Abstract
Osteoarthritis (OA) and other degenerative joint diseases are characterized by articular cartilage destruction, synovial inflammation, sclerosis of subchondral bone, and loss of extracellular matrix (ECM). Worldwide, these diseases are major causes of disability. Cell therapies have been considered to be the best therapeutic strategies for long-term treatment of articular cartilage diseases. It has been suggested that the mechanism of stem cell-based therapy is related to paracrine secretion of extracellular vesicles (EVs), which are recognized as the main secretion factors of stem cells. EVs, and in particular the subclass exosomes (Exos), are novel therapeutic approaches for treatment of cartilage lesions and OA. The results of recent studies have shown that EVs isolated from mesenchymal stem cells (MSCs) could inhibit OA progression. EVs isolated from various stem cell sources, such as MSCs, may contribute to tissue regeneration of the limbs, skin, heart, and other tissues. Here, we summarize recent findings of preclinical and clinical studies on different MSC-derived EVs and their effectiveness as a treatment for damaged cartilage. The Exos isolation techniques in OA treatment are also highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Articular cartilage possesses a decreased natural ability to repair itself after an injury. Once a defect develops, the area underneath the subchondral bone becomes involved and results in an osteochondral defect, which is recognized as osteoarthritis (OA) (Brittberg et al. 2016). An estimated 250 million people currently suffer from cartilage defects, and there is a predicted six–sevenfold increase in OA in the next decade (Mora et al. 2018). Currently, no gold standard clinical treatment exists for articular cartilage defects. Traditional and investigational new drugs for cartilage lesions or OA disease focus on rescue from pain and inflammation, but they lack the capacity to regenerate damaged cartilage (Zhang et al. 2016a). Current surgery based-therapeutic approaches include microfracture and osteochondral grafts; however, they result in the formation of a fibrocartilage type tissue (Medvedeva et al. 2018). Recent cell-based therapeutic approaches, such as chondrocyte or stem cell therapy, are powerful strategies for cartilage tissue regrowth. Long-term analysis of clinical trials for OA treatment by autologous chondrocyte implantation (ACI) indicated that more than 90% of the patients continued to have good function five years post-implantation (Mithoefer et al. 2012). Concerns regarding autologous cell transplantation include loss of chondrocyte phenotype, limited donor availability, and fibrocartilage tissue development (Mithoefer et al. 2012).
Injection of undifferentiated cells, including stem cells, increases the risk for cell migration toward an incorrect site, and they may differentiate into ectopic tissue (Herberts et al. 2011). The use of modified or allogenic cells can induce rejection of the implanted cells by increasing autoimmune reactions and may increase the risk of cancer or other side effects of the cell therapy after cartilage repair (Kim and Cho 2015).
An abundance of evidence has shown that cell-free approaches are the most recent techniques for treatment of cartilage lesions. For instance, extracellular vesicles (EVs) and soluble factors released by mesenchymal stem cells (MSCs) are responsible for the therapeutic effectiveness of stem cells (Gimona et al. 2017). EVs that originate from the endosome (30–150 nm in diameter) contain a membrane that encapsulates mRNAs, microRNAs, proteins, and liposomes. These factors have potential use in development of drug delivery therapeutic biomarkers (Li et al. 2019). Recently, EVs such as Exos have emerged as potent cell-free transfer tools because of their elevated physicochemical strength and biocompatibility.
Three major EV classes secreted by cells include apoptotic bodies, microvesicles (MVs), and Exos. It has been assumed that these vesicle populations are homogeneous in size and density; however, the subtypes are heterogeneous in nature (Kim et al. 2013). Recent evidence confirmed that MSCs release distinct-sized EV sub-populations that have different biophysical, proteomic, and RNA repertoires (Willms et al. 2016). MSCs can secrete many diverse subtypes of vesicles That are composed of various RNA, miRNA, DNA, and proteins (Zhang et al. 2018a).
Risks for embolism or tumorigenesis are not associated with Exos therapies compared to modified cell therapies, and this has made Exos a promising tool for regenerative medicine. Furthermore, their low toxicity and the ability to cross the blood-brain barrier makes them far preferable to other delivery procedures such as microcarriers and synthetic drug carriers (Jiang and Gao 2017).
