Abstract
Human and veterinary pharmaceuticals reach agricultural soils via crop irrigation with treated wastewater and via soil fertilising with biosolids or manure. Compelling evidences on the environmental fate of pharmaceuticals suggest that accumulation of these emerging pollutants in soil is currently a serious risk for soil quality and food security. Currently, engineered remediation methodologies to remove pharmaceuticals from soils as well as those (e.g. aerobic composting) to treat biosolids and manure are not sufficiently efficient to full removal of pharmaceuticals. Moreover, these techniques are often economically prohibitive and may cause adverse side-effects in the environment. Microbes, soil fauna (e.g. earthworms) and their interactions exert a strong control in the organic matter decomposition and nutrient cycling of soil. By taking advantage of these naturally occurring processes, we propose the use of earthworms to clean biosolids and manure (ex situ vermiremediation) and to reduce pharmaceutical bioavailability in soil (in situ vermiremediation). The impact of earthworms on soil physicochemical and biological properties together to the tolerance of these organisms to pharmaceuticals makes these bioremediation strategies viable in soils receiving pharmaceutical-contaminated amendments and water. Additionally, some studies have evidenced that earthworms (Eisenia spp.) accumulate pharmaceuticals in their tissues, thus being an advantageous biological process in the vermicomposting of biosolids and manure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
According to the US Food and Drug Administration [1], the term active pharmaceutical ingredient (API) refers to “any substance or mixture of substances intended to be used in the manufacture of a drug (medicinal) product and that, when used in the production of a drug, becomes an active ingredient of the drug product. Such substances are intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure and function of the body”. Many APIs and their metabolites are currently detected in treated (or reclaimed) wastewater, biosolids (sediments obtained from wastewater treatment plants) and animal manure. In this chapter, we will use the term API to refer to both human and veterinary pharmaceuticals.
The incomplete removal of APIs during wastewater treatment and the high use of veterinary pharmaceuticals in concentrated animal feeding operations (CAFOs) are the main reasons for detecting APIs in treated wastewater, biosolids and manure [2, 3]. In addition, pharmaceuticals’ consumption is significantly high in densely populated areas, particularly in Asian countries, thus leading to discharge API-contaminated wastewater [4]. Likewise, crop irrigation with treated wastewater and the application of biosolids and manure as soil amendments are common agricultural practices in arid and semiarid areas, where, in addition to water scarcity, soils are characterised by a low organic carbon content [5, 6]. Therefore, these agroecosystems have a high risk of contamination by APIs.
Irrigation of agricultural soils with treated wastewater is, therefore, a significant route of continual input of APIs. Indeed, some of them are named “pseudo-persistent” pollutants because of concentrations in soil keep constant via irrigation [7], despite displaying short half-life times [8]. Fertilisation with biosolids/manure is another important source of API contamination. Although APIs are generally detected in treated water [8,9,10], some studies have reported the occurrence of these pollutants in biosolids [11, 12] and manure [13]. Moreover, the application of biosolids to soil has been shown that increases the persistence of certain APIs (triclosan and triclocarban) probably because of organic matter of biosolids that decreases the API bioavailability for microbial degradation [14, 15]. Accordingly, API concentrations in the range of ng/g dry mass are detected in agricultural soils worldwide receiving this form of fertilisation [8, 9, 16], with high potential to be accumulated in edible crops [10, 13, 17].
Environmental fate of APIs largely depends on soil physicochemical and biological processes. A detailed description on physicochemical and transport processes governing API fate in soil is beyond of the scope of this chapter, but some generalisations are shown in Fig. 1. Environmental fate of APIs depends on intrinsic and extrinsic variables. The former are the physicochemical properties of the substance such as water solubility and dissociation of ionisable compound [18]. Some APIs are neutral (e.g. carbamazepine, diazepam, caffeine) and generally display a high capacity to bind to soil organic matter [7], whereas ionic pharmaceuticals (e.g. diclofenac, naproxen, ibuprofen, atorvastatin) tend to be less persistent in soil and their fate depends on soil pH. Among extrinsic variables, photodegradation, hydrolysis and biodegradation significantly contribute to API transformation and dissipation [11, 19]. Furthermore, soil properties also affect transformation and bioavailability of APIs. For example, the organic matter content of soil has a strong influence in the retention of hydrophobic APIs, therefore reducing their bioavailability and biodegradation [20].
It is now widely recognised that API accumulation in agricultural soils may be a serious threat to non-target organisms and natural resources. For example, ketoprofen, carbamazepine and caffeine were detected in groundwater samples from Europe at concentrations of 2.88, 3.60 and 4.50 μg/L, respectively [16], suggesting a high mobility of these chemicals in soil. Likewise, many studies have demonstrated that edible plants growing in API-contaminated soils accumulate and translocate APIs to aerial parts [11]. Furthermore, APIs may cause biochemical and physiological adverse effects in plants, negatively affecting their growth and development [7]. As a result, non-target organisms including human beings may be exposed to API through the consumption of contaminated edible plants. For example, bee exposure to pharmaceuticals accumulated in pollen and nectar from zucchini flowers was modelled for carbamazepine, and outcomes revealed that honeybee colonies as well as the bee behaviour could be seriously affected by moderate hydrophobic APIs [21]. Nevertheless, field surveys are still needed to draw solid conclusions about exposure levels of wildlife to API-contaminated plants.
Soil functioning is also altered by APIs. Although biodegradation is the major dissipation route [22, 23], these chemicals are able to alter soil microbial activity and community [19] and soil enzyme activities [24,25,26]. Because soil enzyme activities catalyse most chemical reactions involved in the transformation and decomposition of organic matter, and nutrient cycling [27], their alteration by APIs could lead to soil degradation. Therefore, affordable mitigating measures and remediation strategies should be taken into account to reduce the potential environmental risks of APIs. In this context, the use of earthworms emerges as a promising strategy for reducing API concentration and toxicity at the source (treatment of biosolids and manure) and in agricultural soils receiving continual input of APIs.
This chapter describes the mechanisms and technical aspects linked to earthworms’ capacity to remediate API-contaminated soils and amendments. The first section makes a brief overview of the earthworm effects on soil functioning, therefore providing insights into the importance of these organisms in API degradation (Sect. 2). The third section provides data on toxic effects of APIs in earthworms: a knowledge needed to propose these organisms as biological vectors of API biodegradation. The fourth and fifth sections consider two options for using earthworms in managing API residues: vermicomposting of organic residues such as biosolids and manure (ex situ vermiremediation) and inoculation of soils with earthworms (in situ vermiremediation). The sixth section discuss how to improve API vermiremediation by using biochar. The last section will identify knowledge gaps that require further research to boost the use of earthworms for enhancing the natural attenuation of agricultural soil against APIs and other organic pollutants.
2 Impact of Earthworms on Soil Quality
The term soil quality defines the “capacity of a specific kind of soil to function, within natural or managed ecosystem boundaries, to sustain plant and animal productivity, maintain or enhance water and air quality, and support human health and habitation” [28]. This capacity is achieved by an exquisite interplay between inherent physicochemical and biological properties and processes, which are originally defined during soil formation or pedogenesis [29]. Many exogenous factors such as land use, agrochemical inputs, global warming and introduction of exotic species, among others, alter soil quality with the risk of causing its degradation (i.e. the loss of actual or potential productivity or utility of soil as a result of natural and anthropogenic factors [30]). Current knowledge on soil biology indicates that biodiversity is a pivotal property in soil quality [31,32,33], and conventional agriculture (defined as the agricultural practices that use synthetic pesticides and fertilisers in short rotation crops [34]) seriously threats it [35]. Therefore, promotion and maintenance of soil biodiversity is determinant to boost sustainable agriculture that ensures reasonably high crop yields and food security.
Earthworms are annelids belonging to soil macrofauna (i.e. organisms of >2 mm in size [36]) and exert a profound impact on soil quality. In general, these organisms alter soil microbial and mesofauna (0.1–2 mm, body size [36]) communities with indirect effects on nutrient cycling and soil biodiversity [37]. The continuous burrowing and feeding activities of earthworms create a complex network of permanent (anecic species) and temporary (endogeic species) galleries [38], which have led these organisms to be considered as “soil engineers” [39]. In fact, earthworms have a significant contribution in soil bioturbation, i.e. “the biological reworking of soils and sediments by all kinds of organisms, including microbes, rooting plants, and burrowing animals” [40], whereby they largely affect microbial population dynamics [41] and facilitate microorganism dispersion in soil [42]. These functional capacities have led earthworms to have a particular interest in agronomy and environmental sciences because of their beneficial effects on plant growth and development [43, 44], control of soil-borne pathogens [45, 46], indirect degradation of organic pollutants [47] and buffering effect in polluted soils [48]. However, the agronomic and ecological benefits depend on feeding habits of earthworms. Soil ecologists classify earthworms into three categories according to preferred soil habitats, feeding habits and morphological traits [36, 49,50,51]: epigeic, anecic and endogeic (Table 1).
Epigeic earthworms are small-medium sized, inhabit soil surface and feeding on decomposing organic residues accumulated on the soil surface (Fig. 2). Epigeic earthworms rarely burrow into the soil and ingest it, so they are little or no exposed to organic pollutants occurring in the mineral soil. Some species of this ecological group such as Eisenia fetida, E. andrei or Lumbricus rubellus are used in the composting of municipal and industrial organic wastes (vermicomposting) [52]. Anecic earthworms are large sized and create long, permanent vertical burrows and feeding on decomposing litter that collect from the soil surface and drag into the burrow or accumulate at the entrance of the burrow, forming a deposit of litter mixed with cast named “midden” [53]. They also ingest mineral soil to obtain particulate organic matter [49]. The middens are considered hotspots of organic matter decomposition and faunal diversity [54, 55].
Functional classification of earthworms. Epigeic earthworms are litter-dwelling which feed on organic matter accumulated on the soil surface. Endogeics are geophagous earthworms which construct subhorizontal, non-permanent burrows (they refilled the burrows with casts). Anecic earthworms built long, vertical, permanent burrows and feed on litter that collect from the soil surface and drag into the burrows. These earthworms also form an accumulation of litter mixed with casts around the burrow’s entrance which is known as middens
Endogeic species are medium sized soil-dwellers and ingest large amounts of soil to obtain nutrients. Earthworms of this ecological group intensively built temporary horizontal burrows in the uppermost 10–15 cm of soil. This group is subdivided, in turn, into polyhumic, mesohumic and oligohumic endogeics, depending on the amount and quality of organic matter in soil [36]. Polyhumic endogeics are small filiform earthworms that live in the topsoil (A horizon) feeding on fine, organic matter-rich soil. Mesohumics are medium-sized endogeic earthworms that ingest soil with no selection (A and B horizon dwellers), whereas oligohumics are large-sized earthworms that live at higher depth soil (30–60 cm, B and C horizon dwellers) feeding on soil with a low amount and quality of organic matter [50, 56, 57].
3 Impact of Earthworms on Environmental Fate of Pharmaceuticals
The impact of earthworms on the environmental fate of APIs will depend on their ecological and biological traits. Many APIs are highly hydrophobic with KOW values around 3.0 [58], so exposure of epigeic earthworms to APIs will be maximum as long as these chemicals are present in biosolids and manure applied to soil, or remain adsorbed to the organic matter-rich A horizon of soil where epigeic earthworms live. However, anecic and endogeic earthworms are suitable organisms to investigate the API transport in soil because of their constant burrowing activity. The feeding behaviour of anecics means a vertical transport of APIs towards deeper soil layers, thus increasing the risk of plant exposure to these compounds via the root system. For example, some studies have reported that anecic (e.g. L. terrestris) and endogeic (e.g. A. caliginosa) earthworms facilitate translocation of water-soluble organic contaminants [59], metals [60], Ag nanoparticles [61] and microplastics [62, 63] from soil surface to deeper soil layers through the bioturbation process. Moreover, anecic earthworms could contribute to environmental fate of APIs via the following three processes: indirect microbial degradation occurring in the burrow walls and middens, vertical transport of APIs from the soil surface towards the deep soil via leaching through the burrows and via burying of API-contaminated litter, and trophic transfer of APIs to earthworm predators (e.g. birds). Past studies with pesticides [59], metals [64] and more recently with microplastics [65] also lead to hypothesise that APIs could be lixiviated by the action of earthworms. Likewise, the high microbial and mesofauna activity and diversity in burrow walls [66, 67] and middens [54, 68] make them hotspots for API biodegradation. In fact, a wide range of soil organisms such as springtails, enchytraeids, mites, nematodes and millipedes are generally found in earthworm casts and in the burrow linings [54, 66, 69].
