Abstract
The monoclonal antibody C3.1 has been found to afford protection against Candida albicans, an opportunistic fungal pathogen. The exceptional inhibition profile of C3.1 has propelled synthetic and immunochemical studies of antibody–oligosaccharide interactions, leading to the development of candidate vaccines. The β1,2-linked mannan of the fungal-cell-wall phosphomannan complex is a protective antigen that exhibits a well-defined conformation. A β1,2-linked trisaccharide conjugated to tetanus toxoid generates protective antibodies in rabbits, and STD-NMR studies show that these antibodies approximate the binding profile of the protective monoclonal antibody. This simple trisaccharide–tetanus toxoid conjugate was a strong immunogen in rabbits, but it was poorly immunogenic in mice. However, when a carbohydrate antigen targeted at dendritic cells was incorporated in this conjugate vaccine, it improved uptake and processing of the antigen and resulted in a five-fold higher mannan-specific antibody response together with a cytokine profile appropriate for an antifungal vaccine. When the same trisaccharide was conjugated to a T-cell peptide derived from Candida cell-wall protein, the resulting glycopeptide–tetanus toxoid conjugate engendered a peptide- and carbohydrate-specific response that afforded protection against live challenge by C. albicans without requiring an adjuvant.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adjuvant-free vaccine
- Antibody reverse engineering of a protective vaccine
- Candida albicans
- Conjugate vaccine
- Dendritic cell targeting of antigen
- Glycopeptide vaccine
- β-mannan
1 Introduction
For more than 80 years it has been known that vaccination of adults with the purified capsular polysaccharide of Streptococcus pneumoniae produces anti-polysaccharide antibodies [1]. These antibodies also confer protective immunity against other S. pneumoniae serotypes that express the same or closely related polysaccharides [2, 3]. These findings subsequently resulted in several licensed polysaccharide vaccines that afforded protection in adults and adolescents against diseases caused by encapsulated bacteria such as S. pneumoniae and Neisseria meningitides. Unfortunately, the immune responses afforded by these polysaccharides to infants under the age of 18 months and to seniors were non-existent or significantly impaired [4–7].
An equally important observation (also in the 1930s) established that capsular polysaccharides conjugated to proteins induced anti-polysaccharide antibodies that could confer active and passive immunity against the homologous strain of S. pneumoniae [8, 9]. It took another 50 years before this knowledge was applied to vaccine development, when the capsular antigen of Haemophilus influenzae type b was conjugated to a fragment of diphtheria toxoid [10]. The resulting vaccine was shown to protect infants against this agent of childhood meningitis [11]. The ensuing 30 years have seen the rapid deployment of a series of conjugate vaccines with annual sales now in the billions of dollars [12]. The use of such vaccines constitutes a highly successful and cost-effective public health measure. These successes have spurred many research initiatives into capsular polysaccharide conjugate vaccines that target a wide spectrum of infectious diseases, including Group B Streptococcus and S. aureus. Other initiatives have investigated conjugates of cell-wall polysaccharides such as lipopolysaccharides from Shigella species.
The bacterial diseases caused by encapsulated bacteria such as S. pneumoniae, H. influenzae type b, and N. meningitides have been countered by licensed vaccines. Major producers of conjugate vaccines now face a dilemma: the cost of the research and development and marketing for new vaccines currently in the pipeline can exceed the financial capacity of even the largest manufacturers. Further, since licensed vaccines address diseases that represent the largest markets, vaccines at the research stage often address diseases that affect a smaller population than those that afford protection against S. pneumoniae and N. meningitides. In this context development of new conjugate vaccines will need to focus on diseases that are the most economically and medically critical. Many of the diseases for which conjugate vaccines have yet to be deployed represent such niche markets.
Vaccines for some microbial diseases – especially those caused by intracellular pathogens and fungal infections – will likely have to address the need for cell-mediated as well as antibody-mediated protection. Recent advances in our understanding of how the immune system processes conjugate vaccines [13] and whether carbohydrate recognition can be exploited for the purpose of cell-mediated immunity together offer intriguing possibilities for the development of chemically defined vaccines and perhaps fully synthetic vaccines.
2 Steps Toward a Vaccine for C. albicans
Numerous reviews have covered polysaccharide [14] and conjugate vaccines [15– 22] including those developed using synthetic oligosaccharides [23–26]. This chapter describes attempts to develop a conjugate vaccine against the fungal pathogen Candida. The investigation evolved from studies of molecular recognition of β-mannans by two monoclonal antibodies that protected mice challenged by live C. albicans. In this sense, much of the initial impetus might be described as reverse engineering these antibody specificities to arrive at a potential conjugate vaccine.
2.1 Reverse Engineering Monoclonal Antibodies
The term “reverse engineering” can have a variety of subtle interpretations. In this case, it refers to using a monoclonal antibody (mAb) known to protect against a given pathogen to guide the development of an immunogen that elicits polyclonal antibodies of related specificity and – most importantly – with the capacity to confer protection following immunization with the inferred antigen [27]. Since the specificity of the mAb can be determined in some detail, molecular recognition of the native antigen can be mapped by various approaches, including saturation transfer difference (STD) NMR, crystallography, and functional group substitutions. The key recognition elements can then be incorporated into a minimally sized antigen. Depending on whether this antigenic determinant is itself immunogenic, it may be necessary to conjugate the determinant to a carrier protein to ensure a robust immune response.
The most intense attempts to reverse engineer an antibody have involved 2G12, an N-linked glycan-specific mAb [28–31]. This broadly neutralizing antibody binds the envelope protein gp120 of human immunodeficiency virus type 1 (HIV-1) [29]. It is a highly unusual antibody, the most unique feature of which is its V H domain-exchanged structure. The multivalent binding surface has two primary glycan binding sites specific for the conserved cluster of oligomannose-type sugars on the surface of gp120. Crystal structures of the 2G12 fragment that contains the antigen-binding region (Fab) and its complexes with the disaccharide Manα(1 → 2)Man and with the oligosaccharide Man9GlcNAc2 revealed that two Fabs assemble into an interlocked V H domain-swapped dimer [28]. This dimeric assembly was not previously observed in hundreds of other Fab structures in the protein data bank. The V H domain swap in 2G12 results in a twist of the variable regions relative to the constant region, putting the two Fabs side-by-side with a 35 Å separation of binding sites that face in the same direction. This arrangement of immunoglobulin G (IgG) binding sites is markedly different from the norm, in which such sites are located at the tips of Y- or T-shaped antibodies. Crystal structures and models derived from them suggest that the dimeric Fab can bind up to four epitopes, two in the usual IgG sites created by the six hypervariable loops of the V L and V H domains and an additional two at the interface between the two V H domains of the Fab dimer. As a result of this multivalency, 2G12 binds the saccharide epitopes with nanomolar avidity.
Heroic efforts have been expended to synthesize and conjugate Man9GlcNAc2 [30–32] and related epitopes to protein carriers. While most of the synthetic constructs have shown strong binding to 2G12, none of these immunogens have so far produced protective antibodies in immunization experiments. An epitope that binds a protective antibody will not necessarily generate an antibody that has both binding specificity and functional protective properties.
Reasons for this lack of success may include the adoption of a unique conformation by the native antigen when it is displayed at the cell surface, and synthetic conjugate antigens may lack these conformational features. Consequently, antibodies induced by immunization with such synthetic antigens may bind the native antigen but lack the essential effector functions.
An additional complication in the reverse engineering of antibodies is the existence of conformational epitopes [33–38]. Several capsular polysaccharide epitopes of this type have been identified. Typically but not exclusively, they have contained neuraminic acid. In addition, only the relatively large oligomers – often within the range of 10–20 saccharide residues – inhibit native antigen–antibody binding, whereas smaller epitopes appear to lack this ability [33]. For conformational epitopes, antigenic oligomers may need to be significantly larger to be capable of raising a protective immune response [39].
In general, one can imagine the binding site as able to capture an antigen and adapt the conformation to satisfy the primary protein–antigen interactions. Although short epitopes may fit the binding site, they may fail to adopt the conformation of the native antigen in its cell wall or membrane environment for the length of time required to induce protective antibodies.
This chapter will discuss in detail the reverse engineering of a protective carbohydrate binding antibody that binds to and protects against the pathogenic fungus C. albicans. The mAb C3.1 and its immunoglobulin M (IgM) counterpart B6.1 both bind the cell-wall β-mannan of C. albicans [40]. This substructure is a relatively small component of the much larger cell-wall phosphomannan complex.
2.2 Antibody Binding Sites
Early work by Kabat sought to determine the size of an oligosaccharide that optimally filled a binding site. These studies looked at polyclonal sera and the inhibitory power of oligomers of increasing length. In this case, the optimum size ranged from trisaccharides to hexasaccharides [41, 42]. Other work examined the size of oligosaccharide epitopes that could generate sera able to recognize the native antigen on the microbial cell wall [43–45] or that could confer protection to live microbial challenge after immunization with a conjugate vaccine prepared from the oligosaccharide [45–47].
Although these studies often used polysaccharides with grossly different characteristics (such as homo- versus heteropolysaccharides), a comparison is of interest. Although inhibition data suggested that short oligosaccharides filled the binding sites, protection data pointed to oligosaccharides that were at least twice or three times larger.
The first crystal structures of oligosaccharides bound to antibodies suggested that tri- and tetrasaccharides filled the binding sites [48, 49]. This was true for many antibodies [48–57], although exceptions were found where a single terminal residue provided nearly all of the binding specificity [58]. In another case, that of the antigen for 2,8-polysialic acid meningococcal serogroup B, the binding site required at least ten monomer residues [59]. This polysaccharide belongs to the class of carbohydrate antigens that are thought to possess a conformational epitope. These conclusions are supported by a detailed body of work [39].
Many anti-carbohydrate antibodies possess a groove-type binding site that allows entry and exit of the polysaccharide chain [50, 51, 53, 57, 59]. Given the repeating-unit character of many bacterial polysaccharides, this feature allows the antibody to bind to internal units as well as terminal epitopes. The polysaccharide can enter and exit a grooved site without impediment. An interesting example of an antibody that binds a branched lipopolysaccharide (LPS) O-antigen demonstrates the importance of this steric requirement. The Salmonella serogroup B O-polysaccharide has a tetrasaccharide repeating unit (Fig. 1a). The crystal structure of the antibody with bound trisaccharide shows the branching monosaccharide, an abequose residue (Abe; 3,6-dideoxy-d-xylo-hexopyranose) to be buried and the two residues of the main chain galactose (Gal) and mannose (Man) essentially lay across the top of the binding pocket (Fig. 1b) [48]. Modeling studies suggested that the next residue, α- l-rhamnopyranose (Rha) unit, does not contact the protein but points its anomeric bond directly toward the protein [60, 61]. This orientation would cause the next residue, a galactose, to clash with protein if the glycosidic linkage from rhamnose to galactose adopts the usual syn conformation (Fig. 2a, c). To avoid a steric clash, the psi angle of this Rha-Gal glycosidic bond was proposed to rotate approximately 180 degrees and adopt the anti glycosidic conformation. NMR experiments on the bound state confirmed this prediction (Fig. 2b, d) [60]. Further experiments using two repeating units of the polysaccharide that could each bind in only one of the frame-shifted modes also supported the requirement for an antigen conformational change on binding (Fig. 2d) [62]. Of course, the adoption of a higher energy conformation comes with an attendant decrease in intrinsic affinity [61].