Although the results of several preclinical studies showed a robust ability of EVs-MSCs as treatments for various diseases, safety considerations in clinical trials should still be addressed. In addition, the standard expansion of large scale GMP-grade Exos-based pharmaceuticals are main current challenges that should be resolved (Navabi et al. 2005). In the MSC culture settings, Exos are harvested according to procedures that need standardization, however, it is expected that mutagenicity and oncogenicity concerns of Exos-based clinical trials will be less when compared to live cell MSC trials.
The purpose of the present review is to summarize the studies with Exos, modified Exos, or those derived from manipulated cells as natural nanocarrier treatments of cartilage lesions or OA. We also discuss different isolation methods used to harvest EVs.
2 Articular Cartilage Structure, Injuries, and Repair
2.1 Articular Cartilage Structure and Function
Articular cartilage is a highly dense tissue characterized by a smooth, lubricated surface (Eckstein et al. 1996). The main role of articular cartilage is to support the surrounding soft tissues, absorb shock, lubricate for the joints and facilitate bone movements, mechanical load-bearing, and protect the subchondral bones from frictional shear forces (Luo et al. 2017). This crucial function is related to the composition of the extracellular matrix (ECM), particularly the arrangement and orientation of the collagen fibers and its connection to the ECM macromolecules (glycosaminoglycan [GAGs] and glycoproteins) (Shen 2005). Remarkably, articular cartilage is an avascular, aneural, alymphatic, and hypocellular tissue that receives its nutrients by a double diffusion barrier via the synovial fluid and subchondral bone (Kwan et al. 1991). Articular cartilage varies in thickness from 1 to 7 mm in humans, depending on the location of the joint. Chondrocytes are one type of specialized cartilage cell that constitutes only 1–5% of the articular cartilage volume; thus, they have no cell-to-cell interactions, and are similar to osteocytes that reside in bone tissue (Kozhemyakina et al. 2015). Chondrocytes are highly specialized cells that are responsible for the synthesis, maintenance, and turnover of the specialized matrix infrastructure that is rich in GAGs and proteoglycans. Proteoglycans and their associated GAGs have important functional roles in tissue remodeling, and they maintain the fluid, uptake of proteins, intracellular signaling, cell migration, and the electrolyte balance in articular cartilage. Despite its high specification, articular cartilage is a thin layer that has a low potential for self-regeneration. Over the past three decades, regeneration of articular cartilage and the underlying mechanisms of cartilage restoration have been primary challenges in clinical and experimental settings. However, current approaches have not shown acceptable outcomes for restoration of articular cartilage function. Nevertheless, it is important to understand current therapeutic strategies and their impacts (Luo et al. 2017; Shen 2005; Kwan et al. 1991; Ryan et al. 2009).
2.2 Articular Cartilage Injuries and their Repair
Chondral lesions are attributed to several factors and their symptoms include swelling, localized pain, and locking. Local cartilage lesions are classified in stages according to the severity of the lesions (Zhang et al. 2018b; Kwon et al. 2019). Chondral damages include clefts, fissures, chondral flaps or tears, and loss of part of the articular cartilage and are triggered by acute or repetitive trauma (Zhang et al. 2018b). In osteochondral lesions, injuries extend into the subchondral bone and cause hemorrhaging and fibrin clot formation. Inflammatory responses may also occur at the injured site in this condition (Buckwalter 2002).
Chondral and osteochondral damages are the most common types of joint injuries and they are graded according to the depth of the lesion. In addition to the size of the lesion, the patient’s age is also an important aspect in non-healing cartilage (Lespasio et al. 2017).
Failure to seek initial treatment along with progressive cartilage degeneration followed by excessive focal stresses on the joints can lead to OA. On the other hand, chondral injuries are progressive and mainly occur because of traumatic or abnormal loading on the joints. In this progressive event, chondrocytes throughout the cartilage thickness undergo apoptosis or necrosis and subsequent damage to the ECM. These crucial conditions lead to secondary hypoxic damage that develops as a result of activation of a cascade of inflammatory factors, tissue necrosis, scar repair, and remodeling. When the full-thickness lesions occur, destruction progresses to the subchondral bone and there is an influx of blood cells that include MSCs and medullary bone elements into the lesion site from medullary bone marrow. Thereafter, the full-thickness defect is filled by a fibrocartilage-like tissue with type I collagen fibers secreted by the MSCs that penetrated into the injured site. However, biochemical, biomechanical, and load-bearing properties of alternative tissues are inferior compared to hyaline cartilage and are not appropriate for articular cartilage (Bhosale and Richardson 2008).
