Abstract
Human induced pluripotent stem (iPS) cells are a promising source of autologous cardiomyocytes to repair and regenerate myocardium for treatment of heart disease. In this study, we describe a method for enhanced cardiomyocyte production from human iPS cells by treating embryoid bodies with a histone deacetylase inhibitor, trichostatin A (TSA), together with activin A and bone morphogenetic protein (BMP)-4. The resulting cardiomyocytes expressed cardiac-specific transcription factors and contractile proteins at both gene and protein levels. Functionally, the contractile embryoid bodies (EBs) displayed calcium cycling and were responsive to the chronotropic agents isoprenaline (0.1 μM) and carbachol (1 μM). The cardiomyocytes derived from human iPS cells may be used to engineer functional cardiac muscle tissue for studying pathophysiology of cardiac disease, for drug discovery test beds, and potentially for generation of cardiac grafts to surgically replace damaged myocardium.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Keywords:
- Trichostatin A
- Epigenetic
- Induced pluripotent stem cells
- Cardiomyocyte
- Differentiation
- Efficiency
- Cardiac tissue engineering
1 Introduction
Induced pluripotent stem (iPS) cells are an ideal source of donor cells for cardiac regenerative therapies because they have the ability to self-renew while retaining the potential to differentiate into all cell types of the body, including cardiomyocytes. Furthermore, patient-specific iPS cells offer an autologous source of cardiomyocytes for personalized therapeutic strategies to circumvent immunological issues such as rejection of transplants (1).
Cardiomyogenesis from iPS cells is a highly organized process mediated by several signaling pathways (2). Spontaneous differentiation of cardiomyocytes occurs from pluripotent cells; however, it is not very efficient, so we and others have used trichostatin A, a histone deacetylase (HDAC) inhibitor, to direct more efficient cardiomyocyte differentiation (3–6), presumably through an epigenetic mechanism. Trichostatin A belongs to the hydroxamic acid class of HDAC inhibitors and was selected because of its high potency and pan-HDAC selectivity (7).
2 Materials
2.1 Cells
-
1.
Human iPS cell lines. iPS(foreskin)-1 and iPS(foreskin)-2 were obtained from WiCell Research Institute (Madison, WI, USA) (8). FA3 is an iPS cell line generated from patients with Friedreich’s ataxia (9).
-
2.
Human newborn foreskin fibroblasts (HFF-1) were obtained from American Type Culture Collection (ATCC, VA, USA).
2.2 Cell Culture Medium and Solutions
-
1.
Dulbecco’s phosphate-buffered saline without Ca2+ and Mg2+ (DPBS-, Invitrogen, 14190-250).
-
2.
Dulbecco’s phosphate-buffered saline with Ca2+ and Mg2+ (DPBS+, Invitrogen, 14040-182).
-
3.
TrypLE Select enzyme (1×) (Invitrogen, 12563-011).
-
4.
Dulbecco’s Modified Eagle’s Medium: Nutrient Mixture F-12 (DMEM/F-12) Glutamax (Invitrogen, 10565-042).
-
5.
β-Mercaptoethanol (Invitrogen, 21985-023).
-
6.
MEM nonessential amino acids (100×) (Invitrogen, 11140-050).
-
7.
Penicillin/streptomycin (10,000 U/mL) (Invitrogen, 15140-122).
-
8.
Human recombinant fibroblast growth factor basic protein (FGF2, Millipore, GF003).
-
9.
Fetal bovine serum (Sigma-Aldrich, 12003C, Lot 7C0029) (see Note 1).
-
10.
KnockOut serum replacement (KSR, Invitrogen, 10828-028).
-
11.
Fibroblast growth medium: DMEM/F-12 Glutamax supplemented with 10 % fetal bovine serum and 50 U/mL penicillin/streptomycin.
-
12.
Undifferentiated human iPS culture medium (iPS medium): DMEM/F-12 Glutamax supplemented with 20 % KSR, 0.1 mM nonessential amino acid, 0.1 mM β-mercaptoethanol, 50 U/mL penicillin/streptomycin, and 20 ng/mL FGF2 (see Note 2).
