Abstract
Better living standards, population growth, and expanding urbanization escalate the energy requirement tremendously. Declining stockpile of nonrenewable fossil fuels and its severe impact on environment have created huge consciousness among government, researchers, and industries to develop alternative renewable energy sources. Bioethanol has been considered as one of the most efficient alternative liquid fuels to replace the existing conventional crude oil-based petrol. Among the different lignocellulosic biomass, agricultural residues especially sugarcane tops (SCT) are becoming a promising feedstock for bioethanol production. However, the presence of high amount of lignin possesses a major hurdle in converting this promising feedstock to bioethanol. Hence, this review paper summarizes the various pretreatment methods, hydrolysis, and fermentation techniques reported in the bioethanol production from underutilized SCT. From the overall studies, it was evident that the SCT can be used as a potential renewable feedstock for the production of fermentable sugars and bioethanol.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
High population growth, industrialization, and urbanization increase the energy requirement tremendously. Nonrenewable energy sources such as coal, crude oil, nuclear power, propane, and natural gas were utilized to meet the ever-increasing energy demand, and these sources were found to be the major contributors of environmental pollution [1]. To overcome these issues, utilization of the most sustainable renewable resources offers an attractive solution to meet the world’s primary energy demand. Biofuels are fuels which are derived from renewable energy resources that provide energy security, strengthen the rural and agricultural economies, and emit no or less toxic gases. Bioethanol is one among the dominant global renewable transport biofuels since it can be produced from a wide range of agricultural residues. Bioethanol produced from sucrose-containing feedstocks (sugarcane, sugar beet, and sweet sorghum) and starch-rich feedstocks (corn, wheat, and cassava) are named as first-generation bioethanol [2]. However, it holds several limitations in producing energy from these substrates owing to its conflicts between fuel and food. Hence, the lignocellulosic biomass has been considered as a potential source for the biofuel production [3, 4].
Biomass is a lignocellulosic-based bio-residue that exists in water-based vegetation, forest or organic waste, crop production, and agro or food industries waste. There are various forms of biomass resources available for the production of biofuels and are mostly available in the form of grasses, woody plants, fruits, vegetables, manures, and aquatic plants. These sources can be majorly classified as residues of an agricultural crop, energy plantation, and municipal and industrial waste [5]. Recently, lignocellulosic biomass especially non-food crops and industrial and municipal waste acquires huge attention among researchers since it has been identified as the low-cost and sustainable feedstock for the production of biofuels and other value-added products [3, 5]. Among various types of lignocellulosic biomass, the agricultural residues or wastes such as rice husks, wheat straw, rice straw, energy grass, corn cobs, sugarcane bagasse, dry sugarcane leaves, etc. are available abundantly. Till date, most of these underutilized agricultural residues are being burnt in the open fields after cultivation of crops thereby creating a severe impact on the environment with huge loss of energy [6].
Sugarcane (Saccharum officinarum) is a species of tall perennial true grasses of the genus Saccharum, tribe Andropogoneae, which has stout jointed fibrous stalks that are rich in sugar and measure 2–6 m tall. Sucrose accumulated in the stalk internodes of sugarcane was extracted and purified in specialized mill factories and is used as raw material in food industries or fermented to produce ethanol. Ethanol is produced on large scale by the Brazilian sugarcane industry and yet the world demand for sugar is the primary driver of sugarcane agriculture [7].
Sugarcane tops or trash (SCT) is regarded as an agricultural residue that represents the top fragment of the sugarcane plant along with its leaves [8], and the one-third portion of sugarcane plant holds SCT (dry mass basis) [9]. Currently, these unutilized residues were dumped and burnt on the fields after sugarcane cultivation. To some extent, the green SCT obtained immediately after the cultivation was used as animal fodder [4, 10]. This cellulose-rich SCT can be employed to extract the bioethanol by taking advantage of its rich cellulosic matter and the huge availability. Extraction of bioethanol from cellulosic SCT will neither affect food supply nor interfere negatively with sugar-based food or juice extracted from stalks of sugarcane plant.
2 Steps Involved in Bioethanol Production
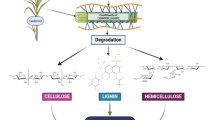
The process of converting lignocellulosic biomass into bioethanol mainly consists of four unit operations such as pretreatment, hydrolysis or saccharification, fermentation, and product recovery [3, 11, 12]. The overall schematic representation of bioethanol production from lignocellulosic biomass is presented in Fig. 1. Prior to pretreatment step, the raw lignocellulosic biomass was subjected to size reduction as shown in Fig. 2. Pretreatment is essential to alter the biomass size and structure and its sub-microscopic chemical composition so that hydrolysis of the carbohydrate-rich solid fraction to monomeric sugars can be achieved more rapidly with greater yields. Hydrolysis involves the processing step that converts the carbohydrate polymers into monomeric sugars. Six carbon sugars (hexoses) are readily fermented to ethanol by various naturally occurring organisms. However, the five carbon sugars (pentoses) were utilized by few native strains to produce ethanol at relatively low yields.
The basic five stages of this process are:
-
1.
Pretreatment step – makes the raw material amenable to hydrolysis
-
2.
Hydrolysis step – to break down the cellulose present in solid fraction into sugars
-
3.
