, it remains possible that Avp might act to reduce aggression by its action in other brain regions or by its action on other receptors (e.g., the Oxtr) to reduce/inhibit aggression. It is also important to recognize that aggression is a complex behavior and may be facilitated by neurochemical systems other than Avp, at least in some cases.
In females, Avp has very different effects on offensive aggression than it does in males. As described above, in male hamsters, injection of Avpr1a antagonists into the AH inhibits offensive aggression and injection of Avp stimulates aggression (Ferris and Potegal 1988; Potegal and Ferris 1990; Caldwell and Albers 2004a; Ferris et al. 1997). In contrast, in female hamsters, an Avpr1a antagonist injected into the AH stimulates offensive aggression and injection of Avp inhibits aggression in the resident–intruder test (Gutzler et al. 2010). More recently, similar sex differences have been seen in the effects of Avp and Avpr1a antagonists on social play behavior in rats (Bredewold et al. 2014; Veenema et al. 2013). For example, injection of Avpr1a antagonists in the LS increases social play in juvenile males and reduces social play in juvenile females. In a related study, a negative correlation was found between Avp mRNA levels in the BNST and social play in male juvenile rats (Paul et al. 2014). As social play is hypothesized to be a precursor to aggressive behavior, these data are consistent with Avp-related sex differences in the regulation of competitive behaviors.
4.3.4 The Vasopressin 1A Receptor and Maternal Aggression
Maternal aggression is an intense form of aggression displayed by lactating mothers confronted by intruders (Lonstein and Gammie 2002). Studies using Avpr1a −/− mice found that maternal aggression does not differ from that seen in wild-type mice (Wersinger et al. 2007b). In contrast, i.c.v. administration of Avp in lactating rats reduces and an Avpr1a antagonist increases aggression toward male intruders (Nephew and Bridges 2008; Nephew et al. 2010). The effects of i.c.v. administration of Avp and an Avpr1a antagonist on maternal aggression were also examined in rat strains that had been selectively bred for high (HAB) or low (LAB) anxiety (Bosch and Neumann 2010). In both strains, Avp was found to increase and an Avpr1a antagonist to decrease maternal aggression, respectively. Other studies have used microdialysis to examine the role of Avp in specific brain regions in the regulation of maternal aggression in HAB and LAB rats (Bosch and Neumann 2010). In HAB, but not LAB, rats, Avp is positively correlated with maternal aggression in the CeA but not the PVN. In addition, administration of an Avpr1a antagonist into the CeA reduces aggression in HAB rats, while administration of Avp into the CeA increases aggression in LAB rats. The ability of Avp to promote maternal aggression by acting in the CeA does not appear to be restricted to HAB rat strains since similar results have been reported in Sprague-Dawley rats (Meddle and Bosch, unpublished; cited in, Bosch 2011). In the future, it will be important to clarify the effects of Avp on maternal aggression , its site(s) of action, and whether the effects of Avp on aggression are related to anxiety levels.
4.3.5 The Vasopressin 1b Receptor and Competitive Behavior
There is compelling evidence that the Avpr1b is essential for displays of aggressive behavior directed toward a conspecific (for review, see Caldwell et al. 2008a, c; Stevenson and Caldwell 2012). In resident–intruder and neutral cage aggression tests, Avpr1b −/− mice display fewer attacks and have longer attack latencies than Avpr1b +/+ controls (Wersinger et al. 2002, 2007a). Further, in a reversal of a resident–intruder test where the experimental mice are intruders rather than residents, Avpr1b −/− mice display normal defensive avoidance behaviors when attacked by a stimulus animal, but are less likely to initiate retaliatory attacks (Wersinger et al. 2007a). Pharmacological studies using the Avpr1b antagonist, SSR149415, support the findings of work in Avpr1b −/− mice. Syrian hamsters orally administered SSR149415 have reductions in the frequency and duration of offensive attacks, in chase behaviors, in flank marking, and in the olfactory investigation that often precedes and accompanies an offensive attack (Blanchard et al. 2005). Mice given SSR149415 display fewer defensive bites when forced to encounter a threatening predator and reductions in the duration of offensive aggression in a resident–intruder test (Griebel et al. 2002).
The deficits in aggressive behaviors observed in Avpr1b −/− mice are not limited to males. Following parturition, female Avpr1b −/− mice have reductions in maternal aggressive behaviors, compared to control mice, as measured by longer attack latencies and fewer attacks, directed toward a male intruder (Wersinger et al. 2007a). Interestingly, the disruption of the Avpr1b does not affect all forms of aggressive behavior. In a nonsocial context, such as the predation of a cricket, Avpr1b −/− and Avpr1b +/+ mice have similar attack latencies (Wersinger et al. 2007a). Based on the genetic and pharmacological data, it has been hypothesized that the disruption of the Avpr1b does not specifically disrupt aggressive behavior, but rather the ability to have the appropriate behavioral response within a given social context (Caldwell et al. 2008c; Young et al. 2006; Stevenson and Caldwell 2012).
With prominent expression within the pyramidal neurons of the CA2 region of the hippocampus, recent work has focused on what the Avpr1b may be doing here. To this end, Pagani et al. 2015 have shown that replacement of the Avpr1b in the dorsal CA2 region of Avpr1b −/− mice restores socially mediated attack behaviors. Further, selective Avpr1b antagonists result in the production of N-methyl-D-aspartic-acid-dependent excitatory postsynaptic responses specifically within the CA2 region of control mice, but not Avpr1b −/− mice (Pagani et al. 2015). While the hippocampus is not currently a recognized node in the SBNN , it is a part of the motivational pathway. The CA2 region is structurally unique, as it does not receive rich mossy fiber input from the dentate gyrus (Tamamaki et al. 1988), and is the only part of the hippocampus to receive input from the posterior hypothalamus and the perforant pathway (Bartesaghi et al. 2006; Borhegyi and Leranth 1997; Vertes and McKenna 2000), which connects the entorhinal cortex to the hippocampal formation (Bartesaghi and Gessi 2004). Further, there is a vasopressinergic projection from the PVN to the CA2 region (Cui et al. 2013). Based on the findings described here, we, and others, have hypothesized that the CA2 region of the hippocampus may aid in the formation and/or recall of accessory olfactory-based memories (Caldwell et al. 2008c; Young et al. 2006). Thus, it seems likely that this is a region that will need to be included in future discussions of the SBNN and its interactions with the mesolimbic DA system.
4.4 Social Communication
While Avp plays a key role in the regulation of social communication in hamsters (see below), Oxt also contributes to its regulation. Hamsters communicate using a form of scent marking called flank marking, and the expression of flank marking is essential for the maintenance of dominant/subordinate relationships (Johnston 1985). After the rapid establishment of dominance, aggressive behavior declines and flank marking increases in dominant hamsters and to a lesser extent in subordinate hamsters (Ferris et al. 1987). In the absence of flank marking, aggression remains high and a stable relationship is not formed.
Oxt injected into the areas extending from the MPOA to the posterior medial and lateral aspects of the AH (referred to from here on as the MPOA-AH) of dominant female hamsters induces flank marking in a dose-dependent manner but only when the dominant hamsters are tested with their subordinate partners (Harmon et al. 2002b). Oxt does not induce flank marking when injected into the MPOA-AH of socially naive female hamsters tested with an opponent or alone. In males, by contrast, Oxt induces flank marking in dominant hamsters when they are tested with their subordinate partner or alone (Harmon et al. 2002b). These data indicate that social experience, social context, and sex interact to regulate the ability of Oxt to stimulate flank marking by its actions in the MPOA-AH in hamsters.
Although Oxt can stimulate flank marking, Avp plays the predominate role in regulating its expression. Avp stimulates high levels of flank marking in male and female hamsters by acting on the Avpr1a in the rostral hypothalamus (Ferris et al. 1984, 1985; Albers et al. 1986). It is of note that the MPOA-AH is substantially larger than the site where Avp can induce aggression (Ferris et al. 1986a). Avp can also induce flank marking following its injection into the LS, BNST, and PAG (Irvin et al. 1990; Hennessey et al. 1992). Gonadal hormones modulate the ability of Avp to stimulate flank marking by regulating the number of hypothalamic Avpr1a (Huhman and Albers 1993; Albers et al. 1988). In the LS, BNST, and PAG, gonadal hormones have only small effects on the ability of Avp to stimulate flank marking, suggesting that the MPOA-AH may be the primary site where gonadal hormones influence the ability of Avp to stimulate flank marking (Albers and Cooper 1995). Interestingly, Avp seems to induce flank marking regardless of the social context. For example, in hamsters with an established dominant/subordinate relationship, injection of Avp produces high levels of flank marking in either the dominant or subordinate hamster during social interactions (Ferris et al. 1986b). Similar results have been obtained in squirrel monkeys where Avp injected i.c.v. induces scent marking in both dominant and subordinate males (Winslow and Insel 1991a).
4.5 Interactions Between Oxytocin, Vasopressin, and Dopamine in the Regulation of Cooperation/Competition
As discussed in detail above, Oxt and Avp act within brain regions considered to be components of the mesolimbic DA system to influence cooperative and competitive behaviors. There is also evidence that DA in the mesolimbic DA system plays important roles in the regulation of both cooperative and competitive behaviors. Non-selective DA antagonists block mating-induced partner preferences in both male and female prairie voles, and treatment with the non-selective DA agonist apomorphine facilitates partner preference in the absence of mating (Aragona et al. 2003; Wang et al. 1999). The NAcc shell, and not the core, appears to be the site of action for these drugs since local administration into the NAcc shell, but not the core, has the same effects as systemic administration. Increases in DA activity within the NAcc that occurs following mating is necessary for the formation of pair bonds in male prairie voles (Aragona et al. 2003). Activation of DA D2 receptors in the NAcc can induce a pair bond in cohabiting voles in the absence of mating, while activation of D1 receptors can block pair bonding (Aragona et al. 2006). In addition, D1 receptors are increased in the NAcc in male prairie voles following the formation of a pair bond. Mate guarding aggression in males can also be inhibited by D1 antagonists injected into the NAcc (Aragona and Wang 2009). Other regions in the network also play a key role in the formation of cooperative behavior. Pair-bonded voles have lower concentrations of D1 receptors and higher concentrations of D2 receptors in the medial prefrontal cortex than non-pair-bonded voles (Smeltzer et al. 2006). Enhancement of DA release in the VTA via administration of GABA or glutamate antagonists can also induce pair bonding in male voles (Curtis and Wang 2005).
There is also considerable evidence that the mesolimbic DA system plays a critical role in the regulation of competitive behavior and in particular aggression (for review, see de Almeida et al. 2005). For instance, the non-selective DA receptor agonist apomorphine stimulates aggression and flank marking in male hamsters (Hyer et al. 2012). Social experience can regulate the expression of DA in several nodes of the SBBN. The amount of the rate-limiting synthetic enzyme for DA, tyrosine hydroxylase, increases significantly in several regions of the SBNN including the LS and BNST as well as within the shell of the NAcc in males trained to fight as compared to controls (Schwartzer et al. 2013). In addition, activation of D1 and D2 receptors in the NAcc influences the expression of aggression and its rewarding properties (Couppis and Kennedy 2008; Miczek et al. 2002); although selective aggression in male voles appears to require only the D1 receptor activation (Aragona et al. 2006). While DA is involved in various aspects of the preparation, expression, and consequences of aggression, its precise role remains elusive.
The importance of interactions between Oxt, Avp, and DA in social motivation has been widely discussed, and yet there are few studies demonstrating a direct link between these neuropeptides and DA in controlling social behavior. Partner preferences induced by the activation of D2 receptors in the NAcc can be prevented by administration of an Oxtr antagonist and partner preference induced by i.c.v. Oxt administration can be blocked by a D2 antagonist administered in the NAcc (Liu and Wang 2003). In addition, overexpression of Avpr1a in the VP of the non-pair-bonded meadow voles results in mating-induced partner preferences that can be blocked by a D2 antagonist given prior to mating (Lim et al. 2004).