The presence of blood vessels is essential for tissue repairs; hyaline cartilage lack blood vessels and, thus, fails to appropriately rebuild or restore the damaged tissue. Strategies for repairing cartilage defects are categorized into two completely different approaches - traditional surgery and novel tissue engineering/cell-based methods.
2.2.1 Traditional Treatment of Cartilage Defects
-
(a)
Microfracture (bone marrow stimulation)
In 1984, Steadman introduced the microfracture technique as the primary surgery method for hyaline cartilage restoration. This method involves orderly removal of all the calcified cartilage covering followed by the generation of small fractures in the underlying bone in order to release bone marrow clots into the site of the cartilage defect, which would induce hyaline-like tissue formation (Xu et al. 2015). Significant improvements were reported in more than 80% of patients after microfracture surgery when compared with the pre-operative condition. Long-term follow-up indicated the formation of fibrous tissue, which primarily consisted of type I collagen, at the defect site. Although this method was reported to be effective by most studies, inconsistency and variable results, along with degradation of newly formed tissue have been reported (Shen 2005; Kwan et al. 1991). Asik et al. used the microfracture technique for cartilage repair in 90 patients who had focal full-thickness articular cartilage defects. The patients reported considerable pain relief and better cartilage performance. Furthermore, a correlation existed between functional performance and prognostic parameters such as age, size of the defect, and body mass index (Asik et al. 2008). Mithoefer et al. stated that knee function was good to excellent for 67% and poor for 8% of 48 study patients with articular cartilage lesions during a short follow-up of 2 years (Mithoefer et al. 2005).
It is believed that the quality and quantity of bone marrow-derived stem cells and patient’s age play a critical role in the microfracture efficacy (Kozhemyakina et al. 2015). The new tissue forms nearly 8 weeks after surgery; thus, the postoperative recovery seems to be effective (Chu et al. 2018).
-
(b)
Arthroscopic debridement and drilling of osteochondral lesions
Debridement of joints was first carried out by Pridi in 1959 on an experimental rabbit model. Magnusson established this procedure more than 60 years ago to treat human knee injuries (Hubbard 1996). Debridement is an arthroscopic surgery in human and veterinary medicine where small holes are generated in the subchondral bone and unstable cartilage and necrotic bone are removed with a curette to the border of healthy tissue. This stimulation causes the release of surface proteoglycans, which can encourage later adhesion of reparative cells from the synovial membrane. Microfracture is often used for full-thickness lesions in joints, whereas drilling is performed for the initial stage of joint lesions where damaged cartilage is less than or equal to 10 mm in diameter. On the other hand, debridement is indicated in cases of necrotic bone, and the overlying cartilage is intact or compromised (Logli et al. 2019; Bexkens et al. 2017). Improvement was observed in 74% of 78 patients after a 1-year follow-up.
-
(c)
Mosaicplasty
Osteochondral autograft transfer mosaicplasty is a common surgical procedure for osteochondral lesions that was first described in 1993 by Matsusue (Matsusue et al. 1993). In this method, cylindrical osteochondral plugs from low-bearing areas of the articular cartilage are grafted into the cartilage defect. Notwithstanding the suitable outcomes, inadequate donor tissue is the main problem of autologous osteochondral grafts. Moreover, the replaced fragments may not integrate with the native hyaline cartilage and, in some cases, may cause cyst formation due to diffusion of the synovial fluid inside the joints (Hangody et al. 1998; Hangody and Fules 2003; Smith et al. 2005).
-
(d)
Soft tissue grafts
In 1990, Homminga engrafted autologous perichondrium as a biomembrane in another regenerative method to repair cartilage lesions (Homminga et al. 1990). The periosteum, which also has both osteogenic and chondrogenic capabilities due to progenitor cells (PCs) that reside on the cambium layer, is another alternative. PCs are maintained within the periosteum and are recruited in response to injury. Therefore, these biomembranes are ideal biological tools for repair of cartilage lesions (Bouwmeester et al. 1997; Duchamp de Lageneste et al. 2018). Accordingly, transplantation of periosteum and perichondrium flaps have been widely used in full-thickness defects of articular cartilage in animal models and in human clinical trials (Bouwmeester et al. 1999; Carranza-Bencano et al. 1999).