Collection of liquid hydrolyzate and detection of inhibitory compounds such as furfurals, hydroxymethylfurfural (HMF), and acetic acid
-
4.
Fermentation of the five and six carbon containing sugar solution
-
5.
Distillation and dehydration step to produce absolute ethanol
3 Different Pretreatment Techniques Employed on SCT
The pretreatment is the most crucial step in biomass conversion as it possesses a huge impact on the efficiency of bioethanol production. Lignocellulosic biomass mainly consists of densely packed cellulose and hemicelluloses along with lignin which serves plant for performing several functions [13]. The aim of the pretreatment is to make cellulose more accessible to the enzymes for the conversion into fermentable sugars which can be done by disrupting the recalcitrant lignin structures using different pretreatment techniques. The removal of lignin mainly depends on the type of pretreatment employed and the optimum conditions maintained. Various pretreatments employed for successful biomass conversion were presented in Fig. 3.
Sindhu et al. [8] performed dilute acid pretreatment on sugarcane tops and observed a decrease in hemicellulose content from 18.9% to 6.16% under the conditions of 3% w/w H2SO4, incubation time of 60 min at 121°C with 15% w/w solid loading. Sindhu et al. [6] experimented surfactant-assisted acid pretreatment on sugarcane tops and perceived 50% lignin removal and 53% hemicellulose removal under 2.5% w/w Triton X-100 and 1.5% sulfuric acid concentration with 30% biomass loading for a period of 45 min at 121°C. Sindhu et al. [14] enhanced the sugar release using a novel method of surfactant-assisted ultrasound pretreatment on sugarcane tops. Authors reported 40% lignin removal and 30.2% hemicellulose removal under the conditions of 20% w/w biomass concentration and 3% w/w surfactant (Tween 40) concentration, incubated at 121°C for 60 min followed by 1 min sonication. Sindhu et al. [11] observed a maximum lignin removal of 89.80% and hemicellulose removal of 45.78% on NaOH pretreated SCT under the optimal conditions of 3% NaOH, 15% biomass loading incubated for 60 min at 121°C.
Sherpa et al. [4] carried out statistical optimization on delignification of sugarcane tops using laccase and observed 73.86% lignin removal under 20% w/v of substrate concentration, 6 h incubation time, pH 7, and 500 IU/mL enzyme titer maintained at 40°C. Maurya et al. [15] observed 67% lignin removal on microwave alkali-pretreated sugarcane tops under the conditions of 2% (w/v) NaOH and 10% biomass loading with microwave operating at 320 W for 10 min. Srinorakutara et al. [1] performed alkali and acid pretreatment on sugarcane trash for ethanol production and reported 2% w/v NaOH concentration, 15% w/v biomass loading, autoclaved at 121°C for 15 min, followed by 2% w/v sulfuric acid concentration autoclaved at 121°C for 15 min with 15 lb./in2. Raghavi et al. [16] developed a novel sequential pretreatment for the bioethanol production from sugarcane trash. Authors observed 2.3% lignin and 47.5% hemicellulose removal under the optimal conditions of 3% v/v glycerol in presence of 1% NaOH and different transition metal concentration of 1% w/w at 121°C for 60 min. Althuri and Banerjee [17] observed 70–80% lignin removal by performing enzymatic pretreatment on SCT using 500 IU/mL laccase maintained at pH 7 with substrate concentration of 30% (w/v) incubated at 35°C for 6 h.
4 Dilute Acid Pretreatment
Pretreatment of the biomass that acts as feedstock is the first step in the production of bioethanol. It helps to alter the structure of the biomass and the chemical composition so that higher yields of monomeric sugars are obtained from the hydrolysis process. The dilute acid pretreatment helps to disrupt the recalcitrant lignin structures in the feedstock such as the covalent bonds present in it which helps further in the hydrolysis process due to more accessibility of the enzymes. It solubilizes the hemicellulose present in the biomass as well as makes the cellulose more accessible [18, 19]. The xylan and glucomannan are relatively acid stable and hence will not be affected during the acid pretreatment. The hydrolyzation of cellulose and the solubilization of the glucose result in an increase in the crystallinity index (CrI) in the biomass which can be obtained from the XRD spectra [13].The type of pretreatment employed and the process parameters plays an important role for the basis of lignin removal. Dilute acid pretreatments are widely chosen for the pretreatment by the industries due to its cost and its corrosion properties. The pretreatment efficiency depends on several process parameters such as incubation temperature, biomass loading, and the acid concentration. All these parameters in combination play an important role to give better yield, and hence the optimization of the parameters is considered to be necessary in order to produce high yield of monomeric sugars.
Pretreatment of SCT with sulfuric acid with mixed size particle and incubation time of 60 min gave higher reducing sugar and improved the hydrolysis efficiency fourfold when compared to the yield given by the native SCT. 3% w/w was found to be more suitable in maximum reducing sugar production of 0.611 g/g. The effect of biomass loading on acid pretreatment showed that 15% w/w is the optimum condition and the efficiency decreased with the further increase in the biomass loading which could be due to decrease in the accessibility of the pretreatment agent [8].