Despite the mounting empirical evidence suggesting that Oxt and Avp can directly interact with DA to influence social behavior, the majority of evidence for this interaction comes from studies showing either that Oxt and/or Avp act within structures that comprise the mesolimbic DA system, or that manipulations of DA can influence the same social behaviors that are influenced by Oxt and Avp. Interestingly, there are very little data on whether Oxt or Avp might contribute the rewarding properties of social behavior. A limited amount of data suggest that Oxt can induce a conditioned place preference (CPP) when given peripherally to male rats or centrally to female mice (Liberzon et al. 1997; Kent et al. 2013). Recently, we investigated whether injection of Oxt or Avp into the VTA could produce a conditioned place preference in male hamsters (Song et al. 2014a). Both Oxt and Avp increase CPP when injected into the VTA. The administration of selective Oxt and Avpr1a agonists and antagonists revealed that the rewarding properties of both Oxt and Avp in the VTA are mediated by the Oxtr and not the Avpr1a.
5 Cooperativity and Competitiveness in Humans
As we all know, cooperation and competition are a hallmark of nearly all human endeavors. Successful cooperation and competition require a set of social skills collectively termed social cognition, which allow an individual to engage in social behaviors that are appropriate for a particular context. Since the emergence of the social cognition field in the late 1960s and early 1970s, there has been a concerted scientific effort to understand the complicated cognitive processes that underlie human social interactions. While social cognition and many disciplines that are brought to bear in this field are interesting, there is accumulating evidence that Oxt and Avp in humans are important to social cognition. This section will highlight what we know about Oxt and Avp in humans, in particular how exogenous administration of these nonapeptides impacts measures of social cognition.
As described throughout this chapter, there is considerable compelling evidence that Oxt promotes social behaviors, at least in specific contexts. In humans, the study of social behaviors includes testing procedures designed to measure trust, the ability to read facial expressions, the memory for socially salient information, such as faces, and more recently measures of empathy. In most human studies focused on the therapeutic effects of Oxt and Avp, they are exogenously administered intranasally. This delivery system is preferable as it is considered noninvasive and some assert that Oxt and Avp are able to cross the blood–brain barrier (Born et al. 2002); however, this latter assertion is questionable given their size, hydrophilic nature, as well as numerous other issues.
5.1 Nonapeptides and Social Cognition in Healthy Humans
5.1.1 Oxytocin and Social Cognition in Humans
Investigation into the role of Oxt in human cognition has dramatically increased over the last decade. More recently, attempts have been made to reconcile the existing data on the role of Oxt into a broader theory and understanding of human cognition. For example, De Dreu proposes that Oxt plays a critical role in the motivation of cooperation and competition in humans (De Dreu and Kret 2015; De Dreu 2012). This hypothesis stems from the idea that humans are social animals and are likely to cooperate with others, even with those genetically unrelated. He suggests that Oxt motivates humans to like and empathize with others in their group, to comply with group norms, and to reciprocate trust with other group members, while competing with out-group members. While De Dreu’s hypothesis is in reference to the endogenous Oxt system, this idea is supported by some recent work using intranasal Oxt. Essentially, intranasal Oxt increases “in-group” favoritism when individuals are asked to use intuitive decision making; however, Oxt has an opposite effect if individuals are asked to use reflective decision making (Ma et al. 2015). These data suggest that an individual’s “cognitive style” may contribute to the effects of Oxt, which has implications for both endogenous and exogenous Oxt.
Given the literature in animal models, and the proposed prosocial effects of Oxt, in recent years, there has been a surge in the number of clinical studies that have administered exogenous Oxt to improve social interactions in healthy individuals. While the data do suggest that intranasal Oxt as a therapeutic agent has some efficacy, long-term and dose–response studies have yet to be completed. In humans, intranasal Oxt may influence the processing of social information in several ways, including selective attention, enhancement of the memory, and/or the appraisal of socially relevant information (Guastella and MacLeod 2012). Intranasal Oxt also increases trust, as measured by an individual’s willingness to accept social risk during a social interaction (Zak et al. 2005; Kosfeld et al. 2005). However, similar to what is observed in other species, the effects of Oxt on trust are nuanced and often sex specific. For instance, if subjects are provided with information that suggests that a trustee is untrustworthy, then intranasal Oxt does not facilitate trust, or as the authors state, “Oxt makes people trusting, not gullible” (Mikolajczak et al. 2010). In females that have had their trust betrayed, intranasal Oxt results in less restoration of trust behavior compared to controls (Yao et al. 2014). However, in males, intranasal Oxt does not alter trust behavior in individuals that have had their trust betrayed, whereas placebo controls decrease their trust in response to betrayal (Baumgartner et al. 2008). Further, when intranasal Oxt treatment in males is coupled with functional magnetic resonance imaging (fMRI), there is a reduction in activity in areas of the brain associated with processing fearful stimuli, such as the amygdala and some areas of the midbrain, and reward feedback, such as the striatum (Baumgartner et al. 2008).
Oxt also has sex-specific effects in the context of reciprocal altruism, as measured using the prisoner’s dilemma game, which examines cooperative exchange (Chen et al. 2015b; Feng et al. 2014; Rilling et al. 2014). Specifically, intranasal Oxt administered to females causes them to treat computer partners more like human partners; this same effect is not observed in males (Rilling et al. 2014). In this same context, there are also striking sex differences in neural activation, as measured by fMRI. In males, Oxt increases activation in the caudate/putamen while decreasing activation in females. The authors suggest that in this context, Oxt may increase the reward or salience of positive social interactions among males, while having the opposite effect in females (Feng et al. 2014; Rilling et al. 2014). Recent work also indicates that Oxt selectively improves kinship recognition in women but not men and that Oxt improves men’s performance in competition recognition (Fischer-Shofty et al. 2013). Taken together, these data suggest that intranasal Oxt does not universally promote social interactions, but rather that the effects are subtle and dependent upon the social context and sex of the individuals. As mentioned previously, there has been a flood of clinical studies using intranasal Oxt, and with those studies, there is evidence that it can improve the ability to infer another individual’s mental state, improves facial recognition memory, alters the processing of faces, and can facilitate empathy (Heinrichs et al. 2009; Rimmele et al. 2009; Domes et al. 2007; Guastella et al. 2008, 2009; Zak et al. 2007; Hurlemann et al. 2010; Unkelbach et al. 2008; Bos et al. 2015).
While the intranasal administration of Oxt can definitely influence aspects of social cognition , it is the individual differences in endogenous Oxt and their association with social cognition that is equally, if not more, important. Not only are individual differences in Oxt genetically heritable (Rubin et al. 2014), but also they appear to be stable (Feldman et al. 2007) and can be modulated by social interactions (Feldman et al. 2010). Further, individual differences in Oxt and Oxt signaling may increase an individual’s vulnerability to certain neuropsychiatric disorders characterized by altered social cognition; see the section titled “Implications for Neuropsychiatric Disorders.” Some recent work suggests that endogenous Oxt may underlie natural variations in social perception (Lancaster et al. 2015). Further, in a more recent study, plasma Oxt is associated with increases in activity in brain areas that are important to social cognition such as the superior temporal sulcus, inferior frontal gyrus, and medial prefrontal cortex (Lancaster et al. 2015). Interpretation of some of the aforementioned data is muddled by our lack of understanding about the functional relationship between peripheral and central Oxt. But, it does follow that peripheral physiology and central physiology (behavioral output) are often going to be coupled, even if the systems are separate. However, while there is some evidence that they can be associated with one another (Landgraf and Neumann 2004), this does not always appear to be the case (Amico et al. 1990; Rosenblum et al. 2002; Winslow et al. 2003; Leng and Ludwig 2015).
5.1.2 Vasopressin and Social Cognition in Humans
The role of Avp in the regulation of social behavior in humans has not been studied as extensively as Oxt, though it is often associated with antisocial rather than prosocial behaviors. In males, intranasal Avp increases electromyogram (EMG) activity to socially neutral facial expressions (Thompson et al. 2004), as well as the memory for happy and angry faces (Guastella et al. 2010b). This suggests that Avp acts to bias an individual to perceive a neutral stimulus as an aggressive or threatening stimulus or enhances the encoding of negative social cues (Thompson et al. 2004; Guastella et al. 2010b). In females, Avp decreases EMG responses to happy and angry faces, suggesting that Avp acts to increase the perception of friendliness (Thompson et al. 2006). These sex-specific responses to Avp are supported by other studies as well (Feng et al. 2014; Rilling et al. 2014; Thompson et al. 2006) and may reflect sex differences in Avp neurochemistry and in the types of behavioral strategies employed during social interactions. Other effects include decreases in emotional recognition in men (Uzefovsky et al. 2012) and increases in empathy concern in individuals who received more “parental warmth” in their early family life (Tabak et al. 2015). It should be noted that the same issues that plague intranasal Oxt studies, such as the separation of central and peripheral Avp, are issues with the aforementioned studies as well.
5.2 Oxytocin, Vasopressin, and the Mesolimbic Dopamine System
Studies employing nasally administered Oxt and Avp have found numerous examples where these peptides alter the activity in various structures of the mesolimbic DA system. For example, Oxt augments the ventral striatum response to viewing the faces of romantic partners and to reciprocated cooperation from human partners (Rilling et al. 2012; Scheele et al. 2013). In women, Oxt increases activity in the VTA in response to both positive and negative facial expressions, but reduces the VTA response to reciprocated cooperation (Groppe et al. 2013; Chen et al. 2015a). While it is clear that Oxt and Avp can significantly alter activity in several structures in the mesolimbic DA system, the functional significance of these responses is not known. It is also possible that these neuropeptides alter social motivation without engaging DA neurons. For example, recent data suggests that Oxt enhances the attractiveness of female faces and correlates with increased activity in the striatum, while having no measureable impact on DA signaling (Striepens et al. 2014). However, more sensitive methods may be needed to detect subtle changes in behaviorally evoked DA release.
6 Implications for Neuropsychiatric Disorders
Oxt and Avp have also been implicated in a variety of neuropsychiatric disorders, particularly those characterized by aberrant social interactions and/or heightened aggression , such as ASD, personality disorder, schizophrenia, and posttraumatic stress disorder (PTSD). Further, many of these neuropsychiatric disorders are also characterized by dysfunctions of the mesolimbic DA system (for review, see Dichter et al. 2012). As many of these disorders are complex and have multiple etiologies, it becomes even more important not only to understand the potential contributions of Oxt and Avp, as well as their therapeutic effects, but also to determine how these systems interact with the DA system.
6.1 Autism Spectrum Disorder
ASD is characterized by repetitive behaviors, communication difficulties, and abnormal sociability (Matson and Nebel-Schwalm 2007a, b). In preclinical models of ASD, specifically Oxt −/− and Oxtr −/− mice, behavioral deficits are observed that are consistent with some of the symptoms of ASD (Crawley et al. 2007; Lee et al. 2008a, b; Macbeth et al. 2009; Wersinger et al. 2008; Winslow and Insel 2002; Ferguson et al. 2000, 2001). Evidence that Oxt may have a role in ASD comes from several sources. There are reports of reduced concentrations of Oxt in the cerebral spinal fluid (CSF) of autistic children, and reduced CSF Oxt is correlated with impairments in social functioning (Modahl et al. 1998). There are also increases in the amount of the Oxt prohormone in the blood of autistic children, which is indicative of incomplete processing of Oxt into its biologically active form (Green et al. 2001). Oxt treatment in adults with ASD results in the reduction of repetitive behaviors and improvements in emotional recognition (Hollander et al. 2003, 2007) and can increase eye gazing, a behavior important to social communication (Auyeung et al. 2015). In youth and adults with ASD, intranasal Oxt enhances emotion recognition (Domes et al. 2013, 2014; Guastella et al. 2010a) and increases social interactions (Andari et al. 2010). Further, in adults with Asperger’s syndrome, Oxt-mediated increases in emotional recognition are associated with an increase in activity in the left amygdala (as measured in fMRI) (Domes et al. 2014).
There are also some genetic and epigenetic links between the Oxt system and ASD. There are data in the Chinese Han population, in Finnish families, in Caucasian children, and in individuals with “high-functioning” ASD, suggesting that portions of the OXTR gene may contain susceptibility loci for ASD (Wu et al. 2005; Ylisaukko-oja et al. 2006; Jacob et al. 2007; Wermter et al. 2010; Nyffeler et al. 2014). Epigenetic modifications of the OXTR gene have also been reported, with hypermethylation of the OXTR promoter found in ASD subjects and subsequent reductions in OXTR mRNA (Gregory et al. 2009). A more recent study focused on whether the levels of DNA methylation of the OXTR could predict individual variability in social perception found that there are significant associations between the degree of OXTR methylation and social perception (Jack et al. 2012). Though the sample sizes in these latter two epigenetic studies are small, the data are provocative and will likely facilitate more research in this area.
