Abstract
Heterocyclic N-oxides have been shown to be useful in energetic materials applications such as propellants, explosives, and pyrotechnics. This chapter provides a survey of a variety of different heterocyclic N-oxides that have been studied for their energetic materials properties. Where possible, information such as heat of formation, density, detonation pressure (P CJ), and detonation velocity (V D ) are provided.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- 1,2,3-Triazole
- 1,2,4-Triazole
- Energetic materials
- Explosives
- Furoxan
- Hypofluorous acid
- Macrocycles
- Oxone
- Pyrazine
- Pyrazole
- Pyridine
- Tetrazine
- Tetrazole
- Triazine
1 Introduction
Heterocyclic N-oxides have received intense interest in the field of energetic materials in the past decade. This is in large part due to the fact that the introduction of an N-oxide functional group on to a heterocyclic system is a strategy that attempts to make use of the interesting properties N-oxide materials tend to display. The zwitterionic nature of the N-oxide bond tends to lead to large dipole moments, which often leads to increases in crystal densities, a property that is highly important with respect to energetic material performance. N-oxides also increase the oxygen balance of a molecule, providing more oxidizer to improve the overall combustion of the fuel in the molecule. Additionally, N-oxides are also known to provide a stabilizing effect for some heterocyclic systems. Introduction of the N-oxide functional group can also lead to reduction in the sensitivity of an energetic material toward destructive stimuli, such as impact, spark, or friction.
The strategy used to introduce N-oxides onto the majority of the heterocycles discussed in this section (with the exception of furoxans) is through oxidation of ring nitrogen atoms. One of the most often used oxidants in this regard is Oxone. Other peracids are used as well, including peracetic acid and peroxytrifluoroacetic acid. For particularly unreactive heterocyclic systems, hypofluorous acid has also been employed. Examples of the formation of heterocyclic N-oxides directly through cyclization or condensation reactions are seen less frequently but are common in the formation of furoxans and some tetrazole and triazole N-oxides.
This chapter is intended to provide an overview of novel, energetic heterocyclic N-oxides that have been prepared over the past 15–20 years, though there are a few examples from earlier work that have been included. This chapter is not intended to be a comprehensive review of all of the heterocyclic N-oxide energetic material work that has been reported, but rather, it is a survey that highlights energetic, heterocyclic N-oxide syntheses and characterizations. This chapter attempts to discuss N-oxide derivative of five-membered heterocycles (pyrazoles, triazoles, tetrazoles, furoxans) and six-membered heterocycles (pyridines, pyrazines, triazines, and tetrazines) as well as some fused combinations of different heterocycles while providing energetic materials properties where the data exists.
2 Pyrazole Oxides
2.1 Dinitropyrazole 1-Oxides
Shevelev reported the synthesis of N-hydroxydinitropyrazoles in 1996 [1]. Both 3,4- and 3,5-dinitropyrazoles were oxidized to the corresponding 1-hydroxy compounds using a buffered KHSO5 solution (Scheme 1). The yields were modest (20–48%). No salts were prepared nor were any explosive properties of these materials reported. While these materials were not converted to N-oxide salts by reaction with bases, the work opened up the possibility for other efforts to proceed along those lines and, thus, is an important contribution to energetic heterocyclic N-oxide chemistry.
2.2 3,4,5-Trinitropyrazole 1-Oxide
Shreeve reported the oxidation of 3,4,5-trinitropyrazole with Oxone in 2012 [2]. Reaction of the ammonium salt of 3,4,5-trinitropyrazole proceeded at 55°C for 3 days to give the 1-oxide (Scheme 2). Acidification provided the free acid, which was then converted to a variety of nitrogenous salts. The most promising salt was the ammonium salt. This material has a density of 1.82 g/cm3, has a heat of formation of 118 kJ/mol, and is thermally stable up to 176°C. It has an excellent oxygen balance and has impact sensitivity similar to conventional high explosives such as RDX (1,3,5-trinitroperhydro-1,3,5-triazine). The explosive performance was predicted to be good (V D = 8,676 km/s, P CJ = 35 GPa).
3 Triazole Oxides
3.1 1,2,3-Triazole 1-Oxides
Furoxans have been shown to react with alkylamines to form triazole 1-oxides [3]. In their work toward the synthesis of tetrazino-1,2,3,4-tetrazine dioxide, Churakov and coworkers found that a variety of alkylamines reacted with 3-tert-butylazoxy-4-aminofuroxan to give the corresponding 1,2,3-triazole 1-oxide (Scheme 3). Treatment of the tert-butyl derivative with trifluoroacetic acid or gaseous HCl easily removes the tert-butyl group.
Direct oxidation of the 1,2,3-triazole was reported for 4,5-dinitro-1,2,3-triazole, which can be oxidized to the 1-oxide derivative with Oxone in the presence of K2HPO4 [4]. After 48 h at 55°C, the free acid of 4,5-dinitro-1,2,3-triazole 1-oxide is formed with 96% conversion (Scheme 4). The acid can be converted to the potassium or ammonium salts with potassium carbonate or ammonia. The crystal density of the ammonium salt was found to be 1.789 g/cm3 and this material was found to be thermally stable up to 195°C.
Treatment of 5,6-diaminofurazano[3,4-b]pyrazine with nitronium ion results in a condensation and cyclization reaction to form 1,2,3-triazolo[4,5-e]furazano[3,4-b]pyrazine 6-oxide (Scheme 5) [5]. The N–H group in this molecule is acidic enough to react with nitrogenous bases, as Shreeve showed in 2014 [6]. The ammonium, hydrazinium, and hydroxylammonium salts were prepared along with some salts with heterocyclic cations. The materials ranged in thermal stability from 141°C for the hydroxylammonium salt to 281°C for the free acid. The free acid also showed the highest density (1.85 g/cm3) and the most promising calculated performance (V D = 8.53 km/s, P CJ = 32.4 GPa). All of the materials were relatively insensitive to impact (30–40 J).
