Abstract
Personal care products (PCPs) have been found in surface water, wastewater, tap water, and swimming pool water. The chlorine used in the disinfection process of water reacts with these compounds generating chlorinated byproducts that may possess enhanced toxicity.
In the case of swimming pool water chlorine also reacts with organic material released by swimmers such as amino acids and other nitrogen compounds yielding chlorinated compounds. Besides this organic material, sunscreen cosmetics used by swimmers are also released into pool water and react with chlorine. UV-Filters 2-ethylhexyl-p-dimethylaminobenzoate (EHDPABA), benzophenone-3 (BP-3), benzophenone-4 (BP-4), 2-ethylhexyl-4-methoxycinnamate (EHMC), and 4-tert-butyl-4′-methoxy-dibenzoylmethane (BDM) are known to suffer an electrophilic aromatic substitution of one or two atoms of hydrogen per one or two chlorine atoms leading to mono- and di-chlorinated byproducts. It has also been observed the presence of halobenzoquinones (HBQs) in pool water that results from the chlorination of UV-filters such as BDM, octocrylene, and terephthalilidene dicamphor sulfonic acid. The chlorination of some parabens has also been studied. It is known that some of the formed chlorinated byproducts are genotoxic. In this chapter we present a review on the work done so far to determine the stability of PCPs in chlorinated water and to identify the chlorinated byproducts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Personal care products (PCPs) have been found in surface water such as lakes, rivers, and sea, wastewater, and tap water [1–4]. The main reason for this is that during the wastewater treatment, the parental compounds are not totally removed and, in several cases, they also suffer biodegradation and biotransformation [5]. Then, the release of these effluents in the environment leads to the occurrence of PCPs and derivatives in the locations above mentioned. PCPs have been also found in bathing waters and swimming pool water due to their use by swimmers [6] by washing bath effect during bathing and swimming activities [7]. The problem is that, as in drinking water, the chlorine used in the disinfection process reacts with these compounds generating chlorinated byproducts that may possess enhanced toxicity [6, 8, 9]. Also body fluids such as urine and sweat mainly constituted by organic compounds can act as disinfection byproducts (DBPs) precursors [10]. Urea, amino acids, uric acid, gluconic acid, and sodium chloride are the major components of urine and sweat released by swimmers [11, 12]. However, waters disinfection is essential to kill microbial pathogens [13] that are mostly introduced into the water by humans [6].
In this chapter we present a review of reports on the chlorination of PCPs.
2 Reaction with Chlorine
2.1 Chlorination of Organic Matter Present in Body Fluids
In 2007, Li and Blatchley III [14] conducted a study to identify DBPs that result from chlorination of organic-nitrogen compounds present in pool waters due to urine and sweat released from human body. For instance, they verified that urea, creatinine, l-histidine, and l-arginine are trichloramine precursors. A few years later, Kanan and Karanfil [15] observed that some amino acids in urine, such as histidine and aspartic acid, are responsible for high formation rates of haloacetic acids (HAA), and that citric acid present both in urine and sweat is a chloroform precursor, just like albumin. All these information is compiled in Table 1.
Concerning amino acids chlorination, it begins with organic mono- or dichloramines formation which depends on chlorine dose and is followed by carbonyl or nitrile compounds production through decarboxylation and deamination (Fig. 1).
The chlorination of body fluids and other compounds is regulated by several factors. The presence of ion bromide (Br−) influences the levels of halogenated DBPs increasing them, because it is more reactive than chlorine in HAA formation. Although its contribution for DBPs formation is complicated and without a defined pattern, the pH also interferes in this reaction. In some situations, such as nitrile formation, low pH acts favoring the DBPs formation [16] but, in another cases, it does exactly the opposite [13]. Water temperature, total organic content, and number of people in the water [6], dose and residual disinfectant available in the water and contact time between reactants [7] also impact DBPs formation.