Data implicating Avp in the etiology of ASD are sparse, but there have been studies suggesting that polymorphisms of the AVPR1A may contribute to ASD (Kim et al. 2002; Wassink et al. 2004; Yirmiya et al. 2006; Kantojarvi et al. 2015; Tansey et al. 2011). Further, two of the polymorphisms, RS3 and RS1, have been linked to differential activation of the amygdala (Meyer-Lindenberg et al. 2009), providing a possible neural substrate with which the Avp system may interact to mediate a genetic risk for ASD.
6.2 Personality Disorder
Personality disorder is characterized by disconnect between an individual’s behavior and cultural norms. Those diagnosed with personality disorder have impairments in at least two of the following areas: (1) cognition, (2) affectivity, (3) interpersonal functioning, and (4) impulse control (American Psychiatric Association 2013). To date, only a handful of studies have examined how exogenous Oxt treatment affects individuals diagnosed with personality disorder, and they have been performed primarily in individuals diagnosed with borderline personality disorder (BPD). Females diagnosed with BPD and treated with intranasal Oxt have reductions in stress reactivity (Simeon et al. 2011), decreases in hypersensitivity to threat, and decreases in amygdala activation in response to angry faces compared to controls; suggesting that Oxt in these individuals may decrease their reaction to a perceived social threat (Bertsch et al. 2013; Brune et al. 2013). This same research group also reports that females with BPD have reduced plasma Oxt concentrations (Bertsch et al. 2012). There are also data that suggest that intranasal Oxt can worsen trust in BPD and that this worsening is correlated with the patients’ history of childhood trauma (Ebert et al. 2013). One issue with all of the aforementioned studies is that BPD patients were not used as controls, only healthy individuals, making it more difficult to parse out what is going on specifically within BPD patients. However, these data do reinforce the importance of past history and social context.
One study has measured Oxt in the CSF of individuals with personality disorder, which includes individuals diagnosed with intermittent explosive disorder, as well as BPD. This study found that while CSF Oxt concentrations are not correlated with having a personality disorder, a life history of suicidal behavior is inversely correlated with Oxt (Lee et al. 2009b). The authors suggest that these data are consistent with the previous work in animal models demonstrating that Oxt can reduce aggressive behaviors (Consiglio and Lucion 1996; Giovenardi et al. 1998; Harmon et al. 2002a; Bales and Carter 2003).
Since individuals diagnosed with a personality disorder often have more impulsive behaviors, which can result in increased aggression , it is not surprising that Avp has been examined in these individuals. However, the data appear to be contradictory. A study by Coccaro et al. (1998) found a positive correlation between Avp in the CSF of personality-disordered individuals that have a life history of aggressive behavior. However, an earlier study found no differences in CSF Avp between violent offenders and controls (Virkkunen et al. 1994). It may be that differences in the populations studied account for the inconsistency in the findings, but it seems that more work in this area is warranted.
6.3 Schizophrenia
There are three broad categories of symptoms that characterize schizophrenia: (1) positive (e.g., hallucinations and delusion), (2) negative (e.g., anhedonia, impaired social behavior), and (3) cognitive/attentional (e.g., impaired memory and executive function) (American Psychiatric Association 2013). However, in humans, while its role has remained controversial, Oxt has been linked to schizophrenia since the 1970s when it was used as an antipsychotic (Bujanow 1974, 1972; for a recent review, see Rich and Caldwell 2015). Altered CSF concentrations of Oxt are reported in patients diagnosed with schizophrenia (Beckmann et al. 1985; Linkowski et al. 1984). However, the data are conflicting with some studies reporting an increase in Oxt and the Oxt carrier protein neurophysin I (Linkowski et al. 1984; Beckmann et al. 1985) and another reporting no change in CSF Oxt concentrations (Glovinsky et al. 1994). However, patients with higher plasma levels of Oxt have less severe positive symptoms and exhibit fewer social deficits (Rubin et al. 2010, 2011).
There are also reports of SNPs in the promotor regions of the OXT and OXTR genes that may contribute to symptom severity and treatment efficacy in schizophrenic patients (Teltsh et al. 2012; Watanabe et al. 2012; Montag et al. 2013). SNPs of the OXTR gene are associated with the severity of symptoms and the improvement of the positive symptoms of schizophrenia following treatment with antipsychotics (Souza et al. 2010a, b). Additionally, postmortem analysis of brain tissue from unmedicated schizophrenia patients has altered immunoreactivity of the Oxt carrier protein, neurophysin I, in the PVN, internal palladium, and substantia nigra (Mai et al. 1993). Most recently, in patients with schizophrenia and polydipsia, decreases in plasma Oxt were found to correlate with the ability to correctly identify facial emotions (Goldman et al. 2008). Thus, it appears that alterations in the Oxt system underlie all three symptom domains. Given the dysregulation of the Oxt system in patients with schizophrenia, Oxt has been studied as a candidate for use as a therapeutic.
Some studies suggest that Oxt may have antipsychotic properties (for review, see Macdonald and Feifel 2012; Bakermans-Kranenburg and van Jzendoorn 2013). Previous work found that injections of Oxt can reduce the symptoms of psychosis and anhedonia in patients with schizophrenia (McEwen 2004; Churchland and Winkielman 2012). In healthy patients, intranasal Oxt increases holistic processing, divergent thinking, and creative cognition (De Dreu et al. 2013), and studies in schizophrenic patients report that intranasal Oxt can be beneficial. Specifically, intranasal Oxt can facilitate social cognition (Davis et al. 2013; Feifel et al. 2010; Pedersen et al. 2011; Averbeck et al. 2012) and alleviate some of the cognitive deficits and positive symptoms (Pedersen et al. 2011). Yet, intranasal Oxt may be most effective as an adjunctive therapy to already prescribed antipsychotics, where chronic Oxt treatment is able to ameliorate some of the negative symptoms and the cognitive deficits, as well as the positive symptoms (Feifel et al. 2010, 2012; Modabbernia et al. 2013). While this research suggests that Oxt treatment has the potential to improve symptoms in all three domains, where in the brain and how these effects are mediated remains unknown.
Support for a potential role for Avp in schizophrenia comes from studies indicating that treatment with neuroleptics improves psychiatric symptoms and reduces (or normalizes) Avp in blood plasma (Peskind et al. 1987; Raskind et al. 1987). In studies using an animal model that lacks Avp, the Brattleboro rat, there are reports of deficits in behaviors associated with schizophrenia, specifically social discrimination and prepulse inhibition of the startle reflex; these deficits can be rescued following treatment with antipsychotics (Feifel and Priebe 2001, 2007; Feifel et al. 2004, 2007, 2009).
Given that schizophrenia is a complicated multiple etiology neuropsychiatric disorder, it may be that Oxt and Avp only contribute to certain types of schizophrenia. Further, it is likely that their action within specific parts of the brain is particularly important. Thus, the use of preclinical models continues to be critical to help improve our understanding of the specific neural substrates where these neuropeptides may act to contribute to the symptoms associated with schizophrenia as well as to determine their therapeutic efficacy (for review, see Rich and Caldwell 2015; Feifel 2011, 2012; Rosenfeld et al. 2010).
6.4 Posttraumatic Stress Disorder
Work examining Oxt and Avp in the context of PTSD has only recently begun, but PTSD is characterized by some symptoms related to Oxt and Avp function. Specifically dysregulation of stress reactivity and problems with intimate relationship interactions, which relies on normal social cognition (American Psychiatric Association 2013; Monson et al. 2009). While there have been no clinical studies looking at Oxt, intranasal Oxt has been proposed to be used as an adjunctive treatment to help enhance psychotherapy, as it may help ultimately reduce the fear response at the level of the amygdala and diminish the hormonal stress response (Koch et al. 2014). In regard to Avp, there has been one clinical study. Intranasal Avp administered to men, but not women, diagnosed with PTSD results in improved social cognition with their heterosexual partner. Further, men’s urinary Avp is negatively correlated with the severity of their PTSD (Marshall 2013). While these data are limited in scope, they do suggest that understanding the role of Avp in modulating social cognition may have clinical relevance in some psychiatric conditions.
7 Conclusions and Future Directions
Oxt and Avp are key neurochemical signals that act throughout the brain to influence socially motivated behaviors, having powerful effects on both cooperative and competitive behaviors mediated by their actions within the SBNN . There is also a substantial body of evidence that the mesolimbic DA system is involved in regulating cooperative and competitive behaviors. More recently, these two networks have been combined into a larger social decision-making network. However, there is a lack of understanding about the neurochemical linkages between the “social” and “motivational” elements within these networks. We propose that the Oxt and Avp systems represent a bridge between the social and motivational elements. More specifically, we propose that Oxt and Avp provide the critical links between specific elements of the SBNN and DA within the motivational network that produce the forces that drive social behaviors. While there is little direct support for this hypothesis, there is ample evidence that Oxt and Avp can act within structures comprising the mesolimbic DA system and that manipulations of DA can influence the same social behaviors as Oxt and Avp.
These interactions also have important implications for neuropsychiatric disorders characterized by aberrant social behaviors, particularly those with known disruptions in Oxt and Avp signaling. Rigorous testing of preclinical models continues to be the best way to uncover the specific mechanisms (i.e., neurochemistry, substrates, and circuits) that are important to socially motivated behaviors. Thus, it is crucial that a variety of species be studied to help determine both the conserved and unique mechanisms of Oxt and Avp actions across development, sexes, and behaviors. Overall, this is an exciting time in the field of socially motivated behaviors, with the advent of new experimental tools and an improvement in our ability to examine entire circuits we are now on the brink of making significant leaps in our understanding of these systems.
References
Acher R, Chauvet J (1995) The neurohypophysial endocrine regulatory cascade: precursors, mediators, receptors, and effectors. Front Neuroendocrinol 16(3):237–289
Acher R, Chauvet J, Chauvet MT (1995) Man and chimera: selective versus neutral oxytocin evolution. Adv Exp Med Biol 395:615–627
Adkins-Regan E (2009) Neuroendocrinology of social behavior. ILAR J 50(1):5–14
Albers HE (2012) The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav 61(3):283–292. doi:10.1016/j.yhbeh.2011.10.007
Albers HE (2015) Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol 36:49–71. doi:10.1016/j.yfrne.2014.07.001
Albers HE, Cooper TT (1995) Effects of testosterone on the behavioral response to arginine vasopressin microinjected into the central gray and septum. Peptides 16(2):269–273
Albers HE, Pollock J, Simmons WH, Ferris CF (1986) A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J Neurosci 6(7):2085–2089
Albers HE, Liou SY, Ferris CF (1988) Testosterone alters the behavioral response of the medial preoptic-anterior hypothalamus to microinjection of arginine vasopressin in the hamster. Brain Res 456:382–386
Albers HE, Rowland CM, Ferris CF (1991) Arginine-vasopressin immunoreactivity is not altered by photoperiod or gonadal hormones in the Syrian hamster (Mesocricetus auratus). Brain Res 539:137–142
Albers HE, Huhman KL, Meisel RL (2002) Hormonal basis of social conflict and communication. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT (eds) Hormones, brain and behavior. Academic Press, Amsterdam, pp 393–433
Albers HE, Dean A, Karom MC, Smith D, Huhman KL (2006) Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Res 1073–1074:425–430
Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Washington, D.C.