-
(e)
Osteotomy
An osteotomy is a controlled surgical break of bone that allows for bone realignment. It is performed to correct primary knee deformities and as a treatment for knee OA. Smith, in 1958, first used this technique to realign the knee joint of early or medial unicompartmental OA (Smith et al. 2005). Tibial and femoral osteotomies are two types of surgical procedures. The osteotomy is a corrective surgical procedure where cutting and realignment of the bone distributes the joint loading and prevents pressure on the cartilage surface. This procedure may reduce pain, enhance function, postpone knee deterioration, and delay the need for a partial or total knee replacement surgery (Brouwer et al. 2014; Schultz and Gobel 1999).
2.2.2 Novel Tissue Engineering and Cell-Based Methods
-
(a)
Engineered cartilage tissue
In the last few years, tissue engineering has been developed as an alternative to traditional procedures. Accordingly, supportive scaffolds that carry cells and guide matrix production are well-known as promising tools for cartilage regeneration (Nam et al. 2018). Scaffold-based techniques is a cutting-edge technology that uses a three-dimensional (3D) structured material to rebuild new tissue that has a high degree of similarity in architecture and function to the native cell environment. Furthermore, they must allow for successful infiltration, and stimulate cellular differentiation and proliferation via providing suitable bioactive molecules. Although there are numerous scaffolds from different origins (synthetics or natural), alignments and structures, an ideal scaffold should have the capability to induce chondrogenesis and ECM formation, it should be biocompatible, biodegradable and absorbable, and non-immunogenic with appropriate mechanical properties comparable to native cartilage (Nam et al. 2018). Moreover, the surface topography, elasticity, mechanical, and biochemical properties of a scaffold play a principal role in cell behavior. Recently, the use of 3D bio-printed scaffolds enabled fabrication of customized structures specific for individual defect sites (Medvedeva et al. 2018). Although this method is a high-resolution strategy to fabricate scaffolds, it is relatively expensive.
The choice of a cell source has enormous impact on the success of cartilage restoration and is one of the foremost challenges related to populating scaffolds. Autologous cell sources such as chondrocytes may avoid immune response, but other crucial factors for choosing the proper cell source include accessibility, reproducibility, responsiveness to growth factors, and not tumorigenic. In order to overcome these limitations, MSCs are considered to be an alternative allogenic cell source for cartilage repair because they lack the limitations associated with chondrocytes. In addition, anti-inflammatory and immunomodulatory properties of MSCs facilitate tissue wound repair and chondrogenesis without the need to suppress inflammation (Solheim et al. 2016).
The use of autologous chondrocyte spheroids (chondrospheres) is a novel scaffold-free approach to regenerate lesions. Its advantages include not interrupting cell-cell interactions and high integration potential with adjacent tissue. In a study of a minipig model, chondrocyte spheroids were well-integrated with the host tissue (Meyer et al. 2012). An ongoing randomized phase III clinical study (CO.Don® AG) is based on chondrospheres and the results are not yet available (Fickert et al. 2012).
In this section, clinical interventions for articular cartilage regeneration were briefly discussed in addition to some emerging technologies that are promising for cartilage rehabilitation. Yet, a well-characterized technology should be developed to address the appropriate bioactivity, integrity, biomechanical, and biological properties of the articular cartilage tissue. In the next section, we discuss in detail the emerging technology of cell therapy for cartilage regeneration and focus on its challenges and potentials.
-
(b)
Cell-based therapies in cartilage regeneration
An emerging technology for articular cartilage regeneration is cell therapy based on autologous or allogenic cells, differentiated or stem cells (Wang et al. 2017a). Stem cells are multipotent cells found in various tissues that have tremendous potential for self-renewal and differentiation.
The ACI procedure is a form of tissue engineering as a treatment for deep focal chondral defects. ACI is the first application of cell therapy for cartilage regeneration, which was developed by Peterson in 1987. A cartilage biopsy is surgically collected from a low-weight-bearing area and chondrocytes are released from the ECM following enzymatic treatment. They undergo large scale expansion in vitro for implantation into the chondral defects (Brittberg et al. 1996). The cartilage defect is covered by a membrane once the chondrocytes are implanted into the defect site. Clinical outcomes have shown that ACI is an effective therapy for large cartilage defects (>4 cm2) (Zhang et al. 2018a; Buckwalter 2002). The longest follow-up investigation showed overall improvement in knee functions in 84% of the patients.