-
13.
Cardiac differentiation medium: DMEM/F-12 Glutamax supplemented with 20 % fetal bovine serum, 0.1 mM nonessential amino acid, 0.1 mM β-mercaptoethanol, and 50 U/mL penicillin/streptomycin.
2.3 Chemicals
-
1.
Mitomycin C from Streptomyces caespitosus (Sigma-Aldrich, M4287).
-
2.
Gelatin from porcine skin (Sigma-Aldrich, G1890). Prepared 0.1 % gelatin in distilled water and autoclaved to solubilize the gelatin. Store at 4 °C for 3–4 months.
-
3.
Trichostatin A (TSA, Sigma-Aldrich, T8552). Prepared aliquots of stock solution 100 μg/mL in dimethyl sulfoxide (DMSO, Sigma-Aldrich, D2650) for long-term storage at −20 °C. A final concentration of 1 ng/mL was prepared with cardiomyocyte differentiation medium before use.
-
4.
Fibronectin from human plasma (Sigma-Aldrich, F2006). Prepared aliquots of stock solution 1 mg/mL in DPBS- for long-term storage at −20 °C. A final concentration of 10 μg/mL fibronectin was prepared with 0.1 % gelatin before use.
-
5.
Recombinant activin A (R&D Systems, 338-AC). Prepared aliquots of stock solution 100 μg/mL in DPBS- containing 0.1 % bovine serum albumin (Sigma-Aldrich, A7030) for long-term storage at −20 °C. A final concentration of 100 ng/mL was prepared with cardiac differentiation medium before use.
-
6.
Recombinant human bone morphogenetic protein-4 (BMP-4, PeproTech). Prepared aliquots of stock solution 20 μg/mL in DPBS- containing 0.1 % bovine serum albumin for long-term storage at −20 °C. A final concentration of 20 ng/mL was prepared with cardiac differentiation medium before use.
2.4 Plasticware
-
1.
T-150 cm2 tissue culture flasks (Falcon).
-
2.
Ultra-low attachment surface flat bottom polystyrene six-well plate (Corning).
-
3.
Center-well organ culture dishes (Falcon).
-
4.
Glass capillary (SDR Clinical Technology), flame-pulled and sterilized with 70 % alcohol.
3 Methods
3.1 Fibroblast Expansion, Inactivation, and Feeder Layer Preparation
-
1.
Human fibroblasts were grown in fibroblast growth medium in T-150 cm2 flasks. Once the cells have reached 90 % confluent, the growth medium is removed by aspiration and the cells are washed twice with DPBS-. Incubate cells with 5 mL of pre-warmed TrypLE solution at 37 °C for 3–5 min to detach cells.
-
2.
Once cells have detached, add 10 mL of fibroblast growth medium to neutralize the action of the TrypLE. Fibroblasts can be divided into new T-150 cm2 flasks at a split ratio of 1:5 for further expansion.
-
3.
At passage 18, arrest cell division by treating 90 % confluent fibroblasts with mitomycin C at a final concentration of 10 μg/mL for 3 h at 37 °C. Following mitomycin C treatment, wash fibroblasts thoroughly with DPBS- twice and perform a cell count using trypan blue dye exclusion (see Note 3).
-
4.
Mitomycin C-inactivated fibroblasts can be aliquoted and cryopreserved in fibroblast growth medium supplemented with 10 % DMSO and used for fibroblast feeder plate preparation upon thawing.
-
5.
To prepare fibroblast feeder plate, mitomycin C-inactivated fibroblasts are thawed quickly and resuspended in pre-warmed fibroblast growth medium. Gently disperse fibroblasts onto the inner well of each organ culture dish at a density of 5.5 × 104 cells/cm2. Add sterile distilled water to the outer well of each organ culture dish to maintain the humidity of the culture environment. Incubate dishes at 37 °C in 5 % CO2 overnight and should be used within 5 days after preparation.
-
6.
On the day of iPS cell expansion, wash new feeder plates with pre-warmed iPS medium once.