Amico JA, Challinor SM, Cameron JL (1990) Pattern of oxytocin concentrations in the plasma and cerebrospinal fluid of lactating rhesus monkeys (Macaca mulatta): evidence for functionally independent oxytocinergic pathways in primates. J Clin Endocrinol Metab 71(6):1531–1535. doi:10.1210/jcem-71-6-1531
Anacker AM, Beery AK (2013) Life in groups: the roles of oxytocin in mammalian sociality. Front Behav Neurosci 7:185. doi:10.3389/fnbeh.2013.00185
Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A (2010) Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA 107(9):4389–4394. doi:10.1073/pnas.0910249107 0910249107 [pii]
Antoni FA (1984) Novel ligand specificity of pituitary vasopressin receptors in the rat. Neuroendocrinology 39:186–188
Aragona BJ, Wang Z (2009) Dopamine regulation of social choice in a monogamous rodent species. Front Behav Neurosci 3:15. doi:10.3389/neuro.08.015.2009
Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang ZX (2003) A critical role for nucleus accumbens dopamine in partner preference formation of male prairie voles (Microtus ochrogaster). J Neurosci 23:3483–3490
Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z (2006) Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci 9(1):133–139. doi:10.1038/nn1613 nn1613 [pii]
Auyeung B, Lombardo MV, Heinrichs M, Chakrabarti B, Sule A, Deakin JB, Bethlehem RA, Dickens L, Mooney N, Sipple JA, Thiemann P, Baron-Cohen S (2015) Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Transl Psychiatry 5:e507. doi:10.1038/tp.2014.146
Averbeck BB, Bobin T, Evans S, Shergill SS (2012) Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med 42(02):259–266. doi:10.1017/S0033291711001413
Bakermans-Kranenburg MJ, van Jzendoorn MH (2013) Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry 3:e258. doi:10.1038/tp.2013.34
Bales KL, Carter CS (2003) Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster). Horm Behav 44(3):178–184
Bamshad M, Cooper TT, Karom M, Albers HE (1996) Glutamate and vasopressin interact to control scent marking in Syrian hamsters (Mesocricetus auratus). Brain Res 731:213–216
Bankir L (2001) Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. CardiovascRes 51(3):372–390
Bartesaghi R, Gessi T (2004) Parallel activation of field CA2 and dentate gyrus by synaptically elicited perforant path volleys. Hippocampus 14(8):948–963
Bartesaghi R, Migliore M, Gessi T (2006) Input-output relations in the entorhinal cortex-dentate-hippocampal system: evidence for a non-linear transfer of signals. Neuroscience 142(1):247–265
Baskerville TA, Douglas AJ (2010) Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther 16(3):e92–123. doi:10.1111/j.1755-5949.2010.00154.x
Baumeister RF, Leary MR (1995) The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol Bull 117(3):497–529
Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E (2008) Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58(4):639–650. doi:10.1016/j.neuron.2008.04.009 S0896-6273(08)00327-9 [pii]
Beckmann H, Lang RE, Gattaz WF (1985) Vasopressin–oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology 10(2):187–191
Beiderbeck DI, Neumann ID, Veenema AH (2007) Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci 26(12):3597–3605. doi:10.1111/j.1460-9568.2007.05974.x
Benelli A, Bertolini A, Poggioli R, Menozzi B, Basaglia R, Arletti R (1995) Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides 28(4):251–255
Berthold AA (1849) Transplantation der Hoden (Transplantation of testes). Arch Ant Physiol Wissenschr Med 42–46
Bertsch K, Schmidinger I, Neumann ID, Herpertz SC (2012) Reduced plasma oxytocin levels in female patients with borderline personality disorder. Horm Behav. doi:10.1016/j.yhbeh.2012.11.013
Bertsch K, Gamer M, Schmidt B, Schmidinger I, Walther S, Kastel T, Schnell K, Buchel C, Domes G, Herpertz SC (2013) Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. Am J Psychiatry 170(10):1169–1177. doi:10.1176/appi.ajp.2013.13020263
Bester-Meredith JK, Marler CA (2001) Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus). Horm Behav 40(1):51–64
Bester-Meredith JK, Young LJ, Marler CA (1999) Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav 36:25–38
Bielsky IF, Young LJ (2004) Oxytocin, vasopressin, and social recognition in mammals. Peptides 25:1565–1574
Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ (2003) Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29(3):483–493
Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ (2005) The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron 47(4):503–513
Blanchard DC, Blanchard RJ (2003) What can animal aggression research tell us about human aggression? Horm Behav 44(3):171–177
Blanchard RJ, Wall PM, Blanchard DC (2003) Problems in the study of rodent aggression. Horm Behav 44(3):161–170
Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC (2005) AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol Biochem Behav 80(1):189–194. doi:10.1016/j.pbb.2004.10.024 S0091-3057(04)00352-1 [pii]
Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID (2008) Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci 27(8):1947–1956. doi:10.1111/j.1460-9568.2008.06184.x
Bluthe RM, Schoenen J, Dantzer R (1990) Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res 519(1–2):150–157
Bluthe RM, Gheusi G, Dantzer R (1993) Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology 18(4):323–335
Borhegyi Z, Leranth C (1997) Distinct substance P- and calretinin-containing projections from the supramammillary area to the hippocampus in rats; a species difference between rats and monkeys. Exp Brain Res Experimentelle Hirnforschung Experimentation Cerebrale 115(2):369–374
Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL (2002) Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 5(6):514–516. doi:10.1038/nn849
Bos PA, Montoya ER, Hermans EJ, Keysers C, van Honk J (2015) Oxytocin reduces neural activity in the pain circuitry when seeing pain in others. NeuroImage 113:217–224. doi:10.1016/j.neuroimage.2015.03.049
Bosch OJ (2011) Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Horm Behav 59(2):202–212. doi:10.1016/j.yhbeh.2010.11.012
Bosch OJ (2013) Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Philos Trans R Soc Lond B Biol Sci 368(1631):20130085. doi:10.1098/rstb.2013.0085
Bosch OJ, Neumann ID (2008) Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci USA 105(44):17139–17144. doi:10.1073/pnas.0807412105
Bosch OJ, Neumann ID (2010) Vasopressin released within the central amygdala promotes maternal aggression. Eur J Neurosci 31(5):883–891. doi:10.1111/j.1460-9568.2010.07115.x
Bosch OJ, Neumann ID (2012) Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav 61(3):293–303. doi:10.1016/j.yhbeh.2011.11.002
Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID (2005) Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci 25(29):6807–6815. doi:10.1523/JNEUROSCI.1342-05.2005 25/29/6807 [pii]
Bredewold R, Smith CJ, Dumais KM, Veenema AH (2014) Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci 8:216. doi:10.3389/fnbeh.2014.00216
Bridges RS (2015) Neuroendocrine regulation of maternal behavior. Front Neuroendocrinol 36:178–196. doi:10.1016/j.yfrne.2014.11.007
Bruce HM (1959) An exteroceptive block to pregnancy in the mouse. Nature 184:105
Brune M, Ebert A, Kolb M, Tas C, Edel MA, Roser P (2013) Oxytocin influences avoidant reactions to social threat in adults with borderline personality disorder. Human Psychopharmacol 28(6):552–561. doi:10.1002/hup.2343
Buijs RM, Swaab DF (1979) Immuno-electron microscopical demonstration of vasopressin and oxytocin synapses in the limbic system of the rat. Cell Tissue Res 204(3):355–365
Buijs RM, De Vries GJ, Van Leeuwen FW, Swaab DF (1983) Vasopressin and oxytocin: distribution and putative functions in the brain. Prog Brain Res 60:115–122
Buijs RM, Gash DM, Boer GJ (1987) Vasopressin localization and putative functions in the brain. Vasopressin: principles and properties. Plenum Press, New York, pp 91–115
Bujanow W (1972) Hormones in the treatment of psychoses. Br Med J 4(5835):298
Bujanow W (1974) Letter: is oxytocin an anti-schizophrenic hormone? Can Psychiatr Assoc J 19(3):323
Calcagnoli F, de Boer SF, Althaus M, den Boer JA, Koolhaas JM (2013) Antiaggressive activity of central oxytocin in male rats. Psychopharmacology (Berl) 229(4):639–651. doi:10.1007/s00213-013-3124-7
Calcagnoli F, Meyer N, de Boer SF, Althaus M, Koolhaas JM (2014) Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Horm Behav 65(4):427–433. doi:10.1016/j.yhbeh.2014.03.008
Calcagnoli F, Kreutzmann JC, de Boer SF, Althaus M, Koolhaas JM (2015a) Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology 51:112–121. doi:10.1016/j.psyneuen.2014.09.019
Calcagnoli F, Stubbendorff C, Meyer N, de Boer SF, Althaus M, Koolhaas JM (2015b) Oxytocin microinjected into the central amygdaloid nuclei exerts anti-aggressive effects in male rats. Neuropharmacology 90:74–81. doi:10.1016/j.neuropharm.2014.11.012
Caldwell HK, Albers HE (2004a) Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Horm Behav 46(4):444–449
Caldwell HK, Albers HE (2004b) Photoperiodic regulation of vasopressin receptor binding in female Syrian hamsters. Brain Res 1002(1–2):136–141
Caldwell HK, Young 3rd WS (2006) Oxytocin and vasopressin: genetics and behavioral implications. In: Lim R (ed) Neuroactive proteins and peptides, vol 3rd. Handbook of Neurochemistry and Molecular Neurobiology, 3rd edn. Springer, New York, pp 573–607
Caldwell HK, Lee HJ, Macbeth AH, Young WS 3rd (2008a) Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol 84(1):1–24. doi:10.1016/j.pneurobio.2007.10.007 S0301-0082(07)00192-X [pii]
Caldwell HK, Smith DA, Albers HE (2008b) Photoperiodic mechanisms controlling scent marking: interactions of vasopressin and gonadal steroids. Eur J Neurosci 27(5):1189–1196. doi:10.1111/j.1460-9568.2008.06071.x EJN6071 [pii]
Caldwell HK, Wersinger SR, Young WS 3rd (2008c) The role of the vasopressin 1b receptor in aggression and other social behaviours. Prog Brain Res 170:65–72. doi:10.1016/S0079-6123(08)00406-8 S0079-6123(08)00406-8 [pii]
Carter CS, Getz LL (1993) Monogamy and the prairie vole. Sci Am 268(6):100–106
Carter CS, DeVries AC, Getz LL (1995) Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev 19(2):303–314
Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW (2008) Oxytocin, vasopressin and sociality. Prog Brain Res 170:331–336. doi:10.1016/S0079-6123(08)00427-5 S0079-6123(08)00427-5 [pii]
Castel M, Morris JF (1988) The neurophysin-containing innervation of the forebrain of the mouse. Neuroscience 24(3):937–966
Cavanaugh J, Mustoe AC, Taylor JH, French JA (2014) Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology 49:1–10. doi:10.1016/j.psyneuen.2014.06.020
Champagne FA (2008) Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol 29(3):386–397. doi:10.1016/j.yfrne.2008.03.003
Champagne F, Diorio J, Sharma S, Meaney MJ (2001) Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA 98(22):12736–12741
Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ (2004) Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci 24(17):4113–4123. doi:10.1523/JNEUROSCI.5322-03.2004
Chen X, Hackett P, DeMarco A, Gautam P, Feng C, Haroon E, Rilling J (2015a) Oxytocin and vasopressin effects on the neural response to social cooperation among women: a within-subject study. Abstract presented at the Organization for Human Brain Mapping
Chen X, Hackett PD, DeMarco AC, Feng C, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK (2015b) Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain Imaging Behav. doi:10.1007/s11682-015-9411-7
Cho MM, DeVries AC, Williams JR, Carter CS (1999) The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav Neurosci 113(1071):1079
Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ (2005) Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 46(4):647–660. doi:10.1016/j.neuron.2005.04.011
Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S (2003) An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci USA 100:6192–6197
Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW (2007) Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci USA 104(11):4670–4675. doi:10.1073/pnas.0700670104 0700670104 [pii]
Churchland PS, Winkielman P (2012) Modulating social behavior with oxytocin: how does it work? What does it mean? Horm Behav 61(3):392–399. doi:10.1016/j.yhbeh.2011.12.003
Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF (1998) Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry 55(8):708–714
Compaan JC, Buijs RM, Pool CW, de Ruiter AJ, Koolhaas JM (1993) Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Res Bull 30(1–2):1–6