All three ecological groups of earthworms will contribute to pollutant degradation in different ways, and the magnitude of this effect largely depends on feeding habits (litter feeders versus geophagous) and the impact on soil microorganisms, which are the major drivers of contaminant biodegradation. Furthermore, the burrow system holds a high microbial activity and biomass [67, 70], which is reflected in the higher enzyme activity of burrow walls respect to that in undisturbed soils [71,72,73]. Dissipation of APIs in earthworms’ biostructures (burrow walls, casts and middens) needs to be further explored to know the impact of both anecic and endogeic earthworms in the environmental fate of APIs in agricultural soils. Recently, Briones and Álvarez-Otero [74] reported marked differences in the cuticle and epidermis thickness of the three ecological groups of earthworms. Anecic species have thickest cuticle (4.03 ± 1.6–5.72 ± 1.7 μm, range of mean ± SD) and epidermis (42.7 ± 16.7–46.3 ± 9.7 μm) than epigeic (cuticle = 1.51 ± 0.4–3.21 ± 1.5 μm, epidermis = 24.7 ± 5.2–39.4 ± 14.5 μm) and endogeic species (cuticle = 0.46 ± 0.15–1.22 ± 0.52 μm, epidermis = 31.1 ± 7.5–38.9 ± 10.5 μm). Beside the taxonomical and ecological meaning, these species-specific differences in the tegument thickness may be relevant in ecotoxicology. Past studies using E. andrei as model already demonstrated that the uptake of organochlorine pollutants takes place across the skin and the gastrointestinal epithelium [75]. Using a three-compartment model (soil-earthworm tissue-gut content), the researchers found that the uptake of organochlorine compounds via gastrointestinal tract was a significant bioaccumulation route for highly hydrophobic chemicals (log KOW > 6) [75]. In addition, the transfer across the skin decreased as the KOW value of the organochlorine compounds increased. Probably, the mucous secretion of skin and the cuticle layer contributed to reduce the uptake of highly hydrophobic pollutants through the skin. The role of the cuticle thickness in API bioaccumulation may be supported by the data in the study by Carter et al. [76]. These researchers compared the bioconcentration factors and uptake rates of four APIs (carbamazepine, diclofenac, fluoxetine and orlistat), and found that L. terrestris had lower uptake rate constants through the skin (0.12–1.35 mL g−1 day−1) than E. fetida (1.48–4.46 mL g−1 day−1). The variation in the cuticle thickness between both species could explain this marked difference in the API uptake rates [74], although contribution of other potential variables linked to experimental procedures (temperature of incubation, soil pH, feeding habit of earthworms) should not be excluded. Indeed, bioconcentration factors and uptake rate constants of APIs largely vary with the type of soil [77].
4 Pharmaceutical Toxicity in Earthworms
There is a huge body of literature dealing with the impact of APIs on soil microorganisms [19, 78]. Alterations in microbial community structure and microbial activity as well as emergence of antibiotic-resistant microorganisms are frequently detected in soil receiving APIs [79, 80]. However, toxicity of these substances on soil macrofauna is still scarce. Most data are obtained from laboratory incubation studies (standardised toxicity testing), which being important in a regulatory context for API marketing authorization [81], the outcomes provide limited information about the real impact on soil macrofauna in an ecological context. For example, the European Medicines Agency (EMA) guidelines recommend that assessment of API adverse effects on terrestrial ecosystems should follow the standardised acute toxicity tests issued by the Organization for Economic Co-operation and Development (OECD), such as OECD 207 [82] and OECD 222 [83], or the International Organization for Standardization (ISO), such as ISO 11268-1 [84], ISO 11268-2 [85] and ISO 17512-1 [86]. The recommended earthworm species in all these tests are Eisenia fetida and E. andrei. These two species display a set of advantages for running standardised toxicity testing such as the high reproduction rate, the ease of measuring the toxicity endpoints (e.g. mortality, body mass change, reproduction rate, behaviour), the low cost of maintenance in laboratory conditions and the availability of individuals from local suppliers (e.g. fishing stores, vermiculture centres).
Toxicity testing has revealed that Eisenia species tolerate API-contaminated soils compared to other soil organisms. For example, E. fetida was used in a standardised multi-test study to identify the ecological risk assessment of the antiparasitic ivermectin [87]. The earthworm was less sensitive to ivermectin with no mortality recorded after 28 days of exposure to soil spiked with 0.47–5.71 mg/kg dry soil respect to collembolan and predatory mites. Similarly, the acute toxicity of fluazuron (an insect growth regulator used to control ticks) was evaluated using E. andrei and Folsomia candida. The acaricide was lethal to earthworms at high concentrations (14d-LC50 = 111.3 mg/kg dry soil), reduced its reproduction rate (50% decrease respect to controls) at concentrations ≥20 mg/kg, and the animals avoided soils contaminated with ≥3.0 mg/kg fluazuron [88]. Likewise, the earthworms were also less sensitive to fluazuron than collembolans. Eisenia andrei and F. candida were also used for testing the acute toxicity of the veterinary pharmaceuticals nicarbazin and monensin [89]. Nicarbazin was not toxic to both species at concentrations between 10 and 1,000 mg/kg dry soil, although monensin was lethal to earthworms (14d-LC50 = 31.6 ± 1.13 mg/kg, mean ± SD) and significantly decreased the reproduction rate of collembolans (28d-EC50 = 95.5 ± 28 mg/kg). The median lethal concentration of monensin for earthworms was similar to that reported in a previous study with E. andrei (28d-LC50 = 49.3 mg/kg dry soil) [90], although the incubation time was double than that of the study by Menezes-Oliveira et al. [89].
However, cautions must be taken when extrapolating outcomes from lab-scale toxicity testing to the field. First, the earthworm ecology and distribution should be considered in the environmental risk assessment of APIs. Eisenia fetida and E. andrei are epigeic earthworms, which mean that they live above the mineral soil surface and feed on plant litter [36]. These species rarely burrow into the soil as anecic and endogeic earthworms do, so exposure of epigeics to API-contaminated mineral soils should be lower than that for geophagous earthworm species [91]. Additionally, because agricultural soils are continually altered by tilling in successive crop seasons, Eisenia spp. are not abundant in these soils. Conversely, anecic and endogeic species are well represented in agroecosystems [92,93,94]. Second, the toxicity tests recommend the use of artificial soils (e.g. OECD soil or LUFA 2.2 soil), which obviously cannot be considered agricultural soils. A myriad of fluctuating variables of field soils may influence API degradation, bioavailability and mobility that are not considered in artificial soils, such as quantity and quality of organic matter content, microbial communities, aggregate distribution, etc. Third, the risk of species confusion in toxicity testing is another potential disturbing variable. In the case of E. fetida and E. andrei, both species can be easily confused with the risk of obtaining non accurate results. They are different species [95], with probably different responses (ecotoxicological biomarkers) to environmental pollutants [96]. Therefore, caution should be taken when using Eisenia spp. in the assessment of API toxicity. Finally, species-specific differences in earthworm sensitivity to environmental contaminants should be also considered when assessing API toxicity. For example, a meta-analysis study revealed that L. terrestris and A. caliginosa are more sensitive to pesticide toxicity than E. fetida, which questions the role of the latter for establishing environmental protection limits [97]. Indeed, earthworm species other than Eisenia spp. are now suggested as model organisms for standardised soil toxicity testing [91, 98, 99]. Therefore, despite the improvements made by EMA on the original guideline document for the environmental risk assessment of APIs [81] – discussed in Whomsley et al. [100] – the inclusion of other earthworm species highly representative of agroecosystems is not considered yet.
Earthworm biomarkers have been also included in toxicity testing as indicators of API bioavailability and to assess the potential adverse effects of APIs. For example, signs of oxidative stress (antioxidant enzyme activities and lipid peroxidation) and genotoxicity (DNA breaks) induced by chlortetracycline were observed in E. fetida incubated in antibiotic-spiked soils for 28 days, although such responses were not dose-dependent [101]. The researchers also found neither dead worms nor significant decrease in reproduction rate (number of juveniles and cocoons) at the highest antibiotic concentrations (100 and 300 mg/kg). Using the contact filter paper test (OECD 1984), McKelvie et al. [102] investigated the nuclear magnetic resonance-based metabolomic profile of E. fetida exposed for 48 days to caffeine (19.3 μg/cm2), carbamazepine (1,000 μg/cm2) and estrone (1,000 μg/cm2). These researchers found that carbamazepine and estrone caused a decrease in the concentration of certain metabolites in the whole earthworm body, although at a level of statistical significance of α = 0.1. Despite the promising potential of metabolomics to elucidate the mode of action of APIs, several questions related to tissue-specific metabolic alterations or whether the natural environment (e.g. soil or organic matter-rich substrates) can modulate the earthworm metabolite profile remain unanswered at present. Genotoxic and oxidative stress have also been evaluated in E. fetida exposed to API-spiked soils by Dong et al. [103]. DNA damage assessed by the comet assay was the only biomarker that provided a consistent dose-dependent relationship with tetracycline, chlortetracycline, and the combination of both antibiotics. The antioxidant enzymes catalase and superoxide dismutase had erratic responses to the antibiotic exposure. The low number of replicates (n = 3 earthworm/treatment) in that study could be a limiting factor in concluding whether tetracycline, and chlortetracycline are oxidative stress inducers in earthworms.
Although the primary scope of ecotoxicological biomarkers is to predict adverse effects at individual and population levels, no study reports consistent data linking sub-individual level responses (e.g. DNA damage, antioxidant enzyme responses) with adverse effects at higher levels of biological organisation. Therefore, the impact of environmentally realistic concentrations of APIs on earthworms remains to be elucidated. Moreover, the functional association between biomarker responses and API toxicity is a challenge when the mechanism of toxic action in non-target organisms as earthworms is unknown. The reader can find a detailed analysis of earthworm biomarker applications in the Chap. 10 in this book.
The range of API concentrations in ecotoxicity testing normally are unrealistic, although they could represent a worst-case scenario defined by a continue input of APIs via biosolids application or irrigation with treated wastewater, low environmental degradation rate of APIs and soils with a high organic matter content. Nevertheless, the effective API concentrations estimated from laboratory toxicity testing are generally higher than those regularly detected in agricultural soils. For example, an acute toxicity testing with 18 pharmaceuticals using E. fetida and the standard OECD artificial soil revealed that only 8 drugs were lethal to earthworms after 14 days of exposure. The 14d-LC50 values were higher than API concentrations frequently found in soil, varying between 64.8 mg/kg (ibuprofen) and 3,298 mg/kg (propranolol) [104]. Therefore, data collected from standardised toxicity tests suggest that environmentally relevant pharmaceutical concentrations in soil, defined in the context of background concentrations reported in the literature, do not represent a serious risk to Eisenia species, at least at short term. However, because these epigeic earthworms are typically used in the aerobic decomposition of solid organic waste (particularly E. andrei [105, 106]), the question arises as: are API concentrations measured in cattle manure or biosolids high enough as to be toxic to composting earthworms, so compromising the vermicomposting process?
Soil-dwelling earthworms have also been used to test API toxicity and, like with epigeic earthworms, the results point out to a certain degree of tolerance. For example, toxic effects from the antibiotics tylosin and oxytetracycline were assessed using the endogeic earthworm A. caliginosa incubated in an agricultural sandy loam soil [107]. The researchers did not find significant effects after 21 days of exposure to the antibiotic-spiked soils (500–5,000 mg/kg dry soil). Therefore, assuming a certain degree of tolerance of soil-dwelling earthworm species to APIs, we propose that inoculation of agricultural soils with earthworms could be an eco-friendly strategy to alleviate potential toxic effects of these chemicals on soil microbial activity, and to reduce the uptake of APIs by plants. The next two sections provide an overview on how earthworms may function as “bioreactors” of API degradation in the feedstocks to be used as soil amendments as well as in agricultural soils.
5 Pharmaceutical-Contaminated Soil Amendments (Ex Situ Vermiremediation)
Fertilisation of agricultural soils with biosolids and treated (or untreated) manure is one of the main routes of soil contamination with APIs. Biosolids are stabilised organic materials resulting from treatment of municipal or industrial sewage that meet regulatory guidelines for its application as a soil amendment [108]. It is now recognised that biosolids application to agricultural lands increase the concentration of APIs in soil, the risk of surface water and groundwater contamination and the uptake of API (and metabolites) by plants [109]. Furthermore, biosolids application is between 5 and 50 times greater in forest and degraded sites than in agricultural soils [109], which represent a high ecological risk for soil biodiversity and soil biological processes.