(a) The biological repeating unit of the O-antigen of the LPS belonging to Salmonella serogroup B: {→2)[α- d-Abe(1 → 3)]α- d-Man(1 → 4)α-L-Rha(1 → 3)α- d-Gal(1−}. In some serogroup B antigens, an acetyl group may occur at O-2 of the abequose residue. (b) The crystal structure of the trisaccharide α- d-Gal(1 → 2)[α- d-Abe(1 → 3)]α- d-Man(1 → OCH3 with the Fab of the monoclonal antibody Se155.4 [48]. The abequose residue is buried in the binding pocket with mannose and galactose lying across the top of the pocket
(a) Extension of the bound trisaccharide shown in Fig. 1 shows that in the expected low-energy conformation, the Man-Rha glycosidic linkage orients the Rha O1 toward the protein. This orientation would result in the Gal residue clashing with the antibody surface. (b) To relieve the steric clash, the Man-Rha glycosidic bond adopts an anti conformation rather than the normal syn conformation. (c) A molecular model showing the Man-Rha linkage in the syn conformation. The Rha O1 points toward the protein. This would cause subsequent polysaccharide residues to clash with the extended antibody surface adjacent to the binding site. (d) A molecular model showing the Man-Rha linkage in the anti conformation. The Rha O1 points away from the protein, allowing the subsequent polysaccharide residues to avoid clashes with the extended antibody surface
These observations are of interest in the context of the Candida work to be discussed. The results with the Salmonella LPS antigens demonstrate that even though a significant conformation change may occur in the antigen, antigen–antibody interactions will still occur (albeit with reduced affinity) provided the energy costs in adopting this conformation are sufficiently small. The crystal structures also show that polysaccharide elements contact protein beyond the formal binding pocket.
2.3 Candida
Candida is a commensal organism that can shift toward pathogenesis with alterations in host immunity, physiology, or normal microflora. The acquisition of novel or hypervirulent factors is not necessary. Invasive C. albicans infection can be a serious clinical complication, especially for patients with an impaired immune system: it has an incidence of between 1.1 and 24 cases per 100,000 individuals and a mortality rate of more than 30% [63]. Antifungal agents have limited impact on the mortality statistics for systemic fungal infections, and new clinical approaches are needed (such as a combination of chemotherapy and immunotherapy). However, the immune response to fungal infections is complex and requires not only the production of protective antibodies but also the recruitment of cell-mediated immunity. The fungal cell wall plays an important role in directing the immune response to fungal infection [40].
Host defense against systemic candidiasis relies on the ingestion and elimination of fungal cells by cells of the innate immune system, especially neutrophils and macrophages [64]. These responses can be promoted by antibodies. Macrophages not only have direct antimicrobial activity against such organisms but also promote the presentation of antigens and the production of cytokines and chemokines. Recognition of C. albicans by monocytes and macrophages is mediated by systems that sense pathogen-associated molecular patterns (PAMPs) of the C. albicans cell wall. These systems include recognition of N-linked mannans by the mannose receptor (MR), O-linked mannans by Toll-like receptor 4 (TLR4), β-glucans by dectin-1/TLR2, β-mannosides by galectin-3/TLR2 complexes [65] and by the C-type lectin known as mincle [66].
2.3.1 The Cell Wall of C. albicans
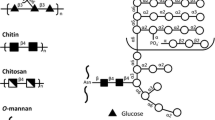
The three major glycans of the C. albicans the cell wall are branched β(1 → 3) and β(1 → 6) glucans, chitin, and a phosphomannan glycoprotein [67, 68]. These polysaccharides function as a robust exoskeleton and scaffold for external protein structures. This outer layer consists of highly glycosylated mannoproteins that are modified by N-linked [69] and O-linked mannose glycans and phosphomannans [64, 69]. The phosphomannan complex has been the subject of detailed structural analyses and immunological studies.
The structural elucidation of the phosphomannan complex represents a major achievement. Unlike bacterial cell-wall polysaccharides, it lacks a regular repeating unit motif and its glycan component possesses marked microheterogeneity as well as variation across different Candida species [70]. An example of the substructures present in the mannan component of C. albicans is shown in Fig. 3. More detailed examples can be found in reviews by the Suzuki group. These structures were determined using acetolysis [71] and partial hydrolysis in conjunction with NMR and mass spectrometry [72–75]. Mannans consist largely of short α(1 → 2)- and α(1 → 3)-d-mannopyranan; less than 10% is O-linked [76]. The complex N-linked components are composed of extended α(1 → 6)-d-mannopyranan backbones containing α(1 → 2)-d-mannopyranan branches, some of which may contain α(1 → 3)-d-mannopyrannose residues. Oligomers of β(1 → 2)-mannopyranan may be attached to these side chains either glycosidically or through a phosphodiester bridge (Fig. 3). The position of attachment of the phosphodiester has yet to be determined. Mannans are heterogeneous, with chain lengths depending on nutritional and environmental conditions [77].
The structure of the glycan chains of C. albicans phosphomannan glycoprotein. The composite structure varies widely with Candida species and with growth conditions. The (1 → 6)-linked α-mannan backbone (blue) is attached to protein. Branching side chains consist of (1 → 2)α-mannan (green) with occasional (1 → 3) linkages. This basic structure is shared by most Candida species. In C. albicans and several other prominent Candida species, (1 → 2)-linked β-mannans (black) can be attached either via a phosphate diester (red), giving acid-labile β-mannan, or glycosidically linked to the α-mannan side chains
The precise antigenic epitopes of β-mannan exist in three different forms. Two forms of the β(1 → 2)-linked oligosaccharides are extensions of the α(1 → 2)-mannose and α(1 → 3)-mannose residues [55]. The third form has the β(1 → 2)-d-mannopyranan attached via the phosphodiester to α(1 → 2)-d-mannopyranan branches [72, 73].
C. albicans strains are assigned to either of two major serogroups, A or B [72, 73, 75, 77]. Serotype A has β-mannose residues attached to α(1 → 2) mannose side-chain residues via glycosidic bonds and via phosphodiesters. Serogroup B lacks β-mannan attached via a glycosidic bond and possesses only phosphodiester-linked β-mannan. Thus, acid-labile β-mannan (linked through a phosphodiester to the phosphomannan side chains) is thought to be a major epitope of both serotypes A and B.
C. tropicalis and C. guilliermondii also express the β-mannan epitope, and it may also be attached via a (1 → 3)-linkage to an α-mannose residue [78–80]. Although β-mannan epitopes larger than a tetrasaccharide have been reported, it appears that (1 → 2)-β-mannopyranan di-, tri-, and tetrasaccharides oligomers predominate [81–84]. Goins and Cutler found that tri- and disaccharide epitopes are most abundant in the β(1 → 2)-mannopyranan oligomers released from the phosphomannan by β elimination [85].
In their structural studies of several Candida species, Shibata et al. employed detailed multidimensional NMR studies and inferred from nuclear Overhauser effect (NOE) data that the β(1 → 2)-linked mannose oligosaccharides possessed a “distorted” structure [73].
2.3.2 Monoclonal Antibody Specific for the β-Mannan of C. albicans
Cutler and colleagues immunized mice with a liposomal preparation of the C. albicans cell-wall phosphomannan complex and produced a series of mAbs [86]. Those that were specific for the α-mannan component failed to protect mice against a live challenge with C. albicans. However, two mAbs (mAbs), C3.1 (IgG3) and B6.1 (IgM), were specific for the (1 → 2)-β-mannan epitope and protected mice against candidiasis in passive protection studies [40, 87–89]. Preliminary binding studies also suggested that these antibodies recognized a trisaccharide epitope [90]. In addition, the Cutler group reported results suggesting that relatively short β-mannan oligosaccharides (di- to tetrasaccharides) were heavily represented on the surface of growing C. albicans cells [85].
2.3.3 Mapping the Size of β-Mannan-Specific Antibody Binding Sites
When the mAbs C3.1 (an IgG3) and B6.1 (an IgM) were evaluated against a set of (1 → 2)-β-mannan oligosaccharide propyl glycosides ranging in size from di- to hexasaccharide, unexpected and dramatic results were observed (Fig. 4) [91]. Oligosaccharide inhibition with carbohydrate-specific antibodies normally shows affinities increasing with oligosaccharide length until a plateau of activity is reached [42, 43]. Further increases in oligosaccharide size produce only very small affinity gains. Dramatic decreases in binding are not seen. Surprisingly, however, inhibitory power for C3.1 and B6.1 mAbs reached a maximum for di- and trisaccharides. Both antibodies exhibited similar binding constants for di- and trimannosides [with half-maximal inhibitory concentration (IC50) values of 44 μM and 38 μM for B6.1 and 8 μM and 16 μM for C3.1]. Larger oligosaccharides were bound progressively more weakly, with the IC50 of a hexamannoside being 844 μM for C3.1 and >1,000 μM for B6.1. It was speculated that the weaker binding of larger oligosaccharides was due to steric restrictions at either end of the (1 → 2)-β-mannans [91].
3 Solution Structure of β-Mannan
3.1 Conformational Studies (1 → 2)-β-Mannan Oligosaccharides
The chemical synthesis of (1 → 2)-β-mannan propyl glycosides 1–5 (Fig. 4) [92] provided a set of oligomers that were not only useful as inhibitors of antibody–antigen interactions but also permitted a thorough analysis of the solution structures of these important C. albicans antigens [91].
The excellent NMR signal dispersion observed for the proton resonances of the (1 → 2)-β-mannopyranan oligomers 1–5 at 800 MHz aided the assignment of resonances by gradient correlation spectroscopy (GCOSY), gradient total correlation spectroscopy (GTOCSY), and heteronuclear multiple quantum coherence (HMQC) experiments (Fig. 5). The discrete chemical shifts of each hexopyranose spin system of pentasaccharide 4 suggested that each hexose is conformationally distinct within a well-defined oligosaccharide conformation (Fig. 5a, b). The distinct 1H chemical shifts observed for the H1 and H2 resonances of each mannose residue of pentasaccharide 4 are in contrast to the near identical shifts observed for H1 resonances of many homo-oligomers, (1 → 2)-α-mannopyranotetrose [93] and maltoheptose [(1 → 4)-α-glucopyranoheptose] [94]. Similarly distinct chemical shifts of anomeric resonances occur for the α- and β-1,2-linked oligomers of glucose [95, 96]. These two oligomers and (1 → 2)-β-mannopyranan were identified by early modeling studies as belonging to a class of homopolymers expected to form relatively stiff and crumpled conformations [97].
After the resonances of the ring protons had been assigned (Fig. 5b), NOE correlations between protons on either side of each glycosidic linkage could be used to establish the residue identity of the spin systems of adjacent pyranose rings and to confirm their sequence within each linear oligomer (Fig. 6) [91].
NOE contacts observed for the pentasaccharide propyl glycoside 4. Three groups of NOE are displayed: ones between adjacent residues n/n + 1, those between residues separated by 1 pyranose ring, and those between residues separated by 2 residue. The mannose residues are labeled A through E as in Fig. 5. Bold numbers are the interproton distances determined by quantitation of the NOE data. Numbers in parentheses are the corresponding interproton distances predicted by molecular dynamics
NOE correlations were then used to define solution conformations of oligosaccharides 1–5. This method is especially effective when there are numerous NOE contacts between hexose residues. NOE correlations were measured from the two-dimensional rotating-frame Overhauser effect spectroscopy (T-ROESY) spectra of the oligomers (1–5). These results revealed numerous contacts between noncontiguous n + 2 and n + 3 residues, as illustrated for pentasaccharide 4 (Fig. 6).
NOE contacts between noncontiguous residues occur often, especially in branch structures such as the A, B and Lewis antigens in human blood [98–100]. A linear homopolysaccharide can achieve the equivalent of vicinal substitution if the hydroxyl group adjacent to the anomeric center is the site for chain extension. Many 1,2-linked homo-oligomers, such as those of the Brucella abortus A antigen, yield few if any NOE contacts between noncontiguous residues [101]. In the conformational analysis of linear oligosaccharides observation of multiple NOE contacts between noncontiguous residues as seen for the (1 → 2)-β-mannopyranans studied here is atypical (Fig. 6). The presence of NOE interactions between residues A and D (n to n + 3 contacts) has limited precedence and is indicative of a compact structure (Fig. 7).
Three-dimensional model of hexasaccharide 5 in solution as determined by NMR. Running from left to right atoms defining the glycosidic linkages C1-O1-C2-O2 trace out a helix shown in yellow. In this conformation the most prominent exposed atoms of each ring are C3 to C6 and their attached hydroxyl groups
The T-ROESY cross-peak volumes for the tri-, tetra-, and pentasaccharide (2–4) were used to derive conformationally averaged interproton distances (Fig. 6) [91]. Similar distances observed across the glycosidic linkages of the oligomers 1–4 are indicative of a compact, repetitive solution structures. The distances also suggest the inferred structure is not just a result of steric interactions between distant, noncontiguous residues but represents a population of low-energy conformations with similar torsional angles about the glycosidic linkages. If steric interactions between noncontiguous residues were limiting the conformations of the oligomers, the shorter oligomers would be expected to have distances that differed from those of their counterparts with higher molecular weights, but this was not the case.