The hybrid pretreatment involving surfactant and dilute acid has improved the dissolution and hence the removal efficiency of lignin. It helps in the removal of both hemicellulose and lignin. Triton X-100 as the surfactant in the presence of dilute sulfuric acid was found to be more efficient in producing reducing sugar. The total lignin content was reduced to 50% during the pretreatment process with the increase in cellulose content to 45.39% which is a twofold increase from the native SCT. Maximum reducing sugar of 0.448 g/g was obtained after pretreatment at optimum conditions like 2.5% (w/w) Triton X-100, 1.5% H2SO4 concentration, and 30% (w/w) of biomass loading [6].
5 Alkaline Pretreatment
Alkaline pretreatments are considered to be among the major chemical pretreatment technology besides the acidic pretreatments. It can either use chemicals like sodium hydroxide, calcium hydroxide, or ammonia as the reagent. Pretreatments including sodium hydroxide have enhanced cellulose digestibility. Alkaline pretreatments require lower temperatures and pressures, but the incubation time is recorded in terms of hours or days. It results in the removal of lignin in the solid fraction which can be recovered using appropriate recovery measures, and the solid fraction contains hemicelluloses and celluloses. The residual alkali obtained can be reused by the chemical recovery process. This pretreatment is based on delignification process which includes a high amount of hemicellulose being solubilized. The major aim is to remove the lignin from the biomasses and thus improve the reactivity of the polysaccharides present in it. Apart from it, this pretreatment also helps to swell the cell wall, thus improving the cell wall accessibility for subsequent enzymatic hydrolysis. The reaction mechanism supposed to take place is the solvation and saponification of the intermolecular ester bonds that cross-link with the hemicellulose and lignin leading to the cleavage of the lignin carbohydrate complex and therefore expose the cellulose microfibrils present in it [20]. Alkali helps in the removal of acetyl groups and various uronic acid substitutes, and hence steric hindrance of the enzymes in the hydrolysis process is reduced, thus increasing their accessibility towards the carbohydrates. The formation of furfural and HMF in the hydrolysates is found to be lower when compared to the dilute acid pretreatment. The degree of polymerization of the cellulose is decreased and hence causes swelling of cellulose leads to increase in its internal surface area [13].
Alkali pretreatment on SCT with 15% w/w biomass loading and 3% NaOH and incubation time of 60 min yielded 0.684 g of reducing sugar after the enzymatic hydrolysis. The compositional analysis proved that the amount of cellulose was almost intact during the pretreatment, but substantial amount of lignin (89.80%) and hemicellulose (46%) was found to be removed. SEM images revealed that the pretreated sample had distorted structure and an increase in the surface area in SCT thus improving the hydrolysis efficiency when compared to the native SCT which had a compact rigid structure. X-ray spectrum of native and pretreated SCT showed that the crystallinity index had increased in the preheated sample being 67.4% when compared to the native sample of 37.4% thereby influencing the enzymatic hydrolysis process [11].
Another study on SCT showed that alkaline followed by acid pretreatment yielded the maximum reducing sugar after enzymatic hydrolysis. It was observed that 2% (w/v) NaOH followed by 2% (w/v) sulfuric acid with autoclaving at 121°C for 15 min produced maximum ethanol of 48.17 g/L [1].
The combination of microwave with alkaline pretreatment studies was also an efficient hybrid method in producing reducing sugars. Pretreatment of SCT using this method was able to remove 67% of the lignin from the biomass and hence increases the cellulose and hemicellulose content making it more accessible for the enzymes for the saccharification process. Furthermore, optimization of hydrolysis process parameters using Box-Behnken design resulted in higher sugar yield of 0.376 g/g which represented 90.24% of the theoretical maximum based on the contents of cellulose and hemicellulose present in the pretreated biomass [15].
6 Ultrasound Pretreatment
Ultrasound pretreatments are considered to be efficient technique due to the fractionation of the lignocelluloses present in the biomass into bioethanol. Most of the other pretreatments including acid, alkali, ionic liquids, and steam are said to form unwanted by-products which then result in the loss of carbohydrates. However ultrasonic irradiation helps in the intensification of various pretreatment methods employed in biomass, the principle behind the ultrasonication is the cavitation, which involves the spontaneous growth and collapse of microsize cavities caused due to the propagation of ultrasonic waves, further causing high temperatures and pressure gradients. This results in the enhanced surface areas and leads to higher transfer rate. It helps to accomplish the necessities for bioethanol production by the formation of reactive cellulose fraction for reducing sugar formation along with reduction in power requirements as well as permitting the usage of sources which are inexpensive. The degree of fractionation can be increased by the combination of ultrasound with conventional pretreatment techniques. The ultrasound when applied to the biomass helps in disrupting the structure of the cell wall, increases the specific surface area, and therefore decreases the degree of polymerization to higher extent thereby enhancing the utilization of lignocellulosic biomass [21].
Yuan et al. [22] showed that the extent of delignification brought about by the ultrasound pre-treatment is 96% as well as 75% removal of the hemicellulose present by the ultrasound-assisted organosolv pretreatment [22].
Studies were also done on SCT based on surfactant-assisted ultrasound pretreatment. Tween-40 being the surfactant produced reducing sugar of 0.661 g/g after enzyme saccharification with process conditions as 3% (w/w) Tween-40 for 60 min, followed by sonication for 1 min and biomass loading at 20% w/w. SEM results showed distorted structure of the pretreated sample since it helps in the removal of some of the fibers present externally which appears to be in the form of a complex [14].