3.2 1,2,4-Triazole 1-Oxides
Oxidation of 3-nitro-1,2,4-triazole was briefly mentioned in a publication by Shevelev where the oxidation of pyrazoles had been studied [1]. KHSO5 was the oxidant found to be the most successful for the 3-nitro-1,2,4-triazole substrate, providing the 1-N-hydroxy derivative. No energetic materials properties for the product were reported, nor were any salts prepared.
A much more challenging triazole to oxidize is 3,5-dinitro-1,2,4-triazole, but a method was reported in 2002 [7]. The ultimate goal of the research was to prepare highly energetic liquid propulsion ingredients. Here again, Oxone was used as the oxidant, but the reaction was found to be slow and inefficient, and pure salts of 3,5-dinitro-1,2,4-triazole 1-oxide were not isolated. In 2012, a different oxidant was used to prepare pure materials [4]. Hypofluorous acid oxidation proceeded within 15 min giving a 65% yield of the potassium salt of 3,5-dinitro-1,2,4-triazole 1-oxide (density = 2.08 g/cm3, T dec = 215°C) (Scheme 6). The ammonium salt had a crystal density of 1.784 g/cm3 and a heat of formation of 25 kcal/mol. The material begins to decompose at 145°C.
Klapoetke and coworkers prepared several energetic salts of 3,3′-dinitrobis(1,2,4-triazole) 1,1′-dioxide [8]. 3,3′-Dinitrobis(1,2,4-triazole) was oxidized with Oxone in the presence of a potassium acetate buffer. When the reaction was complete, the reaction mixture was treated with sulfuric acid and extracted to give the diol. The diol was then treated with a variety of bases (ammonia, hydrazine, hydroxylamine, guanidine, aminoguanidine, triaminoguanidine) (Scheme 7). Oxidation was found to occur only at the 1 and 1′ positions. The diol was found to be thermally stable to 191°C and had gas pycnometry density of 1.91 g/cm3. Conversion to the nitrogenous salts improves the thermal stability in all cases (207–329°C), with the guanidinium salt being the most thermally stable. In general, the densities of the salts are decreased compared to the parent diol compound, with the hydroxylamine salt having the highest density (1.90 g/cm3). All of the salts have low sensitivity to impact (>40–15 J) and are essentially insensitive to friction. The salt with the most promising explosive properties was the hydroxylamine salt, with a predicted detonation pressure of 39 GPa and a detonation velocity of 9.1 km/s, comparable to high-performing conventional explosives such as HMX (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine). No toxicity studies were reported for any of the compounds.
4 Tetrazole Oxides
4.1 5-Nitrotetrazole 2-Oxide
5-Nitrotetrazoles can be oxidized to the corresponding 2-N-oxide with Oxone, in the presence of an acetate buffer (Scheme 8) [7]. No 1-oxide isomer was observed under these reaction conditions. Various salts can be prepared using ion exchange resins. The hydroxylamine salt was reported to have an experimental heat of formation of 40 kcal/mol and a density of 1.85 g/cm3. The explosive performance properties of the energetic salts were reported by Klapoetke [9]. The hydroxylamine salt was predicted to have a detonation pressure of 39 GPa and a detonation velocity of 9.45 km/s. The other salts displayed lower performance. Thermal studies of the hydroxylamine amine salt showed it began to decompose at 157°C at a 5°C heating ramp rate. It was also relatively sensitive to impact (4 J) and friction (60 N). Interestingly, all of the 2-N-oxide salts studied were less sensitive than the corresponding 5-nitrotetrazolate salts, with the exception of the aminoguanidinium salt.
4.2 5-Azidotetrazole 2-Oxide
In 2011, the Klapoetke group attempted to study the oxidation of the extremely sensitive compound 5-azidotetrazole [10]. This compound is quite hazardous to work with and can be considered a contact explosive. 5-Azidotetrazole was oxidized with Oxone in the presence of potassium acetate as a buffer. The reaction proceeded at 40°C for 3 days (Scheme 9). Acidification, extraction with ether, and treatment with ammonia provided the ammonium salt. The silver, potassium, and sodium salts were prepared along with the aminoguanidinium salt. All of the salts displayed lower thermal stability than the corresponding 5-azidotetrazole salts. Here again the sensitivity to impact and friction was less for the 2-N-oxide derivatives compared to respective non-oxide compounds. The density of the ammonium salt was 1.689 g/cm3 and the detonation velocity and pressure were calculated to be 8.92 km/s and 32.5 GPa, respectively.
4.3 5-Aminotetrazole 2-Oxide
An interesting tetrazole N-oxide synthesized by the Klapoetke group is 5-aminotetrazole 1-oxide [11]. Rather than accessing this heterocycle through oxidation of 5-aminotetrazole, it was prepared by reacting cyanogen azide with hydroxylamine. In the presence of excess hydroxylamine, the hydroxylammonium salt can be isolated (Scheme 10). Treatment with HCl, to protonate the oxide, followed by treatment with ammonia provides the ammonium salt. The hydroxylamine salt begins to decompose at 155°C and was shown to have two polymorphs, one with a density of 1.664 g/cm3 and the other with 1.735 g/cm3. The ammonium salt had a much lower density (1.53 g/cm3) and an onset of decomposition of 195°C. These materials were relatively insensitive to impact (>40–10 J) and insensitive to friction (>360 N). The high-density hydroxylammonium salt showed the most promise with respect to explosive performance (P CJ = 35.7 GPa, V D = 9.3 km/s).