2.2 Chlorination of Personal Care Products
On the other hand, pool water also contains PCPs. Inside this category are cosmetic ingredients, food supplements and other products like shampoos, lotions, and sunscreens cosmetics [17]. Sunscreens cosmetics are any cosmetic which contains a UV filter in its formulation to protect human skin from the solar UV radiation since they absorb, reflect and/or scatter UV radiation with a wavelength between 320 and 400 nm for UVA and between 290 and 320 for UVB [7, 18, 19]. There are two types of UV-filters: the organic (or chemical) and the inorganic (or physical) [19]. Inorganic UV-filters category only contains titanium dioxide (TiO2) and zinc oxide (ZnO), which are known to reflect and scatter UV radiation. Regarding organic UV-filters, there are several classes such as para-amino-benzoates, cinnamates, benzophenones, dibenzoylmethanes, camphor derivates, and benzimidazoles and these compounds absorb the UV radiation [7]. There are many UV-filters allowed for use but their maximum concentration depends on legislation. Although European legislation differs from other countries legislation, like the USA and Japan, the usual concentration of UV-filters in cosmetics is between 0.1 and 10% [19].
Most of the organic UV-filters are relatively lipophilic and their structures contain aromatic rings, conjugated with carbon–carbon double bonds [18] and one benzenic moiety (or more) which has an efficient electronic delocalization due to the conjugation with electron releasing and electron acceptors groups located in either ortho or para positions. It is this feature that provides a specific maximum absorbance wavelength to the UV-filters [7].
UV-filters are known to react with chlorine leading to halomethanes, such as chloroform, haloacids, halonitriles, haloaldeydes, haloketones, halonitromethanes, haloamines, haloamides, and haloalcohols [17, 20] and also chlorinated UV-filter structures [18].
2.2.1 UV-Filters Chlorination
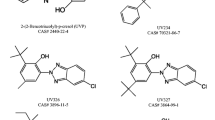
Few papers have been published in order to study both the UV-filters stability in chlorinated waters and to identify the resulting DBPs. In Fig. 2 we represent the UV-filters whose chlorination reaction was already studied.
In 2008, Negreira and co-workers [18] performed a study to assess the reactivity of three UV-filters containing hydroxy or amino groups in chlorinated waters: 2-ethylhexyl salicylate (ES), 2-ethylhexyl-p-dimethylaminobenzoate (EHDPABA), and benzophenone-3 (BP3). They found that the stability of these UV-filters is related with the pH: EHDPABA is more stable at basic water and for BP3 it happens exactly the opposite. ES showed a high stability independent of pH whereby ES halogenated reactions were considered negligible in real-life situations, since in this case there are several organic species competing for available chlorine. The following order of stability for these UV-filters was observed to be: BP3<EHDPABA<ES. However, it was verified that bromide addition, even at low concentrations, reduces the UV-filters stability, especially for EHDPABA. This occurs due to bromide formation which largely reacts with aromatic compounds. Thus, differences among stabilities show the effect of different organic groups on the activation or deactivation of the phenolic ring towards electrophilic substitution reactions [7].
About DBPs, Negreira et al. [18] observed the formation of mono-halogenated species resulting from EHDPABA chlorination and the formation of mono- and di-substituted byproducts from BP3. These DBPs are formed by hydrogen replacement per chlorine in the aromatic rings. Although it is not demonstrated, looking at the parent species structure and considering the activation effects of the hydroxyl and amino groups towards electrophilic substitution reactions, it can be assumed that these replacements occurred at the carbons in ortho- to the amino moiety (EHDPABA) and in ortho- and para- to the hydroxyl group (BP3).
Summarizing, EHDPABA has a relatively simple degradation pathway and the same pattern was also verified for BP3 which resulted in mono- and dihalogenated byproducts: Cl-BP3 (2 isomers) and Cl2-BP3 (1 isomer). However, in the case of BP3, another group of byproducts was detected. Negreira et al. [18] identified halogenated forms of 3-methoxyphenol generated from cleavage of the carbonyl bond between the two aromatic rings in the BP3 molecule followed by methoxyphenol fragment halogenation. Moreover, mono- and dihalogenated BP3 substitution byproducts might also break down rendering different halogenated methoxyphenols. Figure 3 represents the reaction pathway for BP3 proposed by Negreira et al. [18]. All the DBPs of EHDPABA and BP3 showed a considerable stability.
Degradation pathway for BP3 proposed by Negreira et al. [18]
The degradation of EHDPABA was previously studied by Sakkas et al. [21] in distilled, sea, and swimming pool water and the authors found one dichlorinated byproduct of the UV-filter and also mono- and dichlorinated degradation products of EHDPABA.