Consiglio AR, Lucion AB (1996) Lesion of hypothalamic paraventricular nucleus and maternal aggressive behavior in female rats. Physiol Behav 59(4–5):591–596
Consiglio AR, Borsoi A, Pereira GA, Lucion AB (2005) Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav 85(3):354–362. doi:10.1016/j.physbeh.2005.05.002 S0031-9384(05)00161-7 [pii]
Cooper MA, Karom M, Huhman KL, Albers HE (2005) Repeated agonistic encounters in hamsters modulate AVP V1a receptor binding. Horm Behav 48(5):545–551. doi:10.1016/j.yhbeh.2005.04.012
Couppis MH, Kennedy CH (2008) The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacol (Berl) 197(3):449–456. doi:10.1007/s00213-007-1054-y
Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS (2007) Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides 41(3):145–163
Crews D (1997) Species diversity and the evolution of behavioral controlling mechanisms. Ann NY Acad Sci 807:1–21
Cui Z, Gerfen CR, Young WS 3rd (2013) Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J Comp Neurol 521(8):1844–1866. doi:10.1002/cne.23263
Curtis JT, Wang Z (2005) Glucocorticoid receptor involvement in pair bonding in female prairie voles: the effects of acute blockade and interactions with central dopamine reward systems. Neuroscience 134(2):369–376. doi:10.1016/j.neuroscience.2005.04.012
Curtis JT, Liu Y, Aragona BJ, Wang Z (2008) Neural regulation for social behavior in rodents. In: Wolff JO, Sherman PW (eds) Rodent societies: an ecological and evolutionary perspective. University of Chicago Press, Chicago, pp 185–194
Dadds MR, Moul C, Cauchi A, Dobson-Stone C, Hawes DJ, Brennan J, Ebstein RE (2014) Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Dev Psychopathol 26(1):33–40. doi:10.1017/S0954579413000497
Dale HH (1906) On some physiological actions of ergot. J Physiol (Lond) 34:163–206
Darwin C (1871) The descent of man, and selection in relation to sex. John Murray, London
Davis MC, Lee J, Horan WP, Clarke AD, McGee MR, Green MF, Marder SR (2013) Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr Res 147(2–3):393–397. doi:10.1016/j.schres.2013.04.023
de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA (2005) Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol 526(1–3):51–64. doi:10.1016/j.ejphar.2005.10.004
De Dreu CK (2012) Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Horm Behav 61(3):419–428. doi:10.1016/j.yhbeh.2011.12.009
De Dreu CK, Kret ME (2015) Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol Psychiatry. doi:10.1016/j.biopsych.2015.03.020
De Dreu CK, Baas M, Roskes M, Sligte DJ, Ebstein RP, Chew SH, Tong T, Jiang Y, Mayseless N, Shamay-Tsoory SG (2013) Oxytonergic circuitry sustains and enables creative cognition in humans. Soc Cogn Affect Neurosci. doi:10.1093/scan/nst094
De Vries GJ, Buijs RM (1983) The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res 273(2):307–317
De Vries GJ, Buijs RM, Sluiter AR (1984) Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res 298:141–145
Delville Y, Ferris CF (1995) Sexual differences in vasopressin receptor binding within the ventrolateral hypothalamus in golden hamsters. Brain Res 681:91–96
Delville Y, Mansour KM, Ferris CF (1996) Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol Behav 60(1):25–29
Delville Y, De Vries GJ, Ferris CF (2000) Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol 55(2):53–76
Demas GE, Albers HE, Cooper MA, Soma KK (2007) Novel mechanisms underlying neuroendocrine regulation of aggression: a synthesis of bird, rodent and primate studies. In: Blaustein JD (ed) Behavioral neurochemistry, neuroendocrinology and molecular neurobiology. Kluwer Press, Heidelberg, pp 337–372
Dichter GS, Damiano CA, Allen JA (2012) Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodevelopmental Dis 4(1):19. doi:10.1186/1866-1955-4-19
Dluzen DE, Muraoka S, Engelmann M, Landgraf R (1998) The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides 19(6):999–1005
Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC (2007) Oxytocin improves “mind-reading” in humans. Biol Psychiatry 61(6):731–733. doi:10.1016/j.biopsych.2006.07.015 S0006-3223(06)00939-5 [pii]
Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC (2013) Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry 74(3):164–171. doi:10.1016/j.biopsych.2013.02.007
Domes G, Kumbier E, Heinrichs M, Herpertz SC (2014) Oxytocin promotes facial emotion recognition and amygdala reactivity in adults with asperger syndrome. Neuropsychopharmacology 39(3):698–706. doi:10.1038/npp.2013.254
Ebert A, Kolb M, Heller J, Edel MA, Roser P, Brune M (2013) Modulation of interpersonal trust in borderline personality disorder by intranasal oxytocin and childhood trauma. Soc Neurosci 8(4):305–313. doi:10.1080/17470919.2013.807301
Elkabir DR, Wyatt ME, Vellucci SV, Herbert J (1990) The effects of separate or combined infusions of corticotrophin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul Pept 28(2):199–214
Engelmann M, Landgraf R (1994) Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav 55(1):145–149
Engelmann M, Wotjak CT, Ebner K, Landgraf R (2000) Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp Physiol 85(Spec No):125S–130S
Everts HGJ, De Ruiter AJH, Koolhaas JM (1997) Differential lateral septal vasopressin in wild-type rats: correlation with aggression. Horm Behav 31:136–144
Feifel D (2011) Is oxytocin a promising treatment for schizophrenia? Expert Rev Neurother 11(2):157–159. doi:10.1586/ern.10.199
Feifel D (2012) Oxytocin as a potential therapeutic target for schizophrenia and other neuropsychiatric conditions. Neuropsychopharmacology 37(1):304–305. doi:10.1038/npp.2011.184
Feifel D, Priebe K (2001) Vasopressin-deficient rats exhibit sensorimotor gating deficits that are reversed by subchronic haloperidol. Biol Psychiatry 50:425–433
Feifel D, Priebe K (2007) The effects of cross-fostering on inherent sensorimotor gating deficits exhibited by Brattleboro rats. J Gen Psychol 134(2):173–182
Feifel D, Melendez G, Shilling PD (2004) Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology 29(4):731–738 10.1038/sj.npp.13003781300378 [pii]
Feifel D, Melendez G, Priebe K, Shilling PD (2007) The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res 181(2):278–286. doi:10.1016/j.bbr.2007.04.020 S0166-4328(07)00230-6 [pii]
Feifel D, Mexal S, Melendez G, Liu PY, Goldenberg JR, Shilling PD (2009) The brattleboro rat displays a natural deficit in social discrimination that is restored by clozapine and a neurotensin analog. Neuropsychopharmacology 34(8):2011–2018. doi:10.1038/npp.2009.15 npp200915 [pii]
Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A (2010) Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry 68(7):678–680. doi:10.1016/j.biopsych.2010.04.039 S0006-3223(10)00479-8 [pii]
Feifel D, Macdonald K, Cobb P, Minassian A (2012) Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr Res 139(1–3):207–210. doi:10.1016/j.schres.2012.05.018
Feldman R, Weller A, Zagoory-Sharon O, Levine A (2007) Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci 18(11):965–970. doi:10.1111/j.1467-9280.2007.02010.x
Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O (2010) Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology 35(8):1133–1141. doi:10.1016/j.psyneuen.2010.01.013
Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK (2014) Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. doi:10.1007/s11682-014-9333-9
Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT (2000) Social amnesia in mice lacking the oxytocin gene. Nat Genet 25:284–288
Ferguson JN, Aldag JM, Insel TR, Young LJ (2001) Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21(20):8278–8285
Fernald RD (2014) Communication about social status. Curr Opin Neurobiol 28:1–4. doi:10.1016/j.conb.2014.04.004
Ferris CF, Potegal M (1988) Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav 44:235–239
Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE (1984) Vasopressin injected into the hypothalamus triggers a complex stereotypic behavior in golden hamsters. Science 224:521–523
Ferris CF, Pollock J, Albers HE, Leeman SE (1985) Inhibition of flank-marking behavior in golden hamsters by microinjection of a vasopressin antagonist into the hypothalamus. Neurosci Lett 55:239–243
Ferris CF, Meenan DM, Albers HE (1986a) Microinjection of kainic acid into the hypothalamus of golden hamsters prevents vasopressin-dependent flank-marking behavior. Neuroendocrinology 44:112–116
Ferris CF, Meenan DM, Axelson JF, Albers HE (1986b) A vasopressin antagonist can reverse dominant/subordinate behavior in hamsters. Physiol Behav 38:135–138
Ferris CF, Axelson JF, Shinto LH, Albers HE (1987) Scent marking and the maintenance of dominant/subordinate status in male golden hamsters. Physiol Behav 40:661–664
Ferris CF, Foote KB, Meltser HM, Plenby MG, Smith KL, Insel TR (1992a) Oxytocin in the amygdala facilitates maternal aggression. Ann NY Acad Sci 652:456–457
Ferris CF, Pilapil CG, Hayden-Hixson D, Wiley RG, Koh ET (1992b) Functionally and anatomically distinct populations of vasopressinergic magnocellular neurons in the female golden hamster. J Neuroendocrinol 4(2):193–205. doi:10.1111/j.1365-2826.1992.tb00159.x
Ferris CF, Melloni RH Jr, Koppel G, Perry KW, Fuller RW, Delville Y (1997) Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci 17(11):4331–4340
Fischer-Shofty M, Levkovitz Y, Shamay-Tsoory SG (2013) Oxytocin facilitates accurate perception of competition in men and kinship in women. Social Cogn Affective Neurosci 8(3):313–317. doi:10.1093/scan/nsr100
Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ (2004) Epigenetic programming of stress responses through variations in maternal care. Ann NY Acad Sci 1036:167–180. doi:10.1196/annals.1330.011
Francis D, Diorio J, Liu D, Meaney MJ (1999) Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286(5442):1155–1158
Francis DD, Champagne FC, Meaney MJ (2000) Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol 12(12):1145–1148
Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E (2012) Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci 126(1):97–109. doi:10.1037/a0026464
Gil M, Nguyen NT, McDonald M, Albers HE (2013) Social reward: interactions with social status, social communication, aggression, and associated neural activation in the ventral tegmental area. Eur J Neurosci 38(2):2308–2318. doi:10.1111/ejn.12216
Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81(2):629–683
Giovenardi M, Padoin MJ, Cadore LP, Lucion AB (1998) Hypothalamic paraventricular nucleus modulates maternal aggression in rats: effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav 63(3):351–359
Glovinsky D, Kalogeras KT, Kirch DG, Suddath R, Wyatt RJ (1994) Cerebrospinal fluid oxytocin concentration in schizophrenic patients does not differ from control subjects and is not changed by neuroleptic medication. Schizophr Res 11(3):273–276
Gobrogge KL, Liu Y, Young LJ, Wang Z (2009) Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci USA 106(45):19144–19149. doi:10.1073/pnas.0908620106 0908620106 [pii]
Goldman M, Marlow-O’Connor M, Torres I, Carter CS (2008) Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr Res 98(1–3):247–255. doi:10.1016/j.schres.2007.09.019 S0920-9964(07)00427-6 [pii]
Goodson JL, Kingsbury MA (2013) What’s in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm Behav 64(1):103–112. doi:10.1016/j.yhbeh.2013.05.006
Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M (2001) Oxytocin and autistic disorder: alterations in peptide forms. Biol Psychiatry 50(8):609–613 S0006322301011398 [pii]
Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, Langford CF, Worley G, Delong GR, Murphy SK, Cuccaro ML, Persico A, Pericak-Vance MA (2009) Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med 7(1):62. doi:10.1186/1741-7015-7-62 1741-7015-7-62 [pii]
Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P (2002) Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci USA 99(9):6370–6375
Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Grunder G, Spreckelmeyer KN (2013) Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry 74(3):172–179. doi:10.1016/j.biopsych.2012.12.023
Guastella AJ, MacLeod C (2012) A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav 61(3):410–418. doi:10.1016/j.yhbeh.2012.01.002
Guastella AJ, Mitchell PB, Dadds MR (2008) Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry 63(1):3–5. doi:10.1016/j.biopsych.2007.06.026 S0006-3223(07)00617-8 [pii]