One of the environmental risks of CAFOs is the occurrence of veterinary pharmaceuticals (e.g. antibiotics) in manure [110]. The high consumption of antibiotics in CAFOs together to the fact that antibiotics are not completely metabolised by animals [111], lead to their presence in urine and manure. The most frequently antibiotics found in animal manure belong to fluoroquinolones, sulfonamides and tetracyclines [110, 111]. Concentrations of these pharmaceuticals may be so high that direct application of untreated manure to soil is discouraged or forbidden. Accordingly, manure is aerobically or anaerobically treated to reduce the risk of soil contamination by APIs and other environmental contaminants and to obtain thereby value-added organic fertilisers. The most frequent treatments are composting, anaerobic digestion and accumulation in aerobic/anaerobic open-air ponds. Among them, composting provides the most technically easy and low-cost option, but there are still uncertainties about the extent of API biodegradation during composting. Although composting generally removes >90% of APIs [111], some studies show that this technique is not efficient for the full elimination of some types of APIS. For example, 17–31% of the initial concentration of ciprofloxacin (fluoroquinolone) in swine manure was found in the resulting compost [112]. Similarly, composting of turkey litter spiked with some antibiotics led to the full removal of chlortetracycline, whereas reduction of monensin and tylosin varied between 54 and 76% of initial concentration and sulfamethazine was not removed at all [113]. It is postulated that sorption processes seem to be the most feasible elimination pathway for many APIs during composting [110, 111], thus hampering the mineralisation of these chemicals. However, most studies on composting-induced degradation of APIs do not consider the mass evolution of feedstock (e.g. formation of humic substances) during composting and the mechanisms underpinning the API degradation, so leading to inaccurate conclusions on the composting efficiency in the removal of APIs [114]. In addition, the impact of composting on API degradation has been a research topic mainly investigated at lab scale using API-spiked manures, so the aging effect has not been considered. Aging of hydrophobic organic pollutants in soil is a well-known phenomenon whereby pollutant availability and biodegradation decrease as the time that pollutants remain in soil increases [115]. A similar assumption has not been considered in composting studies of API-contaminated feedstocks where organic matter content is higher than that in agricultural soils. Likewise, complementary strategies such as vermicomposting (use of earthworms in composting of solid organic residues) have not been deeply investigated. Indeed, some benefits could be obtained with vermicomposting technology compared to aerobic composting. For example, the quality of compost, in terms of physicochemical properties, produced from green waste (trimmings and litter) was higher with vermicomposting than with composting [116]. Additionally, enzymes such as phosphatase and β-glucosidase showed a higher activity in the vermicompost than in compost, both produced from cattle manure [117]. The impact of vermicompost on soil physicochemical and biological properties was reviewed by Lim et al. [118], who concluded that vermicompost has a higher beneficial impact on plant growth and soil fertility than compost, because the former contains a larger amount of available nutrients and plant growth-stimulating substances (phytohormones), which probably degrade during the thermophilic phase of aerobic composting.
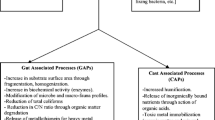
Vermicomposting is an oxidative process mainly driven by earthworms and microorganisms, whereby organic wastes are broken down and transformed into a fine and porous peat-like material named vermicompost [119]. This bio-oxidative process occurs in a mesophilic environment (<30°C) created by the continue activity of epigeic earthworms (e.g. Eisenia spp.), which aerate and facilitate heat dissipation during organic matter decomposition. Vermicomposting of organic waste has been described by Domínguez [106] in two actions: the earthworm gut-associated processes (GAPs) and the cast-associated processes (CAPs) (Fig. 3). The GAPs involve the physical break down (e.g. grinding in gizzard) and biochemical transformations of organic matter ingested by earthworms. Secretion of enzymes from the earthworm gut epithelium and exoenzymes secreted by gut symbionts provide a biochemical cocktail to decompose the organic matter [120, 121]. Nutrients are absorbed at the gut epithelium, and secretion of substances such as mucus, urea and ammonia will form the chemical composition of the egested material (casts). It is interesting to highlight that during GAPs, the initial microbial composition and activity of the ingested material change during the gastrointestinal transit [121, 122]. Some studies have reported that pathogens generally occurring in cattle manure are significantly reduced in the earthworm cast probably as a consequence of the digestive processes occurring in the gastrointestinal tract of earthworms [123, 124]. The CAPs occur in the earthworm casts, and microorganisms and other decomposer fauna (e.g. collembolan) actively participate in the further decomposition of more recalcitrant organic wastes such as lignin, cellulose and hemicellulose (maturation stage). Therefore, CAPs prolong the decomposition of the feedstock although earthworms are no longer present. Indeed, changes in the enzymatic profile, microbial composition and nutrient concentration still happen in the maturation phase (earthworm free) of vermicomposting [125, 126].
Hypothesised model on vermicomposting of active pharmaceutical ingredient (API)-contaminated feedstocks (e.g. biosolids and manure). Fate of APIs during vermicomposting may follow multiple pathways (biodegradation or immobilisation) depending on the physicochemical properties of APIs and the biological cast-associated processes (CAPs) and gut-associated processes (GAPs) occurring during vermicomposting. In CAPs, pharmaceuticals may be bound to the cuticle, cross the earthworm tegument or bound to the organic matter of fresh feedstock, casts and mucus. Likewise, microorganisms of the feedstock and casts may degrade APIs. In GAPs, ingested pharmaceuticals may be breakdown by enzymes released from both symbionts and the earthworm gut epithelium. Additionally, APIs may be co-metabolised by symbionts or cross the gut epithelium. Adapted from Sanchez-Hernandez et al. [167] with permission from Elsevier
In summary, it can be postulated that vermicomposting provides a source of microorganisms and extracellular enzymes with potential capacity for breaking down organic pollutants present in the feedstock (intrinsic remediation potential) and to remediate polluted soils when vermicompost is used as a soil amendment (extrinsic remediation potential).
Vermicomposting of biosolids and manure requires the assessment of three critical issues: (1) earthworm tolerance to APIs, (2) biodegradation of APIs and metabolites and (3) development of resistant microbial strains. For example, vermicomposting of biosolids experimentally contaminated with tetracycline revealed that the concentration of 100 mg/kg had a stimulating effect on earthworm growth and organic matter decomposition, whereas that higher concentrations (500 and 1,000 mg/kg) led to a significant decrease of the decomposition process and to the emergence of antibiotic-resistant genes, thus compromising the quality and environmental safety of the final vermicompost [127]. Similarly, degradation of oxytetracycline and its main metabolites in chicken manure mixed with shredded paper waste was monitored in a co-composting system, which consisted in a first thermophilic composting phase followed by vermicomposting. Results from that study revealed that the additional phase of vermicomposting increased the degradation of oxytetracycline and its metabolite 4-epi-oxytetracycline in the feedstock containing a C:N ratio of 40 [128]. Despite these studies, there are still many unknowns on the efficiency of vermicomposting in reducing the concentration and toxicity of APIs and their metabolites. Furthermore there is no data available on the microorganisms and enzyme activities implied in API biodegradation, so that the vermicomposting process can be externally modified to facilitate removal of APIs.
Earthworms can accumulate biosolids-bound APIs. For example, E. fetida accumulated around 20% of ciprofloxacin and 40% of azithromycin present in soils amended with anaerobically digested biosolids which were contaminated with these antibiotics [26]. Although the study suggests ecological implications of the moderate bioaccumulation of APIs by earthworms, as these organisms may introduce APIs in food webs, their bioaccumulation capacity can be also regarded as an opportunity for removing APIs during biosolids vermicomposting.
6 Pharmaceutical-Contaminated Soils (In Situ Vermiremediation)
Soil bioturbation by earthworms has been exploited as a bioremediation strategy [129]. Earthworms are able to facilitate biodegradation of organic contaminants via three processes: (1) stimulating soil microorganisms, which may co-metabolise pollutants; (2) mobilising contaminants entrapped in soil organomineral complexes, thus rendering them bioaccessible to microbial biodegradation; and (3) altering the soil physicochemical properties (e.g. pH), which may contribute to contaminant degradation. Besides these external degrading processes, the gastrointestinal tract of earthworms contributes to contaminant degradation by the action of the gut symbionts and digestive enzyme secretion [47, 130]. Many studies have shown that earthworm activity in soils contaminated by environmental pollutants such as pesticides, polycyclic aromatic hydrocarbons (PAHs) or polychlorinated biphenyls (PCBs) reduces the initial concentration of these organic pollutants [47]. However, most of these studies have been performed under controlled conditions of laboratory (microcosm), and the real impact of earthworms in soil persistence of contaminants requires field validation [131]. Nevertheless, earthworm activity may also have no effect on contaminant degradation rate. The most reliable explanation for this effect is the change in soil organic matter content and quality (humification) by earthworm activity. However, earthworms exert a positive effect on soil microbial activity and exoenzyme production even in the presence of environmental contaminants [48]. Taken together these studies suggest that inoculation of agricultural soils with earthworms could be a suitable strategy to remove or immobilise APIs, thus reducing the risk of being available to plants.
To date, remediation technology aimed to remove APIs is focused on the treatment of wastewater [2]. In soil remediation, only physical and chemical engineering systems have been tested in API-contaminated soils. For example, the electrokinetic technique, which consists of applying an electric field using two or more electrodes introduced in soil, has been used to remediate soils spiked with a mixture of sulfamethoxazole, ibuprofen, triclosan and caffeine [132]. The soil physicochemical alterations induced by the electric field, mainly on soil pH, caused a significant API degradation (13–85% of initial concentration) within 7 days of continual electrokinetic treatment (10 mA of current intensity). Among the chemical remediation methods, the use of the oxidant chemical persulfate alone or in combination with activating agents (iron), heat, alkaline chemicals or electrokinetic is widely used in the degradation of a variety of environmental contaminants such as PCBs, PAHs, pesticides, phthalates and APIs [133]. For example, ibuprofen (46–48 μM/kg soil) was fully removed from soils after a 60-min treatment with persulfate (20 mM/kg soil) activated by thermal treatment of soil (60°C) [134]. In a similar laboratory study, the antibiotic sulfamethoxazole was almost fully degraded (87.6% of initial concentration) in agricultural soils incubated for 4 h at 30°C with persulfate activated with nanoscale zero-valent iron (nZVI) nanoparticles [135]. However, persulfate-assisted remediation technologies have three main drawbacks: (1) alterations in the soil physicochemical and biological properties with potential adverse consequences to soil quality, (2) the need of external energy supply (e.g. electrokinetic technique and heating-activated persulfate treatment) and (3) the high costs associated with the application of these remediation techniques in real-field scenarios [132]. For example, remediation of ibuprofen-contaminated soils using both Fenton oxidation and nZVI nanoparticle methodologies led to toxic soils showing phytotoxicity [136].
Bioremediation of API-contaminated soils has not been extensively investigated. As with other organic pollutants, API dissipation is mainly due to microorganisms [23]. Additionally, aerobic conditions largely facilitate their degradation [137, 138]. Because anecic and endogeic earthworms continually aerate soil via the creation of burrows, they should be excellent “bioreactors” of API degradation. Table 2 summarises the main advantages and limitations of using soil-dwelling earthworms in the bioremediation of API-contaminated soils as well as some uncertainties that demand further research. The effect of earthworms on API degradation must be seen not only as a biodegradation process but also as a strategy of chemical immobilisation leading to reduce bioavailability and toxicity of these pollutants. Many studies have documented that the earthworm feeding activity and cast deposition on the soil surface and the burrow walls contribute to decrease the degradation rate of certain organic pollutants in these biostructures [71, 154]. However, because of the high organic carbon content and quality (humification) in casts and burrow linings, pollutants may result immobilised. The conceptual model in Fig. 4 explains how earthworms could participate in the bioremediation of API-contaminated soils. Such a bioremediation would consist in two complementary processes [130]: (1) external earthworm-depending inactivating processes and (2) earthworm gut-associated inactivating processes. Here, inactivating processes refer to biodegradation and immobilisation of APIs in soil, both actions rendering them unavailable to edible crops, thus reducing the risk of API exposure to consumers.