3.2 Human iPS Cell Culture and Expansion
-
1.
To passage iPS cells, remove iPS medium by aspiration and replace with pre-warmed DPBS+. Using a flame-pulled glass capillary, dissect undifferentiated colonies of iPS cells manually into ~0.1 mm2 pieces and detach gently from the feeder layer. Wash pieces of iPS colonies with DPBS + twice and transfer onto new feeder plates containing fresh iPS medium (see Note 4).
-
2.
Each feeder plate should contain no more than 12 evenly spaced iPS cell pieces. Culture iPS cells at 37 °C in 5 % CO2 and change media every 2–3 days. Passage iPS cells on a weekly basis.
3.3 Cardiomyocyte Differentiation of Human iPS Cells
-
1.
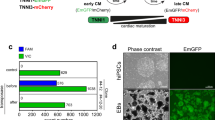
To induce formation of embryoid bodies (EBs), dissect undifferentiated colonies of iPS cells manually into ~0.2 mm2 pieces (Fig. 1a) and detach gently from the feeder layer. Wash pieces of iPS colonies with DPBS + once and transfer to an ultra-low attachment surface six-well plate containing pre-warmed cardiac differentiation medium where they will form EBs (see Note 5) (Fig. 1b).
-
2.
To direct cardiac differentiation during EB formation, supplement cardiac differentiation medium with 100 ng/mL activin A and 1 ng/mL TSA for 1 day (day 0–1); follow by 20 ng/mL BMP-4 and 1 ng/mL TSA for 5 days (day 1–5). Change media at day 1 and 3 (see Notes 6 and 7).
-
3.
After 6 days of EB formation, transfer EBs onto matrix-coated tissue culture plates containing pre-warmed cardiac differentiation medium. Tissue culture plates are coated with 0.1 % gelatin and 10 μg/mL fibronectin for at least 60 min at 37 °C before use (see Note 8).
-
4.
Culture EBs in cardiac differentiation medium at 37 °C in 5 % CO2 and change medium every 2–3 days. Beating cells appear as early as 1 day post-plating and treatment with TSA significantly increased the proportion of EBs beating (Fig. 2).
Fig. 2 Trichostatin A (TSA) enhances the cardiomyogenic effect of activin-A and BMP4 (AB) in all three human iPS cell lines tested: iPS(Foreskin)-1, iPS(Foreskin)-2 and FA3. *p < 0.05, **p < 0.01 and **** p < 0.0001 by two-way ANOVA and Bonferroni post hoc test. Figure modified from (3)
-
5.
Cells from beating EBs can be harvested for various end-point analyses. These include:
-
Immunostaining with antibodies for cardiac progenitor markers (e.g., NKX2.5 and GATA4), cardiac contractile proteins (e.g., cardiac troponin T, cardiac troponin I, α-actinin, and myosin heavy chain), and gap junctions (connexin-43).
-
Quantitative polymerase chain reaction (qPCR) to show the expression of genes encoding the cardiac-restricted transcription factors (e.g., MEF2C, GATA4, and NKX2.5) and the cardiac-specific structural and contractile proteins (e.g., ACTC1, TNNT2, TNNI3, MHL7, and MYL7).
-
Electrophysiological properties of the derived cardiomyocytes with the microelectrode array recordings or patch clamping.
-
Calcium transient imaging with the fluorescent Ca2+ indicator, Fluo-4AM (Invitrogen).
-
4 Notes
-
1.
Fetal bovine serum can vary by source and lot. The batch-to-batch variability of serum may affect the rate of differentiation of human iPS cells into cardiomyocytes. Therefore, the same source and batch of serum should be used throughout the experiment to ensure consistency and reproducibility of experimental result.
-
2.
bFGF should be added freshly to media before use. Multiple freeze/thaw cycles will result in loss of cytokine activity of bFGF and should be avoided. Therefore, aliquots of small volumes of bFGF should be prepared and stored at −20 °C.
-
3.
Avoid excessive treatment with mitomycin C which can induce cytotoxicity and affect the quality of fibroblasts as feeder cells for human iPS cells.