Guastella AJ, Carson DS, Dadds MR, Mitchell PB, Cox RE (2009) Does oxytocin influence the early detection of angry and happy faces? Psychoneuroendocrinology 34(2):220–225. doi:10.1016/j.psyneuen.2008.09.001 S0306-4530(08)00231-X [pii]
Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB (2010a) Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry 67(7):692–694. doi:10.1016/j.biopsych.2009.09.020 S0006-3223(09)01122-6 [pii]
Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB (2010b) Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol Psychiatry 67(12):1220–1222. doi:10.1016/j.biopsych.2010.03.014
Gutzler SJ, Karom M, Erwin WD, Albers HE (2010) Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus). Eur J Neurosci 31(9):1655–1663. doi:10.1111/j.1460-9568.2010.07190.x
Hammock EA (2015) Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology 40(1):24–42. doi:10.1038/npp.2014.120
Hara Y, Battey J, Gainer H (1990) Structure of mouse vasopressin and oxytocin genes. Brain Res Mol Brain Res 8:319–324
Harmon AC, Huhman KL, Moore TO, Albers HE (2002a) Oxytocin inhibits aggression in female Syrian hamsters. J Neuroendocrinol 14(12):963–969
Harmon AC, Moore TO, Huhman KL, Albers HE (2002b) Social experience and social context alter the behavioral response to centrally administered oxytocin in female Syrian hamsters. Neuroscience 109(4):767–772
Hattori T, Kanno K, Nagasawa M, Nishimori K, Mogi K, Kikusui T (2015) Impairment of interstrain social recognition during territorial aggressive behavior in oxytocin receptor-null mice. Neurosci Res 90:90–94. doi:10.1016/j.neures.2014.05.003
Heinrichs M, von Dawans B, Domes G (2009) Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol 30(4):548–557. doi:10.1016/j.yfrne.2009.05.005 S0091-3022(09)00029-6 [pii]
Hennessey AC, Whitman DC, Albers HE (1992) Microinjection of arginine-vasopressin into the periaqueductal gray stimulates flank marking in Syrian hamsters (Mesocricetus auratus). Brain Res 569:136–140
Hernando F, Schoots O, Lolait SJ, Burbach JP (2001) Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology 142(4):1659–1668
Hitti FL, Siegelbaum SA (2014) The hippocampal CA2 region is essential for social memory. Nature 508(7494):88–92. doi:10.1038/nature13028
Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S (2003) Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology 28(1):193–198. doi:10.1038/sj.npp.1300021 1300021 [pii]
Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S (2007) Oxytocin increases retention of social cognition in autism. Biol Psychiatry 61(4):498–503. doi:10.1016/j.biopsych.2006.05.030 S0006-3223(06)00729-3 [pii]
House JS, Landis KR, Umberson D (1988) Social relationships and health. Science 241(4865):540–545
Hu SB, Zhao ZS, Yhap C, Grinberg A, Huang SP, Westphal H, Gold P (2003) Vasopressin receptor 1a-mediated negative regulation of B cell receptor signaling. J Neuroimmunol 135(1–2):72–81
Huber D, Veinante P, Stoop R (2005) Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308(5719):245–248. doi:10.1126/science.1105636
Huchard EC, Cowlishaw G (2011) Female-female aggression around mating: an extra cost of sociality in a multimale primate society. Behav Ecol 22(5):1003–1011. doi:10.1093/beheco/arr083
Huck UW, Lisk RD, McKay MV (1988) Social dominance and reproductive success in pregnant and lactating golden hamsters (Mesocricetus auratus) under seminatural conditions. Physiol Behav 44(3):313–319
Huhman KL, Albers HE (1993) Estradiol increases the behavioral response to arginine vasopressin (AVP) in the medial preoptic-anterior hypothalamus. Peptides 14:1049–1054
Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM (2010) Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci 30(14):4999–5007. doi:10.1523/JNEUROSCI.5538-09.2010 30/14/4999 [pii]
Hyer MM, Rycek LM, Floody OR (2012) Effects of apomorphine on mating behavior, flank marking and aggression in male hamsters. Pharmacol Biochem Behav 101(4):520–527. doi:10.1016/j.pbb.2012.02.019
Insel TR, Hulihan TA (1995) A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci 109:782–789
Insel TR, Shapiro LE (1992) Oxytocin receptor distribution relects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA 89:5981–5985
Insel TR, Gelhard R, Shapiro LE (1991) The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience 43(2–3):623–630
Insel TR, Wang ZX, Ferris CF (1994) Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci 14:5381–5392
Insel TR, Winslow JT, Wang ZX, Young L, Hulihan TJ (1995) Oxytocin and the molecular basis of monogamy. Adv Exp Med Biol 395:227–234
Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF (1990) Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav 48:693–699
Jack A, Connelly JJ, Morris JP (2012) DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Front Hum Neurosci 6:280. doi:10.3389/fnhum.2012.00280
Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH Jr (2007) Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett 417(1):6–9. doi:10.1016/j.neulet.2007.02.001 S0304-3940(07)00135-8 [pii]
Jakab RL, Naftolin F, Leranth C (1991) Convergent vasopressinergic and hippocampal input onto somatospiny neurons of the rat lateral septal area. Neuroscience 40(2):413–421
Jirikowski GF, Caldwell JD, Stumpf WE, Pedersen CA (1990) Topography of oxytocinergic estradiol target neurons in the mouse hypothalamus. Folia Histochem Cytobiol 28:3–9
Johnson AE, Barberis C, Albers HE (1995) Castration reduces vasopressin receptor binding in the hamster hypothalamus. Brain Res 674:153–158
Johnston RE (1985) Communication. In: Seigel HI (ed) The hamster: reproduction and behavior. Plenum Press, New York, pp 121–149
Johnston RE, Peng A (2008) Memory for individuals: hamsters (Mesocricetus auratus) require contact to develop multicomponent representations (concepts) of others. J Comp Psychol 122(2):121–131. doi:10.1037/0735-7036.122.2.121
Jurek B, Slattery DA, Maloumby R, Hillerer K, Koszinowski S, Neumann ID, van den Burg EH (2012) Differential contribution of hypothalamic MAPK activity to anxiety-like behaviour in virgin and lactating rats. PLoS One 7(5):e37060. doi:10.1371/journal.pone.0037060
Kantojarvi K, Oikkonen J, Kotala I, Kallela J, Vanhala R, Onkamo P, Jarvela I (2015) Association and promoter analysis of AVPR1A in finnish autism families. Autism Res Official J Int Soc Autism Res. doi:10.1002/aur.1473
Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ (2015) RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci 1–10. doi:10.1080/17470919.2015.1040893
Kelly AM, Goodson JL (2014) Social functions of individual vasopressin-oxytocin cell groups in vertebrates: what do we really know? Front Neuroendocrinol. doi:10.1016/j.yfrne.2014.04.005
Kent K, Arientyl V, Khachatryan MM, Wood RI (2013) Oxytocin induces a conditioned social preference in female mice. J Neuroendocrinol 25(9):803–810. doi:10.1111/jne.12075
Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook EH Jr, Insel TR (2002) Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry 7(5):503–507
Kim YR, Kim JH, Kim MJ, Treasure J (2014) Differential methylation of the oxytocin receptor gene in patients with anorexia nervosa: a pilot study. PLoS One 9(2):e88673. doi:10.1371/journal.pone.0088673
Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H (1992) Structure and expression of a human oxytocin receptor. Nature 356:526–529
Kleiman DG (1977) Monogamy in mammals. Q Rev Biol 52:39–69
Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V (2012) Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73(3):553–566. doi:10.1016/j.neuron.2011.11.030
Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2014) Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: salience processing and fear inhibition processes. Psychoneuroendocrinology 40:242–256. doi:10.1016/j.psyneuen.2013.11.018
Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005) Oxytocin increases trust in humans. Nature 435(7042):673–676. doi:10.1038/nature03701 nature03701 [pii]
Kremarik P, Freund-Mercier MJ, Stoeckel ME (1993) Histoautoradiographic detection of oxytocin- and vasopressin-binding sites in the telencephalon of the rat. J Comp Neurol 333(3):343–359
Ku CY, Qian A, Wen Y, Anwer K, Sanborn BM (1995) Oxytocin stimulates myometrial guanosine triphosphatase and phospholipase-C activities via coupling to G alpha q/11. Endocrinology 136(4):1509–1515
Kubota Y, Kimura T, Hashimoto K, Tokugawa Y, Nobunaga K, Azuma C, Saji F, Murata Y (1996) Structure and expression of the mouse oxytocin receptor gene. Mol Cell Endocrinol 124(1–2):25–32
Lancaster K, Carter CS, Pournajafi-Nazarloo H, Karaoli T, Lillard TS, Jack A, Davis JM, Morris JP, Connelly JJ (2015) Plasma oxytocin explains individual differences in neural substrates of social perception. Front Hum Neurosci 9:132. doi:10.3389/fnhum.2015.00132
Landgraf R, Neumann ID (2004) Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol 25(2–4):150–176
Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M (1995) V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci 15(6):4250–4258
Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS 3rd (2008a) A conditional knockout mouse line of the oxytocin receptor. Endocrinology 149(7):3256–3263. doi:10.1210/en.2007-1710 en.2007-1710 [pii]
Lee HJ, Caldwell HK, Macbeth AH, Young WS 3rd (2008b) Behavioural studies using temporal and spatial inactivation of the oxytocin receptor. Prog Brain Res 170:73–77. doi:10.1016/S0079-6123(08)00407-X S0079-6123(08)00407-X [pii]
Lee HJ, Macbeth AH, Pagani JH, Young WS 3rd (2009a) Oxytocin: the great facilitator of life. Prog Neurobiol 88(2):127–151. doi:10.1016/j.pneurobio.2009.04.001 S0301-0082(09)00046-X [pii]
Lee R, Ferris C, Van de Kar LD, Coccaro EF (2009b) Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology 34(10):1567–1573. doi:10.1016/j.psyneuen.2009.06.002 S0306-4530(09)00192-9 [pii]
Lee AG, Cool DR, Grunwald WC Jr, Neal DE, Buckmaster CL, Cheng MY, Hyde SA, Lyons DM, Parker KJ (2011) A novel form of oxytocin in New World monkeys. Biology letters 7(4):584–587. doi:10.1098/rsbl.2011.0107
Leng G, Ludwig M (2008) Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol 586(Pt 23):5625–5632. doi:10.1113/jphysiol.2008.159103
Leng G, Ludwig M (2015) Intranasal Oxytocin: myths and delusions. Biol Psychiatry. doi:10.1016/j.biopsych.2015.05.003
Liberzon I, Trujillo KA, Akil H, Young EA (1997) Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology 17(6):353–359. doi:10.1016/S0893-133X(97)00070-5
Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ (2004) Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429(6993):754–757
Linkowski P, Geenen V, Kerkhofs M, Mendlewicz J, Legros JJ (1984) Cerebrospinal fluid neurophysins in affective illness and in schizophrenia. Eur Arch Psychiatry Neurol Sci 234(3):162–165
Liu Y, Wang ZX (2003) Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121:537–544
Liu Y, Curtis JT, Wang ZX (2001) Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav Neurosci 115:910–919
Lolait SJ, O’Carroll AM, Mahan LC, Felder CC, Button DC, Young WS III, Mezey E, Brownstein MJ (1995) Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci USA 92(15):6783–6787
Lonstein JS, Gammie SC (2002) Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev 26(8):869–888
Love TM (2014) Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav 119:49–60. doi:10.1016/j.pbb.2013.06.011
Ludwig M (1998) Dendritic release of vasopressin and oxytocin. J Neuroendocrinol 10(12):881–895
Ludwig M, Leng G (2006) Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7(2):126–136. doi:10.1038/nrn1845
Ma Y, Liu Y, Rand DG, Heatherton TF, Han S (2015) Opposing oxytocin effects on intergroup cooperative behavior in intuitive and reflective minds. Neuropsychopharmacology. doi:10.1038/npp.2015.87
Macbeth AH, Lee HJ, Edds J, Young WS 3rd (2009) Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes Brain Behav 8(5):558–567. doi:10.1111/j.1601-183X.2009.00506.x GBB506 [pii]
Macdonald K, Feifel D (2012) Oxytocin in schizophrenia: a review of evidence for its therapeutic effects. Acta Neuropsychiatrica 24(3):130–146. doi:10.1111/j.1601-5215.2011.00634.x
Mai JK, Berger K, Sofroniew MV (1993) Morphometric evaluation of neurophysin-immunoreactivity in the human brain: pronounced inter-individual variability and evidence for altered staining patterns in schizophrenia. J Hirnforsch 34(2):133–154
Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G (2008) Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res 170:473–512. doi:10.1016/S0079-6123(08)00437-8
Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G (2012) Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol 24(4):609–628. doi:10.1111/j.1365-2826.2012.02303.x