Conceptual model of in situ vermiremediation of agricultural soils contaminated by active pharmaceutical ingredients (APIs). The system exploits the feeding behaviour of anecic and endogeic earthworms to improve soil quality and reduce API uptake by plants. In addition, biochar can be co-applied with earthworms to increase immobilisation of APIs. Fate of APIs is driven by the interplay between biological processes occurring in the earthworm biostructures (sphere 1: burrow walls, casts and middens), and those occurring in the gastrointestinal tract (sphere 2: cross-sectional view of the earthworm). Arrows denote the multiple pathways of API dissipation, which include microbial biodegradation, adsorption to organic matter and biochar, breakdown by exoenzymes, bioaccumulation and metabolism (e.g. in the chloragogen tissue)
External earthworm-depending inactivating processes are mainly driven by microorganisms and mesofauna (e.g. nematodes, springtails, enchytraeids, mites and millipedes) associated with the structures created by earthworms (biostructures) such as middens, casts and the burrow system (Fig. 4). The high nutrient content of these structures boosts microbial proliferation. Moreover, the presence of cutaneous mucus (burrow linings and middens) and gastrointestinal mucus (casts and middens) also provide a C-labile source for microfauna and mesofauna foraging. Many studies have examined the organic carbon dynamic and microbial community structure of earthworm casts [139, 140], burrow linings [72, 139] and middens [54, 55, 68]. All them conclude that these biostructures are hotspots of organic matter decomposition, displaying higher microbial and enzymatic activities respect to undisturbed soil [70, 71, 141]. Therefore, it can be assumed that earthworm biostructures are also microenvironments for API biodegradation. However, because of the organic matter content of biostructures, API may also be immobilised by binding to organic ligands, thus reducing their bioavailability and transport in soil [47, 142, 143]. Extracellular enzymes or exoenzymes represent also a pivotal mechanism of API inactivation. Enzymes such as phenol oxidases (laccases) and peroxidases (manganese peroxidase and lignin peroxidase) are actively involved in the oxidative metabolism of organic contaminants including APIs [144, 145]. For example, laccase from the white-rot fungi (lignin degraders) Trametes versicolor removed 100%, 95% and 85% of diclofenac, trimethoprim and carbamazepine, respectively, from aqueous enzymatic preparations [146]. Similarly, peroxidases from multiple biological sources are also able to degrade (>80%) many APIs such as triclosan, carbamazepine, naproxen and antibiotics [145]. Many other white-rot fungi species degrade anticancer drugs via oxidative reactions catalysed by laccases and peroxidases [147]. These enzymes are produced and excreted to the environment by soil microorganisms [148], and the presence of lignocellulosic-rich organic matter induces their production [149]. Furthermore, laccase activity requires molecular oxygen, so earthworm burrowing activity should facilitate laccase-mediated degradation of organic pollutants [150] because of soil aeration increase. Therefore, API dissipation by these exoenzymes should be a potential biodegradation process, particularly in earthworm biostructures.
Earthworm gut-associated inactivating processes involve gut microbiota and the enzymes secreted by the earthworm gut epithelium (Fig. 4). Many digestive enzymes have been measured in the gastrointestinal content of earthworms such as lipases, esterases, chitinases and cellulases [151,152,153]. Furthermore, laccase activity has also been found in the gastrointestinal content of epigeic and endogeic earthworms, although its activity level is low respect to other digestive enzymes [154], an expected finding if one considers that the earthworm alimentary canal is anoxic [155] and laccases require molecular oxygen. However, laccase activity has been measured in the casts of some earthworm species [141], suggesting that microbial-mediated oxidative metabolism occurs in these biostructures. Carboxylesterases are other group of enzymes with potential to metabolise pharmaceuticals and illicit drugs containing the ester bond such as capecitabine, cilazapril, clopidogrel, cocaine, dabigatran etexilate, enalapril, heroin, imidapril, irinotecan, meperidine, methylphenidate, olmesartan, orlistat, oseltamivir, quinapril, ramipril, temocapril and trandolapril [156]. Some of these compounds are detected in reclaimed wastewater, surface water and groundwater [157, 158]. Interestingly, carboxylesterase activity has been found in the gastrointestinal tract of several earthworm species [153, 159] and in soil disturbed by earthworms [160]. However, it has not been demonstrated if the earthworm-induced carboxylesterase activity hydrolyses ester-containing APIs as mammalian carboxylesterases do [156, 161].
The persistence of exoenzymes largely depend on the organomineral complexes of soil [162]. Binding of exoenzymes to clays and organic matter protect them from physic stress (soil desiccation or high temperature) and microbial foraging [163]. With this premise, biochar technology has been proposed as an environmentally compatible approach to stabilise exoenzymes and concentrate their activity in soil for agronomic and remediating purposes [164]. The next section discusses how biochar may synergistically improve the earthworm-assisted bioremediation of contaminated soils.
7 Biochar-Improved Vermiremediation
In the last decade, biochar technology has emerged as a remediating strategy to eliminate a wide range of both organic and inorganic pollutants from water and soil [165,166,167,168]. Biochar is simply charcoal, but it is used as a soil conditioner instead of being used for energy generation [169]. This carbonaceous material is produced by pyrolysing solid organic feedstocks (e.g. manure, wood chips, pine needles, spent coffee grounds, municipal biosolids, nut shells, corncob, rice straw, switchgrass, and many others) under anoxic environment and temperatures between 250 and 700°C [169, 170]. Biochar has been used in the remediation of API-contaminated wastewater [171, 172]. Some studies even suggest that biochar may be an ideal material in filtering drinking water because of its excellent capacity to adsorb many inorganic and organic pollutants, including APIs [173]. However, the remediation capacity of biochar depends on the type of feedstock and the pyrolysis temperature which, in turn, have a strong influence on the physicochemical and structural properties of biochar [174]. Pyrolysis temperatures above 450°C generally produce biochar suitable to be used in bioremediation of contaminated soils because of its higher specific surface area, open porosity, alkalinity, hydrophobicity, density of aromatic groups and lower oxygenated functional groups on the surface compared to biochar produced at temperatures below 450°C [175]. For example, wheat straw-derived biochar produced at 700°C had a higher adsorption capacity for ketoprofen, atenolol and carbamazepine than biochar produced at 300°C [176]; a marked difference in the specific surface area between both biochars explained the biochar-specific adsorption of these APIs (605 m2/g for 700°C-biochar versus 6.47 m2/g for 300°C-biochar). Moreover, physicochemical properties of biochar other than the specific surface area seem to be involved in API adsorption. For instance, a laboratory study that compared the sorption behaviour of sulfamethoxazole in eight types of biochar (bamboo, Brazilian pepper wood, sugarcane bagasse and hickory wood, produced at both 450 and 600°C) evidenced that only the biochars derived from sugarcane bagasse and bamboo at 450°C had the highest capacity for retaining sulfamethoxazole [177]. This high sorption ability was corroborated in soil column tests (2% w/w biochar), which led to propose those biochars as soil amendments to reduce API leaching potential. Researchers of that study also postulated that the occurrence of functional groups on the biochar surface would explain the high sorption capacity of the biochars produced at 450°C.
The pH is another environmental variable that facilitates API sorption onto biochar surface. Sorption of triclosan and ibuprofen significantly increased in solution of pH between 4.0 and 7.0 [178]. Furthermore, the occurrence of humic substances in the aqueous phase reduced the sorption of APIs to biochar because of two reasons: the binding of APIs to the dissolved humic substances and/or blockage of the open pores of biochar by humic substances, thus hampering the interaction between biochar and APIs [178]. These observations suggest that in alkaline soils or soil with a high organic matter content, biochar may fail in its capacity of binding APIs. Despite these interfering factors, what it seems clear is that pH <7.0 favours adsorption of APIs to biochar surface, irrespectively of the soil type [179].
Biochars produced at low pyrolysis temperatures (<450°C) are more appropriated for soil fertilisation. They generally contain non-pyrolysed organic matter susceptible to be foraged by soil microorganisms; therefore its application causes an increase of soil microbial activity and biomass [175]. This type of biochars has a low specific surface area and porosity, which reduces its capacity to retain agrochemicals such as herbicides [180], therefore not compromising the agronomic purpose of pesticide treatment [181].
The scope of adding biochar to API-contaminated soils is decreasing API bioavailability and toxicity to plants. Indeed, bioaccumulation of APIs by plants is substantially reduced in biochar-amended soils. For example, the application of biochar produced at 700°C to soil (5% w/w) reduced a 86% and 63% the uptake of 5 and 50 mg/kg sulfamethazine, respectively, by lettuce (Lactuca sativa) [182]. Similarly, carbamazepine and propranolol concentrations were markedly lower in Lolium perenne grown in API-spiked soils amended with biochar produced at 450–520°C than plants grown in biochar-free, API-spiked soils [183]. However, the adsorption of APIs on the biochar surface could have two side-effects: (1) an enhanced toxicity on soil microorganisms because of progressive accumulation of APIs on the biochar surface [177] and (2) the failure of API biodegradation because of limited bioaccessibility for microbial degradation [80]. One strategy that could partially solve these biochar-linked side-effects could be the co-application of earthworms and biochar.
Past studies have reported no clear synergistic effects from co-application of earthworms and biochar on soil microbial communities [184] or soil enzyme activities and plant growth [185]. However, a recent investigation evidenced beneficial effects of the co-application of A. caliginosa and willow chip-derived biochar on the abundance of springtails and soil fungal biomass after 6 months of incubation (1% w/w biochar in 2.65 L of soil holding 4 adult earthworms), although such positive interactions depended on the soil type [186]. Moreover, some studies have shown that incubation of earthworms (L. terrestris and A. caliginosa) in the presence of pine needle- or spent coffee ground-derived biochar caused a significant increase of soil extracellular enzymes linked to C-, P-, and S-cycling, which were bound onto biochar surface [187]. The earthworm mucus produced by the skin mucous cells and the gastrointestinal epithelium was postulated as the main mechanism of enzymatic activation of biochar [73]. The functional system created by the co-application of earthworms and biochar was proposed as a strategy for removing organic pollutants from contaminated soils and feedstocks [164]. We propose an identical model for the in situ degradation or immobilisation of APIs in agricultural soils (Fig. 4). Whether or not this bioremediation strategy is viable will depend mainly on the following variables, which require further investigation:
-
1.
Earthworm species and exotic species. Figure 2 illustrates the feeding strategies of epigeic, anecic and endogeic earthworms. Both anecic and endogeic species are soil engineering organisms because of their intensive burrowing activity [39]. Moreover, some laboratory experiments have shown that anecic and endogeic species can co-exist in a limited volume of soil. For example, the burrowing activity of L. terrestris was not affected by the presence of A. caliginosa, although the depth of the burrow system created by the anecic species was shorter than the burrow structure created when the species was incubated alone [188]. Moreover, the burrowing activity of A. caliginosa was favoured by the presence of the anecic earthworm Aporrectodea giardi; the organic matter-rich walls of the burrows created by A. giardi served as a food source to A. caliginosa [188]. These examples suggest that co-application of earthworms of different ecological strategies to soils contaminated with APIs could be the best option for obtaining the maximal benefit from earthworm activity on API dissipation. Environmental fate of APIs should be, therefore, investigated in soils holding a wide representation of the most common earthworms found in agricultural soils [92,93,94], ideally covering the three ecological groups of earthworms (Fig. 2). In our model of in situ vermiremediation, particular concern should be put on the introduction of earthworm exotic species (the term refers to not naturally occurring species, so-called alien species, in the location in which it is found [189]). Indeed, one of the objectives of the United Nations Sustainable Development Goal no. 15 (Life on land, www.undp.org) is “to prevent the introduction and significantly reduce the impact of invasive alien species on land and water ecosystems...”. Therefore, care must be taken when we chose in situ vermiremediation. Endogenous and exogenous features of earthworms such as feeding behaviour (epigeic, endogeic and anecic), tolerance to environmental changes (phenotypic plasticity), reproductive characteristics, morphological characteristics and locomotion as well as environmental variables (edaphic and climatic conditions, presence of predators, and substantial and continue surface litter layers, among others) are important invasiveness traits to be considered before adding earthworms to agricultural soils [70].
-
2.