Amine oxidation of 5-aminotetrazole 1-oxide is possible using basic potassium permanganate resulting in the bis-potassium salt of the azo-coupled product (Scheme 11) [11]. Treatment of the potassium salt with acid followed by reaction with nitrogen bases gives access to the hydroxylammonium, ammonium, and hydrazinium salts. The nitrogenous salts displayed impact sensitivities in the range of 3–15 J and friction sensitivities in the range of 20–160 N. The densities ranged from 1.725 g/cm3 for the hydrazinium salt to 1.80 g/cm3 for the hydroxylammonium salt. Amazingly the ammonium salt was quite thermally stable (decomposition onset 250°C), while the other salts decomposed at about 190°C. The best performer was the hydroxylammonium salt (P CJ = 37.5 GPa, V D = 9.35 km/s).
4.4 Dihydroxylammonium 5,5′-Bistetrazole 1,1′-Dioxide
One of the most promising energetic N-oxide heterocycles discovered recently is the dihydroxylammonium salt of 5,5′-bistetrazole 1,1′-dioxide (TKX-50) [12]. TKX-50 is described as a molecule that is easily prepared and very powerful but with the required thermal stability, low toxicity, and safety properties to be used as an RDX replacement. Two different approaches were used to access the TKX-50 structure. The first was oxidation of 5,5′-bitetrazole using potassium acetate-buffered Oxone. Unfortunately, the method resulted in the production of both the 1,1′-di-N-oxide (11%) and the 2,2′-di-N-oxide (58%) (Scheme 12). A different route to TKX-50 is based upon the work of Tselinksi [13]. This method involves the reaction of sodium azide with dichloroglyoxime, followed by treatment with HCl in ether to access the TKX-50 precursor diol.
TXK-50 was found to have an impact sensitivity of 20 J and a friction sensitivity of 120 N. These values compare very favorably with conventional energetic materials such as RDX (impact = 7.5 J, friction = 120 N). It is also thermally stable up to 221°C; TKX-50 has a density of 1.877 g/cm3 at 298 K and a heat of formation calculated to be 446.6 kJ/mol. These data taken together were used to predict the explosive performance of TKX-50. The detonation velocity was predicted to be 9.7 km/s and a detonation pressure of 42.4 GPa. These data were compared to other standard explosives to show that TKX-50 was predicted to outperform most other conventional explosives with respect to detonation velocity and was only surpassed by CL-20 with respect to detonation pressure. Toxicity studies were also performed on Vibrio fischeri, and the results showed that TKX-50 was less toxic to this microorganism than RDX.
4.5 Amino-Hydroximoyl-Tetrazole 2-Oxides
Another example of the use of the N-oxide to improve the performance of tetrazole compounds is seen in the synthesis of amino-hydroximoyl-tetrazole 2-oxides [14]. The N-oxide was installed prior to the formation of the aminohydroximoyl group by oxidizing cyanotetrazole, which provided cyanotetrazole-2-oxide (Scheme 13). Subsequent treatment with hydroxylamine provided the amino-hydroximoyl-tetrazole-2-N-hydoxy compound. Treatment with nitrogenous bases allows access to a variety of salts (hydroxylammonium, guanidinium, aminoguanidinium, ammonium, triaminoguanidinium). In the report, the hydroxylammonium salt was compared to the hydroxylammonium salt of the unoxidized amino-hydroximoyl-tetrazole. Here it was shown that the N-oxide improves the insensitivity toward impact (>40 vs 10 J), while the thermal stability is reduced slightly (T dec = 164 vs 171°C). A slight decrease in the heat of formation (282 vs 294 kJ/mol) and a significant increase in density (1.704 vs 1.639 g/cm3) lead to an increase in calculated detonation velocity by 3.6% (8.934 vs 8.643 km/s) and an increase in calculated detonation pressure by 12% (30.9 vs 27.2 GPa).
5 Furoxans
5.1 4,4′-Dinitro-3,3′-Diazofuroxan
A very high-energy furoxan was reported by Binnikov in 1999, namely, 4,4′-dinitro-3,3′-diazofuroxan [15]. This molecule was synthesized by azo coupling of 4-amino-3-azidocarbonylfuroxan. A Curtius rearrangement was used to access the corresponding 4,4′-diamino compound. Oxidation using Na2WO4 in the presence of sulfuric acid and 90% peroxide provided 4,4′-dinitro-3,3′-diazofuroxan (Scheme 14). An experimental detonation velocity was determined to be 10 km/s, with a crystal density of 2.02 g/cm3. This is one of the fastest detonation velocities ever reported. The high density is due in large part to very efficient crystal packing.
5.2 Dinitrofurazanofuroxan
3,4-bis(3-nitrofurazan-4-yl)furoxan (BNFF) is a promising furoxan derivative that displays good performance and physical properties. The first appearance of BNFF in the literature was in a report by Sheremetev and coworkers [16]. Subsequently, another report was published, with few synthesis details [17]. In 2010, Chung published a detailed synthesis procedure for the preparation of BNFF, a portion of which is included in Scheme 15. The synthesis includes diazotization of the amide oxime compound in the presence of HCl to provide the oximyl chloride, treatment of the oximyl chloride with base to produce a nitrile oxide intermediate that dimerizes to provide 3,4-bis(3-aminofurazan-4-yl)furoxan (BAFF). Oxidation with peroxytrifluoroacetic acid (PTFA) provided BNFF.
BAFF can be crystallized from water or ethanol, which results in two different polymorphs with densities of 1.745 and 1.737 g/cm3, respectively. The high-density material has a predicted detonation velocity of 8.1 km/s and an experimental detonation velocity of 7.18 km/s at a density of 1.53 g/cm3. It was reported to be insensitive to impact, spark, and friction [18].
BNFF is a powerful explosive with a relatively low melting point (108–110°C) and a performance similar to that of HMX [19]. It is reported to have a density of 1.937 g/cm3 and a detonation velocity of 9.25 km/s. Because of these properties, BNFF has been considered for melt-cast explosives applications [20]. It has also been shown to form eutectics with a variety of other energetic materials such as TNT, pentaerythritol tetranitrate, and trinitroazetidine.