Potential immune issues are avoided with ACI because the patient’s own cells are used (Bhosale and Richardson 2008; Xu et al. 2015). Matrix-induced autologous chondrocyte implantation (MACI) is a refined version of ACI in which isolated autologous chondrocytes are cultured on type I or III collagen membranes (Shen 2005). Despite promising results, ACI and MACI have donor limitations and complications that include donor site morbidity and graft failure. Chondrocyte hypertrophy in response to in vitro expansion is another challenge. Limitations such as the need for an additional operation and dedifferentiation potential during in vitro cultivation should be addressed (Fisher et al. 2017).
Recently, various commercial chondrocyte-based samples prepared under good manufacturing practice (GMP) conditions are on the market and in different clinical trial phases. However, once the specialized cells are implanted, immunosuppressive agents must be administered to prevent graft rejection (Ebrahimi et al. 2014). Some studies have used ACI for cartilage disorders treatments (Peterson et al. 2003; Brittberg et al. 1994; Steinwachs 2009).
In parallel, nasal septum chondrocytes are an alternative terminally differentiated cell for cartilage regeneration. An ongoing study by the University Hospital of Basel is using nasal chondrocytes (Nose2Knee), and has completed a phase I clinical trial after successful outcomes on the safety and feasibility of this procedure (Onuora 2016).
MSCs have been considered as an alternative allogenic cell source for cartilage repair because they do not have the limitations associated with chondrocyte. MSCs are multipotent stromal cells found in various tissues and organs such as the bone marrow, umbilical cord blood, adipose tissue, and synovial fluid. The chondrogenic potential of MSCs depends on the tissue source. Yoshimura et al. conducted a comparison study between rat MSCs derived from bone marrow, synovium, periosteum, adipose tissue, and muscle (Yoshimura et al. 2007). It has been reported that the synovium-derived MSCs have a 100-fold higher colony number per nucleated cells than bone marrow-derived MSCs (BmMSCs). Moreover, synovium-derived MSCs have the highest potential for proliferation and chondrogenesis. An advantage of MSCs over terminally differentiated chondrocytes is their easier in vitro expansion (Nam et al. 2018).
Currently, articular cartilage regeneration by transplantation of autologous MSCs is a widely used procedure (Negoro et al. 2018). Intra-articular administration of MSCs is a minimally invasive method in articular cartilage regeneration due to the presence of synovial fluid with less tissue damage (Nasiri et al. 2019). However, it is important to investigate the fate and homing of the cells. A research group in Germany established a reliable tracking method of genetically labeled MSCs in distant organs of rat models after injection into articular knee (Zwolanek et al. 2017; Satue et al. 2019). Although a few MSCs were spotted in the lungs of one rat 1 day after the injection, there was no other evidence of donor cells observed in the distant organs during a 6-month observation period. The injected MSCs improved cartilage regeneration and supported the safety and efficacy of an intra-articular injection of MSCs. In a completed phase I-II clinical trial, 15 patients with chronic OA each received a single injection of intra-articular autologous BmMSCs. The patients were observed for 12 months after the injection (Soler et al. 2016). Both the regenerative and anti-inflammatory results supported the feasibility and safety of this procedure. Re-Join is another MSC therapy for OA that is based on autologous adipose-derived MSCs (ADMSCs). The results of a phase II clinical trial (Lu et al. 2019) that enrolled 26 patients who received Re-Join injections showed significant improvements in terms of joint function and cartilage regeneration after 12 months of follow-up.