Earthworm tolerance to biochar. Many studies have investigated the potential toxicity of biochar upon earthworms. Doses of biochar ≤2.0% (w/w) generally are tolerated by different earthworm species as indicated by the absence of significant avoidance response to biochar-amended soils [190]. However, signs of oxidative stress are frequently found at those biochar doses in E. fetida [191] and L. terrestris [192], although some studies have reported no oxidative damage in E. fetida exposed at doses of biochar >2% [193]. Despite these contrasting results, further research is still needed to know long-term effects of earthworm inhabiting biochar-amended soils. For example, a 6-month mesocosm study with A. caliginosa incubated in two different soils evidenced that the synergistic effects of earthworms and biochar (1% w/w) increased the abundance of other soil organisms such as springtails and fungi, beside to improve soil fertility and plant growth [186]. Similarly, a 2-year field experiment examined the impact of biochar applied on topsoil (10 cm depth) at application rates of 10, 25 and 50 t/ha (corresponding to 0.6, 1.5 and 3% w/w, respectively) on both soil macrofauna and mesofauna [194]. The study revealed that, although the abundance of earthworms decreased as the concentration of biochar increased, biochar did not cause a significant impact on earthworm community structure, and the dose of 0.6% did not alter earthworm species richness compared to that of control (biochar-free) soils. Conversely, it was found a significant increase in the abundance of enchytraeids, mites and collembolans at the highest doses of biochar. In other field study, researchers observed that biochar applied at 5 and 10 t/ha was no toxic to macrofauna and also caused an attraction effect to earthworms after 2 years of application [195], thus recording a twofold density of earthworms in the soils that received 10 t/ha biochar respect to control (biochar-free) soils. Factors such as type of biochar and application rate, type of soil, climatic conditions, time of exposure and microbial community generally modulate the earthworm response to biochar-amended soils. Taken together these studies encourage biochar application rates of around 1% (w/w) on topsoil to be compatible with fauna diversity and abundance, and to exploit the potential synergistic effects of earthworms and biochar to immobilise or degrade APIs.
-
3.
Pharmaceutical toxicity and accumulation in earthworms. To date, most of toxicity tests with APIs have been performed using E. fetida and E. andrei as model organisms (discussed in Sect. 4 of the chapter), and data show that these earthworm species tolerate high API concentrations compared with other soil organisms (e.g. [87]). Therefore, the use of epigeic earthworms in the vermicomposting of API-contaminated feedstocks could be a workable strategy. However, the sensitivity of anecic and endogeic earthworms (Fig. 2) to APIs should be explored in order to apply them in the in situ vermiremediation strategy (Fig. 4). In addition, API toxicity has been generally evaluated using a single chemical, and API mixture or even API molecules mixed with other environmental contaminants commonly detected in agricultural soils have not been investigated. As discussed in previous sections, a wide variety of APIs is generally found in reclaimed wastewater and biosolids, so exposure of soil fauna to an API mixture is probably the most real scenario. Similarly, API biodegradation should be also studied in the context of multiple environmental contaminants co-existing in agricultural soil.
-
4.
Toxicity of API metabolites. Biodegradation of APIs in soil not necessarily lead to full mineralization. For example, a laboratory study reported that mineralisation of triclosan (1, 10 and 100 mg/kg) in soils varied between 5.8 and 6.5% (cumulative recovery of 14CO2) over a period of 42 days [80]. The finding suggests that metabolites may persist in soil with potential toxicity on soil organisms and soil function. For example, triclosan is photochemically decomposed into the toxic metabolites 2,8-dichlorodibenzo-p-dioxin (2,8-DCDD) and 2,4-dichlorophenol (2,4-DCP), which are very unstable in aqueous solutions [196], but their organic carbon-adsorption coefficients (KOC) suggest a high affinity for the soil organic matter (log KOC = 3.2 for 2,8-DCDD and log KOC = 2.8 for 2,4-DCP; estimated values generated using the EPISuite™ software, USEPA, www.chemspider.com).
-
5.
Synergistic effects of APIs and other environmental contaminants. A vast variety of organic and inorganic pollutants may occur in agricultural soils. For example, PAHs, PCBs, polybrominated diphenyl ethers and phthalates are frequently detected in agricultural soils irrigated with reclaimed wastewater or fertilised with biosolids or municipal composts [197,198,199,200]. Additionally, chemical control of agricultural pests may lead to accumulation of pesticides in soil. Therefore, toxic effects and degradation of APIs should be investigated in a context of pollutant mixture, which is the most realistic scenario in the agroecosystem. Furthermore, the high capacity of biochar to retain environmental pollutants, including APIs [176, 201], may result in toxic biochar at long term because of high concentrations of pollutants onto its surface. Therefore, this concern must be clarified in detail to know whether biochar could behave as a secondary source of soil pollution under specific soil conditions (e.g. changes in pH, moisture or biodiversity).
-
6.
Life cycle assessment for earthworm-biochar bioremediation technology. Life cycle assessment (LCA) consists of a set of standardised and robust tools for appraising the efficiencies of methodologies and processes aimed to attend the decision-making related to environment protection and efficiency of the process (ISO14040:2006, ISO 2006). In the case of bioremediation of contaminated sites, LCA has been used to identify adverse impacts from the application of remediation strategies and consequently to take alternative remediation actions [202]. LCA can be used before initiating the remediation action (predictive) to select the best option according to technical, economic and environmental variables or when the remediation action is completed (prospective LCA). In the latter case, the scope of LCA is to know the environmental impacts derived from the applied remediation technology. For example, an LCA study of systems for biochar production revealed that some issues such as costs related to the pyrolysis process as well as feedstock selection, management and transportation hampered the economic viability of biochar technology, therefore compromising its affordability as a strategy for climate change mitigation [203]. The systematic review by Matustík et al. [204] on LCA of biochar technology evidenced that although the application of biochar to agricultural soils provides important environmental and economic benefits, there are still some issues that require further understanding and improvements such as the mechanisms underpinning the biochar effects on soil quality and crop yield and the use of low-tech pyrolysis systems (e.g. Kon-tiki flame curtain kilns [205, 206]) accessible to small-scale rural farming. A detailed step-by-step description of LCA is beyond the scope of this chapter but can be found in the handbook by Hauschild et al. [207], and several reviews [202, 208] in which cases study are discussed.
8 Conclusions
Crops need healthy soils, but their fertility is under permanent threat of degradation by multiple environmental stressors (e.g. high agrochemical input, nutrient imbalance, loss of soil biodiversity, salinisation and decrease of organic matter). Additionally, water consumption for crop irrigation is a serious challenge in the coming years because of the global climate change, particularly in areas of arid and semiarid climates. The use of by-products derived from wastewater treatment plants such as biosolids and treated wastewater seems an affordable solution to alleviate the water and organic matter demands in the agriculture. However, both biosolids and treated wastewater contain significant amounts of APIs that pose a serious threat to soil functioning and human health.
One of the strategies for removal APIs at the source or in agricultural soils is the vermiremediation (i.e. use of earthworms to remove environmental pollutants). Earthworms provide multiple ecosystem benefits, from improve soil quality and fertility up to be used in the recycling of solid organic waste (vermicomposting). All these ecosystem services require the intervention of microorganisms. Indeed, microbes, earthworms and their interactions are proposed as a vermiremediation strategy to remove APIs. Many ecotoxicological studies with earthworms indicate that these organisms may contribute to contaminant degradation by stimulating microbial degraders, or may reduce contaminant mobility and bioavailability by facilitating sorption of contaminants to soil organic-mineral complexes. Likewise, certain earthworm species (epigeic earthworms) are commonly used in the aerobic composting of solid organic residues to produce organic fertilisers (vermicompost). Data in the literature reveal that vermicomposting may be also a viable strategy for removing organic contaminants occurring in raw materials such as biosolids and manure. Based on this knowledge, we propose two bioremediation strategies to reduce the risk of API uptake by plants and the potential adverse effects on soil microorganisms. The first system consists of vermicomposting of API-contaminated biosolids and manure (ex situ vermiremediation), whereas the second one involves the inoculation of agricultural soils with earthworms (in situ vermiremediation). In the last decade, biochar has emerged as an eco-friendly strategy for fighting against soil pollution. Because recent studies indicate that the co-application of earthworms and biochar improve soil quality in terms of microbial proliferation and soil detoxification, the in situ vermiremediation system considers also the synergistic effects of soil-dwelling earthworms and biochar in the removal or immobilisation of APIs. Main advantages, drawbacks and uncertainties in the use of earthworms in API inactivation are summarised in Table 2 in an attempt to encourage future research in this field of bioremediation.
References
ICH (2016) Good manufacturing practice guide for active pharmaceutical ingredients: guidance for industry. U.S. Department of Health and Human Services Food and Drug Administration. Revision 1, 52 p
Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 473-474:619–641
Bartelt-Hunt S, Snow DD, Damon-Powell T, Miesbach D (2011) Occurrence of steroid hormones and antibiotics in shallow groundwater impacted by livestock waste control facilities. J Contam Hydrol 123:94–103
Jameel Y, Valle D, Kay P (2020) Spatial variation in the detection rates of frequently studied pharmaceuticals in Asian, European and north American rivers. Sci Total Environ 724:137947
Aguilera E, Díaz-Gaona C, García-Laureano R, Reyes-Palomo C, Guzmán GI, Ortolani L, Sánchez-Rodríguez M, Rodríguez-Estévez V (2020) Agroecology for adaptation to climate change and resource depletion in the Mediterranean region. A review. Agr Syst 181:102809
Jiménez-de-Santiago DE, Lidón A, Bosch-Serra ÀD (2019) Soil water dynamics in a rainfed mediterranean agricultural system. Water 11:799
Fu Q, Malchi T, Carter LJ, Li H, Gan J, Chefetz B (2019) Pharmaceutical and personal care products: from wastewater treatment into agro-food systems. Environ Sci Technol 53:14083–14090
Qin Q, Chen X, Zhuang J (2015) The fate and impact of pharmaceuticals and personal care products in agricultural soils irrigated with reclaimed water. Crit Rev Environ Sci Technol 45:1379–1408
Kinney CA, Furlong ET, Werner SL, Cahill JD (2006) Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ Toxicol Chem 25:317–326
Calderón-Preciado D, Jiménez-Cartagena C, Matamoros V, Bayona JM (2011) Screening of 47 organic microcontaminants in agricultural irrigation waters and their soil loading. Water Res 45:221–231