The nitro groups on BNFF are labile and reactive toward nucleophiles such as amines and azides. Treatment of BNFF with sodium azide in acetonitrile results in the bisazido compound DAZTF (Scheme 16) [21]. DAZTF has a density of 1.743 g/cm3, a melting point of 50–52°C, and a thermal decomposition onset temperature of 204°C. The detonation velocity was estimated to be 8.5 km/s.
5.3 Macrocyclic Furoxans
In 2012, Chavez and coworkers reported the synthesis of a macrocycle prepared from the oxidation of bis(aminofurazanyl)furoxan using trichloroisocyanuric acid as the oxidant (Scheme 17) [22]. The resulting product has the possibility of forming two isomers.
Mahkova and coworkers synthesized a similar set of furoxan macrocycles [23]. The starting furoxan substrates are based on N-oxide isomers of 3-amino-4-(aminofurazanyl)furoxan. The position of the N-oxide was either internal or external to the heterocycle–heterocycle bond. Scheme 18 displays the resulting heterocycles formed after oxidation. In the case of the external N-oxide, only one macrocycle was isolated, when the diamine was oxidized with dibromoisocyanuric acid (DBI). With the internal N-oxide, oxidation with DBI resulted in a 12-membered macrocycle, but the assignment of which isomer was formed was not determined. These macrocycles were formed in low yield due to numerous other oligomeric products that are formed in the process.
Treatment of bis(nitrofurazanyl)furoxan (BNFF) with sodium carbonate in acetonitrile leads to cyclization to the bifurazano[3,4-b:3′,4′-f]furoxano[3″,4″-d]oxacycloheptatriene (BFFO) (Scheme 19) [24]. The product melts at 97°C and thus has potential as a melt-cast material. X-ray crystallographic analysis showed that the crystal density of the BFFO monohydrate was 1.866 g/cm3. Solvate-free crystals could not be obtained but were predicted to have a density of 1.9 g/cm3 and a heat of formation of 275.2 kJ/mol. BFFO was predicted to have a detonation velocity of 8.6 km/s and a detonation pressure of 34.6 GPa. The material was also described as less sensitive to impact than HMX.
Treatment of bis(nitrofurazanyl)furoxan (BNFF) with ammonia in acetonitrile leads to cyclization to the bifurazano[3,4-b:3′,4′-f]furoxano[3″,4″-d]azacycloheptatriene (BFFO), while treatment with hydrazine provides the N-amino derivative (Scheme 20) [25]. The azepine compound is hygroscopic and forms a monohydrate crystal with a density of 1.817 g/cm3 and a measured detonation velocity of 7.9 km/s. The N-amino compound is much more sensitive to impact and friction than the N–H azepine, which is thought to be due to the relative weakness of the N–N bond [26].
5.4 Other Furoxan Derivatives
The two isomers of 3,3′-diamino-4,4′-bifuroxan can be converted to the bisnitramine compounds by treatment with 100% nitric acid (Scheme 21) [27]. While these derivatives display poor thermal stability (T d < 95°C), they could be converted to nitrogenous salts by treatment with ammonia, hydroxylamine, or hydrazine. The bisnitramine with the N-oxides external to bisfuroxan bond was also converted to the guanidine, diaminoguanidine, and triaminoguanidine salts. All of the materials prepared were characterized with respect to their energetic materials safety and performance properties. None of the salts displayed thermal stability above 168°C. The most promising salts were the hydroxylammonium compounds, which displayed promising safety properties and detonation performance properties (V D = 9.5–9.8 km/s, P CJ = 41.5–46.4 GPa).
In 2005, Hiskey and coworkers reported the synthesis of a fused, tricyclic furoxan, 7-nitrotetrazolo[1,5-f]furazano[4,5-b]pyridine 1-oxide [28]. The synthesis began with the nitration of 2,6-dimethoxy pyridine, which gave 3,5-dinitro derivative, followed by subsequent treatment with hydrazine and diazotization to give 2,6-diazido-3,5-dinitropyridine (Scheme 22). Azidoazine compounds are known to participate in azido-tetrazolo tautomerization. In the case of 2,6-diazido-3,5-dinitropyridine, dissolution in deuterated acetonitrile resulted in tautomerization to the tetrazolo form, followed by loss of dinitrogen and the formation of the furoxan ring. The diazido compound undergoes the transformation more quickly in polar solvents and converts to the product in the solid state over 8–10 days. X-ray crystallography showed that the product has at least two polymorphs (alpha (density = 1.853 g/cm3), beta (density = 1.828 g/cm3)). The material began to decompose at 160°C, was insensitive to electrostatic discharge, displayed moderate sensitivity to friction, and was slightly more sensitive to impact than HMX (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine).
5.5 3-Oxyfuroxan
3-Oxyfuroxan is predicted to have higher heat of formation than furoxan while also having improved oxygen balance [29]. Thus, energetic derivatives of 3-furoxan may show promise as high-performing materials. Chen and coworkers recently published the preparation of energetic metal organic frameworks incorporating a 3-oxyfuroxan. In the work, a bis(oximylchloride)furoxan was treated with dinitrogen pentoxide, and this resulted in a mixture of the bis(dinitrochloromethyl)furoxan and bis(dinitrochloromethyl)-3-oxyfuroxan (Scheme 23). Treatment of this mixture with KI in methanol followed by reaction with silver nitrate produced a metal organic framework in which the 3-oxyfuroxan derivative can be identified. No properties of the pure 3-oxyfuroxan compound were reported.