BP3 belongs to the benzophenones class of UV-filters approved by European legislation, which contains only another filter: benzophenone-4 (BP4) (Fig. 2). The stability of BP4 and its chlorination as well as its DBPs were also determined by Negreira et al. [22]. BP4 shows a low stability which decreases even more with pH increasing. As it happens with BP3, bromide addition decreases BP4 stability for the same reason of the first one.
The reaction between BP4 (C14H12O6S) and chlorine yields three DBPs designated as B1 (C14H11O6SCl), B2 (C14H11O7SCl), and B3 (C14H10O7SCl2) by Negreira et al. [22]. B1 results from an electrophilic substitution of hydrogen per chlorine and this reaction is similar to the BP3 chlorination described above. The difference between B1 and B2 is one atom of oxygen which occurs due to the oxidation of the carbonyl group to an ester moiety (known as the Baeyer–Villiger reaction) with loss of a benzoyl moiety and ester bond established between the carbonyl group and the BP4 phenolic ring. Regarding B3, a dichlorinated byproduct, it is formed when B2 suffers electrophilic substitution of hydrogen per chlorine in carbon number 6 of the phenolic ring. Although the presence of hydroxyl- and methoxyl-functionalities in carbons located in meta-position deactivates this type of reaction, there exists an atom of oxygen in ortho- to carbon number 6 due to the Baeyer–Villiger reaction, which increases the probability of a electrophilic attack by chlorine [22].
Re-evaluating the BP3 chlorination with the methodology used in BP4 studies, Negreira [22] observed two other BP3 byproducts which had empirical formula C14H10Cl2O4 and C14H9Cl3O4. The first one is formed when the UV-filter undergoes its most important reaction pathway: two successive electrophilic substitutions of hydrogen per chlorine in carbons located at positions number 3 and 5 in the phenolic ring [18] but only when chlorine level is 0.03 μg/mL and at long reactions [22]. However, this byproduct is also compatible with oxidation of the carbonyl bridge in the molecule of BP3 to an ester group but only after the first reaction. The second byproduct (C14H9Cl3O4) appears due to further electrophilic substitution of hydrogen per chlorine in carbon number 6 of the C14H10Cl2O4 at chlorine concentrations above 2 μg/mL [22].
So, it can be said that the most favorable reaction pathway of both BP3 and BP4 with free chlorine consists of electrophilic substitutions of hydrogen per chlorine in carbon numbers 3 and 5 (ortho- and para- to the 2-hydroxyl moiety). Only after this reaction or when these carbons are already attached to other functionalities, the carbonyl group is converted into an ester moiety which links the two aromatic rings of these UV-filters. Finally, the aromatic ring bonded to the atom of oxygen in the ester group might undergo a further electrophilic substitution reaction [22]. Figure 4 represents the reaction pathway of this BP4 with free chlorine proposed by Negreira et al. [22].
Chlorination reaction for BP4 proposed by Negreira et al. [22]
Chloroform was also found as stable byproduct in the chlorination of BP3 and another benzophenone: benzophenone-8 (BP8) (Fig. 2) [20]. Chloroform formation is a function of pH and occurs in the presence of excess chlorine. However, BP3 and BP8 exhibited different chloroform formation behavior depending on pH: for the first one, chloroform formation decreases when pH increases from 6 to 10. This behavior is generally not only due to the speciation of aqueous chlorine (HOCl to Cl−) but also due to the speciation of BP3 to the phenolate form, since chloroform/phenol molar yields have pH 8 as average for phenols and substituted phenols. Therefore, there is less HOCl to react with BP3. Concerning BP8, chloroform formation increases as pH increases from 6 to 10, probably due to 3-methoxy and the ortho- substituted phenolic moieties in BP8 molecular structure being less reactive with aqueous chlorine than BP3. Despite all of this, 3-methoxyphenol moiety appears to be the primary function group responsible for chloroform formation for both UV-filters [20].
There are two other UV-filters which are typically together in many commercial sunscreens: 2-ethylhexyl-4-methoxycinnamate (EHMC) and 4-tert-butyl-4′-methoxydibenzoylmethane (BDM). The first one has absorption capacity in the UVB range and the second one in UVA. Therefore, these two UV-filters combined offer UV protection over a wider range of wavelengths. Although EHMC and BDM are present in sunscreens as the isomer E for the first one and as enol form for the second one, under irradiation EHMC suffers isomerization from E to Z form (Fig. 5a) and BDM tautomerizes from enol to keto form (Fig. 5b) [7].