A study of dose selection of ADSC injections explored the impact of cell dosage on cartilage regeneration in 18 patients with severe knee OA (Pers et al. 2016). The phase I clinical trial outcomes showed no major adverse effect and patients had significantly improved pain levels and cartilage function at the low-dose MSC injection (cells) after 6 months of follow-up. A similar, recent study of 50 selected patients who had knee OA assessed three doses of intra-articular autologous MSC injections and compared them with platelet-rich plasma (PRP) injections. After 12 months of follow-up, patients who received the PRP injections reported no significant improvement, whereas radiological and arthroscopic examinations showed improved hyaline cartilage regeneration with the mid-dose MSC (5 × 107 cells) injection (Filardo et al. 2015). Ozeki et al. investigated the effect of single-dose or multiple-dose injections of synovial MSCs on rat OA models (Ozeki et al. 2016). It was concluded that intra-articular injected green fluorescent protein (GFP)-labeled MSCs mostly migrated into the synovium while maintaining their undifferentiated state. These MSCs expressed anti-inflammatory and chondroprotective proteins TSG-6, PRG4, and BMPs that hindered OA progression. The number of MSCs in the synovium decreased over time; thus, a weekly injection of cells for up to 12 weeks maintained the long-term effects of the procedure. In addition to autologous MSC therapy, allogeneic treatments have been conducted in animals with promising outcomes. In a recent study, canine models were subjected to an intra-articular injection of either hyaluronic acid (HA) (2 mL, 1%) or allogeneic BmMSCs (1 × 107cells) in conjunction with HA (2 mL, 1%) (Li et al. 2018). After 28 weeks, the animals were sacrificed and examined in terms of cartilage regeneration or emerging adverse effects. According to histological staining and immunohistochemistry, allogeneic MSCs plus HA resulted in more cartilaginous tissue than HA alone. In a similar study, OA rabbits received intra-articular allogeneic BmMSCs (1 × 106) in combination with HA (0.4 mL, 1%) (Chiang et al. 2016). It was concluded that histological scores and inhibition of OA were significantly higher in the animals injected with allogeneic MSCs plus HA. Stempeucel® is a biologic product based on allogeneic pooled human BmMSCs for OA treatment. In a clinical study, 60 patients with knee OA received an intra-articular injection of either 25, 50, 75, or 150 × 106 cells of Stempeucel® in combination with HA (2 mL, 1%) (Gupta et al. 2016). Adverse events of pain and swelling were observed at the higher doses of MSCs (above 50 × 106 cells). Although Stempeucel® was safe at the lowest dose and an improving trend was observed for cartilage repair and pain relief, the MRI score revealed no significant improvements compared with the placebo (PLASMA-LYTE) group. This finding suggests that additional, thorough investigations are essential. Another promising approach for cartilage regeneration via cell therapy is the combined injection of MSCs and chondrocytes. A phase I/II clinical trial on IMPACT by University Medical Center Utrecht was based on the intra-articular injection of autologous chondrocytes (10–20%) in combination with allogeneic MSCs (80–90%) (de Windt et al. 2017). After 12 months’ follow-up, there were no adverse effects observed in the patients. MRI scans indicated that the defects were filled in patients with cartilage tissue, and tissue biopsies showed elevated levels of proteoglycans and collagen type II. Short tandem repeat (STR) analysis revealed that after 12 months, the biopsy tissues had only autologous DNA and no allogeneic DNA was identified. No significant difference was observed in 10% or 20% of the chondrocytes. Thus, it can be concluded that transplantation of allogeneic or autologous MSCs for articular cartilage repair is effective in terms of pain relief and short-term tissue restoration. However, long-term assessment is crucial to confirm the safety and efficacy of the underlying procedure.