Marshall AD (2013) Posttraumatic stress disorder and partner-specific social cognition: a pilot study of sex differences in the impact of arginine vasopressin. Biol Psychol 93(2):296–303. doi:10.1016/j.biopsycho.2013.02.014
Martinez M, Guillen-Salazar F, Salvador A, Simon VM (1995) Successful intermale aggression and conditioned place preference in mice. Physiol Behav 58(2):323–328
Mateo JM, Johnston RE (2003) Kin recognition by self-referent phenotype matching: weighing the evidence. Animal Cogn 6(1):73–76. doi:10.1007/s10071-003-0165-z
Matson JL, Nebel-Schwalm M (2007a) Assessing challenging behaviors in children with autism spectrum disorders: a review. Res Dev Disabil 28(6):567–579. doi:10.1016/j.ridd.2006.08.001 S0891-4222(06)00073-4 [pii]
Matson JL, Nebel-Schwalm MS (2007b) Comorbid psychopathology with autism spectrum disorder in children: an overview. Res Dev Disabil 28(4):341–352. doi:10.1016/j.ridd.2005.12.004 S0891-4222(06)00049-7 [pii]
Maybauer MO, Maybauer DM, Enkhbaatar P, Traber DL (2008) Physiology of the vasopressin receptors. Best Pract Res Clin Anaesthesiol 22(2):253–263
Mayes CR, Watts AG, McQueen JK, Fink G, Charlton HM (1988) Gonadal steroids influence neurophysin II distribution in the forebrain of normal and mutant mice. Neuroscience 25(3):1013–1022
McEwen BB (2004) Brain-fluid barriers: relevance for theoretical controversies regarding vasopressin and oxytocin memory research. Adv Pharmacol 50(531–592):655–708. doi:10.1016/S1054-3589(04)50014-5
Meisel RL, Joppa MA (1994) Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol Behav 56(5):1115–1118
Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, Weinberger DR (2009) Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry 14(10):968–975. doi:10.1038/mp.2008.54 mp200854 [pii]
Michopoulos V, Checchi M, Sharpe D, Wilson ME (2011) Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Horm Behav 59(4):528–535. doi:10.1016/j.yhbeh.2011.02.002
Michopoulos V, Higgins M, Toufexis D, Wilson ME (2012) Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology 37(7):1071–1085. doi:10.1016/j.psyneuen.2011.12.004
Miczek KA, Fish EW, De Bold JF, De Almeida RM (2002) Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl) 163(3–4):434–458. doi:10.1007/s00213-002-1139-6
Mikolajczak M, Gross JJ, Lane A, Corneille O, de Timary P, Luminet O (2010) Oxytocin makes people trusting, not gullible. Psychol Sci 21(8):1072–1074. doi:10.1177/0956797610377343
Miller TV, Caldwell HK (2015) Oxytocin during development: possible organizational effects on behavior. Front Endocrinol 6:76. doi:10.3389/fendo.2015.00076
Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, Hosseini SM, Tajdini M, Ghaleiha A, Akhondzadeh S (2013) Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia : an 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs 27(1):57–65. doi:10.1007/s40263-012-0022-1
Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H (1998) Plasma oxytocin levels in autistic children. Biol Psychiatry 43(4):270–277 S0006322397004393 [pii]
Monson CM, Taft CT, Fredman SJ (2009) Military-related PTSD and intimate relationships: from description to theory-driven research and intervention development. Clin Psychol Rev 29(8):707–714. doi:10.1016/j.cpr.2009.09.002
Montag C, Brockmann EM, Bayerl M, Rujescu D, Muller DJ, Gallinat J (2013) Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: a case-control study. World J Biol Psychiatry Official J World Fed Soc Biol Psychiatry 14(7):500–508. doi:10.3109/15622975.2012.677547
Nephew BC, Bridges RS (2008) Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiol Behav 95(1–2):182–186. doi:10.1016/j.physbeh.2008.05.016
Nephew BC, Byrnes EM, Bridges RS (2010) Vasopressin mediates enhanced offspring protection in multiparous rats. Neuropharmacology 58(1):102–106. doi:10.1016/j.neuropharm.2009.06.032
Neumann ID (2008) Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol 20(6):858–865. doi:10.1111/j.1365-2826.2008.01726.x JNE1726 [pii]
Newman SW (1999) The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann NY Acad Sci 877:242–257
Nyffeler J, Walitza S, Bobrowski E, Gundelfinger R, Grunblatt E (2014) Association study in siblings and case-controls of serotonin- and oxytocin-related genes with high functioning autism. J Molecul Psychiatry 2(1):1. doi:10.1186/2049-9256-2-1
O’Connell LA, Hofmann HA (2011a) Genes, hormones, and circuits: an integrative approach to study the evolution of social behavior. Front Neuroendocrinol 32(3):320–335. doi:10.1016/j.yfrne.2010.12.004
O’Connell LA, Hofmann HA (2011b) The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol 519(18):3599–3639. doi:10.1002/cne.22735
Ostrowski NL, Lolait SJ, Bradley DJ, O’Carroll A, Brownstein MJ, Young WS 3rd (1992) Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology 131(1):533–535
Ostrowski NL, Lolait SJ, Young Iii WS (1994) Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology 135(4):1511–1528
Ott I, Scott JC (1910) The action of infundibulin upon the mammary secretion. Proc Soc Exp Biol (NY) 8:48–49
Pagani JH, Zhao M, Cui Z, Avram SK, Caruana DA, Dudek SM, Young WS (2015) Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol Psychiatry 20(4):490–499. doi:10.1038/mp.2014.47
Paul MJ, Terranova JI, Probst CK, Murray EK, Ismail NI, de Vries GJ (2014) Sexually dimorphic role for vasopressin in the development of social play. Front Behav Neurosci 8:58. doi:10.3389/fnbeh.2014.00058
Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL (2011) Intranasal oxytocin reduces psychotic symptoms and improves theory of mind and social perception in schizophrenia. Schizophr Res 132(1):50–53. doi:10.1016/j.schres.2011.07.027
Peskind ER, Raskind MA, Leake RD, Ervin MG, Ross MG, Dorsa DM (1987) Clonidine decreases plasma and cerebrospinal fluid arginine vasopressin but not oxytocin in humans. Neuroendocrinology 46(5):395–400
Petrulis A (2009) Neural mechanisms of individual and sexual recognition in Syrian hamsters (Mesocricetus auratus). Behav Brain Res 200(2):260–267. doi:10.1016/j.bbr.2008.10.027 S0166-4328(08)00594-9 [pii]
Popik P, Van Ree JM (1991) Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol 1:555–560
Potegal M, Ferris CF (1990) Intraspecific aggression in male hamsters is inhibited by intrahypothalamic vasopressin-receptor antagonist. Aggressive Behav 15:311–320
Puglia MH, Lillard TS, Morris JP, Connelly JJ (2015) Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc Natl Acad Sci USA 112(11):3308–3313. doi:10.1073/pnas.1422096112
Ragen BJ, Bales KL (2013) Oxytocin and vasopressin in non-human primates. In: Choleris E, Pfaff DW, Kavaliers M (eds) Oxytocin, vasopressin and related peptides in the regulation of behavior. Cambridge University Press, Cambridge, pp 288–308
Ragnauth AK, Goodwillie A, Brewer C, Muglia LJ, Pfaff DW, Kow LM (2004) Vasopressin stimulates ventromedial hypothalamic neurons via oxytocin receptors in oxytocin gene knockout male and female mice. Neuroendocrinology 80:92–99
Raskind MA, Courtney N, Murburg MM, Backus FI, Bokan JA, Ries RK, Dorsa DM, Weitzman RE (1987) Antipsychotic drugs and plasma vasopressin in normals and acute schizophrenic patients. Biol Psychiatry 22(4):453–462 0006-3223(87)90167-3 [pii]
Rich ME, Caldwell HK (2015) A role for oxytocin in the etiology and treatment of schizophrenia. Front Endocrinol 6:90. doi:10.3389/fendo.2015.00090
Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G (2012) Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology 37(4):447–461. doi:10.1016/j.psyneuen.2011.07.013
Rilling JK, Demarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G (2014) Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 39:237–248. doi:10.1016/j.psyneuen.2013.09.022
Rimmele U, Hediger K, Heinrichs M, Klaver P (2009) Oxytocin makes a face in memory familiar. J Neurosci 29(1):38–42. doi:10.1523/JNEUROSCI.4260-08.2009 29/1/38 [pii]
Rodriguez-Laso A, Zunzunegui MV, Otero A (2007) The effect of social relationships on survival in elderly residents of a Southern European community: a cohort study. BMC Geriatr 7:19. doi:10.1186/1471-2318-7-19
Rosenblum LA, Smith EL, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, Gorman JM, Coplan JD (2002) Differing concentrations of corticotropin-releasing factor and oxytocin in the cerebrospinal fluid of bonnet and pigtail macaques. Psychoneuroendocrinology 27(6):651–660
Rosenfeld AJ, Lieberman JA, Jarskog LF (2010) Oxytocin, dopamine, and the amygdala: a neurofunctional model of social cognitive deficits in schizophrenia. Schizophr Bull. doi:10.1093/schbul/sbq015 sbq015 [pii]
Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ (2009a) Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 162(4):892–903. doi:10.1016/j.neuroscience.2009.05.055 S0306-4522(09)00965-8 [pii]
Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ (2009b) Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci 29(5):1312–1318. doi:10.1523/JNEUROSCI.5039-08.2009 29/5/1312 [pii]
Rosvall KA (2011) Intrasexual competition in females: evidence for sexual selection? Behav Ecol Official J Int Soc Behav Ecol 22(6):1131–1140. doi:10.1093/beheco/arr106
Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM (2010) Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr Res 124(1–3):13–21. doi:10.1016/j.schres.2010.09.014 S0920-9964(10)01543-4 [pii]
Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, Maki PM (2011) Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr Res 130(1–3):266–270. doi:10.1016/j.schres.2011.06.002
Rubin LH, Carter CS, Bishop JR, Pournajafi-Nazarloo H, Drogos LL, Hill SK, Ruocco AC, Keedy SK, Reilly JL, Keshavan MS, Pearlson GD, Tamminga CA, Gershon ES, Sweeney JA (2014) Reduced levels of vasopressin and reduced behavioral modulation of oxytocin in psychotic disorders. Schizophr Bull 40(6):1374–1384. doi:10.1093/schbul/sbu027
Saito M, Sugimoto T, Tahara A, Kawashima H (1995) Molecular cloning and characterization of rat V1b vasopressin receptor: evidence for its expression in extra-pituitary tissues. Biochem Biophys Res Commun 212:751–757
Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B (2011) Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry 69(9):875–882. doi:10.1016/j.biopsych.2010.12.022 S0006-3223(10)01314-4 [pii]
Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H (2008) Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology 33(3):368–374. doi:10.1016/j.psyneuen.2007.12.004
Sawchenko PE, Swanson LW (1982) Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 205(3):260–272
Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, Maier W, Hurlemann R (2013) Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci USA 110(50):20308–20313. doi:10.1073/pnas.1314190110
Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS (2010) Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci 30(24):8274–8284. doi:10.1523/JNEUROSCI.1594-10.2010
Schwartzer JJ, Ricci LA, Melloni RH Jr (2013) Prior fighting experience increases aggression in Syrian hamsters: implications for a role of dopamine in the winner effect. Aggress Behav 39(4):290–300. doi:10.1002/ab.21476
Silk JB (2007) The adaptive value of sociality in mammalian groups. Philos Trans R Soc Lond B Biol Sci 362(1480):539–559. doi:10.1098/rstb.2006.1994 Q05478288G021600 [pii]
Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL (2009) The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc Biol Sci 276(1670):3099–3104. doi:10.1098/rspb.2009.0681 rspb.2009.0681 [pii]
Simeon D, Bartz J, Hamilton H, Crystal S, Braun A, Ketay S, Hollander E (2011) Oxytocin administration attenuates stress reactivity in borderline personality disorder: a pilot study. Psychoneuroendocrinology 36(9):1418–1421. doi:10.1016/j.psyneuen.2011.03.013
Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, Lehtimaki T, Binder EB, Young LJ (2014) Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc Natl Acad Sci USA 111(5):1987–1992. doi:10.1073/pnas.1302985111
Smeltzer MD, Curtis JT, Aragona BJ, Wang Z (2006) Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett 394(2):146–151. doi:10.1016/j.neulet.2005.10.019 S0304-3940(05)01183-3 [pii]
Sofroniew MV (1983) Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res 60:101–114
Sofroniew MV (1985) Vasopressin- and neurophysin-immunoreactive neurons in the septal region, medial amygdala, and locus coeruleus in colchicine-treated rats. Neuroscience 15(2):347–358
Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE (2015) DHEA effects on brain and behavior: insights from comparative studies of aggression. J Steroid Biochem Mol Biol 145:261–272. doi:10.1016/j.jsbmb.2014.05.011