Santos et al. [23] observed six byproducts resulted from EHMC chlorination: two of them are dichlorinated products (C18H24O3Cl2) and the rest of them are monochlorinated byproducts (C18H25O3Cl). Both types of byproducts are probably the result of hydrogen replacement by chlorine in the benzene ring of EHMC in the same way already described above. Regarding BDM byproducts it was observed one monochlorinated byproduct (C20H21O3Cl) and one dichlorinated (C20H20O3Cl2). However, a similar reaction pattern is observed for these two UV-filters because the substitution of hydrogen atoms by chlorine can only occur in the benzene ring containing methoxy group, since chlorination in the benzene ring containing the t-Bu group is highly prohibitive due to the large volume of this group.
The reaction between chlorine and each of these UV-filters is regulated by some factors, such as pH, chlorine concentration, temperature, dissolved organic matter (DOM), and irradiation time. The principal factor affecting the EHMC chemical transformation is pH since the lower is the pH, the higher is the transformation percentages of EHMC. The explanation for this fact is that the main chlorine species present at low pH is HOCl (in contrast with at higher pH, where the hypochlorite anion (OCl−) is prevalent) which is more reactive towards EHMC, resulting in higher degradation. Nevertheless, higher temperature values also lead to higher transformation percentages and this is almost independent of the pH. Concerning BDM, chlorine concentration is the principal factor affecting its transformation percentage, since higher concentrations of chlorine will favor chlorine attack and the incorporation of chlorine in the UV filter structure even at high pH values. However, in presence of DOM, transformation percentages of BDM are low probably due a competition process between the UV-filter and DOM for the available chlorine [23].
2.2.1.1 Halobenzoquinones Formation
It was also observed the presence of halobenzoquinones (HBQs) in pool water that resulted from sunscreens chlorination. Aromatic structures in these PCPs such as phenols and quinones are likely to be the precursors of HBQs as well as some common ingredients of lotions, like benzyl alcohol, lecithin, parabens, and fragrances. UV-filters such as avobenzone, octocrylene (2-ethylhexyl-2-cyano-3,3-diphenyl-2-propenoate, OCT), and terephthalilidene dicamphor sulfonic acid may also be HBQ precursors [24]. Wang et al. [24] observed the formation of 2,6-dichloro-1,4-benzoquinone from the reaction between chlorine and four sunscreens containing organic and inorganic UV-filters. Although warm water provides a comfortable environment for swimmers, this fact may accelerate the chlorination reaction to produce more HBQs [24].
Besides 2,6-dichloro-1,4-benzoquinone, 2,6-dichloro-3-methyl-1,4-benzoqui-none, 2,3,6-trichloro-1,4-benzoquinone, and 2,6-di-bromo-1,4-benzoquinone also are common DBPs in chlorinated water [25].
2.2.2 Parabens Chlorination
Besides sunscreens, other PCPs such as parabens may also be present in pool water. Parabens belong to a group of bactericides and preservative agents in PCPs and they are continuously released in aquatic media through domestic wastewater and, although they are almost completely removed during sewage water treatments, they have been detected in rivers at low ng L−1 level. Considering the extensive employment of the compounds in PCPs, activities like showering and bathing constitute a source of dermal exposition to parabens DBPs [26]. The potential degradation of four alkylated parabens (methyl, ethyl, propyl, and butyl paraben) and the formation of DBPs were investigated by Canosa et al. [26]. Five transformation species were detected for each parent paraben corresponding to mono- and dichlorinated compound. Similar to some UV-filters, they are formed by a substitution of one or two atoms of hydrogen per chlorine in the aromatic ring and this chlorination occurs in both carbons in ortho- to the phenolic group, since the para- position is blocked with the ester moiety. In tap water, the chlorine content is usually enough to produce significant amounts of these DBPs in few minutes. However, the dichlorinated byproducts are rather resistant to undergo further chlorine substitution reactions or cleavage of the aromatic ring, even in presence of relatively high concentrations of chlorine. So, if they are generated in real-life situation, their presence in the aquatic environment is feasible [26].