6 Energetic Pyridine N-Oxides
6.1 Aminonitropyridine N-Oxides
Energetic pyridine N-oxides have been reported by Wilson [30]. 2,6-Diamino-3,5-dinitropyridine can be oxidized with 30% hydrogen peroxide in acetic acid (Scheme 24). The reaction proceeds to give an 84% yield of the N-oxide product. This material is very thermally stable and does not begin to decompose until 340°C. X-ray crystallography experiments showed that the density of the N-oxide is 1.878 g/cm3. Treatment of the N-oxide with hydroxylamine in the presence of potassium hydroxide gives 2,4,6-triamino-3,5-dinitropyridine N-oxide. The density of the material remains nearly the same (1.876 g/cm3) and yet is slightly less thermally stable (308°C). The authors of this study also attempted to prepare the 2,6-dinitro-3,5-diaminopyridine 1-oxide but were unsuccessful.
7 Pyrazine N-Oxides
7.1 2,6-Diamino-3,5-Dinitropyrazine 1-Oxide
In the 1990s, scientists at the Lawrence Livermore National Laboratory described the synthesis of 2,6-diamino-3,5-dinitropyrazine 1-oxide (LLM-105) [31]. LLM-105 displays a crystal density of 1.919 g/cm3 [32] and is described as an insensitive energetic material, with a detonation velocity of 8.73 km/s and a detonation pressure of 35.9 GPa [33].
The synthesis route is displayed in Scheme 25. The synthesis began with the nitration of 2,6-dimethoxypyrazine using mixed acid nitration conditions. The 2,6-dimethoxy-3,5-dinitropyrazine is then treated with ammonium hydroxide to provide 2,6-diamino-3,5-dinitropyrazine (ANPZ). Conversion to the N-oxide is performed using peroxytrifluoroacetic acid (PTFA). Unfortunately, the ANPZ and LLM-105 have low solubility in most solvents, and as the oxidation progresses, starting material becomes occluded in the N-oxide product. The overall result is a product that is 92–94% LLM-105, with the remained being unoxidized ANPZ. The ANPZ impurity does lead to a slightly lower explosive performance.
In 2010, an alternative route to LLM-105 was reported in a patent application [34]. The new route involves the treatment of bis(cyanomethyl)nitrosamine with hydroxylamine (Scheme 26), resulting in a cyclization process that provides 2,6-diaminopyrazine 1-oxide (DAPO) as the product. The material is then nitrated using mixed acid to provide LLM-105. The DAPO route now allows for LLM-105 to be free from the ANPZ impurity.
8 1,2,4-Triazine Oxides
8.1 4-Amino-3,7-Dinitrotriazolo[5,1-c][1,2,4]Triazine 4-Oxide
Examples of energetic 1,2,4-triazine N-oxides are rare. Recently, Piercey reported the synthesis of an energetic triazolo-1,2,4-triazine 4-oxide [35]. The synthesis involves a very interesting cyclization reaction that proceeds after condensation of a diazonium salt with nitroacetonitrile, resulting in the 4-amino-3,7-dinitrotriazolo[5,1-c] [1,2,4]triazine (Scheme 27). Oxidation of this substrate proved challenging, as strong oxidants such as peroxytrifluoroacetic acid and Oxone were unsuccessful. Ultimately, the use of hypofluorous acid was effective at installing an N-oxide at the 4-position of the bicyclic ring system. The material was predicted to have a density of 1.904 g/cm3 and a heat of formation of 378 kJ/mol. The crystal structure of the dihydrate or nitromethane solvate shows that the triazine ring bonds to the triazole have lengthened in the N-oxide compound, and this may explain the large drop in thermal stability (T dec = 138 vs 232°C). This triazine N-oxide was predicted to be a high-performing material that is less sensitive to impact and friction than conventional high-performance explosives (V D = 8.97 km/s, P CJ = 35.4 GPa).
9 1,2,3,4-Tetrazine N-Oxides
9.1 1,2,3,4-Tetrazine 1-Oxide
The synthesis of 1,2,3,4-tetrazine 1-oxide was reported by Piercey and coworkers in 2015 [36]. The synthesis involves the nitrene insertion of the N-amino-1,2,3-triazole compound shown in Scheme 28. Treatment of this compound with MnO2 resulted in the 1,2,3,4-tetrazine-1-benzyloxy p-toluenesulfonate salt. Subsequent treatment with hydrogen gas in the presence of Pd/C produced 1,2,3,4-tetrazine 1-oxide. The compound was confirmed by 13C NMR and by mass spectrometry (DEI+).
9.2 1,2,3,4-Tetrazine Di-N-Oxides
A review of work regarding annulated 1,2,3,4-tetrazine di-N-oxides has been published previously [37]. Since then, some very interesting work on annulated and non-annulated 1,2,3,4-tetrazine di-N-oxides has been published. In 2015, Churakov reported the thermolysis of poly azido benzotetrazine 1,3-dioxide in toluene. This results in the non-annulated dicyano-1,2,3,4-tetrazine 1,3-dioxide as the product (Scheme 29) [38]. Similarly, when 6,7- or 6,8-dimethoxybenzotetrazine 1,3-dioxides were treated with ozone, non-annulated 1,2,3,4-tetrazine 1,3-dioxides were produced (Scheme 30).
In 2014 Churakov reported the synthesis of 1,2,3,4-tetrazine 1,3-dioxides annulated with 1,2,3-triazoles and 1,2,3-triazole 1-oxides [39]. In this work, the tetrazine 1,3-dioxides are installed by condensing a tert-butylazoxy functional group with an amino group in the presence of sulfuric acid, nitric acid, and acetic anhydride (Scheme 31).
To access the 1,2,3-triazole 1-oxide tetrazine di-N-oxide derivatives, the same reaction conditions were employed using 1,2,3-triazole 1-oxide substrates (Scheme 32). Interestingly, non-alkylated 1,2,3-traizoles could also be used as substrates. The product, with an acidic proton, was predicted to be most stable in the 1-oxide 3-NH form. The materials were reported to display thermal stability between 154 and 199°C for the 1,2,3-triazoles derivatives and 180–230°C for the 1,2,3-triazole 1-oxide derivatives. No energetic materials properties for these derivatives were reported.