3.2 Molecular Modeling
Unrestrained molecular dynamics modeling was carried out for oligomers 2–4 [90]. The resulting model for pentasaccharide 4 is discussed below.
Comparisons of the ten structures of the pentasaccharide obtained from a simulated annealing protocol showed a single family of low-energy conformations. A static model of the pentasaccharide in one of these conformations shows the helical nature of this oligosaccharide (Fig. 7). The three-dimensional repeating unit approximates three mannose residues.
Theoretical interproton distances were calculated from a 5 ns molecular dynamics run at 300 K. The resulting data compared well with distances determined from experimental NOEs (Fig. 6 distances in parentheses). During the dynamics model run, the oligosaccharide stayed within the range of glycosidic torsional angles represented by the family of conformations generated by simulated annealing.
A comparison of the conformational space sampled by the oligosaccharides indicates that the tri-, tetra-, and pentasaccharides 2–4 explore very similar torsional angles across all their linkages [91]. The family of conformations sampled is consistent with a model that imparts helical character to this glycan chain (Fig. 7). The repeating unit is approximately three residues long, but due to the inherent flexibility about glycosidic torsional angles, the overlap of residues n and n + 3 is only approximate. In this family of conformations, hydroxyls are oriented out into the solution and a hydrophobic core forms from the α-faces of the mannose rings. Interestingly, the Crich group reported a crystal structure of a (1 → 2)-β-mannopyranan tetrasaccharide with attached organic protecting groups [102] and the gross features of this molecule were closely related to the aqueous solution structure inferred from NMR-derived constraints.
4 Molecular Recognition of β-Mannan by Protective mAb
4.1 Mapping the C3.1 Antibody Site
Inhibition data for oligosaccharides 1–5 established that the mAb C3.1 binding site could optimally accommodate a di- or trisaccharide, but larger oligosaccharides were progressively more difficult to accommodate. To establish which hydroxyl groups were essential for binding a disaccharide epitope, the C3.1 binding site was chemically mapped. The principle of hydroxyl group replacement developed by Lemieux was adopted [103]. Synthesis of monodeoxy- and mono-O-methyl mannoside derivatives followed by their use in inhibition assays identified hydroxyl groups that made energetically important contacts with the mAb binding site [104, 105]. Hydroxyl groups that are involved in hydrogen bonds deep within the binding site cannot be deoxygenated or O-methylated without a significant loss of binding affinity. Those involved in hydrogen bonds at the periphery of the binding site may exhibit a range of changes in affinity upon deoxygenation, whereas O-methylation of such groups generally leads to a significant loss of affinity due to the challenge of accommodating the relatively bulky O-methyl group.
Each hydroxyl group of the disaccharide 6 (the methyl glycoside analog of the propyl glycoside 1) was replaced to produce the disaccharide congeners 7–19 (Fig. 8) [104, 105]. Important hydrogen bonds were identified as those from the C3-, C4-, and C4′-OH groups, since the 3-deoxy (7), 3- O-methyl (8), 4-deoxy (9), and 4- O-methyl (10) congeners and the 4′-deoxy analog 16 were inactive (Table 1). We inferred that these three were buried hydroxyl groups that make essential hydrogen bonds to the antibody binding site (Fig. 9). The weak activity of the 4′- O-methyl disaccharide 17 is consistent with C4′ hydroxyl being relatively exposed and also a hydrogen bond acceptor. Its weak activity suggests C4′-OH is exposed at the periphery of the binding site, where it likely accepts a hydrogen bond from the protein and perhaps donates a hydrogen bond to water. If this hydroxyl group were buried in the site, the larger O-methyl group could not be accommodated and an inactive compound would be expected. The weak activity of the C6′-modified congeners 18 and 19, which were also not completely inactive, suggests that the C6′-OH also forms hydrogen bonds near the periphery of the site. The gluco configuration of the terminal residue is well tolerated (compound 20) suggesting little antibody–oligosaccharide interaction in this region of the epitope.
Also of interest is the only slightly higher activity [Δ(ΔG) = −0.4 kcal/mol] of trisaccharide 21 (Table 1 and Fig. 9) relative to disaccharide 6, which showed that a third mannose residue contributes minimal additional binding affinity. This conclusion is supported by the activity of trisaccharide 22 with a β-glucose residue at the nonreducing end. These data suggest that the minimal epitope that might induce antibodies specific to β-mannan could be a disaccharide or trisaccharide.
4.2 Antigen Frameshift Revealed by Chemical Mapping
Studies with modified trisaccharide derivatives were carried out to investigate the possibility of frameshifting (Fig. 10) [106], which is the exchange of epitope binding between chemically identical binding sites on a homopolymer (assuming that these sites are also conformationally identical). Since the minimal oligosaccharide epitope appeared to be small and since β-mannan is a homopolymer, any compound consisting of more than two mannose units theoretically had the possibility of frameshifting. Monodeoxygenation data described above guided the synthesis of trisaccharides 23 and 24 in which the essential hydroxyl group at C4 of the reducing mannose or C4′ of the nonreducing mannose was deoxygenated, respectively, preventing binding to either the internal (23) or terminal (24) disaccharide epitopes [106]. Trisaccharide 23 showed the most dramatic decrease in binding affinity with Δ(ΔG) = 1.2 kcal/mol relative to the native trisaccharide 6 while 24 showed a decrease of 0.68 kcal/mol (Table 2). These results suggest that an internal disaccharide epitope is the recognition element preferred by mAb C3.1.
Two β-mannan trisaccharide motifs are present in C. albicans phosphomannan: phosphodiester linked (a) and glycosidically linked (b) β-mannan. The possibility of frame shifting is indicated for the two modes of binding a disaccharide epitope in each of the motifs. Compounds 23 and 24 are deoxygenated at the reducing end and nonreducing end. Since deoxygenation abrogates binding of the residue in which that modification occurs 23 and 24, respectively, provide “non-reducing” terminal and “reducing” internal disaccharide epitopes
4.3 Correlation of Solution Structure with Inhibition Data
The unusual pattern of inhibition of compounds 2–5 with C3.1 [91] was consistent with a binding site that could accommodate a trisaccharide and possibly even a fourth residue without a huge loss of binding energy. The slightly higher activity of a trisaccharide over a disaccharide suggested that a trisaccharide provided the optimum fit even though the primary polar contacts are located within a disaccharide. The well-ordered solution conformation of oligosaccharide raised the possibility that tetra-, penta-, and hexasaccharides might have to undergo a conformational change – most probably of glycosidic torsional angles – to avoid clashes with the surface of the binding site, as was seen in a Salmonella O-antigen-specific mAb [60, 61]. For a binding site with strict size constraints, such imposed conformational changes would be progressively more costly energetically with each additional hexose residue beyond a trisaccharide. These data also pointed to the potential of a small oligosaccharide epitope as a B-cell epitope that might be used to raise protective polyclonal sera.
4.4 NMR Studies of Bound Oligosaccharides
Observations of the oligosaccharide interfaces with the C3.1 antibody binding site were made for compounds 1–3, 23, and 24 using STD-NMR spectroscopy [57]. These data were consistent with and extended the conclusions of the inhibition data [91, 105]. STD-NMR experiments can provide an epitope map, with the strongest enhancements corresponding to oligosaccharide protons in close contact with protons of the antibody combining site [107]. STD-NMR patterns for compounds 2, 3, and 6 are shown in Fig. 11. The intensity of the 1H resonances that experience the largest STD effect is seen to increase. Di- and trisaccharides 6 and 3 showed the strongest enhancements in residue A, followed by residue B. Enhancements in residue C of 6 were weaker. These results confirmed that an internal disaccharide is recognized by mAb C3.1. Further, within this disaccharide epitope, the internal monosaccharide residue (residue A) is more strongly bound than the terminal residue. Disaccharide 6 and trisaccharide 2 had similar binding modes, although some subtle differences were detectable, such as a greater enhancement of H2A over H1A in 1.
STD spectra for the disaccharide methyl glycoside 6 (trace A), trisaccharide propyl glycoside 2 (trace B), and tetrasaccharide propyl glycoside 3 (trace C). The labels A through D refer to the saccharide residues identified as in Fig. 6. The associated digits refer to the hydrogen atoms bonded to saccharide or propyl carbon atoms numbered in the standard manner. The tetrasaccharide binds in a frame-shifted mode such that anomeric resonance H1B rather than H1A receives the strongest STD effect
In contrast to the results for disaccharide 6 and trisaccharide 2, tetrasaccharide 3 (a 5-fold weaker inhibitor than trisaccharide 2) showed maximal enhancements for residue B, followed by the other residues in the order A > C ≈ D. These observations (Fig. 11) are interpreted as a frameshift. A frameshift results from the sequence redundancy of the homopolymer and the steric constraints of the combining site. This hypothesis is supported by the increased STD enhancement in the propyl aglycone (non-sugar portion) of 3 relative to 6, suggesting that the increasing oligosaccharide length of 3 leads to additional contacts with the antibody at the reducing end.
Additional NMR studies based on the transferred nuclear Overhauser effect (trNOE) allowed us to determine the bound conformations of 6, 2, and 3. Helical conformations with short distances between monosaccharides separated by one residue were found to be bound by the antibody. These conformations are similar to those adopted by unbound oligosaccharides in solution, suggesting that the antibody recognizes conformations similar to the solution global minimum [91, 108].
NMR data collected for the deoxygenated trisaccharide 24 (H replacing the OH at C4 on the C residue) showed similar STD enhancements to the trisaccharide glycoside 2. Since 24 cannot bind via the B–C disaccharide, this compound provides an essentially pure representation of binding to the internal epitope (the A–B disaccharide) (Fig. 12). That the STD pattern of 24 was only minimally different from that of 2 showed that contributions from a secondary, frame-shifted binding mode (where B–C is recognized) are not very significant in the binding of the native trisaccharide.
Graphical representation of STD-NMR enhancements for the oligosaccharides 6, 2, 3, 23, and 24 binding to the IgG C3.1. The enhancement magnitudes of each resonance (isolated resonances only) are indicated by colored circles (red 67–100%; green 34–66%; blue 0–33%); all magnitudes are relative to the most strongly enhanced resonance in each spectrum [78]. (© American Society for Biochemistry and Molecular Biology)
In contrast, the trisaccharide 23 (deoxygenated at the 4-position of the A residue) showed a different STD enhancement pattern relative to 2: strong enhancements of the B residue, somewhat increased enhancements of the C residue, and increased enhancements of the aglycone (Fig. 12). These observations are consistent with a frameshift similar to the pattern observed for the tetrasaccharide glycoside 3. In total the STD data support the interpretation that molecular recognition of C. albicans (1 → 2)-β-mannan oligosaccharides by a protective monoclonal antibody reveals the immunodominance of internal saccharide residues.
No significant changes in the bound conformations were observed by trNOE for the deoxygenated trisaccharides, suggesting that frameshifting is not accompanied by any noteworthy conformational changes. As we discuss later, structures larger than a tetrasaccharide cannot be accommodated without significant conformational changes and resulting diminution of the binding free energy, ΔG.
4.5 Molecular Modeling of the C3.1 Binding Site
A model of the C3.1 binding site was constructed by homology modeling and a new computational procedure was developed to dock oligosaccharides into the site [57]. This procedure used the previously developed chemical mapping and NMR results to rank computer-generated binding modes of disaccharides. Larger oligosaccharides were then superimposed in the same binding modes, and additional molecular dynamics refinement was used to generate the final model [57]. To avoid producing false-positive binding modes with the computational docking software, it was necessary to omit residues that were known to have no strong interactions with the binding site, such as pyranose C of 2 or C/D of tetramannoside 3; the docking routine would otherwise attempt to produce interactions for these residues as well. We based our approach on disaccharide 1 and used subsequent molecular dynamics rounds to model the larger oligomers. The resulting model explained the unusual binding observations in this system, not only revealing a binding site that optimally accommodates only two mannoside residues but also explaining the preference for a disaccharide epitope (Fig. 13). In agreement with the earlier observations, residue A was found to be in closest contact with the base of the binding groove. The C3- and C4-OH hydroxyl groups of A, which are essential for binding, are near the potential hydrogen bond donors Ala H97 NH and Trp L91 NE1, and the C6-OH group forms hydrogen bonds with the Asp L50 carboxylate side chain. Residue B is partly solvent-exposed, but as predicted from the chemical mapping data, its C6-OH and C4-OH can form hydrogen bonds – the former with Trp H33 N, Asn H95 OD1, and ND1 and the latter with Asn H31 O. Residue C is highly solvent-exposed and makes only minimal contact with the antibody, consistent with the chemical mapping and NMR data.