7 Enzymatic Pretreatment
Lignocellulosic biomass being the key building block of plant cell wall comprises of cellulose, hemicellulose, lignin, extractives, pectin, and ash. These fractions of components may vary with the plant type, species, their age, and the particular climatic conditions. The plant cell wall is a complex structure comprising of several β-glucan chains that are bound by hydrogen bonds to form elementary fibrils which are further bundled together to form microfibrillar structures. Studies done on biomasses that are pretreated using enzymes have shown that it does not require additional washing due to the absence of inhibitors and pH neutralization steps as needed in other chemical or physicochemical pretreatment methods since it is processed at pH conditions of 11–12. Further it helps to enhance the cellulases and xylanases accessibility to the polysaccharides present in the delignified biomass. Laccase is the enzyme usually used for the enzymatic pretreatment since it is said to fit well with the feedstocks containing higher hemicellulosic content because of the maximum recovery being achieved when compared to other pretreatment techniques. Laccase pretreatment of the biomasses is non-hazardous to the environment and also non-corrosive to the bioreactors with no issues regarding the waste disposal and the recycling of the toxic chemicals, therefore reducing the operational costs to a great extent.
Laccase-mediated pretreatment studies done on SCT using central composite design (CCD) based on response surface methodology (RSM) showed that maximum delignification of 79.1% was obtained when the operational process parameters were processed at temperature of 40°C, pH at 7, biomass loading of 21% (w/v), enzyme titer of 430.3 IU/mL, and incubation time of 6 h [4].
Other studies done on a combination of biomasses including Ricinus communis (RC), Saccharum officinarum (SCT) top, Saccharum spontaneum (KG), Lantana camara (LC), Ananas comosus (PA) leaf wastes, and Bambusa bambos (BB) where laccase enzyme of 500 IU/ml, with substrate concentration of 30% (w/v), 1 g of mixed substrate at 35°C, and incubation time of 6 h followed by simultaneous saccharification and fermentation proved to be efficient in the production of bioethanol of 1.396 g/L/h [17].
8 Alkaline Hydrogen Peroxide (AHP) Pretreatment
AHP pretreatment is one of the most effective pretreatment for delignification and a variety of lignocellulosic agricultural residues such as rice husks or hulls [23, 24], rapeseed straw [25], wheat straw [26, 27], sugarcane bagasse [28,29,30], sweet sorghum bagasse [31], cashew apple bagasse [32], bamboo [33], olive tree biomass [34], poplar [35], Jerusalem artichoke stalk [36], and seaweed Ulva prolifera [37]. AHP has oxidative action on the cell wall of biomass and breaks ester linkages with less sugar degradation and increased digestibility. No or very less secondary product formation was reported during the AHP pretreatment [25, 38, 39]. Thus, it can be employed effectively to achieve higher lignin removal during the pretreatment.
The use of H2O2 for the pretreatment of lignocellulosic biomass is based on the chemical reactions that this oxidizing agent undergoes in the alkaline liquid medium [39]. Its dissociation generates the hydroperoxide anion (HOO−) through Eq. (1)
In the alkaline medium, the hydroperoxide anion can react with H2O2, leading to the formation of superoxide and hydroxyl radical, as expressed in Eq. (2).
9 Hydrolysis Employed in SCT
Bioethanol production from lignocellulosic biomasses includes processes like pretreatment, hydrolysis, and fermentation. Pretreatment helps to alter the biomass structure and chemical composition that aids in rapid and efficient hydrolysis of the carbohydrates into reducible sugars. Hydrolysis is a process that converts the polysaccharides into monomeric sugars which is further taken into fermentation. It is considered as one of the most important steps in the production of fermentable sugars. Fermentation is carried out by organisms that can utilize this monomeric sugar and produce ethanol wherein the organisms can be either naturally grown or genetically modified. Cellulose in the biomass is organized into microfibrils containing thousands of glucose residues. These celluloses can be hydrolytically broken down into glucose either by enzymes such as cellulases or by chemicals like sulfuric or other acids.
Hemicellulose also present in the biomass is composed of pentose and hexose sugars being 5-carbon and 6-carbon, respectively, and they can be hydrolyzed by enzymes such as hemicellulases or by acids to release the sugars which include xylose, arabinose, glucose, galactose, or mannose. There are certain process configurations being carried out in order to obtain better yield of bioethanol. These processes include separate hydrolysis and fermentation (SHF), simultaneous saccharification and fermentation (SSF), simultaneous saccharification and co-fermentation (SSCF), and consolidated bioprocessing (CBP). The processes adopted is based on the feedstock and the other process parameters which further helps in giving higher yield of bioethanol. There are two major types of hydrolysis processes being carried out which includes chemical reaction using acids and enzymatic reaction.