Wu X, Dodgen LK, Conkle JL, Gan J (2015) Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: a review. Sci Total Environ 536:655–666
Kinney CA, Furlong ET, Zaugg SD, Burkhard MR, Werner SL, Cahill JD, Jorgensen GR (2006) Survey of organic wastewater contaminants in biosolids destined for land application. Environ Sci Technol 40:7207–7215
Hu X, Zhou Q, Luo Y (2010) Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ Pollut 158:2992–2998
Fu Q, Sanganyado E, Ye Q, Gan J (2016) Meta-analysis of biosolid effects on persistence of triclosan and triclocarban in soil. Environ Pollut 210:137–144
Walters E, McClellan K, Halden RU (2010) Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Res 44:6011–6020
Li WC (2014) Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut 187:193–201
Carter LJ, Harris E, Williams M, Ryan JJ, Kookana RS, Boxall AB (2014) Fate and uptake of pharmaceuticals in soil-plant systems. J Agric Food Chem 62:816–825
Thiele-Bruhn S (2003) Pharmaceutical antibiotic compounds in soils – a review. J Plant Nutr Soil Sci 166:145–167
Barra Caracciolo A, Topp E, Grenni P (2015) Pharmaceuticals in the environment: biodegradation and effects on natural microbial communities. A review. J Pharm Biomed Anal 106:25–36
Pullagurala VLR, Rawat S, Adisa IO, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL (2018) Plant uptake and translocation of contaminants of emerging concern in soil. Sci Total Environ 636:1585–1596
Carter LJ, Agatz A, Kumar A, Williams M (2020) Translocation of pharmaceuticals from wastewater into beehives. Environ Int 134:105248
Grossberger A, Hadar Y, Borch T, Chefetz B (2014) Biodegradability of pharmaceutical compounds in agricultural soils irrigated with treated wastewater. Environ Pollut 185:168–177
Thelusmond JR, Kawka E, Strathmann TJ, Cupples AM (2018) Diclofenac, carbamazepine and triclocarban biodegradation in agricultural soils and the microorganisms and metabolic pathways affected. Sci Total Environ 640-641:1393–1410
Cycoń M, Borymski S, Żołnierczyk B, Piotrowska-Seget Z (2016) Variable effects of non-steroidal anti-inflammatory drugs (NSAIDs) on selected biochemical processes mediated by soil microorganisms. Front Microbiol 7:1969
Molaei A, Lakzian A, Datta R, Haghnia G, Astaraei A, Rasouli-Sadaghiani M, Ceccherini MT (2017) Impact of chlortetracycline and sulfapyridine antibiotics on soil enzyme activities. Int Agrophys 31:499–505
Sidhu H, O’Connor G, Ogram A, Kumar K (2019) Bioavailability of biosolids-borne ciprofloxacin and azithromycin to terrestrial organisms: microbial toxicity and earthworm responses. Sci Total Environ 650:18–26
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234
Karlen DL, Ditzler CA, Andrews SS (2003) Soil quality: why and how. Geoderma 114:145–156
Karlen DL, Andrews SS, Doran JW (2001) Soil quality: current concepts and applications. Adv Agron 74
Lal R (1997) Degradation and resilience of soils. Philos Trans R Soc Lond B Biol Sci 352:997–1010
Bender SF, Wagg C, van der Heijden MGA (2016) An underground revolution: biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol Evol 31:440–452
Nielsen UN, Wall DH, Six J (2015) Soil biodiversity and the environment. Ann Rev Environ Resour 40:63–90
Wagg C, Bender SF, Widmer F, van der Heijden MG (2014) Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci U S A 111:5266–5270
Shennan C, Krupnik TJ, Baird G, Cohen H, Forbush K, Lovell RJ, Olimpi E (2017) Organic and conventional agriculture: a useful framing? Ann Rev Environ Res 42:317–346
Tsiafouli MA, Thébault E, Sgardelis SP, de Ruiter PC, van der Putten WH, Birkhofer K, Hemerik L, de Vries FT, Bardgett RD, Brady MV, Bjornlund L, Jørgensen HB, Christensen S, Hertefeldt TD, Hotes S, Gera Hol WH, Frouz J, Liiri M, Mortimer SR, Setälä H, Tzanopoulos J, Uteseny K, Pižl V, Stary J, Wolters V, Hedlund K (2015) Intensive agriculture reduces soil biodiversity across Europe. Glob Chang Biol 21:973–985
Briones MJI (2014) Soil fauna and soil functions: a jigsaw puzzle. Front Environ Sci 2. https://doi.org/10.3389/fenvs.2014.00007
Liu T, Chen X, Gong X, Lubbers IM, Jiang Y, Feng W, Li X, Whalen JK, Bonkowski M, Griffiths BS, Hu F, Liu M (2019) Earthworms coordinate soil biota to improve multiple ecosystem functions. Curr Biol 29:3420–3429.e5
Capowiez Y, Sammartino S, Michel E (2014) Burrow systems of endogeic earthworms: effects of earthworm abundance and consequences for soil water infiltration. Pedobiologia 57:303–309
Jouquet P, Dauber J, Lagerlöf J, Lavelle P, Lepage M (2006) Soil invertebrates as ecosystem engineers: intended and accidental effects on soil and feedback loops. Appl Soil Ecol 32:153–164
Meysman FJR, Middelburg JJ, Heip CHR (2006) Bioturbation: a fresh look at Darwin’s last idea. Trends Ecol Evol 21:688–695
Medina-Sauza RM, Álvarez-Jiménez M, Delhal A, Reverchon F, Blouin M, Guerrero-Analco JA, Cerdán CR, Guevara R, Villain L, Barois I (2019) Earthworms building up soil microbiota, a review. Front Environ Sci 7. https://doi.org/10.3389/fenvs.2019.00081
Yang P, van Elsas JD (2018) Mechanisms and ecological implications of the movement of bacteria in soil. App Soil Ecol 129:112–120
Scheu S (2003) Effects of earthworms on plant growth: patterns and perspectives. Pedobiologia 47:846–856
van Groenigen JW, Lubbers IM, Vos HM, Brown GG, De Deyn GB, van Groenigen KJ (2014) Earthworms increase plant production: a meta-analysis. Sci Rep 4:6365
Oldenburg E, Kramer S, Schrader S, Weinert J (2008) Impact of the earthworm Lumbricus terrestris on the degradation of Fusarium-infected and deoxynivalenol-contaminated wheat straw. Soil Biol Biochem 40:3049–3053
Wolfarth F, Schrader S, Oldenburg E, Weinert J (2011) Contribution of the endogeic earthworm species Aporrectodea caliginosa to the degradation of deoxynivalenol and Fusarium biomass in wheat straw. Mycotoxin Res 27:215–220
Rodriguez-Campos J, Dendooven L, Alvarez-Bernal D, Contreras-Ramos SM (2014) Potential of earthworms to accelerate removal of organic contaminants from soil: a review. Appl Soil Ecol 79:10–25
Sanchez-Hernandez JC, Notario Del Pino J, Capowiez Y, Mazzia C, Rault M (2018) Soil enzyme dynamics in chlorpyrifos-treated soils under the influence of earthworms. Sci Total Environ 612:1407–1416
Brown GG, Barois I, Lavelle P (2000) Regulation of soil organic matter dynamics and microbial activity in the drilosphere and the role of interactions with other edaphic functional domains. Eur J Soil Biol 36:177–198
Orgiazzi A, Bardgett RD, Barrios E, Behan-Pelletier V, Briones MJI, Chotte J-L, De Deyn GB, Eggleton P, Fierer N, Fraser T, Hedlund K, Jeffery S, Johnson NC, Jones A, Kandeler E, Kaneko N, Lavelle P, Lemanceau P, Miko L, Montanarella L, Moreira FMS, Ramirez KS, Scheu S, Singh BK, Six J, van der Putten WH, Wall DH (2016) Global soil biodiversity atlas. European Commission, Publications Office of the European Union, Luxembourg
Fierer N (2019) Earthworms’ place on earth. Science 366:425–426
Edwards CA, Arancon NQ, Sherman RL (2011) Vermiculture technology: earthworms, organic wastes, and environmental management. CRC Press, Boca Raton
Brown GG (1995) How do earthworms affect microfloral and faunal community diversity. Plant and Soil 170:209–231
Stroud JL, Irons DE, Carter JE, Watts CW, Murray PJ, Norris SL, Whitmore AP (2016) Lumbricus terrestris middens are biological and chemical hotspots in a minimum tillage arable ecosystem. Appl Soil Ecol 105:31–35
Nuutinen V, Butt KR, Hyväluoma J, Ketoja E, Mikola J (2017) Soil faunal and structural responses to the settlement of a semi-sedentary earthworm Lumbricus terrestris in an arable clay field. Soil Biol Biochem 115:285–296
Lavelle P, Barois I, Blanchart E, Brown G, Brussaard L, Decaëns T, Fragoso C, Jimenez JJ, Kajondo K, Martínez MA, Moreno A, Pashanasi B, Senapati B, Villenave C (1998) Earthworms as a resource in tropical agroecosystems. Nat Res 34:26–41
Römbke J, Jänsch S, Didden W (2005) The use of earthworms in ecological soil classification and assessment concepts. Ecotoxicol Environ Saf 62:249–265
Kinney CA, Furlong ET, Kolpin DW, Burkhardt MR, Zaugg SD, Werner SL, Bossio JP, Benotti MJ (2008) Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environ Sci Technol 42:1863–1870
Sigua GC, Isensee AR, Sadeghi AM, Im GJ (1995) Distribution and transport of atrazine as influenced by surface cultivation, earthworm population and rainfall pattern. Chemosphere 31:4237–4242
Covey AK, Furbish DJ, Savage KS (2010) Earthworms as agents for arsenic transport and transformation in roxarsone-impacted soil mesocosms: a μXANES and modelling study. Geoderma 156:99–111
Baccaro M, Harrison S, van den Berg H, Sloot L, Hermans D, Cornelis G, van Gestel CAM, van den Brink NW (2019) Bioturbation of Ag2S-NPs in soil columns by earthworms. Environ Pollut 252:155–162
Rillig MC, Ziersch L, Hempel S (2017) Microplastic transport in soil by earthworms. Sci Rep 7:1362
Zhang L, Sintim HY, Bary AI, Hayes DG, Wadsworth LC, Anunciado MB, Flury M (2018) Interaction of Lumbricus terrestris with macroscopic polyethylene and biodegradable plastic mulch. Sci Total Environ 635:1600–1608
Zorn MI, Van Gestel CAM, Eijsackers H (2005) The effect of Lumbricus rubellus and Lumbricus terrestris on zinc distribution and availability in artificial soil columns. Biol Fertil Soils 41:212–215
Yu M, van der Ploeg M, Lwanga EH, Yang X, Zhang S, Ma X, Ritsema CJ, Geissen V (2019) Leaching of microplastics by preferential flow in earthworm (Lumbricus terrestris) burrows. Environ Chem 16:31
Andriuzzi WS, Ngo P-T, Geisen S, Keith AM, Dumack K, Bolger T, Bonkowski M, Brussaard L, Faber JH, Chabbi A (2016) Organic matter composition and the protist and nematode communities around anecic earthworm burrows. Biol Fertil Soils 52:91–100
Hoang DTT, Pausch J, Razavi BS, Kuzyakova I, Banfield CC, Kuzyakov Y (2016) Hotspots of microbial activity induced by earthworm burrows, old root channels, and their combination in subsoil. Biol Fertil Soils 52:1105–1119
Aira M, McNamara NP, Piearce TG, Domínguez J (2009) Microbial communities of Lumbricus terrestris L. middens: structure, activity, and changes through time in relation to earthworm presence. J Soil Sediment 9:54–61
Tiunov AV, Bonkowski M, Tiunov JA, Scheu S (2001) Microflora, Protozoa and Nematoda in Lumbricus terrestris burrow walls: a laboratory experiment. Pedobiologia 45:46–60
Stromberger ME, Keith AM, Schmidt O (2012) Distinct microbial and faunal communities and translocated carbon in Lumbricus terrestris drilospheres. Soil Biol Biochem 46:155–162
Hoang DTT, Razavi BS, Kuzyakov Y, Blagodatskaya E (2016) Earthworm burrows: kinetics and spatial distribution of enzymes of C-, N- and P-cycles. Soil Biol Biochem 99:94–103
Athmann M, Kautz T, Banfield C, Bauke S, Hoang DTT, Lüsebrink M, Pausch J, Amelung W, Kuzyakov Y, Köpke U (2017) Six months of L. terrestris L. activity in root-formed biopores increases nutrient availability, microbial biomass and enzyme activity. Appl Soil Ecol 120:135–142
Sanchez-Hernandez JC, Cares XA, Pérez MA, del Pino JN (2019) Biochar increases pesticide-detoxifying carboxylesterases along earthworm burrows. Sci Total Environ 667:761–768
Briones MJI, Álvarez-Otero R (2018) Body wall thickness as a potential functional trait for assigning earthworm species to ecological categories. Pedobiologia 67:26–34
Jager T, Fleuren RHLJ, Hogendoorn EA, de Korte G (2003) Elucidating the routes of exposure for organic chemicals in the earthworm, Eisenia andrei (Oligochaeta). Environ Sci Technol 37:3399–3404
Carter LJ, Ryan JJ, Boxall AB (2016) Does uptake of pharmaceuticals vary across earthworm species. Bull Environ Contam Toxicol 97:316–322
Carter LJ, Ryan JJ, Boxall ABA (2016) Effects of soil properties on the uptake of pharmaceuticals into earthworms. Environ Pollut 213:922–931
Cizmas L, Sharma VK, Gray CM, McDonald TJ (2015) Pharmaceuticals and personal care products in waters: occurrence, toxicity, and risk. Environ Chem Lett 13:381–394
Thelusmond JR, Strathmann TJ, Cupples AM (2019) Carbamazepine, triclocarban and triclosan biodegradation and the phylotypes and functional genes associated with xenobiotic degradation in four agricultural soils. Sci Total Environ 657:1138–1149