(a) An end-on view of the C3.1 binding site with a docked trisaccharide ligand 6 and (b) a cutaway side view of the C3.1 binding site with a docked hexasaccharide. The residues A, B, and C displayed in Fig 13 a are labeled and these corresponding residues are also labeled A, B, and C in 2.13b. The cutaway side view 2.13b shows that the residue to the right of A may be accommodated without a clash with the antibody surface but not the next residue. The residue to the left of C cannot be accommodated due to a clash with protein. This view clearly shows that irrespective of frame shifting penta and hexasaccharide will experience serious obstacles to binding, thereby accounting for their weak inhibitory power
Attempting to dock the tetramannoside 3 in the same binding mode as the trisaccharide 2 combining site did not allow room at the nonreducing end for the D residue, because of steric interactions with Trp H33 (Fig 13b). Instead, 3 binds in a frame-shifted mode where the B-C-D trisaccharide takes a position corresponding to that of the A-B-C trisaccharide in 2 (Fig. 13a). This model therefore explains the frameshifting we observed for 3 [106], which presumably must occur for any oligomer larger than a trisaccharide to bind.
The steric constraints of the binding site in the area of both the reducing and nonreducing end of the oligomer are quite strict, and when frameshifting, the ligands must avoid unfavorable interactions with Met H98, Tyr H32, and the protein backbone of CDRs H1 and H3. The shape of the binding site at the reducing end is not complementary to a longer β(1 → 2)-linked oligosaccharide, and hence frameshifting by more than one residue without significant conformational changes is not possible. The binding of oligosaccharides larger than tetrasaccharide 3 would require several energetically unfavorable changes to glycosidic torsional angles to compensate for the lack of shape complementarity (Fig 13b), which explains the drop in binding affinity observed for penta- and hexasaccharides [91].
Taken together, these results are promising for the development of a vaccine based on an epitope of very small size, namely a di- or trisaccharide. However, we still needed to confirm the biological relevance of our hypothesis that reverse engineering the specificity of C3.1 is a valid basis for vaccine design and for establishing the structural models described here. To this end, STD-NMR experiments were performed with trisaccharide 2 and sera derived from rabbit immunization experiments with a trisaccharide-BSA (bovine serum albumin) conjugate 37. Similar to the data for C3.1, the polyclonal sera spectra showed strongest enhancements for the A residue, with weaker enhancements for the B and C residues. The aglycone enhancement strength was also similar for both cases, as were those of H6 and H6′. Compared to the C3.1 case, however, increases were observed in the enhancement strengths of H5 and other degenerate multiplets. This observation is not surprising given that polyclonal sera contain a mixture of different antibodies. The observed STD-NMR trends thus indicate not only a detectable prevalence of C3.1-like binding but also a persistent preference for an internal epitope in experimental immunizations, which points to the biological importance of this mAb [78].
4.6 The β-Mannan to α-Mannan Phosphodiester Linkage
As discussed above, most structural and synthetic efforts have focused on acid-stable β-mannan epitopes. Recently, we completed synthetic and modeling studies of a C. albicans serotype B acid-labile β-mannan epitope that incorporates the phosphodiester: tetrasaccharide 25 (Fig. 14) [109]. This tetrasaccharide, synthesized via an anomeric H-phosphonate method, binds to C3.1 with an affinity essentially identical to that of the β-trimannoside 21.
Modeling of 25 was carried out based on results for the β-mannosides. As described above, the C3.1 binding groove is unable to accommodate β-mannan oligosaccharides larger than a tetrasaccharide without requiring energetically unfavorable conformational adjustments to avoid steric clashes with the antibody. However, it appears that the introduction of an α-linkage at residue B in 25 allows the clashes with the narrowest part of the binding site to be avoided. The phosphodiester and the other α-linked monosaccharide, residue A, provide additional shape complementarity.
The phosphate group does not appear to engage in specific interactions; for example, no salt bridges or strong hydrogen bonds are predicted. Residues C and D of 25 can bind in a similar orientation and conformation to the residues A and B of 2; therefore, they are predicted to participate in similar intermolecular interactions. This is consistent with the inhibition data, which show that 24 does not bind any more strongly than the β-mannotrioside 2. Considering the greater shape complementarity of 25, it is perhaps surprising that it is not more active than 2. However, the introduction of charged groups at the periphery of binding sites has been shown to produce unpredictable effects, as in the case of the GS-IV lectin binding analogs of the Lewis b tetrasaccharide. In these examples, significant enthalpy and entropy changes were observed but the Δ(ΔG) binding energy changes were close to zero [110].
We were then interested in which compound would provide a more successful conjugate vaccine [57, 111]. Our model predicts that 25 would be more complementary to the C3.1 binding site than are β-mannosides. Tetrasaccharide 25 should also contact a more diverse set of amino acid residues than the β-mannosides. This additional complementarity is provided by the arrangement of the phosphate group and residue A. These observations could mean that this compound may more efficiently stimulate the production of C3.1-like antibodies, despite having no greater binding affinity to C3.1 than propyl β-mannotrioside 2. Because C3.1 is protective and C3.1-like binding modes are observable in experimental animal sera, C3.1-like antibodies may be desirable in a vaccine strategy. On the other hand, our candidate vaccine studies to date have been driven by inhibition studies that showed β-mannotriosides to be the most active inhibitors of C3.1 [91]. The latter approach is also favored based on synthetic efficiency as well as chemical and possibly conformational stability [91, 112]. Consequently, immunological studies have to date dealt only with β-mannosides. Syntheses and immunological studies of glycoconjugates of 25 are now in progress, however, and should provide more insight into the immunological correlations of these structural findings.
Our modeling studies with tetrasaccharide 25 also suggest that a (1 → 2)-β-mannotriose joined via its α-anomer to a tether not only would provide the required specificity to β-mannans but also would allow the tether to exit the binding site unrestricted [57]. The component trisaccharide Man(β1–2)Man(β1–2)Man(α1- is common to both acid-stable and acid-labile β-mannan epitopes, and therefore might represent a common phosphomannan epitope of multiple Candida species and growth stages [70, 78]. This antigen would allow us to eliminate the synthetically challenging phosphate group at the same time [109]. Though some complementarity to the C3.1 binding site is predicted to be lost, this loss may be outweighed by the potential advantages of tethering the trisaccharide to other structures such as carrier proteins. The activities of vaccine constructs that incorporate some of these features have been investigated by glycopeptide antigen work (see Sect. 5.6).
5 Synthetic Conjugate Vaccines
5.1 Glycoconjugate Vaccines
Our initial vaccine design was based on the oligosaccharide inhibition data (Fig. 4) [91]. We interpreted this data as suggesting that a trisaccharide or disaccharide epitope conjugated to protein should induce antibodies able to recognize the cell-wall phosphomannan. The first test would be with phosphomannan extracted and coated on ELISA plates; the second would be against phosphomannan as it is displayed on the fungal cell wall. Provided these criteria were satisfied, the final step would be live challenge experiments.
5.2 Immunization and Challenge Experiments in Rabbits
A trisaccharide functionalized for conjugation to a carrier protein employed an allyl group. A selectively protected allyl mannoside 26 (Scheme 1a) was glycosylated by the glucosyl imidate 27. With persistent benzyl protection at C3, C4, and C6, the acetyl group at C2 guided efficient β-glycoside formation to yield 28. Removal of acetate to yield 29 followed by an oxidation–reduction sequence gave the disaccharide alcohol 30, which could be subjected to a similar sequence of glycosylation by 27 to give 31. Then transesterification gave 32, and the same oxidation–reduction sequence was used again to obtain the protected target trisaccharide 33. The fully protected trisaccharide allyl glycoside 33 was reacted with cysteamine to yield an amino-terminated tether 34 after a debenzylation step (Scheme 1b) [92]. This glycoside 34 was activated for conjugation with protein by first reacting with p-nitrophenyl adipic acid diester 35 [112]. The half ester 36 was then reacted with protein to afford either BSA or tetanus toxoid glycoconjugates 37 and 38. Employing a different approach, a disaccharide was similarly conjugated to chicken serum albumin [105, 113]. Both the trisaccharide and disacccharide conjugates were used to immunize rabbits.
(a) Assembly of the protected β-mannan trisaccharide 33 from monosaccharide building blocks. (b) Conversion of trisaccharide allyl glycoside 33 to the deprotected and amino-terminated tether trisaccharide 34. Conjugation to BSA and tetanus toxoid to give antigens 37 and 38 employs a homobifunctional coupling agent 35
When rabbits were immunized with trisaccharide vaccine 38 (administered with alum, an adjuvant approved for use in humans), ELISA titers to the immunizing oligosaccharide conjugated to a heterologous protein exceeded 100,000 [112, 114]. Titers against the cell-wall mannan were approximately 2–3 fold lower (Fig. 15). Serum from rabbits immunized by vaccine 38 generated antibodies that stained C. albicans cells more intensely in fluorescent labeling studies [114] than antibodies from rabbits immunized with a disaccharide conjugate [105].
(a) The antibody responses of rabbits to the conjugate vaccine 38. (b) C. albicans colony count in lung, spleen, liver, and kidney of rabbits in the vaccinated and control groups. Accumulated data were collected for 16 rabbits in the vaccinated group and 13 rabbits in the control group. Colony-forming unit (CFU) counts were performed on the organs of each rabbit, and the data presented are averaged for 16 rabbits vaccinated with the conjugate vaccine and 13 rabbits vaccinated with tetanus toxoid. The statistical treatment of data with the Generalized Estimating Equations (GEE) method revealed that the reduction of Candida CFU was statistically significant in the kidney (average reduction in log counts 4.4, p = 0.016) and liver (average reduction in log counts 3.6, p = 0.033). The effect of vaccination was not observed in lungs and spleen (increase in log counts 0.002, p = 0.99; 0.411, p = 0.80, respectively) [114].
The two vaccines were also evaluated in live challenge experiments [99, 114]. Since C. albicans is a commensal organism, it is necessary to evaluate the vaccine in rabbits that are immunocompromised [114]. The disaccharide vaccine was studied in a smaller sample of rabbits [105], and therefore those results lack the statistical significance of the larger study performed with the trisaccharide vaccine [114]. Vaccine was administered prior to the onset of immunosuppression, allowing a persistent level of antibody directed toward the cell-wall β-mannan. Both vaccines reduced C. albicans counts in vital organs, but it was evident that the vaccines alone were insufficient to provide 100% protection [114].
5.3 Immunization and Challenge Experiments in Mice
When the trisaccharide–tetanus toxoid conjugate vaccine 38 was evaluated in mice, we consistently observed only modest ELISA titers recorded on plates coated with trisaccharide-BSA conjugate [115–117]. Only in 10–20% of immunized mice did titers exceed 1:10,000, which itself was a level of antibody that we have observed to confer almost no protection to live challenge with C. albicans. Several different vaccine constructs were synthesized and tested [115–117]. Some approaches attempted to cluster epitopes [115], while others employed novel carrier constructs [117]. In all of these cases, the immune response as determined by antibody titer was judged to be insufficient to proceed to challenge experiments.
In work that will be described in Sect. 5.4, collaboration with the Dr. Jim Cutler and Dr. Hong Xin established that dendritic cell priming could improve the immune response to low-molecular-weight glycopeptides [118]. These data – together with accumulating literature evidence for the importance of dendritic cells in processing and presenting antigens [119–122] – suggested that targeting dendritic cells with a vaccine construct might improve the immune response to our conjugate vaccine [118].