10 Acid Hydrolysis
Hydrolytic treatment of lignocellulosic biomasses with acid at higher temperatures will improve the efficiency in producing higher yield of fermentable sugars. Sulfuric acid is the mostly used acid, and it is operated under high temperature and low acid concentration. Higher acid concentration makes it highly corrosive and dangerous. But as a disadvantage, they produce large amounts of gypsum during the neutralization process, thereby increasing the investment and operational costs. Dilute acid hydrolysis is a commonly used method among the chemical pretreatment methods. This method can be used either as a pretreatment of lignocelluloses for the subsequent enzymatic hydrolysis or as a method of hydrolyzing into fermentable sugars. At a higher temperature (140–190°C) and lower acid concentration (0.1–1% sulfuric acid), they can result at high reaction rates and thus improve cellulose hydrolysis and along with it 100% hemicellulose removal. It can be performed in short retention time of 5 min at higher temperature of 180°C or in longer retention time of 30–90 min but at lower temperatures of 120°C. In olive tree biomass when used as the biomass, 75% of maximum total sugars were obtained when it was pretreated by dilute acid at 180°C with 1% sulfuric acid concentration and maximum hemicellulose recovery of 83% [40]. The major drawback is the formation of different types of inhibitors such as carboxylic acids, furans, and phenolic compounds which will inhibit the microbial growth during the fermentation process and results in lesser amount of yield and productivity of ethanol [41].
Dilute acid hydrolysis usually occurs in two stages. The first stage being performed at a lower temperature to maximize the hemicellulose yield, and the second stage involves higher temperature at optimized conditions for the cellulose hydrolysis. Mild process conditions (0.7% H2SO4, 463 K) are opted in the first stage to recover the five carbon sugars, whereas in the second stage, the remaining solids containing more resistant cellulose undergo harsher conditions (488 K, but a milder 0.4% H2SO4) that help to recover the six carbon sugars. In order to allow adequate acid penetration, the reduction of the size of the feedstocks is necessary where the maximum particle dimension is in the range of a few millimeters. When considering concentrated acid hydrolysis process, it involves an acid (dilute or concentrated) pretreatment to help liberate the hemicellulosic sugars present in the biomass, while the subsequent stage requires the pretreated biomass to be dried followed by the addition of concentrated sulfuric acid (70–90%). The concentration of the acid used in concentrated acid hydrolysis process ranges between 10 and 30%. Reaction times are found to be much longer than the dilute acid process. This process provides a complete and rapid conversion of cellulose to glucose sugars and hemicelluloses to five carbon sugars with degradation level being very less. The critical factors needed to be considered to make this process economically viable are the optimization of the sugar recovery and cost-effectiveness during the acid recovery taken for recycling. The concentrated acid process offers more potential for cost reductions and leads to little sugar degradation than the dilute acid process. However, environment and corrosion problems as well as the high cost of acid consumption and recovery present major barriers to economic success [2].
11 Enzymatic Hydrolysis
Hydrolysis catalyzed by enzymes that helps in favoring 100% selective conversion of cellulose to glucose is known as enzymatic hydrolysis. This process is considered to be a very slow process due to some factors like structural parameters of the substrate, surface area, and the cellulose crystallinity. Utility cost of enzymatic hydrolysis is low when compared to chemical hydrolysis technique since they are usually conducted at mild conditions (pH 4.8) and temperature (318–323 K) and do not produce corrosion. The enzymatic hydrolysis has resulted in high yields (75–85%) with improvements being still developed. It is an environmentally friendly process that involves enzymes like cellulases and hemicellulases to hydrolyze or degrade the carbohydrates into fermentable sugars. Cellulose is typically hydrolyzed by an enzyme called cellulase which is being produced by several microorganisms, like bacteria and fungi. Cellulase is the most commercially used enzymes that synergistically hydrolyze cellulose that involves synergistic actions by endoglucanses, exoglucanases, and β-glucosidases. These enzymes together help to hydrolyze intramolecular β-1, 4-glucosidic bonds present in the cellulose to release soluble cellobiose or glucose and further hydrolyze cellobiose to glucose in order to eliminate cellobiose inhibition. The different process factors affect the enzymatic hydrolysis of cellulose, like the substrates chosen, cellulase activity, reaction conditions (temperature, pH, as well as other parameters), and product inhibition. Therefore, optimization of the hydrolysis process is necessary to improve the yield and rate of enzymatic hydrolysis [2]. Three different enzymes present in cellulase hydrolysis ß-1, 4 glucosidic linkages in the cellulose chains thus converting them into simple sugars. Three steps involved in enzyme hydrolysis are shown in Fig. 4.
Sindhu et al. [8] observed a fermentable sugar yield of 0.775 g/g under enzymatic saccharification using 50 FPU cellulase enzyme with 11.25% w/w biomass loading and 0.2% (Tween-80) surfactant concentration incubated at 50°C for 42 h. Sherpa et al. [3] performed enzymatic hydrolysis under the conditions of 14% w/v biomass loading and 19.33 IU/ml enzyme titer with pH maintained at 5 having an incubation time of 7 h with temperature ranging from 45° to 55°C.
Maurya et al. [15] observed 0.376 g/g sugar yield under enzymatic hydrolysis using cellulase enzyme with 10% w/w biomass loading, 0.04% surfactant concentration, and 100 FPU/g enzyme concentration incubating for 72 h. Srinorakutara et al. [1] reported 0.509 g/g sugar yield with 15% w/w biomass loading and 50 FPU/g enzyme titer with pH 5 incubated for 48 h. Raghavi et al. [16] observed 0.796 g/g sugar yield under enzymatic hydrolysis using cellulase having 80 FPU/g activity with 5% w/w biomass loading and 0.05% surfactant concentration incubated for 24 h.