Song Z, Larkin T, Albers HE (2014a) Microinjection of arginine-vasopressin (AVP) in the ventral tegmental area (VTA) enhances conditioned place preference and social interaction. Abstract presented at the Society for Neuroscience
Song Z, McCann KE, McNeill JKt, Larkin TE, 2nd, Huhman KL, Albers HE (2014b) Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology 50C:14–19. doi:10.1016/j.psyneuen.2014.08.005
Song Z, Larkin T, O’Malley M, Albers HE (2015) Both oxytocin and arginine vasopressin enhance social recognition by acting on oxytocin receptors. Abstract presented at the Society for Behavioral Neuroendocrinology
Souza RP, de Luca V, Meltzer HY, Lieberman JA, Kennedy JL (2010a) Schizophrenia severity and clozapine treatment outcome association with oxytocinergic genes. Int J Neuropsychopharmacol 13(6):793–798. doi:10.1017/S1461145710000167
Souza RP, Ismail P, Meltzer HY, Kennedy JL (2010b) Variants in the oxytocin gene and risk for schizophrenia. Schizophr Res 121(1–3):279–280. doi:10.1016/j.schres.2010.04.019 S0920-9964(10)01295-8 [pii]
Stanyon R, Bigoni F (2014) Sexual selection and the evolution of behavior, morphology, neuroanatomy and genes in humans and other primates. Neurosci Biobehav Rev 46P4:579–590. doi:10.1016/j.neubiorev.2014.10.001
Stemmelin J, Lukovic L, Salome N, Griebel G (2005) Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V(1b) receptor antagonist SSR149415. Neuropsychopharmacology 30:35–42
Stevenson EL, Caldwell HK (2012) The vasopressin 1b receptor and the neural regulation of social behavior. Horm Behav 61(3):277–282. doi:10.1016/j.yhbeh.2011.11.009
Stockley P, Bro-Jorgensen J (2011) Female competition and its evolutionary consequences in mammals. Biol Rev Cambridge Philos Soc 86(2):341–366. doi:10.1111/j.1469-185X.2010.00149.x
Striepens N, Matusch A, Kendrick KM, Mihov Y, Elmenhorst D, Becker B, Lang M, Coenen HH, Maier W, Hurlemann R, Bauer A (2014) Oxytocin enhances attractiveness of unfamiliar female faces independent of the dopamine reward system. Psychoneuroendocrinology 39:74–87. doi:10.1016/j.psyneuen.2013.09.026
Szot P, Bale TL, Dorsa DM (1994) Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Brain Res Mol Brain Res 24(1–4):1–10
Tabak BA, Meyer ML, Castle E, Dutcher JM, Irwin MR, Han JH, Lieberman MD, Eisenberger NI (2015) Vasopressin, but not oxytocin, increases empathic concern among individuals who received higher levels of paternal warmth: a randomized controlled trial. Psychoneuroendocrinology 51:253–261. doi:10.1016/j.psyneuen.2014.10.006
Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K (2005) Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA 102(44):16096–16101
Tamamaki N, Abe K, Nojyo Y (1988) Three-dimensional analysis of the whole axonal arbors originating from single CA2 pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique. Brain Res 452(1–2):255–272
Tansey KE, Hill MJ, Cochrane LE, Gill M, Anney RJ, Gallagher L (2011) Functionality of promoter microsatellites of arginine vasopressin receptor 1A (AVPR1A): implications for autism. Mol Autism 2(1):3. doi:10.1186/2040-2392-2-3
Teltsh O, Kanyas-Sarner K, Rigbi A, Greenbaum L, Lerer B, Kohn Y (2012) Oxytocin and vasopressin genes are significantly associated with schizophrenia in a large Arab-Israeli pedigree. Int J Neuropsychopharmacol/Official Sci J Collegium Int Neuropsychopharmacologicum 15(3):309–319. doi:10.1017/S1461145711001374
Thompson R, Gupta S, Miller K, Mills S, Orr S (2004) The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology 29(1):35–48
Thompson RR, George K, Walton JC, Orr SP, Benson J (2006) Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci USA 103(20):7889–7894
Timmer M, Cordero MI, Sevelinges Y, Sandi C (2011) Evidence for a role of oxytocin receptors in the long-term establishment of dominance hierarchies. Neuropsychopharmacology 36(11):2349–2356. doi:10.1038/npp.2011.125
Tribollet E, Barberis C, Arsenijevic Y (1997) Distribution of vasopressin and oxytocin receptors in the rat spinal cord: sex-related differences and effect of castration in pudendal motor nuclei. Neuroscience 78(2):499–509
Unkelbach C, Guastella AJ, Forgas JP (2008) Oxytocin selectively facilitates recognition of positive sex and relationship words. Psychol Sci 19(11):1092–1094. doi:10.1111/j.1467-9280.2008.02206.x
Uzefovsky F, Shalev I, Israel S, Knafo A, Ebstein RP (2012) Vasopressin selectively impairs emotion recognition in men. Psychoneuroendocrinology 37(4):576–580. doi:10.1016/j.psyneuen.2011.07.018
Vaccari C, Lolait SJ, Ostrowski NL (1998) Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology 139:5015–5033
van den Pol AN (2012) Neuropeptide transmission in brain circuits. Neuron 76(1):98–115. doi:10.1016/j.neuron.2012.09.014
van Leeuwen FW, Swaab DF, de Raay C (1978) Immunoelectronmicroscopic localization of vasopressin in the rat suprachiasmatic nucleus. Cell Tissue Res 193(1):1–10
Veenema AH, Neumann ID (2008) Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res 170:261–276. doi:10.1016/S0079-6123(08)00422-6 S0079-6123(08)00422-6 [pii]
Veenema AH, Bredewold R, De Vries GJ (2013) Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology 38(11):2554–2561. doi:10.1016/j.psyneuen.2013.06.002
Veinante P, Freund-Mercier MJ (1997) Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol 383(3):305–325
Vertes RP, McKenna JT (2000) Collateral projections from the supramammillary nucleus to the medial septum and hippocampus. Synapse 38(3):281–293
Virkkunen M, Kallio E, Rawlings R, Tokola R, Poland RE, Guidotti A, Nemeroff C, Bissette G, Kalogeras K, Karonen SL (1994) Personality profiles and state aggressiveness in finnish alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry 51(1):28–33
Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R (2011) Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333(6038):104–107. doi:10.1126/science.1201043
Wacker DW, Ludwig M (2012) Vasopressin, oxytocin, and social odor recognition. Horm Behav 61(3):259–265. doi:10.1016/j.yhbeh.2011.08.014
Wang Z, Zhou L, Hulihan TJ, Insel TR (1996) Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol 366(4):726–737
Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR (1999) Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav Neurosci 113(3):602–611
Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, Sheffield VC (2004) Examination of AVPR1a as an autism susceptibility gene. Mol Psychiatry 9(10):968–972
Watanabe Y, Kaneko N, Nunokawa A, Shibuya M, Egawa J, Someya T (2012) Oxytocin receptor (OXTR) gene and risk of schizophrenia: case-control and family-based analyses and meta-analysis in a Japanese population. Psychiatry Clin Neurosci 66(7):622. doi:10.1111/j.1440-1819.2012.02396.x
Watters JJ, Poulin P, Dorsa DM (1998) Steroid homone regulation of vasopressinergic neurotransmission in the central nervous system. Prog Brain Res 119:247–261
Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ (2004) Epigenetic programming by maternal behavior. Nat Neurosci 7(8):847–854. doi:10.1038/nn1276
Wermter AK, Kamp-Becker I, Hesse P, Schulte-Korne G, Strauch K, Remschmidt H (2010) Evidence for the involvement of genetic variation in the oxytocin receptor gene (OXTR) in the etiology of autistic disorders on high-functioning level. Am J Med Genet B Neuropsychiatr Genet 153B(3):629–639. doi:10.1002/ajmg.b.31032
Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS III (2002) Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry 7(9):975–984
Wersinger SR, Caldwell HK, Christiansen M, Young WS 3rd (2007a) Disruption of the vasopressin 1b receptor gene impairs the attack component of aggressive behavior in mice. Genes Brain Behav 6(7):653–660. doi:10.1111/j.1601-183X.2006.00294.x
Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS 3rd (2007b) Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav 6(6):540–551
Wersinger SR, Temple JL, Caldwell HK, Young WS 3rd (2008) Inactivation of the oxytocin and the vasopressin (Avp) 1b receptor genes, but not the Avp 1a receptor gene, differentially impairs the Bruce effect in laboratory mice (Mus musculus). Endocrinology 149(1):116–121. doi:10.1210/en.2007-1056 en.2007-1056 [pii]
Williams JR, Catania KC, Carter CS (1992) Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav 26(3):339–349 0018-506X(92)90004-F [pii]
Williams JR, Insel TR, Harbaugh CR, Carter CS (1994) Oxytocin administered centrally facilitates formation of a partner preference in prairie voles (Microtus ochrogaster). J Neuroendocrinol 6:247–250
Windle RJ, Shanks N, Lightman SL, Ingram CD (1997) Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 138:2829–2834
Winslow J, Insel TR (1991a) Vasopressin modulates male squirrel monkeys’ behavior during social separation. Eur J Pharmacol 200(1):95–101
Winslow JT, Insel TR (1991b) Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J Neurosci 11(7):2032–2038
Winslow JT, Insel TR (2002) The social deficits of the oxytocin knockout mouse. Neuropeptides 26(2–3):221–229
Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR (1993) A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365:545–548
Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR (2003) Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology 28(5):910–918. doi:10.1038/sj.npp.1300128
Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D (2005) Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry 58(1):74–77. doi:10.1016/j.biopsych.2005.03.013 S0006-3223(05)00310-0 [pii]
Yao S, Zhao W, Cheng R, Geng Y, Luo L, Kendrick KM (2014) Oxytocin makes females, but not males, less forgiving following betrayal of trust. Int J Neuropsychopharmacol 17(11):1785–1792. doi:10.1017/S146114571400090X
Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, Dina C, Ebstein RP (2006) Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol Psychiatry 11(5):488–494. doi:10.1038/sj.mp.4001812 4001812 [pii]
Ylisaukko-oja T, Alarcon M, Cantor RM, Auranen M, Vanhala R, Kempas E, von Wendt L, Jarvela I, Geschwind DH, Peltonen L (2006) Search for autism loci by combined analysis of autism genetic resource exchange and finnish families. Ann Neurol 59(1):145–155. doi:10.1002/ana.20722
Young LJ, Huot B, Nilsen R, Wang Z, Insel TR (1996) Species differences in central oxytocin receptor gene expression: comparative analysis of promoter sequences. J Neuroendocrinol 8(10):777–783
Young LJ, Winslow JT, Nilsen R, Insel TR (1997) Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci 111(3):599–605
Young LJ, Wang Z, Cooper TT, Albers HE (2000) Vasopressin (V1a) receptor binding, mRNA expression and transcriptional regulation by androgen in the Syrian hamster brain. J Neuroendocrinol 12(12):1179–1185
Young LJ, Lim MM, Gingrich B, Insel TR (2001) Cellular mechanisms of social attachment. Horm Behav 40(2):133–138. doi:10.1006/hbeh.2001.1691 S0018-506X(01)91691-5 [pii]
Young WS, Li J, Wersinger SR, Palkovits M (2006) The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience 143(4):1031–1039
Young KA, Liu Y, Wang Z (2008) The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol C Toxicol Pharmacol 148(4):401–410. doi:10.1016/j.cbpc.2008.02.004 S1532-0456(08)00032-X [pii]
Young KA, Gobrogge KL, Liu Y, Wang Z (2011) The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol 32(1):53–69. doi:10.1016/j.yfrne.2010.07.006 S0091-3022(10)00055-5 [pii]
Zak PJ, Kurzban R, Matzner WT (2005) Oxytocin is associated with human trustworthiness. Horm Behav 48(5):522–527. doi:10.1016/j.yhbeh.2005.07.009 S0018-506X(05)00175-3 [pii]
Zak PJ, Stanton AA, Ahmadi S (2007) Oxytocin increases generosity in humans. PLoS One 2(11):e1128. doi:10.1371/journal.pone.0001128
Ziegler C, Dannlowski U, Brauer D, Stevens S, Laeger I, Wittmann H, Kugel H, Dobel C, Hurlemann R, Reif A, Lesch KP, Heindel W, Kirschbaum C, Arolt V, Gerlach AL, Hoyer J, Deckert J, Zwanzger P, Domschke K (2015) Oxytocin receptor gene methylation: converging multilevel evidence for a role in social anxiety. Neuropsychopharmacology 40(6):1528–1538. doi:10.1038/npp.2015.2
Zingg HH, Laporte SA (2003) The oxytocin receptor. Trends Endocrinol Metab 14(5):222–227
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Caldwell, H.K., Albers, H.E. (2015). Oxytocin, Vasopressin, and the Motivational Forces that Drive Social Behaviors. In: Simpson, E., Balsam, P. (eds) Behavioral Neuroscience of Motivation. Current Topics in Behavioral Neurosciences, vol 27. Springer, Cham. https://doi.org/10.1007/7854_2015_390
Download citation
DOI: https://doi.org/10.1007/7854_2015_390
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26933-7
Online ISBN: 978-3-319-26935-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)