Scientists at Novartis have shown that conjugate vaccines consisting of different β-glucans (either oligo- or polysaccharides) conjugated to CRM197, a variant of the diphtheria toxin were highly effective against fungal diseases [123, 124]. Antibodies to the β-glucan were responsible for the protection, but it is of interest to note that 1,3-β-glucans are ligands for dectin-1 [125], a C-type lectin present on the surface of dendritic cells [125–130]. In the expectation that β-glucan would deliver vaccine to dendritic cells we elected to attach β-glucan to our β-mannotriose-tetanus toxoid vaccine to examine the effect on antibody levels.
The preparation of the three-component vaccine involved unique chemistry (Scheme 2) [131]. In the first step, tetanus toxoid was reacted with a diazo transfer reagent, imidazole-1-sulfonyl azide hydrochloride, to convert amino groups into azide groups [132]. Removing amino groups in this way avoids the tendency of proteins to form intramolecular or intermolecular lactams, thus permitting efficient use of carbodiimide- or amide-forming coupling reagents. Following activation by 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride of the aspartic acid and glutamine side-chain carboxylic acids of the azido tetanus toxoid, coupling with the amino-terminated trisaccharide hapten 34 yields 39. The resulting conjugate was divided into two portions. One half was reduced by trimethyl phosphine to convert the azide groups back to amines, affording a control vaccine 40 that was designated TS-TT (trisaccharide–tetanus toxoid). The second half of 39 was conjugated to propargylated laminarin 41 using click chemistry [133, 134]. The residual azide groups of the resulting conjugate 42 were reduced back to the original amino groups by trimethyl phosphine to give vaccine 43, designated TS-TT-Lam [131].
The use of azido transfer reagent imidazole-1-sulfonyl azide hydrochloride converts amino groups to azides, allowing amide bond formation between 34 and the carboxylic acid residues of tetanus toxoid employing 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride. Trimethylphosphine is used to reduce protein azide groups back to amino groups. Circular dichroism measures suggest the tetanus toxoid retains its tertiary structure during these transformations
Immunization of mice with the TS-TT-Lam vaccine resulted in an improved immune response, as manifested by high titers of antibody recognizing C. albicans β-mannan antigen when compared to mice immunized with TS-TT vaccine [131]. TS-TT-Lam vaccine also affected the distribution of IgG subclasses, showing that targeting the dectin-1 receptor can augment and immunomodulate the immune response. In more detailed immunological investigation of the two vaccine constructs, a macrophage cell line expressing dectin-1 was employed to reveal the binding and activation of the dectin-1 signal transduction pathway by the TS-TT-Lam vaccine. Antigen binding to dectin-1 resulted in (1) activation of Src-family-kinases and SYK, as revealed by their recruitment and phosphorylation in the vicinity of the bound conjugate, and (2) translocation of NF-κB to the nucleus. Treatment of immature bone-marrow-derived dendritic cells (BMDCs) with tricomponent or control vaccine confirmed that the vaccine containing β-glucan exerted its enhanced activity by virtue of dendritic cell targeting and uptake. Immature primary cells stimulated by the tricomponent vaccine showed activation of BMDCs, but those stimulated by the β-mannan tetanus toxoid vaccine did not. Moreover, treated BMDCs secreted increased levels of several cytokines, including TGF-β and IL 6; these cytokines activate Th17 helper T-cells, a cell type known to be important in antifungal responses.
The vaccination experiments described to this point suggest that (1) a simple trisaccharide conjugated to a potent protein carrier can reduce C. albicans counts in live challenge experiments, (2) targeting dendritic cells significantly augments the β-mannan-specific antibody levels, and (3) targeting dendritic cells also elevates cytokine responses, which in turn activate T-cell subsets that are important to antifungal immune responses.
5.4 Synthesis and Evaluation of Glycopeptide Vaccines
Protection against infections by C. albicans and other Candida species requires the involvement of cellular immunity, including cells of the innate immune system [135]. However, it was only recently appreciated that humoral immunity could also play a role in protection against C. albicans [136–138]. Consequently, fungal cell-wall peptides were sought that would serve as carriers and to provide an additional protective epitope [118]. The latter attribute would induce protection against fungal strains not producing the carbohydrate epitope, especially because that moiety is not essential for C. albicans growth [82, 83] and only 80% of isolates of C. glabrata (another important agent of candidiasis) produce β-linked mannosides [70]. To satisfy these features, efficient conjugation chemistry was developed (Scheme 3). The oligosaccharide B-cell epitope was provided in the form of trisaccharide 34, which was acylated by the NHS ester of the acryloyl polyethylene glycol (PEG) derivative 44 to produce 45. This allowed the facile conjugation of unprotected β-(Man)3 with six candidate peptide carriers derived from the cell wall of C. albicans [118]. The conjugation strategy involved Michael addition of 45 to synthetic peptides that had been modified at their N-terminus by 3-thiopropionic acid. These structures allowed the efficient coupling of the two deprotected components – the antigenic β-(Man)3 and the specific TH peptide epitopes – as the last step of the synthesis [139]. This is shown for the Fba peptide 46, which is first converted to 47 and then conjugated in fairly good yield to give the glycopeptide 48 (Scheme 3). This strategy permits the screening of a range of peptide carriers and facilitates the identification of the most suitable peptides. The heterobifunctional coupling reagent 44 is based on a water-soluble triethylene glycol core functionalized at one end with an N-hydroxysuccinimide ester and a sulfhydryl-reactive acrylate moiety at the other [139]. This linker permits efficient coupling of biomolecules under aqueous conditions while still affording, as far as possible, an immunologically silent tether.
C. albicans carrier proteins were selected from cell-wall proteins expressed during pathogenesis of human-disseminated candidiasis [140, 141]. The six candidate carriers were fructose-bisphosphate aldolase (Fba), methyltetrahydropteroyltriglutamate (Met6), hyphal wall protein-1 (Hwp1), enolase (Enol), glyceraldehyde-3-phosphate dehydrogenase (Gap1), and phosphoglycerate kinase (Pgk1). An epitope-finding algorithm (GenScript, Antigen Design Tool, OptimumAntigen) was used to select putative epitopes composed of 14 amino acids each. Locations near the N-terminus were chosen, as it was presumed that this location was more likely to be accessible to the host immune system. Each peptide shows 100% homology only with C. albicans and none with mammalian proteins. Peptides Fba, Met6, and Pgk1 contain obligatory P1 anchor residues and are predicted to bind the human leukocyte MHC (major histocompatibility complex) class II cell surface receptor and multiple MHC class I binding sites are predicted for all six peptides [142, 143].
Antigen-pulsed dendritic-cell-based vaccination was used in all immunizations for development of antibodies in mice. Immune sera were tested for antibodies specific for the whole conjugates, synthetic β-(Man)3, synthetic peptide carriers and fungal cell surface phosphomannoprotein (PMP) extract from C. albicans cell walls [118]. All six conjugates were immunogenic, as shown by high titers of antibodies specific to each of the test antigens. Each antiserum – but not the negative control sera – reacted directly with yeast and hyphal forms of the fungus, as evidenced by indirect immunofluorescence microscopy. The antibody response to the glycoconjugates was several-fold greater than that of sera from control groups injected with dendritic-cell adjuvant without antigen. The presence of β-(Man)3 antibodies in the sera of vaccinated animals was confirmed by dose–response ELISA inhibition [118].
The conjugates were assessed for their potential in a vaccination strategy for disseminated candidiasis. Fourteen days after the second immunization, mice were challenged intravenously with a lethal dose (5 × 105) of viable yeast forms of C. albicans that eventually killed all control mice. Conjugate immunized animals, except for those that received the β-(Man)3-Pgk1, survived longer than the control groups. The immunized groups that received the β-(Man)3-Hwp1, β-(Man)3-Fba, or β-(Man)3-Met6 conjugates showed 80–100% survival throughout the 120-day post-challenge observation period. Mice that received either β-(Man)3-Enol or β-(Man)3-Gap1 showed 40–80% survival. However, control groups and mice given the β-(Man)3-Pgk1 conjugate had median survival times of 5, 6, 14, 16, and 11 days, respectively [118]. Importantly, compared with animals that succumbed, the survivors had greatly reduced or even nondetectable viable fungal colony-forming units (CFUs) in kidneys, a target organ in disseminated candidiasis. The antibody dependency of protection was evidenced by the protection transferred to naive mice by immune serum, but not by serum pre-absorbed with C. albicans [118].
An advantage of this approach is that the peptide carrier is derived from the infectious agent of interest, providing the possibility of a dual-protective immune response against both the glycan epitope and the peptide carrier. For the trisaccharide-Fba-glycopeptide, both β-mannan and peptide antibodies contributed to protection. These experiments showed the potential for chemical synthesis of vaccines of relatively low molecular weight that induce protection against more than one unrelated epitope expressed by the infectious disease agent of interest. However, ex vivo dendritic cell stimulation is not a generally useful approach: a modified approach would be required for the practical incorporation of glycopeptides into a viable vaccine construct.
5.5 Self-Adjuvanting Glycopeptide Conjugate Vaccine
To improve immunogenicity and allow the incorporation of an adjuvant suitable for human use, we modified the β-(Man)3-Fba conjugate by conjugating it to tetanus toxoid [144]. This necessitated a modified conjugation strategy designed to conjugate glycopeptide to the carrier protein via the C-terminus of the glycopeptide moiety. To this end, the peptide was synthesized with an additional lysine residue at the C-terminus of the peptide (Scheme 4a), and this was linked to the Fba peptide via a triethylene glycol spacer to give the peptide 49. The target glycoconjugate vaccine 50 was obtained by reaction of 45 and 49. Bromoacetate groups were introduced on approximately 20 of the lysine residues present in tetanus toxoid to give 52 (Scheme 4b) and reaction of 50 with 52 was expected to give the target vaccine 53. However, under the basic conditions used to react 49 with 45, it was found that the acetyl group on the C-terminal thioglycolic acid residue used to acylate the ε-amino group of lysine can undergo an intermolecular migration to the 3-thiopropionic acid residue. This set up the conditions whereby two glycopeptides 50 and 51 were formed in the ratio 1:2 rather than a single product 50. Subsequently, 51 also reacts with 52 to give a second vaccine construct 54 [145]. Thus, the study of a self-adjuvanting vaccine [144] unintentionally used a 1:2 mixture of the two constructs 53 and 54 and not the cited single vaccine 53.
(a) Modification of the solid-phase peptide synthesis approach shown in Scheme 3 installs a C-terminal lysine (K) to allow conjugation to tetanus toxoid. The crucial peptide intermediate possesses two thiol groups. The thiolglycolic acid residue at the C-terminus is protected as an acetate 49. The basic conditions used to conjugate 45 with 49 results in acetate transfer and the formation of the two glycopeptides 50 and 51. (b) Tetanus toxoid containing approximately 20 bromoacetyl groups reacts with the latent thiol groups of 50 and 51 (released under basic conjugation conditions) to form 53 and 54, respectively
This mixture of the new glycopeptide vaccine conjugate 53, β-(Man)3-Fba-TT together with the C-terminal conjugated trisaccharide 54 proved to be highly immunogenic, as it induced robust antibody responses when administered with either alum or monophosphoryl lipid A as adjuvants [144]. Moreover, prior to the second booster dose, an isotype switch occurred from IgM to IgG antibodies for both the β-(Man)3 and Fba peptide epitopes. This result indicated a possible memory cell response and, perhaps, a vaccine that induces long-term immunity. Most importantly, the (β-Man)3-Fba-TT conjugates 53 and 54 administered with either alum or MPL induced protection against disseminated candidiasis on a par with the high level of protection observed with the original dendritic-cell/complete Freunds adjuvant immunization approach [118]. In previous work involving the latter approach, we found that the Fba peptide itself was immunogenic, inducing not only a robust antibody response but also a protective response against disseminated candidiasis [118]. We were surprised, therefore, to find that the Fba-TT conjugate in adjuvant did not perform well in mice [144]. We were even more surprised to discover that mice that received the β-(Man)3-Fba-TT without any adjuvant performed as well or better than mice that received the vaccine with adjuvant. All three groups vaccinated with glycoconjugate with or without either of the two adjuvants, alum or MPL, showed a high level of protection against a lethal challenge with C. albicans: survival times were significantly increased and kidney fungal burdens were reduced or nondetectable at the time of sacrifice compared to control groups that received only adjuvants or buffer prior to challenge [144]. Furthermore, sera from mice immunized with the conjugates 53 and 54 transferred protection against disseminated candidiasis to naive mice, whereas C. albicans pre-absorbed immune sera did not; this confirmed that the induced antibodies were protective. These results demonstrated that the addition of tetanus toxoid to the vaccine conjugate provided sufficient self-adjuvanting activity without the need for additional adjuvant. As far as we are aware, this is the first report of a self-adjuvanting glycopeptide vaccine against any infectious disease [144].