Sugarcane tops pretreated with different methods such as dilute acid, surfactant-assisted acid, and surfactant-assisted ultrasound were subjected to enzymatic hydrolysis, and the optimum conditions of 11.25% (w/w) biomass concentration, 50 FPU enzyme concentration, 0.2% of surfactant concentration, and 60 h incubation time yielded the maximum reducing sugars such as 0.665 g/g [8], 0.711 g/g [6], and 0.649 g/g [14], respectively. Alkaline-pretreated sugarcane tops were subjected to enzymatic hydrolysis with optimum conditions of 11.25% (w/w) biomass concentration, enzyme concentration of 50 FPU, 0.2% of surfactant concentration, and incubation time of 42 h; the yield of fermentable sugars was about 0.779 g/g [11]. Enzymatic hydrolysis of microwave-pretreated SCT with optimum conditions such as biomass concentration of 10% (w/w), cellulase loading of 100 FPU/g, surfactant concentration of 0.04% (w/w), and incubation time of 72 h yielded 0.376 g/g glucose [15].
Enzymatic-pretreated SCT was hydrolyzed with 15% w/v substrate loading; 50 FPU/g enzyme loading maintained at pH 5 for 48 h yielded 117.16 g/L of reducing sugar [1]. Enzymatic hydrolysis on the sequential pretreated SCT showed a maximum reducing sugar yield of 0.796 g/g with 5% (w/w) biomass loading, 80 FPU cellulase loading, 0.25% (w/w) surfactant concentration, and 48 h incubation time [16]. Enzymatic saccharification of laccase enzyme-pretreated SCT yielded 508 mg/g of reducing sugar with the optimal conditions of 14% (w/v) solid loading, 50°C temperature, 7 h incubation time, and 19.33 IU/ml enzyme titer at pH 5 [3]. Enzymatic (laccase)-pretreated SCT showed 3.3-fold increase in fermentable sugars after delignification [4].
12 Fermentation Process
Fermentation is the final step in the ethanol production. A variety of microorganisms like bacteria, fungi, and yeast can ferment the monomeric sugars to ethanol. In general, fermentation is carried out under anaerobic conditions leading to the glycosylation of one molecule of glucose into two moles of ethanol and two moles of carbon dioxide as shown in Eqs. (3) and (4).
Saccharomyces cerevisiae is the most commonly used yeast for fermentation, and it is substrate specific. The efficiency of the fermentation depends on the concentration and nature of the substrate, the methods followed for the pretreatment and hydrolysis, and the nature of the organism. It is necessary to maintain proper process conditions such as temperature and pH for the optimal yeast growth. Yeast shows tolerance to high sugar concentrations, is resistant to adverse conditions generated by the presence of ethanol, and is stable at higher temperature [41].
S. cerevisiae can ferment only hexose; co-culturing with other microorganism having the capability to ferment pentose can increase the yield of ethanol. Fermentation of SCT-derived fermentable sugars obtained after dilute acid pretreatment and enzymatic saccharification, with 18 h-old culture of S. cerevisiae incubated at 28 ± 2°C for 72 h, yielded high amount of ethanol of 11.365 g/L [8].
Alkali (NaOH) followed by dilute acid (H2SO4)-pretreated SCT yielded 48.17 g/L of ethanol by using 107 cells/mL of S. cerevisiae TISTR 5596 strain [1]. Fermentation of the non-detoxified hydrolyzate obtained from the sequential pretreated SCT using S. cerevisiae produced 31.928 g of bioethanol/g of dry biomass [16]. Fermentation of liquid hydrolyzate obtained after enzymatic-saccharified SCT using S. cerevisiae yielded 27.2 g/L of ethanol [3]. Fermentation of liquid hydrolyzate obtained from separate hydrolysis (SHF) and simultaneous saccharification (SSF) of pretreated lignocellulosic mixture revealed higher ethanol productivity of about 1.396 g/L/h in SSF [17].
13 Conclusion
From the reported studies, it is evident that different pretreatment technologies have been applied to SCT for efficient delignification and hemicellulose solubilization. The application of combined pretreatment technologies needs attention in order to convert the SCT into bioethanol. Also, the developed pretreatment should be effective in delignification with low consumption of energy. From various reports, it was proved that hydrolysis using enzyme efficiently converts the delignified SCT into fermentable sugars. However, most of the SCT-based reports utilized the yeast S. cerevisiae to ferment the sugars to ethanol. Application of co-culturing techniques involving different microorganisms should be encouraged for further enhancement of bioethanol yield. Furthermore, limited reports are available regarding the acid hydrolysis, commercial scale production, and economic analysis on bioethanol production from SCT.