Many strategies have been proposed to efficiently induce chondrogenic differentiation of iPSCs through formation of embryoid bodies (Umeda et al. 2012; Lee et al. 2015), differentiation into intermediate MSCs (Nejadnik et al. 2015; Chijimatsu et al. 2017), co-culture with primary chondrocytes (Wei et al. 2012; Qu et al. 2013), or the use of growth factors (Cheng et al. 2014; Saito et al. 2015); however, no solid, reproducible protocol has been developed. Although iPSCs are superior in proliferation rate and chondrogenic potential compared to MSCs, other limitations restrict their use in therapeutic applications (Ko et al. 2014). Autologous iPSC therapy is very expensive and allogeneic therapy encounters safety and immunological issues (Lo Monaco et al. 2018). Major challenges in the chondrogenic differentiation of iPSCs include obtaining a purified and homogeneous population of cells and the risk of tumorigenesis. Kotaka et al. provided a strategy for iPSCs delivery to the defect site that used magnetic-labeled cells. Briefly, human fetal lung cell-derived iPSCs were labeled with iron nanoparticles and purified by an external magnetic field (Kotaka et al. 2017). Then, 18 nude rats with patellar defects were treated with a suspension of magnetic-labeled iPSCs in 3% atelocollagen at 107 cells/ml. At 8 weeks after the transplantation, all of the defects were covered by a smooth surface hyaline-like cartilage and no tumors were observed. However, a follow-up study of more than 8 weeks would be needed to prove the safety of this procedure. Saito et al. established a 2 week chondrogenic differentiation protocol of human neonatal dermal fibroblast-derived iPSCs and cultured the differentiated cells on a permeable membrane for 1 week in vitro (Saito et al. 2015). The membranes were subsequently transplanted into full-thickness femoral condyle defects in 36 mice. After 8 or 16 weeks, the femurs were collected and examined for chondrogenic differentiation and tumorigenesis. After 8 weeks no tumor was seen, but after 16 weeks, an immature teratoma was observed in one mouse, which indicated that increasing the follow-up duration might show an increase in the risk of tumorigenesis. Hence, there is an emphasis on the significance of avoiding tumorigenesis in clinical applications of iPSCs.
Despite the diverse cell therapy strategies for cartilage regeneration, there is no universally approved, applicable protocol that fully restores tissue structure and function. The use of terminally differentiated chondrocytes or iPSCs as promising cell sources for cartilage repair necessitate additional research in cell fate determination to prevent dedifferentiation or tumor formation. Among many cell therapies, injections of allogeneic MSCs has achieved the most reliable outcomes in animal and preclinical studies because of its immunomodulatory effects, chondrogenic potential, and paracrine properties. Although the risk of tumorigenesis and rejection of MSCs has not been solved. It is inferred that paracrine cues like TGF-β superfamily growth factors play significant roles in the mechanism of action of MSCs in cartilage regeneration (Bobick et al. 2009). Moreover, MSC-derived EVs (EV-MSCs) induce the formation of a cartilaginous matrix (Zhang et al. 2016b). Therapeutic evidence shows that MSCs-secreted EVs and soluble factors are effective. Therefore, cell-free therapy using EV-MSCs might constitute an alternative point of view for researchers (Kotaka et al. 2017). In the next section, we provide a detailed description of the role of Exos in cartilage repair.
Although, in many preclinical experiments or ongoing clinical trials, MSC therapy appears to be a promising strategy to treat cartilage lesions because of their immunomodulatory and paracrine properties, the risks of tumorigenesis and rejection have not been determined. Scientists are optimistic about the results obtained from EV-MSCs therapy for cartilage defects from OA or rheumatoid arthritis (RA); however, the putative therapeutic effects and mechanism of EV-MSCs on inflammation-induced alignment remains unknown.
3 Exosomes (Exos) as a Promising Substitute for Cell Therapy
Recently, the therapeutic effects of MSCs have been attributed to the paracrine secretion of trophic factors such as EVs. EV-mediated tissue regeneration, as a novel cell-free therapeutic approach, has generated renewed optimism for tissue repair. EV therapy may overcome the complications related to stem cell therapy (Musial-Wysocka et al. 2019; Lukomska et al. 2019). The results of numerous studies have suggested that EVs are the most important mediator of cellular information exchange, which are present in the MSCs secretome (Nooshabadi et al. 2018). Despite therapeutic effects of EV-MSCs in facilitating tissue repair in liver disease (La Greca et al. 2018; Di Rocco et al. 2016), cancer (Ren 2019), myocardial infraction (Bang and Kim 2019; Wang et al. 2008; Muller et al. 2018; Ong and Wu 2015), and Alzheimer’s disease (AD) (Iranifar et al. 2019), the mechanism of action and effect of EVs on cartilage regeneration has not been fully investigated.
Exos are nanometer-sized vesicles of about 30–100 nm that are enclosed in a bilayer membrane and are secreted by various cell types. EVs contain an active cargo comprised of proteins, mRNA and a wide range of mRNAs and metabolites that could regulate inflammatory responses, angiogenesis, and immune-modulation (Nooshabadi et al. 2018). To date, different types of vesicles found in cells, including micro-vesicle bodies (VBs), Exos, and apoptotic bodies are categorized according to their morphology, size, biogenesis, potential release pathways, and content. Many cell types are known to secrete EVs and these include immune cells such as macrophages, mast cells, B and T lymphocytes, dendritic cells, all types of stem cells (adult, embryonic, and cord blood), and chondrocytes. Unlike cells, EVs do not elicit acute immune rejection, and they can be produced at a large scale and stored until needed (Lu et al. 2017).