Phandanouvong-Lozano V, Sun W, Sanders JM, Hay AG (2018) Biochar does not attenuate triclosan’s impact on soil bacterial communities. Chemosphere 213:215–225
EMA EU (2006) Guideline on the environmental risk assessment of medicinal products for human use. EMEA/CHMP/SWP/4447/00 Committee for Medicinal Products for Human Use (CHMP), London, UK
OECD (1984) OECD guideline for testing of chemicals: earthworm, acute toxicity test. Guideline for testing chemicals no. 207. Paris, France
OECD (2016) OECD guideline for the testing of chemicals: earthworm reproduction test (Eisenia fetida/Eisenia andrei). Guideline for testing chemicals no. 222. Paris, France
ISO (2012) Soil quality – effects of pollutants on earthworms – part 1: determination of acute toxicity to Eisenia fetida/Eisenia andrei. ISO 11268-1. Geneva, Switzerland
ISO (2012) Soil quality – effects of pollutants on earthworms – part 2: determination of effects on reproduction of Eisenia fetida/Eisenia andrei. ISO 11268-2. Geneva, Switzerland
ISO (2008) Soil quality – avoidance test for determining the quality of soils and effects of chemicals on behaviour – part 1: test with earthworms (Eisenia fetida and Eisenia andrei). ISO 17512–1.Geneva, Switzerland
Römbke J, Krogh KA, Moser T, Scheffczyk A, Liebig M (2010) Effects of the veterinary pharmaceutical ivermectin on soil invertebrates in laboratory tests. Arch Environ Contam Toxicol 58:332–340
Alves PRL, Bandeira FO, Giraldi M, Presotto R, Segat JC, Cardoso EJBN, Baretta D (2019) Ecotoxicological assessment of Fluazuron: effects on Folsomia candida and Eisenia andrei. Environ Sci Pollut Res Int 26:5842–5850
Menezes-Oliveira V, Loureiro S, Amorim MJB, Wrona F, Soares AMVM (2018) Hazard assessment of the veterinary pharmaceuticals monensin and nicarbazin using a soil test battery. Environ Toxicol Chem 37:3145–3153
Zižek S, Hrženjak R, Kalcher GT, Srimpf K, Semrov N, Zidar P (2011) Does monensin in chicken manure from poultry farms pose a threat to soil invertebrates. Chemosphere 83:517–523
Lopes Alves PR, Niemeyer JC, Nogueira Cardoso JB (2017) The use of non-standardized invertebrates in soil ecotoxicology. In: Larramendy ML (ed) Ecotoxicology and genotoxicology: non-traditional terrestrial models, vol 32. The Royal Society of Chemistry, Cambridge, pp 3–30
Whalen JK, Fox CA (2006) Diversity of lumbricid earthworms in temperate agroecosystems. In: Benckiser G, Schnell S (eds) Biodiversity in agricultural production systems. Taylor & Francis, CRC Press, Boca Raton
Dinter A, Oberwalder C, Kabouw P, Coulson M, Ernst G, Leicher T, Miles M, Weyman G, Klein O (2013) Occurrence and distribution of earthworms in agricultural landscapes across Europe with regard to testing for responses to plant protection products. J Soil Sediment 13:278–293
Baldivieso-Freitas P, Blanco-Moreno JM, Gutiérrez-López M, Peigné J, Pérez-Ferrer A, Trigo-Aza D, Sans FX (2018) Earthworm abundance response to conservation agriculture practices in organic arable farming under Mediterranean climate. Pedobiologia 66:58–64
Domínguez J, Velando A, Ferreiro A (2005) Are Eisenia fetida (Savigny, 1826) and Eisenia andrei (Oligochaeta, Lumbricidae) different biological species. Pedobiologia 49:81–87
Römbke J, Aira M, Backeljau T, Breugelmans K, Domínguez J, Funke E, Graf N, Hajibabaei M, Pérez-Losada M, Porto PG, Schmelz RM, Vierna J, Vizcaíno A, Pfenninger M (2016) DNA barcoding of earthworms (Eisenia fetida/andrei complex) from 28 ecotoxicological test laboratories. App Soil Ecol 104:3–11
Pelosi C, Joimel S, Makowski D (2013) Searching for a more sensitive earthworm species to be used in pesticide homologation tests – a meta-analysis. Chemosphere 90:895–900
Brami C, Glover AR, Butt KR, Lowe CN (2017) Avoidance, biomass and survival response of soil dwelling (endogeic) earthworms to OECD artificial soil: potential implications for earthworm ecotoxicology. Ecotoxicology 26:576–579
Bart S, Amossé J, Lowe CN, Mougin C, Péry ARR, Pelosi C (2018) Aporrectodea caliginosa, a relevant earthworm species for a posteriori pesticide risk assessment: current knowledge and recommendations for culture and experimental design. Environ Sci Pollut Res Int 25:33867–33881
Whomsley R, Brendler-Schwaab S, Griffin E, Jensen J, Moermond C, Scholz B, Nilssen LS, Stemplewski H, Roennefahrt I (2019) Commentary on the draft revised guideline on the environmental risk assessment of medicinal products for human use. Environ Sci Eur 31:17
Lin D, Zhou Q, Xu Y, Chen C, Li Y (2012) Physiological and molecular responses of the earthworm (Eisenia fetida) to soil chlortetracycline contamination. Environ Pollut 171:46–51
McKelvie JR, Wolfe DM, Celejewski MA, Alaee M, Simpson AJ, Simpson MJ (2011) Metabolic responses of Eisenia fetida after sub-lethal exposure to organic contaminants with different toxic modes of action. Environ Pollut 159:3620–3626
Dong L, Gao J, Xie X, Zhou Q (2012) DNA damage and biochemical toxicity of antibiotics in soil on the earthworm Eisenia fetida. Chemosphere 89:44–51
Pino MR, Val J, Mainar AM, Zuriaga E, Español C, Langa E (2015) Acute toxicological effects on the earthworm Eisenia fetida of 18 common pharmaceuticals in artificial soil. Sci Total Environ 518-519:225–237
Chatelain M, Mathieu J (2017) How good are epigeic earthworms at dispersing? An investigation to compare epigeic to endogeic and anecic groups. Soil Biol Biochem 111:115–123
Dominguez J (2011) The microbiology of vermicomposting. In: Edwards CA, Arancon NQ, Sherman R (eds) Vermiculture technology: earthworms, organic wastes, and environmental management. CRC Press, Taylor & Francis Group, Boca Raton, FL, pp 53–66
Baguer AJ, Jensen J, Krogh PH (2000) Effects of the antibiotics oxytetracycline and tylosin on soil fauna. Chemosphere 40:751–757
Liu Z, Mayer BK, Venkiteshwaran K, Seyedi S, Raju ASK, Zitomer D, McNamara PJ (2020) The state of technologies and research for energy recovery from municipal wastewater sludge and biosolids. Curr Opin Environ Sci Health 14:31–36
Kinney CA, Heuvel BV (2020) Translocation of pharmaceuticals and personal care products after land application of biosolids. Curr Opin Environ Sci Health 14:23–30
Spielmeyer A (2018) Occurrence and fate of antibiotics in manure during manure treatments: a short review. Sustain Chem Pharm 9:76–86
Van Epps A, Blaney L (2016) Antibiotic residues in animal waste: occurrence and degradation in conventional agricultural waste management practices. Curr Pollut Reports 2:135–155
Selvam A, Zhao Z, Wong JWC (2012) Composting of swine manure spiked with sulfadiazine, chlortetracycline and ciprofloxacin. Bioresour Technol 126:412–417
Dolliver H, Gupta S, Noll S (2008) Antibiotic degradation during manure composting. J Environ Qual 37:1245–1253
Ezzariai A, Hafidi M, Khadra A, Aemig Q, El Fels L, Barret M, Merlina G, Patureau D, Pinelli E (2018) Human and veterinary antibiotics during composting of sludge or manure: global perspectives on persistence, degradation, and resistance genes. J Hazard Mater 359:465–481
Hatzinger PB, Alexander M (1995) Effect of aging of chemicals in soil on their biodegradability and extractability. Environ Sci Technol 29:537–545
Cai L, Gong X, Sun X, Li S, Yu X (2018) Comparison of chemical and microbiological changes during the aerobic composting and vermicomposting of green waste. PLoS One 13:e0207494
Lazcano C, Gómez-Brandón M, Domínguez J (2008) Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Chemosphere 72:1013–1019
Lim SL, Wu TY, Lim PN, Shak KPY (2015) The use of vermicompost in organic farming: overview, effects on soil and economics. J Sci Food Agric 95:1143–1156
Sanchez-Hernandez JC, Domínguez J (2019) Dual role of vermicomposting in relation to environmental pollution. In: Sanchez-Hernandez JC (ed) Bioremediation of agricultural soils. CRC Press, Taylor & Francis Group, Boca Raton, pp 217–236
Aira M, Bybee S, Pérez-Losada M, Domínguez J (2015) Feeding on microbiomes: effects of detritivory on the taxonomic and phylogenetic bacterial composition of animal manures. FEMS Microbiol Ecol 91:fiv117
Gómez-Brandón M, Aira M, Lores M, Domínguez J (2011) Changes in microbial community structure and function during vermicomposting of pig slurry. Bioresour Technol 102:4171–4178
Aira M, Monroy F, Domínguez J (2007) Earthworms strongly modify microbial biomass and activity triggering enzymatic activities during vermicomposting independently of the application rates of pig slurry. Sci Total Environ 385:252–261
Hait S, Tare V (2011) Vermistabilization of primary sewage sludge. Bioresour Technol 102:2812–2820
Lv B, Xing M, Yang J (2018) Exploring the effects of earthworms on bacterial profiles during vermicomposting process of sewage sludge and cattle dung with high-throughput sequencing. Environ Sci Pollut Res Int 25:12528–12537
Castillo JM, Romero E, Nogales R (2013) Dynamics of microbial communities related to biochemical parameters during vermicomposting and maturation of agroindustrial lignocellulose wastes. Bioresour Technol 146:345–354
Ghosh S, Goswami AJ, Ghosh GK, Pramanik P (2018) Quantifying the relative role of phytase and phosphatase enzymes in phosphorus mineralization during vermicomposting of fibrous tea factory waste. Ecol Eng 116:97–103
Xia H, Chen J, Chen X, Huang K, Wu Y (2019) Effects of tetracycline residuals on humification, microbial profile and antibiotic resistance genes during vermicomposting of dewatered sludge. Environ Pollut 252:1068–1077
Ravindran B, Mnkeni PNS (2017) Identification and fate of antibiotic residue degradation during composting and vermicomposting of chicken manure. Int J Environ Sci Technol 14:263–270
Hickman ZA, Reid BJ (2008) Earthworm assisted bioremediation of organic contaminants. Environ Int 34:1072–1081
Sanchez-Hernandez JC (2019) Bioremediation of pesticide-contaminated soils by using earthworms. In: Sanchez-Hernandez JC (ed) Bioremediation of agricultural soils. CRC Press, Taylor & Francis Group, Boca Raton, pp 165–192
Morillo E, Villaverde J (2017) Advanced technologies for the remediation of pesticide-contaminated soils. Sci Total Environ 586:576–597
Guedes P, Lopes V, Couto N, Mateus EP, Pereira CS, Ribeiro AB (2019) Electrokinetic remediation of contaminants of emergent concern in clay soil: effect of operating parameters. Environ Pollut 253:625–635
Zhou Z, Liu X, Sun K, Lin C, Ma J, He M, Ouyang W (2019) Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: a review. Chem Eng J 372:836–851
Liu Y, Wang S, Wu Y, Chen H, Shi Y, Liu M, Dong W (2019) Degradation of ibuprofen by thermally activated persulfate in soil systems. Chem Eng J 356:799–810
Zhou Z, Ma J, Liu X, Lin C, Sun K, Zhang H, Li X, Fan G (2019) Activation of peroxydisulfate by nanoscale zero-valent iron for sulfamethoxazole removal in agricultural soil: effect, mechanism and ecotoxicity. Chemosphere 223:196–203
Rede D, Santos LHMLM, Ramos S, Oliva-Teles F, Antão C, Sousa SR, Delerue-Matos C (2016) Ecotoxicological impact of two soil remediation treatments in Lactuca sativa seeds. Chemosphere 159:193–198
Koba O, Golovko O, Kodešová R, Klement A, Grabic R (2016) Transformation of atenolol, metoprolol, and carbamazepine in soils: the identification, quantification, and stability of the transformation products and further implications for the environment. Environ Pollut 218:574–585
Biel-Maeso M, González-González C, Lara-Martín PA, Corada-Fernández C (2019) Sorption and degradation of contaminants of emerging concern in soils under aerobic and anaerobic conditions. Sci Total Environ 666:662–671
Lipiec J, Frąc M, Brzezińska M, Turski M, Oszust K (2016) Linking microbial enzymatic activities and functional diversity of soil around earthworm burrows and casts. Front Microbiol 7:1361
Aira M, Lazcano C, Gómez-Brandón M, Domínguez J (2010) Ageing effects of casts of Aporrectodea caliginosa on soil microbial community structure and activity. App Soil Ecol 46:143–146
Mora P, Miambi E, Jiménez JJ, Decaëns T, Rouland C (2005) Functional complement of biogenic structures produced by earthworms, termites and ants in the neotropical savannas. Soil Biol Biochem 37:1043–1048