9.3 Tetrazinotetrazine 1,3,6,8-Tetraoxide
Two isomers of the fused bicyclic heterocycle, tetrazinotetrazine tetraoxide (TTTO), have been the targets of energetic materials synthesis chemist for decades (Fig. 1). The interest in these materials stems from the potential for these materials to display high densities and promising energetic materials properties. Additionally, these materials belong to a class known as alternating charge compounds. These materials have been predicted to display enhanced stability [40]. TTTO has been predicted to have a heat of formation of 206 kcal/mol and a predicted density of 1.98 g/cm3. Iso-TTTO was also predicted to display similar properties [41]. Given these predicted properties, both materials are predicted to have a detonation velocity around 9.7 km/s and a detonation pressure of 432 kbar.
In 2016, Churakov and coworkers reported the first synthesis of TTTO [41]. They used a sequential ring closure strategy to install both ring systems by employing the process of annulating neighboring amino and (tert-butyl-NNO-azoxy) groups. The reported synthesis starts with bis (tert-butyl-NNO-azoxy)-cyano methane, which is converted to the cyclization precursor in seven steps (Scheme 33). The precursor undergoes the first annulation through treatment with BF3·Et2O. Subsequent treatment with ammonia, followed by treatment with mixed acid in the presence of acetic anhydride, produces TTTO.
Unfortunately, TTTO was isolated as the minor product in the last step (23%). TTTO was obtained as a yellow powder with a melting point of 183–186°C. The structure was proven using X-ray crystallographic analysis of the benzene solvate of TTTO. Interestingly, the hydrolytic stability of TTTO and its benzene solvate is poor. While it is stable in air for a short time, it hydrolyzes in 50% aqueous ethanol after 2 h.
10 1,2,4,5-Tetrazine N-Oxides
10.1 3,6-Diaminotetrazine 1,4-Dioxide
One of the earliest heterocyclic N-oxides considered for energetic materials applications was 3,6-diamino-1,2,4,5-tetrazine 1,4-dioxide, often referred to as LAX-112 [42]. LAX-112 was synthesized by the oxidation of 3,6-diamino-1,2,4,5-tetrazine using Oxone as the oxidant (Scheme 34). The material has a crystal density of 1.86 g/cm3 and a heat of formation of 39 kcal/mol. It is thermally stable up to 206°C, is insensitive to friction, and is relatively insensitive toward impact (26.2 J). LAX-112 can be formulated with a binder such as Kel-F or Viton A. A 95:5 mixture of LAX-112:Viton A produces a material with a measured detonation velocity of 8.1 km/s and a detonation pressure of 30.1 kbar (unpublished results).
10.2 3-Amino-6-Nitrotetrazine N-Oxides
If 3,6-diamino-1,2,4,5-tetrazine is oxidized with peroxytrifluoroacetic acid (PTFA) instead, the mono-N-oxide is formed, while LAX-112 was not observed. It is believed that the PTFA was a strong enough acid to protonate the tetrazine ring of the mono-N-oxide and inhibit further oxidation to LAX-112. This material has a density of 1.76 g/cm3 and is less thermally stable than LAX-112 (T dec = 150°C) (Scheme 35). The experimental detonation performance of the material was not determined, but it is predicted to have a detonation velocity of 8.92 km/s and a detonation pressure of 32.5 GPa. A minor product was also isolated from the PTFA oxidation, namely 3-amino-6-nitro-1,2,4,5-tetrazine 2,4-dioxide and was likely to have arisen from a different oxidation pathway, involving oxidation of the amino group as the first step. This material was reported to have a density of 1.919 g/cm3 but began to decompose at a much lower temperature (110°C). This material was predicted to have a detonation velocity of 9.33 km/s and a detonation pressure of 40.2 GPa (unpublished results).
In 2008, Mahkova and Ovchinnikov prepared 3-amino-6-nitro-1,2,4,5-tetrazine 2,4-dioxide through an alternative synthesis route, by diazotizing 3,6-diamino-1,2,4,5-tetrazine (Scheme 36) [43]. The method involved nonstandard diazotization conditions (DMSO, NaNO2, HCl) to provide 3-amino-6-nitro-1,2,4,5-tetrazine followed by treatment with PTFA to give the di-N-oxide product. If less than a stoichiometric amount of PTFA is used, a mixture of the 2,4-dioxide and 2-oxide is formed in a 9:1 ratio. The use of an excess of oxidant results in only the 2,4-dioxide product. Interestingly, this study found that 3-amino-6-nitro-1,2,4,5-tetrazine 2,4-dioxide was stable up to 191°C. No sensitivity or performance properties were reported for the mono-N-oxide compound.
In 1999, Hiskey and coworkers investigated hypofluorous acid as an oxidizer for 3,6-diamino-1,2,4,5-tetrazine [44]. Hypofluorous acid is one of the most powerful oxidizers and oxygen transfer reagents known [45]. The reaction proceeds to provide 3-amino-6-nitro-1,2,4,5-tetrazine 2,4-dioxide and 3-amino-6-nitro-1,2,4,5-tetrazine 1,4-dioxide, a new isomer (Scheme 37). This isomer was found to have a higher density (1.97 g/cm3) and was found to be thermally stable up to 168°C. The predicted detonation velocity for this material is 9.81 km/s and the predicted detonation pressure is 42 GPa.
10.3 Other Amino-1,2,4,5-Tetrazine N-Oxides
The susceptibility of 1,2,4,5-tetrazine heterocycles toward oxidation strongly depends on the substituents at the 3 and 6 positions. If at least one of the tetrazine substituents is an amino group, the other substituent can be a hydrogen or a chlorine and oxidation can still occur with PTFA (Scheme 38) [44].