References
Srinorakutara T, Suttikul S, Butivate E, Panphan V, Boonvitthya N (2014) Optimization on pretreatment and enzymatic hydrolysis of sugarcane trash for ethanol production. J Food Sci Eng 4:148–154
Balat M (2011) Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manag 52:858–875. https://doi.org/10.1016/j.enconman.2010.08.013
Sherpa KC, Ghangrekar MM, Banerjee R (2017) Optimization of saccharification of enzymatically pretreated sugarcane tops by response surface methodology for ethanol production. Biofuels 7269:1–8. https://doi.org/10.1080/17597269.2017.1409058
Sherpa KC, Ghangrekar MM, Banerjee R (2018) A green and sustainable approach on statistical optimization of laccase mediated delignification of sugarcane tops for enhanced saccharification. J Environ Manag 217:700–709. https://doi.org/10.1016/j.jenvman.2018.04.008
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7:163–173. https://doi.org/10.1016/j.jrras.2014.02.003
Sindhu R, Kuttiraja M, Binod P, Preeti VE, Sandhya SV, Vani S et al (2012) Surfactant-assisted acid pretreatment of sugarcane tops for bioethanol production. Appl Biochem Biotechnol 167:1513–1526. https://doi.org/10.1007/s12010-012-9557-3
de Carvalho Macedo I (2005) Sugar cane’s energy: twelve studies on Brazilian sugar cane agribusiness and its sustainability. UNICA, São Paulo
Sindhu R, Kuttiraja M, Binod P, Janu KU, Sukumaran RK, Pandey A (2011) Dilute acid pretreatment and enzymatic saccharification of sugarcane tops for bioethanol production. Bioresour Technol 102:10915–10921. https://doi.org/10.1016/j.biortech.2011.09.066
Pandey A, Biswas S, Sukumaran R, Kaushik N (2009) Study on availability of Indian biomass resources for exploitation: a report based on a nation-wise survey. TIFAC, New Delhi
Sukumaran RK, Surender VJ, Sindhu R, Binod P, Janu KU, Sajna KV et al (2010) Lignocellulosic ethanol in India: prospects, challenges and feedstock availability. Bioresour Technol 101:4826–4833. https://doi.org/10.1016/j.biortech.2009.11.049
Sindhu R, Kuttiraja M, Binod P, Sukumaran RK, Pandey A (2014) Physicochemical characterization of alkali pretreated sugarcane tops and optimization of enzymatic saccharification using response surface methodology. Renew Energy 62:362–368. https://doi.org/10.1016/j.renene.2013.07.041
Thongkheaw S, Pitiyont B (2011) Enzymatic hydrolysis of acid-pretreated sugarcane shoot. World Acad Sci Eng Technol 60:454–458
Xu Z, Huang F (2014) Pretreatment methods for bioethanol production. Appl Biochem Biotechnol 174:43–62. https://doi.org/10.1007/s12010-014-1015-y
Sindhu R, Kuttiraja M, Elizabeth Preeti V, Vani S, Sukumaran RK, Binod P (2013) A novel surfactant-assisted ultrasound pretreatment of sugarcane tops for improved enzymatic release of sugars. Bioresour Technol 135:67–72. https://doi.org/10.1016/j.biortech.2012.09.050
Maurya DP, Vats S, Rai S, Negi S (2013) Optimization of enzymatic saccharification of microwave pretreated sugarcane tops through response surface methodology for biofuel. Indian J Exp Biol 51:992–996
Raghavi S, Sindhu R, Binod P, Gnansounou E, Pandey A (2016) Development of a novel sequential pretreatment strategy for the production of bioethanol from sugarcane trash. Bioresour Technol 199:202–210. https://doi.org/10.1016/j.biortech.2015.08.062
Althuri A, Banerjee R (2017) Separate and simultaneous saccharification and fermentation of a pretreated mixture of lignocellulosic biomass for ethanol production. Biofuels 7269:1–12. https://doi.org/10.1080/17597269.2017.1409059
Binod P, Sindhu R, Singhania RR, Vikram S, Devi L, Nagalakshmi S, Kurien N, Sukumaran RK, Pandey A (2010) Bioethanol production from rice straw: an overview. Bioresour Technol 101:4767–4774
Jeong T, Um B, Kim J, Oh K (2010) Optimizing dilute acid pretreatment of rape seed straw for extraction of hemicellulose. Appl Biochem Biotechnol 161:22–33
Zheng Y, Pan Z, Zhang R (2009) Int J Agric Biol Eng 2:51–68
Subhedar PB, Gogate PR (2016) Use of ultrasound for pretreatment of biomass and subsequent hydrolysis and fermentation. In: Biomass fractionation technologies for a lignocellulosic feedstock based biorefinery. Elsevier, Amsterdam, pp 127–149. https://doi.org/10.1016/B978-0-12-802323-5.00006-2
Yuan TQ, Xu F, He J, Sun RC (2010) Structural and physico-chemical characterization of hemicelluloses from ultrasound assisted extractions of partially delignified fast-growing poplar wood through organic solvent and alkaline solutions. Biotechnol Adv 28:583–593
Saha BC, Cotta MA (2007) Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzym Microb Technol 41:528–532. https://doi.org/10.1016/j.enzmictec.2007.04.006