3 Toxic Effects of UV-Filters and Its Chlorination Byproducts
It is known that byproducts formed from reaction between chlorine and natural organic matter of water, such as chloroform as well other trihalomethanes, nitrosamines, haloacetic acids, etc., have toxic effects like carcinogenic effects in animals and human beings [27]. Now, it is mandatory to assess the toxicity of DBPs formed from PCPs chlorination. The knowledge of this subject is still poor but there are already a few papers published in order to study the toxicity of some of these compounds.
Bladder cancer has been associated with exposure to chlorination byproducts in drinking water, and experimental evidence suggests that exposure also occurs through inhalation and dermal absorption during swimming in pools because certain DBPs have high volatility and dermal permeability. Villanueva et al. [28] observed that subjects who had ever swum in a pool showed an increased risk of bladder cancer compared with those who had never swum in pools and former and current smokers present an excess risk of bladder cancer. This study also revealed a duration-response relation for cumulative time spent in swimming pools. To evaluate the genotoxicity of swimming pool water in swimmers, Kogevinas and co-workers [29] examined some biomarkers of genotoxicity in an experimental study in which adults swam for 40 min in a chlorinated, indoor swimming pool, comparing the biomarker results with the concentrations of four THMs (bromoform, bromodichloromethane, chloroform, and chlorodibromomethane) in exhaled breath. It was observed increases in two biomarkers of genotoxicity (micronuclei in peripheral blood lymphocytes and urinary mutagenicity). Although only brominated THMs showed genotoxicity, all four are carcinogenic in rodents.
It was also verified that recreational pool waters are more genotoxic [30] and cytotoxic than tap water and this elevated genotoxicity and cytotoxicity are associated with many classes of nitrogenous-DBPs (N-DBPs) [10]. The higher genotoxicity of the recreational pools compared to the tap water source could reflect prolonged disinfectant contact times [30].
Furniture conditions, such as illuminations condition, also affect the cytotoxicity of pool water [10, 30]: The pool water under indoor conditions was more cytotoxic (LC50 = 24.2×) than when it was operated as an outdoor pool (LC50 = 181.4×). The outdoor pool exposed to sunlight featured lower cytotoxicity than the same pool under indoor conditions which indicate that either the compounds responsible for the cytotoxicity, or their precursors, may be photolabile [10] or have increased volatilization [30]. Physical activity appears to enhance the absorption of DBPs [31].
UV-filters have high lipophilicity (mostly with log K ow 4–8) whereby they have been shown to accumulate in the food chain and in human milk fat. However, at present, there is a scarcity of data on environmental concentrations of UV-filters [32, 33]. Moreover, concentrations reported fluctuate significantly as a function of sample location, size of the system under study (e.g., lakes and swimming pools), frequency and type of recreational activities, season of the year, and hour of the day. Still, maximum concentrations reported have corresponded to mid-day on warm summer days, as expected [33]. Among UV-filters, octocrylene is of great concern since it has a high lipophilicity (K ow 6.88). Actually, this UV-filter has already detected in liver tissues of dolphins (Pontoporia blainvillei) with concentrations in the range 89–782 ng/g lw and there is evidence that maternal transfer may occur trough placenta and likely also through breast milk [34].
4 Conclusions and Further Researches
Disinfection of drinking water is important for public health but many people are exposed to chlorination byproducts not only through ingestion but also through other activities such as showering, bathing, and swimming [35]. So, future studies should evaluate more completely the uptake and potential effects of a range of DBPs present in pool water [29]. Although the mixture of the byproducts may differ by geographic area and time, studies are needed to examine the potential effects of these mixtures [35]. Furthermore, it is important to examine the various exposure pathways and routes other than ingestion in more detail.
Reports on the occurrence of sunscreen agents in natural waters have so far been scarce and have mainly focused on bathing waters in closed systems (e.g., swimming pools or small lakes). A great deal of additional data is needed to understand the significance of UV-filters in the aquatic environment. It is also necessary to increase knowledge of their bioaccumulation in humans and wildlife [33]. It is also important that further researches take into account pool operation/maintenance. Pool disinfection is essential to preventing exposure to pathogens; still, DBP formation can be reduced with proper disinfectant use along with known engineering solutions. Unhygienic practices enhance the amount of organic matter released by swimmers through urine and other body fluids. So, substantial investments into education and outreach will be necessary to affect these behaviors and practices. By improving disinfection practices and reducing the input of contaminants both chemical and biological, the goal of healthier pools and healthier people can be achieved [6]. For example, showering and using toilet facilities, washing off sunscreen lotions, and applying water-tight diapers can reduce the bather load and help to reduce the potential for DBP formation [36]. If swimmers take showers frequently, DBPs will be removed on skin preventing them from deeper penetration [37].