Oxidation of 3,6-bis(guanidinyl)-1,2,4,5-tetrazine with Caro’s acid proceeds to provide the 1,4-dioxide product [46]. The guanidinyl functional groups of the product maintain enough basicity to form salts with strong acids (HNO3 and HClO4) (Scheme 39). The dinitrate salt was only stable to 157°C and was not studied further, but the perchlorate salt was stable up to 197°C. The perchlorate salt has a measured density of 1.94 g/cm3 and a heat of formation estimated at −250 kJ/mol. The impact sensitivity was determined to be 3.7 J, and the friction sensitivity was 68 N. The detonation velocity of the perchlorate salt was experimentally determined to be 7.85 km/s.
3,6-bis((tetrazol-5-yl)amino)-1,2,4,5-tetrazine can also be oxidized with Caro’s acid [46]. The structure was confirmed by X-ray crystallography to be the 1,4-dioxide (Scheme 40). Unfortunately, the thermal stability was poor (134°C) and further investigation was not performed.
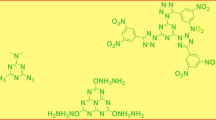
In 2014, Shreeve reported the synthesis of 11 new 1,2,4,5-tetrazine mono- and di-N-oxides [47]. Figure 2 displays four of the more energetic compounds prepared in this work. The guanidine derivative has a density of 1.91 g/cm3 and a calculated heat of formation of 325 kJ/mol. The explosive performance was predicted to be high (V D = 9.1 km/s, P CJ = 37.5 GPa), while the material was relatively insensitive (impact = 20 J, friction = 240 N).
10.4 Oxides of 3,3′-Azobis(1,2,4,5-Tetrazine)
The oxidation of 3,3′-azobis(6-amino-1,2,4,5-tetrazine) with excess PTFA results in a complex mixture of oxides [46]. Due to the insolubility of the product, characterization was limited to elemental analysis. Overall the product was found to contain 3.5 oxygens on average. The structures of those N-oxides are displayed in Scheme 41. The nitrogen atoms most likely to be converted to N-oxide are those adjacent to the amino groups as well as one of the two nitrogen atoms in the azo group. Unfortunately, the insolubility of the material precluded 15N NMR analysis from being performed. The material displayed a gas pycnometry density of 1.88 g/cm3 and a heat of formation of 150 kcal/mol. With these data, the predicted explosive performance is 9.0 km/s and 35.9 GPa. The combustion properties of this material were determined. The material displayed exceptionally high burn rates and a low pressure dependence, two properties which are very important in propulsion applications. The burn rate was determined to be 6.6 cm/s at 14.4 MPa and the pressure dependence was found to be 0.27. At that time, it was the fastest burning material known in the literature. Because of these promising properties, the material has been studied extensively as a monopropellant in microthruster applications [48].
11 1,2,3,5-Tetrazine N-Oxides
11.1 Triazolo-1,2,3,5-Tetrazine 2-Oxide
1,2,3,5-Tetrazines are much less common than 1,2,4,5-tetrazines, and examples of energetic 1,2,3,5-tetrazine N-oxides are even more scarce. Zhou reported the synthesis of a novel energetic structure, 7-nitro-4-oxo-4,8-dihydro [1,2,4]triazolo[5,1-d] [1,2,3,5]tetrazine 2-oxide [49]. The compound was synthesized by treating 3-nitro-1-(2H-tetrazol-5-yl)-1H-1,2,4-triazol-5-amine with oleum and fuming nitric acid (Scheme 42). The proposed mechanism involves the formation of an intermediate nitramine, loss of hydrazoic acid, and subsequent condensation to form the product. The product is relatively acidic and can be converted to numerous nitrogen-based salts such as ammonium, hydroxylammonium, guanidinium, and others. The free acid was reported to be hygroscopic and its energetic and safety properties were not reported. All of the salts displayed thermal stability above 200°C, except for the hydroxylamine salt, which began to decompose at 197°C. The hydroxylamine salt also displayed the most promising properties, as it was reported to be insensitive to impact and friction, yet was predicted to be a high-performing compound (V D = 9.0 km/s, P CJ = 39.5 GPa). The high performance was in part due to the density (1.97 g/cm3) measured using gas pycnometry.