5.6 Variants of Glycopeptide Vaccines
Our modeling studies of various ligands in the binding site of mAb C3.1 suggested that if the terminal reducing residue of a trisaccharide ligand adopted the α configuration. This orientation could be favorable for allowing the extended phosphomannan to avoid contact with the antibody surface. Thus, a trisaccharide attached to a tether via an α-mannopyranosyl linkage might be as active as the β-linked analog 53. However, it would be a synthetically preferred construct for a vaccine, since forming an α-mannopyranosyl linkage involves fewer steps. We set out to synthesize a glycopeptide ligand of this type to investigate this hypothesis and to simplify the conjugation chemistry as summarized in Scheme 5.
(a) An alternative conjugation strategy for unambiguous attachment of glycopeptides to protein. An azido lysine residue is introduced at the C-terminus of the Fba peptide 55. Conjugation of trisaccharide 56 to the peptide follows the same approach described in Schemes 3 and 4a. (b) Propargyl groups are introduced into tetanus toxoid to give 59, and click chemistry is used to conjugate glycopeptides 58 with the modified tetanus toxoid 59 to produce 60
As in the synthesis of peptide derivative 49, peptide 55 was synthesized on solid support. This time, however, azidolysine was attached to the resin instead of lysine and the Fba peptide was elaborated with a diethylene glycol spacer to give 55. The trisaccharide 2-aminoethyl glycoside 56 [145] was bromoacetylated to give 57. Conjugation to the 3-thiopropionic acid residue of 55 by 57 gave glycopeptide 58, which in turn was conjugated to propargylated tetanus toxoid (TT) derivative 59 to afford the target vaccine 60.
Mice were immunized with 60 using the protocol described for vaccines 53 and 54. Sera were titrated against copovidone polymer bearing the epitope 56 and the Fba peptide [146]. While strong antibody titers of ~1:100,000 for the Fba peptide were observed, most mice exhibited only weak titers of <1:1,000–5,000 against the β-mannan.
These data raise questions about the optimum length of tether employed to attach the β-mannan epitope to Fba peptide in order to secure a strong immune response. The data from this experiment combined with other unpublished work using conjugates of glycopeptides and tetanus toxoid containing the α-linked mannan also support the conclusion that the trisaccharide epitope represented by 34 or analogs also β-linked to tether are the preferred structures to elicit protective antibodies with a binding profile of the C3.1 mAb type. This idea is consistent with the profile of antibodies observed in the sera of rabbits immunized with the simple conjugate vaccine 38.
6 Summary, Conclusions, and Outlook
The observed binding profile of mouse mAb C3.1 with homo-oligomers of the C. albicans β-1,2-mannan is in stark contrast with a 40-year-old paradigm of oligosaccharide-antibody binding. This surprising finding prompted a thorough investigation of β-1,2-mannan–antibody interactions and led to the discovery of protective vaccines based on a trisaccharide epitope.
A β-1,2-mannan trisaccharide conjugated to tetanus toxoid gave high titers of antibodies specific to C. albicans in rabbits. Immunized rabbits rendered neutropenic and challenged intravenously with live C. albicans showed significantly reduced fungal burden in vital organs. However, the same conjugate vaccine was poorly immunogenic in mice.
This conjugate may be converted to a much improved vaccine by conjugation with a second carbohydrate antigen, a β-glucan, which is also a ligand for dectin-1 (a dendritic cell lectin). This vaccine is directed to antigen processing cells and induces a much improved immune response, with a cytokine profile well suited to protection against fungal infection.
A third vaccine construct is the trisaccharide conjugated to a T-cell peptide, which was conjugated in turn to tetanus toxoid. This vaccine did not require the use of an adjuvant to exhibit potent immunogenicity: it conferred protection by inducing combined carbohydrate- and peptide-specific responses.
The results summarized here suggest that highly effective and completely synthetic vaccines should be feasible by incorporating ligands targeting dendritic cells in a construct that includes B- and T-cell epitopes.
Abbreviations
- Abe:
-
Abequose (3,6-dideoxy-d-xylo-hexose)
- Ac:
-
Acetyl
- All:
-
Allyl
- ′Bn:
-
Benzyl
- Boc:
-
tert-Butoxycarbonyl
- BSA:
-
Bovine serum albumin
- Bu:
-
Butyl
- DMF:
-
Dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
- Enol:
-
Enolase
- Fab:
-
Fragment (of an antibody) that contains the antigen-binding region
- Fba:
-
Fructose-bisphosphate aldolase
- Gal:
-
Galactose
- Gap1:
-
Glyceraldehyde-3-phosphate dehydrogenase
- Glc:
-
Glucose
- GlcNAc:
-
N-acetylglucosamine
- Hwp1:
-
Hyphal wall protein-1
- IC50 :
-
Half-maximal inhibitory concentration
- LPS:
-
Lipopolysaccharide
- mAb:
-
Monoclonal antibody
- Man:
-
Mannose
- Me:
-
Methyl
- Met6:
-
Methyltetrahydropteroyltriglutamate
- NOE:
-
Nuclear Overhauser effect (NMR)
- Pgk1:
-
Phosphoglycerate kinase 1
- Pr:
-
Propyl
- Rha:
-
Rhamnose
- STD-NMR:
-
Saturation transfer difference nuclear magnetic resonance
- t-Bu:
-
tert-Butyl
- Tf:
-
Trifluoromethanesulfonyl (triflyl)
- THF:
-
Tetrahydrofuran
- TMSOTf:
-
Trimethylsilyl trifluoromethanesulfonate
- Tr:
-
Triphenylmethyl (trityl)
- trNOE:
-
Transferred nuclear Overhauser effect (NMR)
- T-ROESY:
-
Two-dimensional rotating-frame Overhauser effect spectroscopy
References
Francis T Jr, Tillett WS (1930) Cutaneous reactions in pneumonia: the development of antibodies following the intradermal injection of type-specific polysaccharide. J Exp Med 52:573–585
MacLeod CM, Hodges RG, Heidelberger M, Bernhard WG (1945) Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med 82:445–465
Heidelberger M, MacLeod CM, Di Lapi MM (1948) The human antibody response to simultaneous injection of six specific polysaccharides of pneumococcus. J Exp Med 88:369–372
Parke JC Jr, Schneerson R, Robbins JB, Schlesselman JJ (1977) Interim report of a controlled field trial of immunization with capsular polysaccharides of Haemophilus influenzae type b and group C Neisseria meningitidis in Mecklenburg County, North Carolina (March 1974–March 1976). J Infect Dis 136(Suppl):S51–S56
Peltola H, Mäkelä H, Käyhty H, Jousimies H, Herva E, Hällström K, Sivonen A, Renkonen OV, Pettay O, Karanko V, Ahvonen P, Sarna S (1977) Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med 297:686–691
Peltola H, Käyhty H, Sivonen A, Mäkelä PH (1977) Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccines 3 months to 5 Years of age in Finland. Pediatrics 60:730–737
Eskola J, Takala A, Käyhty H, Peltola H, Mäkelä PH (1991) Experience in Finland with Haemophilus influenzae type b vaccines. Vaccine S14–S16; discussion S25
Goebel WF, Avery OT (1931) Chemo-immunological studies on conjugated carbohydrate-proteins. IV. The synthesis of the p-aminobenzyl ether of the soluble specific substance of Type III Pneumococcus and its coupling with protein. J Exp Med 54:431–436
Avery OT, Goebel WF (1931) Chemo-immunological studies on conjugated carbohydrate-proteins. V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of Type III Pneumococcus with foreign protein. J Exp Med 54:437–447
Madore DV, Johnson CL, Phipps DC, Popejoy LA, Eby R, Smith DH (1990) Safety and immunologic response to Haemophilus Influenzae type b oligosaccharide-CRM197 conjugate vaccine in 1- to 6-month-old infants. Pediatrics 85:331–337
Yogev R, Arditi M, Chadwick EG, Amer MD, Sroka PA (1990) Haemophilus influenzae type b conjugate vaccine (meningococcal protein conjugate): immunogenicity and safety at various doses. Pediatrics 85:690–693
Gambillara V (2012) The conception and production of conjugate vaccines using recombinant DNA technology. BioPharm Int 25:28–32
Avci FY, Li X, Tsuji M, Kasper DL (2011) A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 17:1602–1609
Jennings HJ (1983) Capsular polysaccharides as human vaccines. Adv Carbohydr Chem Biochem 41:155–208
Finco O, Rappuoli R (2014) Designing vaccines for the twenty-first century society. Front Immunol 5:12
Robbins JB, Schneerson R, Szu SC, Fattom A, Yang Y, Lagergard T, Chu C, Sørensen US (1989) Prevention of invasive bacterial diseases by immunization with polysaccharide-protein conjugates. Curr Top Microbiol Immunol 146:169–180
Lesinski GB, Westerink MA (2001) Vaccines against polysaccharide antigens. Curr Drug Targets Infect Disord 1:325–334
Paoletti LC, Kasper DL (2003) Glycoconjugate vaccines to prevent group B streptococcal infections. Expert Opin Biol Ther 3:975–984
Pichichero M (2013) Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother 9:2505–2523
Goldblatt DJ (1998) Recent developments in bacterial conjugate vaccines. Med Microbiol 47:563–567
Beurret M, Hamidi A, Kreeftenberg H (2012) Development and technology transfer of Haemophilus influenzae type b conjugate vaccines for developing countries. Vaccine 30:4897–4906
Terra VS, Mills DC, Yates LE, Abouelhadid S, Cuccui J, Wren BW (2012) Recent developments in bacterial protein glycan coupling technology and glycoconjugate vaccine design. J Med Microbiol 61:919–926
Pozsgay V (2000) Oligosaccharide-protein conjugates as vaccine candidates against bacteria. Adv Carbohydr Chem Biochem 56:153–199
Pozsgay V (2008) Recent developments in synthetic oligosaccharide-based bacterial vaccines. Curr Top Med Chem 8:126–140
Lepenies B, Seeberger PH (2010) The promise of glycomics, glycan arrays and carbohydrate-based vaccines. Immunopharmacol Immunotoxicol 32:196–207
Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ (2005) Synthetic carbohydrate-based antitumor vaccines: challenges and opportunities. Expert Rev Vaccines 4:677–685
Bundle DR, Costello C, Nycholat C, Lipinski T, Rennie R (2012) Designing a Candida albicans conjugate vaccine by reverse engineering protective monoclonal antibodies. In: Kosma P, Muller-Loennies S (eds) Anticarbohydrate antibodies: from molecular basis to clinical application. Springer, Vienna, pp 121–146
Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KR, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA (2003) Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065–2071
Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR (2002) The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1 → 2 mannose residues on the outer face of gp120. J Virol 76:7306–7321
Astronomo RD, Lee H-K, Scanlan CN, Pantophlet R, Huang C-Y, Wilson IA, Blixt O, Dwek RA, Wong C-H, Burton DR (2008) A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J Virol 82:6359–6368
Wang LX (2006) Toward oligosaccharide- and glycopeptide-based HIV vaccines. Curr Opin Drug Discov Devel 9:194–206
Aussedat B, Vohra Y, Park PK, Fernández-Tejada A, Alam SM, Dennison SM, Jaeger FH, Anasti K, Stewart S, Blinn JH, Liao HX, Sodroski JG, Haynes BF, Danishefsky SJ (2013) Chemical synthesis of highly congested gp120 V1V2 N-glycopeptide antigens for potential HIV-1-directed vaccines. J Am Chem Soc 135:13113–13120