Ayeni AO, Daramola MO, Sekoai PT, Adeeyo O, Garba MJ, Awosusi AA (2018) Statistical modelling and optimization of alkaline peroxide oxidation pretreatment process on rice husk cellulosic biomass to enhance enzymatic convertibility and fermentation to ethanol. Cellulose 25:2487–2504. https://doi.org/10.1007/s10570-018-1714-6
Karagöz P, Rocha IV, Özkan M, Angelidaki I (2012) Alkaline peroxide pretreatment of rapeseed straw for enhancing bioethanol production by same vessel saccharification and co-fermentation. Bioresour Technol 104:349–357. https://doi.org/10.1016/j.biortech.2011.10.075
Qiu J, Ma L, Shen F, Yang G, Zhang Y, Deng S et al (2017) Pretreating wheat straw by phosphoric acid plus hydrogen peroxide for enzymatic saccharification and ethanol production at high solid loading. Bioresour Technol 238:174–181. https://doi.org/10.1016/j.biortech.2017.04.040
Yuan Z, Wen Y, Li G (2018) Production of bioethanol and value added compounds from wheat straw through combined alkaline/alkaline-peroxide pretreatment. Bioresour Technol 259:228–236. https://doi.org/10.1016/j.biortech.2018.03.044
Ramadoss G, Muthukumar K (2016) Mechanistic study on ultrasound assisted pretreatment of sugarcane bagasse using metal salt with hydrogen peroxide for bioethanol production. Ultrason Sonochem 28:207–217. https://doi.org/10.1016/j.ultsonch.2015.07.006
Verardi A, Blasi A, Marino T, Molino A, Calabrò V (2018) Effect of steam-pretreatment combined with hydrogen peroxide on lignocellulosic agricultural wastes for bioethanol production: analysis of derived sugars and other by-products. J Energy Chem 27:535–543. https://doi.org/10.1016/j.jechem.2017.11.007
Rabelo SC, Filho RM, Costa AC (2008) A comparison between lime and alkaline hydrogen peroxide pretreatments of sugarcane bagasse for ethanol production. Appl Biochem Biotechnol 148:45–58. https://doi.org/10.1007/s12010-008-8200-9
Cao W, Sun C, Qiu J, Li X, Liu R, Zhang L (2016) Pretreatment of sweet sorghum bagasse by alkaline hydrogen peroxide for enhancing ethanol production. Korean J Chem Eng 33:873–879. https://doi.org/10.1007/s11814-015-0217-5
da Correia JAC, Júnior JEM, Gonçalves LRB, Rocha MVP (2013) Alkaline hydrogen peroxide pretreatment of cashew apple bagasse for ethanol production: study of parameters. Bioresour Technol 139:249–256. https://doi.org/10.1016/j.biortech.2013.03.153
Yuan Z, Wen Y, Kapu NS (2018) Ethanol production from bamboo using mild alkaline pre-extraction followed by alkaline hydrogen peroxide pretreatment. Bioresour Technol 247:242–249. https://doi.org/10.1016/j.biortech.2017.09.080
Martínez-Patiño JC, Ruiz E, Romero I, Cara C, López-Linares JC, Castro E (2017) Combined acid/alkaline-peroxide pretreatment of olive tree biomass for bioethanol production. Bioresour Technol 239:326–335. https://doi.org/10.1016/j.biortech.2017.04.102
Zhang L, You T, Zhang L, Yang H, Xu F (2014) Enhanced fermentability of poplar by combination of alkaline peroxide pretreatment and semi-simultaneous saccharification and fermentation. Bioresour Technol 164:292–298. https://doi.org/10.1016/j.biortech.2014.04.075
Li K, Qin JC, Liu CG, Bai FW (2016) Optimization of pretreatment, enzymatic hydrolysis and fermentation for more efficient ethanol production by Jerusalem artichoke stalk. Bioresour Technol 221:188–194. https://doi.org/10.1016/j.biortech.2016.09.021
Li Y, Cui J, Zhang G, Liu Z, Guan H (2016) Optimization study on the hydrogen peroxide pretreatment and production of bioethanol from seaweed Ulva prolifera biomass. Bioresour Technol. https://doi.org/10.1016/j.biortech.2016.04.090
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9. https://doi.org/10.3390/ijms9091621
Dutra ED, Santos FA, Ribeiro B, Alencar A, Libanio A, Reis S (2017) Alkaline hydrogen peroxide pretreatment of lignocellulosic biomass : status and perspectives. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-017-0277-3
Cara C, Ruiz E, Oliva JM, Saez F, Castro E (2007) Conversion of olive tree biomass into fermentable sugars by dilute acid pretreatment and enzymatic saccharification. Bioresour Technol 99:1869–1876
Rabelo SC, Andrade RR, Maciel Filho R, Costa AC (2014) Alkaline hydrogen peroxide pretreatment, enzymatic hydrolysis and fermentation of sugarcane bagasse to ethanol. Fuel 136:349–357. https://doi.org/10.1016/j.fuel.2014.07.033
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Niju, S., Swathika, M. (2020). Lignocellulosic Sugarcane Tops for Bioethanol Production: An Overview. In: Jerold, M., Arockiasamy, S., Sivasubramanian, V. (eds) Bioprocess Engineering for Bioremediation. The Handbook of Environmental Chemistry, vol 104. Springer, Cham. https://doi.org/10.1007/698_2020_621
Download citation
DOI: https://doi.org/10.1007/698_2020_621
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57910-4
Online ISBN: 978-3-030-57911-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)