Environmental chemistry studies should also focus on strategies to minimize the formation of chlorinated byproducts of UV-filters by the development of new sunscreen formulations that prevent the release of UV-filters into chlorinated water [23].
Haloquinones have been proving to be more toxic than the regulated halomethanes [25]. The potential toxic effects of these compounds warrant further investigations into the occurrence, human exposure, and management of haloquinones in chlorinated water [25].
Regarding other cosmetics ingredients further studies are needed to evaluate potential human health risks and ecotoxicological effects of halogenated byproducts and to know their fate in the environment [26, 27].
References
Poiger T, Buser H-R, Balmer ME et al (2004) Occurrence of UV filter compounds from sunscreens in surface waters: regional mass balance in two Swiss lakes. Chemosphere 55:951–963
Giokas DL, Sakkas VA, Albanis TA (2004) Determination of residues of UV-filters in natural waters by solid-phase extraction coupled to liquid chromatography-photodiode array detection and gas chromatography–mass spectrometry. J Chromatogr A 1026:289–293
Sui Q, Huang J, Deng S et al (2011) Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in different biological wastewater treatment processes. Environ Sci Technol 45:3341–3348
Westerhoff P, Yoon Y, Snyder S, Wert E (2005) Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ Sci Technol 39:6649–6663
Onesios KM, Yu JT, Bouwer EJ (2009) Biodegradation and removal of pharmaceuticals and personal care products in treatment systems: a review. Biodegradation 20:441–466
Lakind JS (2010) The good, the bad, and the volatile: can we have both healthy pools and healthy people? Environ Sci Technol 44:3205–3210
Santos AJ, Miranda MS, Esteves da Silva JCG (2012) The degradation products of UV-filters in aqueous and chlorinated aqueous solutions. Water Res 46:3167–3176
Buth JM, Arnold WA, McNeill K (2007) Unexpected products and reaction mechanisms of the aqueous chlorination of cimetidine. Environ Sci Technol 41:6228–6233
Richardson SD, DeMarini DM, Kogevinas M et al (2010) What’s in the pool? A comprehensive identification of disinfection byproducts and assessment of mutagenicity of chlorinated and brominated swimming pool water. Environ Health Perspect 118:1523–1530
Plewa MJ, Wagner ED, Mitch WA (2011) Comparative mammalian cell cytotoxicity of water concentrates from disinfected recreational pools. Environ Sci Technol 45:4159–4165
Barbot E, Moulin P (2008) Swimming pool water treatment by ultrafiltration-adsorption process. J Membr Sci 314:50–57
Anipsitakis GP, Tufano TP, Dionysiou DD (2008) Chemical and microbial decontamination of pool water using activated potassium peroxymonosulfate. Water Res 42:2899–2910
Bond T, Goslan EH, Parsons SA, Jefferson B (2012) A critical review of trihalomethane and haloacetic acid formation from natural organic matter surrogates. Environ Technol Rev 1:93–113
Li J, Blatchley ER III (2007) Volatile disinfection byproduct formation resulting from chlorination of organic-nitrogen precursors in swimming pools. Environ Sci Technol 41:6732–6739
Kanan A, Karanfil T (2011) Formation of disinfection byproducts in indoor swimming pool water: the contribution from filling water natural organic matter and swimmer body fluids. Water Res 45:926–932
Shah AD, Mitch WA (2011) Halonitroalkanes, halonitriles, haloamides, and n-itrosamines: a critical review of nitrogenous disinfection byproduct formation pathways. Environ Sci Technol 46:119–131
Shen R, Andrews SA (2011) Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection. Water Res 45:944–952
Negreira N, Canosa P, Rodriguéz I et al (2008) Study of some UV-filters stability in chlorinated water and identification of halogenated by-products by gas chromatography–mass spectrometry. J Chromatogr A 1178:206–214