Michon F, Brisson JR, Jennings HJ (1987) Conformational differences between linear alpha (2 → 8)-linked homosialooligosaccharides and the epitope of the group B meningococcal polysaccharide. Biochemistry 26:8399–8405
Wessels MR, Kasper DL (1989) Antibody recognition of the type 14 pneumococcal capsule. Evidence for a conformational epitopein a neutral polysaccharide. J Exp Med 169:2121–2131
Paoletti LC, Kasper DL, Michon F, DiFabio J, Jennings HJ, Tosteson TD, Wessels MR (1992) Effects of chain length on the immunogenicity in rabbits of group B Streptococcus type III oligosaccharide-tetanus toxoid conjugates. J Clin Invest 89:203–209
Kasper DL, Paoletti LC, Wessels MR, Guttormsen HK, Carey VJ, Jennings HJ, Baker CJ (1996) Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Invest 98:2308–2314
Laferriere CA, Sood RK, de Muys JM, Michon F, Jennings HJ (1998) Streptococcus pneumoniae type 14 polysaccharide-conjugate vaccines: length stabilization of opsonophagocytic conformational polysaccharide epitopes. Infect Immun 66:2441–2446
Zou W, Mackenzie R, Thérien L, Hirama T, Yang Q, Gidney MA, Jennings HJ (1999) Conformational epitope of the type III group B Streptococcus capsular polysaccharide. J Immunol 163:820–825
Jennings HJ (2012) The role of sialic acid in the formation of protective conformational bacterial polysaccharide epitopes. In: Kosma P, Muller-Loennies S (eds) Anticarbohydrate antibodies: from molecular basis to clinical application. Springer, Vienna, pp 55–74
Han Y, Riesselman MH, Cutler JE (2000) Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody. Infect Immun 68:1649–1654
Kabat EA (1956) Heterogeneity in extent of the combining regions of human antidextran. J Immunol 77:377–385
Kabat EA (1960) Upper limit for the size of the human antidextran combining site. J Immunol 84:82–85
Luderitz O, Westphal O, Staub AM, Le Minor L (1960) Preparation and immunological properties of an artificial antigen with colitose (3-deoxy-1-fucose) as the determinant group. Nature 188:556–558
Kleinhammer G, Himmelspach K, Westphal O (1973) Synthesis and immunological properties of an artificial antigen with the repeating oligosaccharide unit of Salmonella illinois as haptenic group. Eur J Immunol 3:834–838
Lindberg AA, Wollin R, Bruse G, Ekwall E, Svenson SB (1983) Immunology and immunochemistry of synthetic and semisynthetic Salmonella O-antigen-specific glycoconjugates. Am Chem Soc Symp Ser 231:83–118
Svenson SB, Lindberg AA (1981) Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun 32:490–496
Svenson SB, Nurminen M, Lindberg AA (1979) Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun 25:863–872
Cygler M, Rose DR, Bundle DR (1991) Recognition of a cell surface oligosaccharide epitope of pathogenic Salmonella by an antibody Fab fragment. Science 253:442–446
Jeffrey PD, Bajorath J, Chang CY, Yelton D, Hellström I, Hellström KE, Sheriff S (1995) The X-ray structure of an anti-tumour antibody in complex with antigen. Nat Struct Biol 2:466–471
Rose DR, Przybylska M, To RJ, Kayden CS, Oomen RP, Vorberg E, Young NM, Bundle DR (1993) Crystal structure to 2.45 Å resolution of a monoclonal Fab specific for the Brucella A cell wall polysaccharide antigen. Protein Sci 2:1106–1113
Vyas NK, Vyas MN, Chervenak MC, Johnson MA, Pinto BM, Bundle DR, Quiocho FA (2002) Molecular recognition of oligosaccharide epitopes by a monoclonal Fab specific for Shigella flexneri Y lipopolysaccharide: X-ray structures and thermodynamics. Biochemistry 41:13575–13586
van Roon AMM, Pannu NS, de Vrind JPM, van der Marel GA, van Boom JH, Hokke CH, Deelder AM, Abrahams JP (2004) Structure of an anti-Lewis X Fab fragment in complex with its Lewis X antigen. Structure 12:1227–1236
Vulliez-Le Normand B, Saul FA, Phalipon A, Bélot F, Guerreiro C, Mulard LA, Bentley GA (2008) Structures of synthetic O-antigen fragments from serotype 2a Shigella flexneri in complex with a protective monoclonal antibody. Proc Natl Acad Sci U S A 105:9976–9981
Murase T, Zheng RB, Joe M, Bai Y, Marcus SL, Lowary TL, Ng KK (2009) Structural insights into antibody recognition of mycobacterial polysaccharides. J Mol Biol 392:381–392
Evans DW, Müller-Loennies S, Brooks CL, Brade L, Kosma P, Brade H, Evans SV (2011) Structural insights into parallel strategies for germline antibody recognition of lipopolysaccharide from Chlamydia. Glycobiology 21:1049–1059
Blackler RJ, Müller-Loennies S, Brade L, Kosma P, Brade H, Evans SV (2012) Antibody recognition of Chlamydia LPS: Structural insights of inherited immune responses. In: Kosma P, Muller-Loennies S (eds) Anticarbohydrate antibodies: from molecular basis to clinical application. Springer, Vienna, pp 75–120
Johnson MA, Cartmell J, Weisser NE, Woods RJ, Bundle DR (2012) Molecular recognition of Candida albicans (1 → 2)-β-mannan oligosaccharides by a protective monoclonal antibody reveals the immunodominance of internal saccharide residue. J Biol Chem 287:18078–18090
Villeneuve S, Souchon H, Riottot M-M, Mazié J-C, Lei P-S, Glaudemans CPJ, Kováč P, Fournier J-M, Alzari PM (2000) Crystal structure of an anti-carbohydrate antibody directed against Vibrio cholerae O1 in complex with antigen: molecular basis for serotype specificity. Proc Natl Acad Sci U S A 97:8433–8438
Evans SV, Sigurskjold BW, Jennings HJ, Brisson J-R, To R, Altman E, Frosch M, Weisgerber C, Kratzin H, Klebert S, Vaesen M, Bitter-Suermann D, Rose DR, Young NM, Bundle DR (1995) Evidence for the extended helical nature of polysaccharide epitopes. The 2.8 Å resolution structure and thermodynamics of ligand binding of an antigen biding fragment specific for α-(2 → 8)-polysialic acid. Biochemistry 34:6737–6744
Milton MJ, Bundle DR (1998) Observation of the anti- conformation of a glycosidic linkage in an antibody-bound oligosaccharide. J Am Chem Soc 120:10547–10548
Bundle DR (1998) Recognition of carbohydrate antigens by antibody binding sites. In: Hecht S (ed) Carbohydrates. Oxford University Press, Oxford, pp 370–440
Baumann H, Altman E, Bundle DR (1993) Controlled acid hydrolysis of an O-antigen fragment yields univalent heptasaccharide haptens containing one 3,6-dideoxyhexose epitope. Carbohydr Res 247:347–354
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317
Netea MG, Brown GD, Kullberg BJ, Gow NAR (2008) An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 6:67–78
Netea MG, Gow NAR, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, Van der Meer JWM, McKinnon A, Odds FC, Brown AJP, Kullberg B (2006) Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 6:1642–1650
Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, Schroder K, Ma B, Orr S, Stewart L, Lebus D, Sobieszczuk P, Hume DA, Stow J, Blanchard H, Ashman RB (2008) The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 180:7404–7413
Chaffin WL, López-Ribot JL, Casanova M, Gozalbo D, Martínez JP (1998) Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev 62:130–180
Bishop CT, Blank F, Gardner PE (1960) The cell wall polysaccharides of Candida albicans: glucan, mannan, and chitin. Can J Chem 38:869–881
Cutler JE (2001) N-Glycosylation of yeast, with emphasis on Candida albicans. Med Mycol 39:75–86
Shibata N, Kobayashi H, Suzuki S (2012) Immunochemistry of pathogenic yeast, Candida species, focusing on mannan. Proc Jpn Acad Ser B Phys Biol Sci 88:250–265
Shibata N, Kobayashi H, Takahashi S, Okawa Y, Hisamichi K, Suzuki S, Suzuki S (1991) Structural study on a phosphorylated mannotetraose obtained from the phosphomannan of Candida albicans NIH B-792 strain by acetolysis. Arch Biochem Biophys 290:535–542
Shibata N, Arai M, Haga E, Kikuchi T, Najima M, Satoh T, Kobayashi H, Suzuki S (1992) Structural identification of an epitope of antigenic factor 5 in mannans of Candida albicans NIH B-792 (serotype B) and J-1012 (serotype A) as beta-1,2-linked oligomannosyl residues. Infect Immun 60:4100–4110
Shibata N, Hisamichi K, Kikuchi T, Kobayashi H, Okawa Y, Suzuki S (1992) Sequential nuclear magnetic resonance assignment of beta-1,2-linked mannooligosaccharides isolated from the phosphomannan of the pathogenic yeast Candida albicans NIH B-792 strain. Biochemistry 31:5680–5686
Kobayashi H, Shibata N, Nakada M, Chaki S, Mizugami K, Ohkubo Y, Suzuki S (1990) Structural study of cell wall phosphomannan of Candida albicans NIH B-792 (serotype B) strain, with special reference to 1H and 13C NMR analyses of acid-labile oligomannosyl residues. Arch Biochem Biophys 278:195–204
Shibata N, Ikuta K, Imai T, Satoh Y, Richi S, Suzuki A, Kojima C, Kobayashi H, Hisamichi K, Suzuki S (1995) Existence of branched side chains in the cell wall mannan of pathogenic yeast Candida albicans. Structure-antigenicity relationship between the cell wall mannans of Candida albicans and Candida parapsilosis. J Biol Chem 270:1113–1122
Cassone A (1989) Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol 3:248–314
Chaffin WL, Ringler L, Larsen HS (1988) Interactions of monospecific antisera with cell surface determinants of Candida albicans. Infect Immun 56:3294–3296
Kobayashi H, Shibata N, Suzuki S (1992) Evidence for oligomannosyl residues containing both beta-1,2 and alpha-1,2 linkages as a serotype A-specific epitope(s) in mannans of Candida albicans. Infect Immun 60:2106–2109
Kobayashi H, Takahashi S, Shibata N, Miyauchi M, Ishida M, Sato J, Maeda K, Suzuki S (1994) Structural modification of cell wall mannans of Candida albicans serotype A strains grown in yeast extract-Sabouraud liquid medium under acidic conditions. Infect Immun 62:968–973
Shibata N, Akagi R, Hosoya T, Kawahara K, Suzuki A, Ikuta K, Kobayashi H, Hisamichi K, Okawa Y, Suzuki S (1996) Existence of novel branched side chains containing beta-1,2 and alpha-1,6 linkages corresponding to antigenic factor 9 in the mannan of Candida guilliermondii. J Biol Chem 271:9259–9266
Shibata N, Onozawa M, Tadano N, Hinosawa Y, Suzuki A, Ikuta K, Kobayashi H, Suzuki S, Okawa Y (1996) Structure and antigenicity of the mannans of Candida famata and Candida saitoana: comparative study with the mannan of Candida guilliermondii. Arch Biochem Biophys 336:49–58
Shibata N, Kobayashi H, Tojo M, Suzuki S (1986) Characterization of phosphomannan-protein complexes isolated from viable cells of yeast and mycelial forms of Candida albicans NIH B-792 strain by the action of Zymolyase-100 T. Arch Biochem Biophys 251:697–708
Kobayashi H, Giummelly P, Takahashi S, Ishida M, Sato J, Takaku M, Nishidate Y, Shibata N, Okawa Y, Suzuki S (1991) Candida albicans serotype A strains grow in yeast extract-added Sabouraud liquid medium at pH 2.0, elaborating mannans without beta-1,2 linkage and phosphate group. Biochem Biophys Res Commun 175:1003–1009
Faille C, Wieruszeski JM, Lepage G, Michalski JC, Poulain D, Strecker G (1991) 1H-NMR spectroscopy of manno-oligosaccharides of the beta-1,2-linked series released from the phosphopeptidomannan of Candida albicans VW-32 (serotype A). Biochem Biophys Res Commun 181:1251–1258
Goins TL, Cutler JE (2000) Relative abundance of oligosaccharides in Candida species as determined by fluorophore-assisted carbohydrate electrophoresis. J Clin Microbiol 38:2862–2869