-
4.
The washing step is important to reduce the transfer of fibroblasts to new feeder plates.
-
5.
The size of EB can affect the rate of cardiac differentiation. To ensure differentiation experiments are reproducible, EBs that are uniform in size and shape can be generated with commercially available products such as AggreWell from Stem Cell Technologies.
-
6.
The effective time window of TSA is narrow. In a dose-response study, we have shown that the cardiomyogenic effect of TSA is most effective at 1 ng/mL in the three human iPS cell lines tested (3). At high concentration (>10 ng/mL), TSA induced cytotoxicity. We recommend a dose-response experiment should be performed to determine the optimal dose of TSA for each new pluripotent cell line. Similarly, optimization of activin A and BMP-4 growth factor concentrations may be required to achieve optimal efficiency of cardiomyogenic differentiation.
-
7.
Duration of treatment with growth factors, activin A and BMP-4, is crucial to achieve efficient and reproducible cardiomyogenic differentiation. Therefore, record the time when growth factors were added and change media according to the treatment duration, i.e., 24 h for activin A and 5 days for BMP-4.
-
8.
The tissue culture plates can be coated with 0.1 % gelatin alone. We found that addition of 10 μg/mL fibronectin promotes EB attachment.
References
van der Heyden MA, Jonsson MK (2012) Personalized medicine and the role of induced pluripotent stem cells. Cardiovasc Res 95:395–396
Verma V, Purnamawati K, Manasi et al (2013) Steering signal transduction pathway towards cardiac lineage from human pluripotent stem cells: a review. Cell Signal 25:1096–1107
Lim SY, Sivakumaran P, Crombie DE et al (2013) Trichostatin A enhances differentiation of human induced pluripotent stem cells to cardiogenic cells for cardiac tissue engineering. Stem Cells Transl Med 2:715–725
Hosseinkhani M, Hasegawa K, Ono K et al (2007) Trichostatin A induces myocardial differentiation of monkey ES cells. Biochem Biophys Res Commun 356:386–391
Kawamura T, Ono K, Morimoto T et al (2005) Acetylation of GATA-4 is involved in the differentiation of embryonic stem cells into cardiac myocytes. J Biol Chem 280:19682–19688
Kaichi S, Hasegawa K, Takaya T et al (2010) Cell line-dependent differentiation of induced pluripotent stem cells into cardiomyocytes in mice. Cardiovasc Res 88:314–323
Vanhaecke T, Papeleu P, Elaut G et al (2004) Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: toxicological point of view. Curr Med Chem 11:1629–1643
Yu J, Vodyanik MA, Smuga-Otto K et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Liu J, Verma PJ, Evans-Galea MV et al (2011) Generation of induced pluripotent stem cell lines from Friedreich ataxia patients. Stem Cell Rev 7:703–713
Acknowledgment
These studies were supported by grants from the National Heart Foundation and National Health and Medical Research Council of Australia (1024817; 1056589). GJD is a Principal Research Fellow of NHMRC and AP is a Career Development Fellow of NHMRC. Support is also provided by the JR and JO Wicking Trust, Friedreich’s Ataxia Research Association (research grant and 2012 Keith Michael Andrus Cardiac Research Award), Tony and Gwyneth Lennon Foundation, and the Victorian State Government’s Department of Innovation, Industry and Regional Development’s Operational Infrastructure Support Program.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this protocol
Cite this protocol
Lim, S.Y., Sivakumaran, P., Crombie, D.E., Dusting, G.J., Pébay, A., Dilley, R.J. (2014). Enhancing Human Cardiomyocyte Differentiation from Induced Pluripotent Stem Cells with Trichostatin A. In: Turksen, K., Nagy, A. (eds) Induced Pluripotent Stem (iPS) Cells. Methods in Molecular Biology, vol 1357. Humana Press, New York, NY. https://doi.org/10.1007/7651_2014_160
Download citation
DOI: https://doi.org/10.1007/7651_2014_160
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3054-8
Online ISBN: 978-1-4939-3055-5
eBook Packages: Springer Protocols