Alekseeva T, Besse P, Binet F, Delort AM, Forano C, Josselin N, Sancelme M, Tixier C (2006) Effect of earthworm activity (Aporrectodea giardi) on atrazine adsorption and biodegradation. Eur J Soil Sci 57:295–307
Worrall F, Parker A, Rae JE, Johnson AC (1997) The role of earthworm burrows in pesticide transport from ploughlands. Toxicol Environ Chem 61:211–222
Bilal M, Rasheed T, Nabeel F, Iqbal HMN, Zhao Y (2019) Hazardous contaminants in the environment and their laccase-assisted degradation - a review. J Environ Manage 234:253–264
Morsi R, Bilal M, Iqbal HMN, Ashraf SS (2020) Laccases and peroxidases: the smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci Total Environ 714:136572
Alharbi SK, Nghiem LD, van de Merwe JP, Leusch FDL, Asif MB, Hai FI, Price WE (2019) Degradation of diclofenac, trimethoprim, carbamazepine, and sulfamethoxazole by laccase from Trametes versicolor: transformation products and toxicity of treated effluent. Biocatal Biotransformation 37:399–408
Pereira CS, Kelbert M, Daronch NA, Michels C, de Oliveira D, Soares HM (2020) Potential of enzymatic process as an innovative technology to remove anticancer drugs in wastewater. Appl Microbiol Biotechnol 104:23–31
Rao MA, Scelza R, Acevedo F, Diez MC, Gianfreda L (2014) Enzymes as useful tools for environmental purposes. Chemosphere 107:145–162
Kües U (2015) Fungal enzymes for environmental management. Curr Opin Biotechnol 33:268–278
Ba S, Vinoth Kumar V (2017) Recent developments in the use of tyrosinase and laccase in environmental applications. Crit Rev Biotechnol 37:819–832
Drake HL, Horn MA (2007) As the worm turns: the earthworm gut as a transient habitat for soil microbial biomes. Annu Rev Microbiol 61:169–189
Nozaki M, Ito K, Miura C, Miura T (2013) Examination of digestive enzyme distribution in gut tract and functions of intestinal caecum, in megascolecid earthworms (Oligochaeta: Megascolecidae) in Japan. Zoolog Sci 30:710–715
Sanchez-Hernandez JC, Mazzia C, Capowiez Y, Rault M (2009) Carboxylesterase activity in earthworm gut contents: potential (eco)toxicological implications. Comp Biochem Physiol 150C:503–511
Fujii K, Ikeda K, Yoshida S (2012) Isolation and characterization of aerobic microorganisms with cellulolytic activity in the gut of endogeic earthworms. Int Microbiol 15:121–130
Horn MA, Schramm A, Drake HL (2003) The earthworm gut: an ideal habitat for ingested N2O-producing microorganisms. Appl Environ Microbiol 69:1662–1669
Di L (2019) The impact of Carboxylesterases in drug metabolism and pharmacokinetics. Curr Drug Metab 20:91–102
Lesser LE, Mora A, Moreau C, Mahlknecht J, Hernández-Antonio A, Ramírez AI, Barrios-Piña H (2018) Survey of 218 organic contaminants in groundwater derived from the world’s largest untreated wastewater irrigation system: Mezquital Valley, Mexico. Chemosphere 198:510–521
Pal R, Megharaj M, Kirkbride KP, Naidu R (2013) Illicit drugs and the environment – a review. Sci Total Environ 463–464:1079–1092
Sanchez-Hernandez JC, Aira M, Domínguez J (2014) Extracellular pesticide detoxification in the gastrointestinal tract of the earthworm Aporrectodea caliginosa. Soil Biol Biochem 79:1–4
Sanchez-Hernandez JC, Notario del Pino J, Domínguez J (2015) Earthworm-induced carboxylesterase activity in soil: assessing the potential for detoxification and monitoring organophosphorus pesticides. Ecotoxicol Environ Saf 122:303–312
Hatfield MJ, Umans RA, Hyatt JL, Edwards CC, Wierdl M, Tsurkan L, Taylor MR, Potter PM (2016) Carboxylesterases: general detoxifying enzymes. Chem Biol Interact 259:327–331
Nannipieri P, Trasar-Cepeda C, Dick RP (2018) Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol Fertil Soils 54:11–19
Nannipieri P, Sequi P, Fusi P (1996) Humus and enzyme activity. In: Piccolo A (ed) Humic substances in terrestrial ecosystems. Elsevier, Amsterdam, pp 293–328
Sanchez-Hernandez JC, Ro KS, Díaz FJ (2019) Biochar and earthworms working in tandem: research opportunities for soil bioremediation. Sci Total Environ 688:574–583
Shaaban M, Van Zwieten L, Bashir S, Younas A, Núñez-Delgado A, Chhajro MA, Kubar KA, Ali U, Rana MS, Mehmood MA, Hu R (2018) A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J Environ Manage 228:429–440
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282
Tang J, Zhu W, Kookana R, Katayama A (2013) Characteristics of biochar and its application in remediation of contaminated soil. J Biosci Bioeng 116:653–659
Liu Y, Lonappan L, Brar SK, Yang S (2018) Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: a review. Sci Total Environ 645:60–70
Lehmann J (2015) Biochar for environmental management: an introduction. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science, technology and implementation. Routledge, Oxon
Han L, Ro KS, Wang Y, Sun K, Sun H, Libra JA, Xing B (2018) Oxidation resistance of biochars as a function of feedstock and pyrolysis condition. Sci Total Environ 616:335–344
Inyang M, Dickenson E (2015) The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: a review. Chemosphere 134:232–240
Rocha LS, Pereira D, Sousa É, Otero M, Esteves VI, Calisto V (2020) Recent advances on the development and application of magnetic activated carbon and char for the removal of pharmaceutical compounds from waters: a review. Sci Total Environ 718:137272
Palansooriya KN, Yang Y, Tsang YF, Sarkar B, Hou D, Cao X, Meers E, Rinklebe J, Kim K-H, Ok YS (2020) Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: a review. Crit Rev Environ Sci Technol 50:549–611
Weber K, Quicker P (2018) Properties of biochar. Fuel 217:240–261
Sizmur T, Fresno T, Akgül G, Frost H, Moreno-Jiménez E (2017) Biochar modification to enhance sorption of inorganics from water. Bioresour Technol 246:34–47
Wu L, Bi E (2019) Sorption of ionic and neutral species of pharmaceuticals to loessial soil amended with biochars. Environ Sci Pollut Res 26:35871–35881
Yao Y, Gao B, Chen H, Jiang L, Inyang M, Zimmerman AR, Cao X, Yang L, Xue Y, Li H (2012) Adsorption of sulfamethoxazole on biochar and its impact on reclaimed water irrigation. J Hazard Mater 209-210:408–413
Oh S-Y, Seo Y-D (2016) Sorption of halogenated phenols and pharmaceuticals to biochar: affecting factors and mechanisms. Environ Sci Pollut Res 23:951–961
Vithanage M, Rajapaksha AU, Zhang M, Thiele-Bruhn S, Lee SS, Ok YS (2015) Acid-activated biochar increased sulfamethazine retention in soils. Environ Sci Pollut Res Int 22:2175–2186
Graber ER, Tsechansky L, Gerstl Z, Lew B (2012) High surface area biochar negatively impacts herbicide efficacy. Plant Soil 353:95–106
Graber ER, Kookana RS (2015) Biochar and retention/efficacy of pesticides. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science, technology and implementation, 2nd edn. Earthscan, London
Rajapaksha AU, Vithanage M, Lim JE, Ahmed MB, Zhang M, Lee SS, Ok YS (2014) Invasive plant-derived biochar inhibits sulfamethazine uptake by lettuce in soil. Chemosphere 111:500–504
Williams M, Martin S, Kookana RS (2015) Sorption and plant uptake of pharmaceuticals from an artificially contaminated soil amended with biochars. Plant and Soil 395:75–86
Paz-Ferreiro J, Liang C, Fu S, Mendez A, Gasco G (2015) The effect of biochar and its interaction with the earthworm Pontoscolex corethrurus on soil microbial community structure in tropical soils. PLoS One 10:e0124891
Paz-Ferreiro J, Fu S, Méndez A, Gascó G (2014) Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities. J Soil Sediment 14:483–494
Garbuz S, Camps-Arbestain M, Mackay A, DeVantier B, Minor M (2020) The interactions between biochar and earthworms, and their influence on soil properties and clover growth: a 6-month mesocosm experiment. App Soil Ecol 147:103402
Sanchez-Hernandez JC (2018) Biochar activation with exoenzymes induced by earthworms: a novel functional strategy for soil quality promotion. J Hazard Mater 350:136–143
Jégou D, Capowiez Y, Cluzeau D (2001) Interactions between earthworm species in artificial soil cores assessed through the 3D reconstruction of the burrow systems. Geoderma 102:123–137
Hendrix PF, Callaham MA, Drake JM, Huang C-Y, James SW, Snyder BA, Zhang W (2008) Pandora’s box contained bait: the global problem of introduced earthworms. Annu Rev Ecol Evol Syst 39:593–613
Prodana M, Silva C, Gravato C, Verheijen FGA, Keizer JJ, Soares AMVM, Loureiro S, Bastos AC (2019) Influence of biochar particle size on biota responses. Ecotoxicol Environ Saf 174:120–128
Huang C, Wang W, Yue S, Adeel M, Qiao Y (2020) Role of biochar and Eisenia fetida on metal bioavailability and biochar effects on earthworm fitness. Environ Pollut 263:114586
Sanchez-Hernandez JC, Ríos JM, Attademo AM, Malcevschi A, Andrade Cares X (2019) Assessing biochar impact on earthworms: implications for soil quality promotion. J Hazard Mater 366:582–591
Li D, Hockaday WC, Masiello CA, Alvarez PJJ (2011) Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol Biochem 43:1732–1737
Briones MJI, Panzacchi P, Davies CA, Ineson P (2020) Contrasting responses of macro- and meso-fauna to biochar additions in a bioenergy cropping system. Soil Biol Biochem 145:107803
Kamau S, Karanja NK, Ayuke FO, Lehmann J (2019) Short-term influence of biochar and fertilizer-biochar blends on soil nutrients, fauna and maize growth. Biol Fertil Soils 55:661–673
Latch DE, Packer JL, Stender BL, VanOverbeke J, Arnold WA, McNeill K (2005) Aqueous photochemistry of triclosan: formation of 2, 4-dichlorophenol, 2, 8-dichlorodibenzo-p-dioxin, and oligomerization products. Environ Toxicol Chem 24:517–525
Gaylor MO, Mears GL, Harvey E, La Guardia MJ, Hale RC (2014) Polybrominated diphenyl ether accumulation in an agricultural soil ecosystem receiving wastewater sludge amendments. Environ Sci Technol 48:7034–7043
Andrade NA, McConnell LL, Torrents A, Ramirez M (2010) Persistence of polybrominated diphenyl ethers in agricultural soils after biosolids applications. J Agric Food Chem 58:3077–3084
Lü H, Mo CH, Zhao HM, Xiang L, Katsoyiannis A, Li YW, Cai QY, Wong MH (2018) Soil contamination and sources of phthalates and its health risk in China: a review. Environ Res 164:417–429
Sun J, Pan L, Tsang DCW, Zhan Y, Zhu L, Li X (2018) Organic contamination and remediation in the agricultural soils of China: a critical review. Sci Total Environ 615:724–740
Caban M, Folentarska A, Lis H, Kobylis P, Bielicka-Giełdoń A, Kumirska J, Ciesielski W, Stepnowski P (2020) Critical study of crop-derived biochars for soil amendment and pharmaceutical ecotoxicity reduction. Chemosphere 248:125976
Suèr P, Nilsson-Påledal S, Norrman J (2004) LCA for site remediation: a literature review. Soil Sediment Contam Int J 13:415–425
Roberts KG, Gloy BA, Joseph S, Scott NR, Lehmann J (2010) Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential. Environ Sci Technol 44:827–833
Matuštík J, Hnátková T, Kočí V (2020) Life cycle assessment of biochar-to-soil systems: a review. J Clean Prod 259:120998
Schmidt HP, Taylor P, Eglise A, Arbaz C (2014) Kon-Tiki flame curtain pyrolysis for the democratization of biochar production. Biochar J:14–24
Pandit NR, Mulder J, Hale SE, Schmidt HP, Cornelissen G (2017) Biochar from “Kon Tiki” flame curtain and other kilns: effects of nutrient enrichment and kiln type on crop yield and soil chemistry. PLoS One 12:e0176378
Hauschild MZ, Rosenbaum RK, Olsen SI (2018) Life cycle assessment: theory and practice. Springer, Cham
Lemming G, Hauschild MZ, Bjerg PL (2010) Life cycle assessment of soil and groundwater remediation technologies: literature review. Int J Life Cycle Assess 15:115–127
Acknowledgements
We thank the Spanish Ministerio de Ciencia y Tecnología (Grant no. PGC2018-098851-B-I00) for the financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sanchez-Hernandez, J.C. (2020). Vermiremediation of Pharmaceutical-Contaminated Soils and Organic Amendments. In: Pérez Solsona, S., Montemurro, N., Chiron, S., Barceló, D. (eds) Interaction and Fate of Pharmaceuticals in Soil-Crop Systems. The Handbook of Environmental Chemistry, vol 103. Springer, Cham. https://doi.org/10.1007/698_2020_625
Download citation
DOI: https://doi.org/10.1007/698_2020_625
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-61289-4
Online ISBN: 978-3-030-61290-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)