Salvador A, Chisvert A (2005) Sunscreen analysis. A critical survey on UV-filters determination. Anal Chim Acta 537:1–14
Duirk SE, Bridenstine DR, Leslie DC (2013) Reaction of benzophenone UV-filters in the presence of aqueous chlorine: kinetics and chloroform formation. Water Res 47:579–587
Sakkas VA, Giokas DL, Lambropoulou DA et al (2003) Aqueous photolysis of the sunscreen agent octyl-dimethyl-p-aminobenzoic acid. Formation of disinfection byproducts in chlorinated swimming pool water. J Chromatogr A 1016:211–222
Negreira N, Rodriguéz I, Rodil R, Cela R (2012) Assessment of benzophenone-4 reactivity with free chlorine by liquid chromatography quadrupole time-of-flight mass spectrometry. Anal Chim Acta 743:101–110
Santos AJ, Crista DMA, Miranda MS et al (2013) Degradation of UV-filters 2-ethylhexyl-4-methoxycinnamate and 4-tert-butyl-4′-methoxydibenzoylmethane in chlorinated water. Environ Chem 10:127–134
Wang W, Qian Y, Boyd JM et al (2013) Halobenzoquinones in swimming pool waters and their formation from personal care products. Environ Sci Technol 47:3275–3282
Zhao Y, Qin F, Boyd JM et al (2010) Characterization and determination of chloro- and bromo-benzoquinones as new chlorination disinfection byproducts in drinking water. Anal Chem 82:4599–4605
Canosa P, Rodríguez I, Rubí E et al (2006) Formation of halogenated byproducts of parabens in chlorinated water. Anal Chim Acta 575:106–113
Hrudey SE (2009) Chlorination disinfection by-products, public health risk tradeoffs and me. Water Res 43:2057–2092
Villanueva CM, Cantor KP, Grimalt JO et al (2007) Bladder cancer and exposure to water disinfection byproducts through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol 165:148–156
Kogevinas M, Villanueva CM, Font-Ribera L et al (2010) Genotoxic effects in swimmers exposed to disinfection byproducts in indoor swimming pools. Environ Health Perspect 118:1531–1537
Liviac D, Wagner ED, Mitch WA et al (2010) Genotoxicity of water concentrates from recreational pools after various disinfection methods. Environ Sci Technol 44:3527–3532
Lourencetti C, Grimalt JO, Marco E et al (2012) Trihalomethanes in chlorine and bromine disinfected swimming pools: air-water distributions and human exposure. Environ Int 45:59–67
Gago-Ferrero P, Alonso MB, Bertozzi CP et al (2013) First determination of UV-filters in marine mammals. Octocrylene levels in franciscana dolphins. Environ Sci Technol 47:5619–5625
Díaz-Cruz MS, Llorca M, Barceló D (2008) Organic UV-filters and their photodegradates, metabolites and disinfection byproducts in the aquatic environment. Trends Anal Chem 27:873–887
Subedi B, Du B, Chambliss CK et al (2012) Occurrence of pharmaceuticals and personal care products in German fish tissue: a national study. Environ Sci Technol 46:9047–9054
Nieuwenhuijsen MJ, Martinez D, Grellier J et al (2010) Chlorination disinfection byproducts in drinking water and congenital anomalies: review and meta-analyses. Environ Health Perspect 117:1486–1493
Zwiener C, Richardson SD, de Marini DM et al (2007) Drowning in disinfection byproducts? Assessing swimming pool water. Environ Sci Technol 41:363–372
Xiao F, Zhang X, Zhai H et al (2012) New halogenated disinfection byproducts in swimming pool water and their permeability across skin. Environ Sci Technol 46:7112–7119
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
de Oliveira e Sá, M.M., Miranda, M.S., Esteves da Silva, J.C.G. (2014). Occurrence of Personal Care Products and Transformation Processes in Chlorinated Waters. In: Díaz‐Cruz, M., Barceló, D. (eds) Personal Care Products in the Aquatic Environment. The Handbook of Environmental Chemistry, vol 36. Springer, Cham. https://doi.org/10.1007/698_2014_263
Download citation
DOI: https://doi.org/10.1007/698_2014_263
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18808-9
Online ISBN: 978-3-319-18809-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)