References
Vinogradov VM, Dalinger IL, Ugrak BI, Shevelev SA (1996) Mendeleev Commun 6:139–140
Zhang Y, Parrish DA, Shreeve JMJ (2012) Mater Chem 22:12659–12665
Zelenov VP, Voronin AA, Churakov AM, Strelenko YA, Tartakovsky VA (2014) Russ Chem Bull Int Ed 63:123–129
Petrie MA, Koolpe G, Malhotra R, Penwell P (2012) Office of Naval Research Grant No.: N00014-08-0894. http://www.dtic.mil/get-tr-doc/pdf?AD=ADA561743
Starchenkov IB, Andrianov VG, Mishnev AF (1997) Chem Heterocycl Compd 33:1355–1359
Thottempudi V, Yin P, Zhang J, Parrish DA, Shreeve JM (2014) Chem A Eur J 20:542–548
Bottaro JC, Petrie MA, Penwell PE (2002) Integrated high payoff rocket propulsion technology III: advanced solution propellant technology, contract number: F04611-98-C-0018, final report
Dippold AA, Klapoetke TM (2013) J Am Chem Soc 135:9931–9938
Gobel M, Karaghiosoff K, Klapotke TM, Piercey DG, Stierstorfer JJ (2010) Am Chem Soc 132:17216–17226
Klapoetke TM, Piercey DG, Stierstorfer J (2011) Chem A Eur J 17:13068–13077
Fischer D, Klapoetke TM, Piercey DG, Stierstorfer J (2013) Chem A Eur J 19:4602–4613
Fischer N, Fischer D, Klapoetke TM, Piercey DG, Stierstorfer JJ (2012) Mater Chem 22:20418–20422
Tselinski IV, Mel’nikova SF, Romanova TV (2001) Russ J Org Chem 37:430–436
Klapoetke TM, Kurz MQ, Schmid PC, Stierstorfer J (2015) Energy Mater 33:201–215
Binnikov AN, Kulikov AS, Mahkova NN, Ochinnikov OV, Pivina TS (1999) 4-Amino-3-azidocarbonyl furoxan as a universal synthon for the synthesis of energetic compounds of the furoxan series. In: 30th international annual conference of ICT, Karlsrruhe, Germany, 58/1-58/10
Sheremetev AB, Ivanova EA, Spiridonova NP, Melnikova SF, Tselinsky IV, Suponitsky KY, Antipin MY (2005) J Heterocycl Chem 42:1237–1242
Zhao F-Q, Hen P, Hu R-Z, Luo Y, Zhang Z-Z, Zhou Y-S, Yang X-W, Gao Y, Gao S-L, Shi Q-Z (2004) J Hazard Mater A 113:67
Wang J, Li J, Liang Q, Huang Y, Dong H (2008) Propellants Explos Pyrotech 33:347–352
Stepanov AI, Dashko DV, Astrat’ev AA (2012) Cent Eur J Energetic Mater 9:329–342
Wang Q (2003) Chin J Explos Propellants 3:57–59
Zhou Y, Zhang Z, Li J, Guan X, Huang X, Zhou C (2010) Chin J Org Chem 30:1044–1050
Chavez DE, Parrish DA, Leonard P (2012) Synlett 23:2126–2128
Epishina MA, Kulikov AS, Mahkova NN (2008) Russ Chem Bull Int Ed 57:644–651
Zhou Y, Xu K, Wang B, Zhang H, Qui Q, Zhao F (2012) Bull Kor Chem Soc 33:3317–3320
Astrat’ev AA, Dashko DV, Stepanov AI (2012) Cent Eur J Energetic Mater 10:1087–1094
Sheremetev AB, Yudin IL (2003) Russ Chem Bull 72:87–100
He C, Tang Y, Mitchell LA, Parrish DA, Shreeve JMJ (2016) Mater Chem A 4:8969–8973
Huynh MHV, Hiskey MA, Chavez DE, Gilardi RDJ (2005) Energy Mater 23:99–106
Zhai L, Qu X, Wang B, Bi F, Chen S, Fan X, Xie G, Qing W, Gao S (2016) ChemPlusChem. doi:10.1002/cplu.201600287
Hollins RA, Merwin LH, Nissan RA, Wilson WSJ (1996) Heterocycl Chem 33:895–904
Pagoria PF, Mitchell AR, Schmidt RD, Simpson RL, Garcia F, Forbes J, Cutting J, Lee R, Swansiger R, Hoffman DM (1998) Synthesis scale-up and experimental testing of LLM-105 (2,6-diamino-3,5-dinitro-pyrazine-1-oxide). National Defense Industrial Association, San Diego
Pagoria PF (2016) Propellants Explos Pyrotech 41:452–469
Sabatini JJ, Oyler KD (2016) Crystals 6:5. doi:10.3390/cryst6010005
Pagoria PF, Zhang MX. US20100267955
Piercey DG, Chavez DE, Scott BL, Imler GH, Parrish DA (2016) Angew Chem Int Ed 55:15315–15318
Piercey DG, Chavez DE, Heimsch S, Kirst C, Klapoetke TM, Stierstorfer J (2015) Propellants Explos Pyrotech 40:491–497
Churakov AM, Tartakovsky VA (2004) Chem Rev 104:2601–2616
Klenov MS, Churakov AM, Strelenko YA, Ananyev IV, Lyssenko KA, Tarakovsky VA (2015) Tetrahedron Lett 56:5437–5440
Voronin AA, Zelenov VP, Churakov AM, Strelenko YA, Feyanin IV, Tartakovsky VA (2014) Tetrahedron 70:3018–3022
Christe KO, Dixon DA, Vasilliu M, Wagner RI, Haiges R, Boatz JA, Ammon HL (2015) Propellants Explos Pyrotech 40:463–468
Klenov MS, Guskov AA, Anikin OV, Churakov AM, Strelenko YA, Fedyanin IV, Lyssenko KA, Tartakovsky VA (2016) Angew Chem Int Ed 55:11472–11475
Coburn MD, Buntain GA, Harris BW, Hiskey MA, Lee K-Y, Ott DG (1991) J Heterocycl Chem 28:2049
Ovchinnikov IV, Mahkova NN (2008) In: New trends in research of energetic material 2008, Pardubice, Czech Republic, pp 713–718
Chavez DE, Hiskey MAJ (1999) Energy Mater 17:357–377
Rozen S, Brand M (1986) Angew Chem Int Ed 25:554
Chavez DE, Naud DL, Hiskey MA (2004) Propellants Explos Pyrotech 29:209–215
Wei H, Gao H, Shreeve JM (2014) Chem A Eur J 20:16943–16952
Ali AN, Son SF, Hiskey MA, Naud DL (2004) J Propuls Power 20:120
Bian C, Dong X, Zhang X, Zhou Z, Li CJ (2015) Mater Chem A 3:3594–3601
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Chavez, D.E. (2017). Energetic Heterocyclic N-Oxides. In: Larionov, O. (eds) Heterocyclic N-Oxides. Topics in Heterocyclic Chemistry, vol 53. Springer, Cham. https://doi.org/10.1007/7081_2017_5
Download citation
DOI: https://doi.org/10.1007/7081_2017_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-60686-6
Online ISBN: 978-3-319-60687-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)