Han Y, Cutler JE (1995) Antibody response that protects against disseminated candidiasis. Infect Immun 63:2714–2719
Han Y, Cutler JE (1997) Assessment of a mouse model of neutropenia and the effect of an anti-candidiasis monoclonal antibody in these animals. J Infect Dis 175:1169–1175
Han Y, Morrison RP, Cutler JE (1998) A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun 66:5771–5776
Han Y, Kozel TR, Zhang MX, MacGill RS, Carroll MC, Cutler JE (2001) Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J Immunol 167:1550–1557
Han Y, Kanbe T, Cherniak R, Cutler JE (1997) Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun 65:4100–4107
Nitz M, Ling CC, Otter A, Cutler JE, Bundle DR (2002) The unique solution structure and immunochemistry of the Candida albicans β-1,2-mannopyranan cell wall antigen. J Biol Chem 277:3440–3446
Nitz M, Bundle DR (2001) Synthesis of di- to hexasaccharide 1,2-linked beta-mannopyranan oligomers, a terminal S-linked tetrasaccharide congener and the corresponding BSA glycoconjugates. J Org Chem 66:8411–8423
Ogawa T, Yamamoto H (1982) Synthesis of linear d-mannotetraose and d-mannohexaose, partial structures of the cell-surface d-mannan of Candida albicans and Candida utilis. Carbohydr Res 104:271–283
Sugiyama H, Toyohiko N, Horii M, Motohashi K, Sakai J, Usui T, Hisamichi K, Ishiyama J (2000) The conformation of α-(1 → 4)-linked glucose oligomers from maltose to maltoheptaose and short-chain amylose in solution. Carbohydr Res 325:177–182
Ogawa T, Takanashi Y (1983) Synthesis of β- d-(1 → 2)-linked d-glucopentaose, a part of the structure of the exocellular β- d-glucan of Agrobacterium tumefaciens. Carbohydr Res 123:C16–C18
Pozsgay V, Robbins JB (1995) Synthesis of a pentasaccharide fragment of Polysaccharide II of Mycobacterium tuberculosis. Carbohydr Res 277:51–66
Rees DA, Scott WE (1971) Polysaccharide conformation. Part VI. Computer model-building for linear and branched pyranoglycans. Correlations with biological function. Preliminary assessment of inter-residue forces in aqueous solution. Further interpretation of optical rotation in terms of chain conformation. J Chem Soc B 469–479
Lemieux RU, Bock K, Delbaere LTJ, Koto S, Rao V (1980) The conformations of oligosaccharides related to the ABH and Lewis human blood group determinants. Can J Chem 58:631–653
Otter A, Lemieux RU, Ball RG, Venot AP, Hindsgaul O, Bundle DR (1999) Crystal state and solution conformation of the B blood group trisaccharide α- l-Fucp-(1 → 2)-[α- d-Galp]-(1 → 3)]-β- d-Galp-OCH3 Eur J Biochem 259:295–303
Zierke M, Smieško M, Rabbani S, Aeschbacher T, Cutting B, Allain FH-T, Schubert M, Ernst B (2013) Stabilization of branched oligosaccharides: Lewisx benefits from a non-conventional C–H · · · O hydrogen bond. J Am Chem Soc 135:13464–13472
Peters T, Brisson J-R, Bundle DR (1990) Conformational analysis of key disaccharide components of Brucella A and M antigens. Can J Chem 68:979–988
Crich D, Li H, Yao Q, Wink DJ, Sommer RD, Rheingold AL (2001) Direct synthesis of β-mannans. A hexameric [→3)-β- d-Man-(1 → 4)-β- d-Man-(1]3 subunit of the antigenic polysaccharides from Leptospira biflexa and the octameric (1 → 2)-linked β- d-mannan of the Candida albicans phospholipomannan. X-ray crystal structure of a protected tetramer. J Am Chem Soc 123:5826–5828
Nikrad PV, Beierbeck H, Lemieux RU (1992) Molecular recognition X. A novel procedure for the detection of the intermolecular hydrogen bonds present in a protein · oligosaccharide complex. Can J Chem 70:241–253
Nycholat CM, Bundle DR (2009) Synthesis of mono-deoxy and mono-O-methyl congeners of methyl β- d-mannopyranosyl-(1 → 2)-β- d-mannopyranoside for epitope mapping of anti-Candida albicans antibodies. Carbohydr Res 344:555–569
Bundle DR, Nycholat C, Costello C, Rennie R, Lipinski T (2012) Design of a Candida albicans disaccharide conjugate vaccine by reverse engineering a protective monoclonal antibody. ACS Chem Biol 7:1754–1763
Costello C, Bundle DR (2012) Synthesis of three trisaccharide congeners to investigate frame shifting of β1,2-mannan homo-oligomers in an antibody binding site. Carbohydr Res 357:7–15
Meyer B, Peters T (2003) NMR spectroscopy techniques for screening and identifying ligand binding to protein receptors. Angew Chem Int Ed Engl 42:864–890
Nitz M, Bundle DR (2002) The unique solution structure and immunochemistry of the Candida albicans β1,2-mannopyranan cell wall antigen. In: Jiménez-Barbero J, Peters T (eds) NMR spectroscopy of glycoconjugates. Wiley-VCH Verlag, Weinheim, pp 145–187
Dang A-T, Johnson MA, Bundle DR (2012) Synthesis of a Candida albicans tetrasaccharide spanning the β1,2-mannan phosphodiester α-mannan junction. Org Biomol Chem 10:8348–8360
Lemieux RU, Du M-H, Spohr U (1994) Relative effects of ionic and neutral substituents on the binding of an oligosaccharide by a protein. J Am Chem Soc 116:9803–9804
Johnson MA, Bundle DR (2013) Designing a new antifungal glycoconjugate vaccine. Chem Soc Rev 42:4327–4344
Wu X, Bundle DR (2005) Synthesis of glycoconjugate vaccines for Candida albicans using novel linker methodology. J Org Chem 70:7381–7388
Lipinski T, Luu T, Kitov PI, Szpacenko A, Bundle DR (2011) A structurally diversified linker enhances the immune response to a small carbohydrate hapten. Glycoconj J 28:149–164
Lipinski T, Wu X, Sadowska J, Kreiter E, Yasui Y, Cheriaparambil S, Rennie R, Bundle DR (2012) A β-mannan trisaccharide conjugate vaccine aids clearance of Candida albicans in immunocompromised rabbits. Vaccine 30:6263–6269
Wu X, Lipinski T, Carrell F, Bailey JJ, Bundle DR (2007) Synthesis and immunochemical studies on a Candida albicans clustered glycoconjugate vaccine. Org Biomol Chem 5:3477–3485
Wu X, Lipinski T, Paszkiewicz E, Bundle DR (2008) Synthesis and immunochemical characterization of S-linked glycoconjugate vaccines against Candida albicans. Chem Eur J 14:6474–6482
Lipinski T, Kitov P, Szpacenko A, Paszkiewicz E, Bundle DR (2011) Synthesis and immunogenicity of a glycopolymer conjugate. Bioconjug Chem 22:274–281
Xin H, Dziadek S, Bundle DR, Cutler J (2008) Synthetic glycopeptide vaccines combining β-mannan and peptide epitopes induce protection against candidiasis. Proc Natl Acad Sci U S A 105:13526–13531
Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, Franzusoff A, Duke RC, Wilson CC (2001) Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med 7:625–629
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Steinman RM, Banchereau J (2007) Taking dendritic cells into medicine. Nature 449:419–426
Tacken PJ, Figdor CG (2011) Targeted antigen delivery and activation of dendritic cells in vivo: steps towards cost effective vaccines. Semin Immunol 23:12–20
Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A (2005) A novel glycoconjugate vaccine against fungal pathogens. J Exp Med 202:597–606
Bromuro C, Romano M, Chiani P, Berti F, Tontini M, Proietti D, Mori E, Torosantucci A, Costantino P, Rappuoli R, Cassone A (2010) Beta-glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine 28:2615–2623
Brown GD, Herre J, Williams DL, Willment JA, Marshall A, Gordon S (2003) Dectin-1 mediates the biological effects of beta-glucans. J Exp Med 197:1119–1124
Curtis MM, Way SS (2009) Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 126:177–185
LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C (2007) Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 8:630–638
Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reise e Sousa C (2008) DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol 38:3274–3281
Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE Jr, Spellberg B (2009) Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 5:e1000703
Gaffen SL, Hernandez-Santos N, Peterson AC (2011) IL-17 signaling in host defense against Candida albicans. Immunol Res 50:181–187
Lipinski T, Fitieh A, St. Pierre J, Ostergaard HL, Bundle DR, Touret N (2013) Enhanced immunogenicity of a tricomponent mannan tetanus toxoid conjugate vaccine targeted to dendritic cells via Dectin-1 by incorporating β-glucan. J Immunol 190:4116–4128
Goddard-Borger ED, Stick RV (2011) An efficient, inexpensive, and shelf-stable diazotransfer reagent: imidazole-1-sulfonyl azide hydrochloride. Org Lett 13:2514–2514
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl 41:2596–2599
Tornøe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67:3057–3064
Fidel PL Jr, Sobel JD (1994) The role of cell-mediated immunity in candidiasis. Trends Microbiol 2(6):202–206
Casadevall A, Cassone A, Bistono F, Cutler JE, Magliani W, Murphy JW, Polonelli L, Romani L (1998) Antibody and/or cell-mediated immunity, protective mechanisms in fungal disease: an ongoing dilemma or an unnecessary dispute? Med Mycol 36(Suppl 1):95–105
Casadevall A (1995) Antibody immunity and invasive fungal infections. Infect Immun 63:4211–4218
Cutler JE, Deepe GS Jr, Klein BS (2007) Advances in combating fungal diseases: vaccines on the threshold. Nat Rev Microbiol 5:13–28
Dziadek S, Jacques S, Bundle DR (2008) A novel linker methodology for the synthesis of tailored conjugate vaccines composed of complex carbohydrate antigens and specific TH-cell peptide epitopes. Chem Eur J 14:5908–5917
Pitarch A, Abian J, Carrascal M, Sánchez M, Nombela C, Gil C (2004) Proteomics-based identification of novel Candida albicans antigens for diagnosis of systemic candidiasis in patients with underlying hematological malignancies. Proteomics 4:3084–3106
Clancy CJ, Nguyen ML, Cheng S, Huang H, Fan G, Jaber RA, Wingard JR, Cline C, Nguyen MH (2007) Immunoglobulin G responses against a panel of Candida albicans antigens as accurate and early markers for the presence of systemic candidiasis. J Clin Microbiol 46:1647–1654
Singh H, Raghava GPS (2001) Propred: Predication of HLA-DR binding sites. Bioinformatics 17:1236–1237
Sing H, Raghava GPS (2003) Propred1: prediction of promiscuous MHC class-I binding sites. Bioinformatics 19:1009–1014
Xin H, Cartmell J, Bailey JJ, Dziadek S, Bundle DR, Cutler JE (2012) Self-adjuvanting glycopeptide conjugate vaccine against disseminated candidiasis. PLoS One 7:e35106
Cartmell J, Paszkiewicz E, Tam P-H, Luu T, Sarkar S, Lipinski T, Bundle DR (2014) Synthesis of antifungal vaccines by conjugation of β-1,2 trimannosides with T-cell peptides and covalent anchoring of neoglycopeptide to tetanus toxoid. Carbohydr Res. doi:10.1016/j.carres.2014.06.24
Bundle DR, Tam P-H, Tran H-A, Paszkiewicz E, Cartmell J, Sadowska JM, Sarkar S, Joe M, Kitov PI (2014) Oligosaccharides and peptide displayed on an amphiphilic polymer enable solid phase assay of hapten specific antibodies. Bioconjug Chem 25:685–697
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Bundle, D.R. (2014). The Evolution of a Glycoconjugate Vaccine for Candida albicans . In: Seeberger, P., Rademacher, C. (eds) Carbohydrates as Drugs. Topics in Medicinal Chemistry, vol 12. Springer, Cham. https://doi.org/10.1007/7355_2014_60
Download citation
DOI: https://doi.org/10.1007/7355_2014_60
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-08674-3
Online ISBN: 978-3-319-08675-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)