However, biodistribution and in vivo tracking techniques should be investigated in order to increase therapeutic efficacy and avoid the possible off-target effects of EVs (Mitchell et al. 2019). The different therapeutic effects of EVs derived from various cell sources are strictly related to the parental cell origin. On the other hand, the therapeutic effects of EVs depend on their content and include inflammatory mediators, tropic factors, signaling molecules, and nucleic acids. EVs derived from cultured cells are functionally and therapeutically dissimilar to in vivo derived EVs because of signals received from the microenvironment to the parental cells. Furthermore, mimicking the in vivo condition with specific mediators may improve therapeutic outcomes (Seo et al. 2019). Despite advances in transgenic cell therapy, the use of genetically modified cells is limited regenerative medicine because gene therapy is principally a viral vector-based treatment.
The results of numerous studies have shown that MSC-derived conditional medium has positive effects on various diseases such as myocardial infarction, renal diseases, and complete hepatic destruction (Qin et al. 1996; Rota et al. 2019; Nicolas et al. 2016). Thereafter, for the first time, EVs isolated from cardiac PCs (CPCs) hold great cardiac regeneration potential and can be an alternative to stem cell therapies (Galieva et al. 2019; Rovira et al. 2017; Luo et al. 2018). The therapeutic potential of EVs has been shown in the repair of intervertebral disc degeneration. Exos derived from BmMSCs and nucleus pulposus cells (NPCs) have been functionally evaluated. Exos-NPC stimulated BmMSC migrated and differentiated to a nucleus pulposus phenotype after they were taken up by cells (Jin et al. 2018). ADSC vesicles and soluble proteins stimulate skeletal muscle regeneration (Watson et al. 2016). EV-MSCs could also act as immunomodulatory mediators of immune related diseases to prevent the difficulties associated with traditional cell therapies (Haraszti et al. 2018). EVs have been successfully applied for nerve disorders where preclinical studies have shown promising results for diseases such as AD (Reza-Zaldivar et al. 2018), Parkinson’s disease (PD) (Yu et al. 2020), amyotrophic lateral sclerosis (ALS) (Ferrara et al. 2018), multiple sclerosis (MS) (Blonda et al. 2018), stroke and neurotrauma (Colao et al. 2018).
EVs have been used for acute and chronic renal injuries and ureteral strictures (Yan et al. 2018; Li et al. 2017). In a preclinical study on rats with acute liver failure, the EVs released from human ADSCs (hADMSCs) had an enhanced survival rate in the experimental group compared to the control group (Greening et al. 2015).
Despite the therapeutic abilities of EVs, numerous issues remain unsolved such as the lack of distinct manufacturing processes and contaminating endogenous exosomes (Exos) of the serum. These issues necessitate large scale processing of the medium.
Recently, bioreactor cultures are appropriate alternatives for large scale production of EVs for clinical applications. Hollow fiber bioreactors have been used to produce EVs and the results showed that the bioreactor culture yielded ~40-fold more EV per mL of conditioned medium as compared to a conventional T flask cell culture (Watson et al. 2016). The 3D cell cultures based on microcarriers are widely used to grow adherent cells. Tangential flow filtration (TFF) is a method for concentrating proteins or viruses from large quantities of cell culture media. Microcarrier-based 3D culture and TFF allow for scalable production of biologically active Exos from MSCs (Haraszti et al. 2018; Corso et al. 2017; Huang and He 2017). Herein, we explain the current protocols for EVs isolation and characterization.
3.1 Isolation and Characterization of Exosomes (Exos)
Recent Exos isolation methods have been reported that use conditioned media of cultured cells with biological body fluids such as blood and plasma (Properzi et al. 2013). The functional properties, biodistribution, and membrane integrity of Exos is mainly related to the isolation approach. Exos biomarkers are often used as diagnostic and prognostic tools for different cancers; however, Exos fractions might become contaminated by other co-isolated membranous vesicles and lipoproteins. Therefore, the collected conditioned medium should be carefully inspected to ensure that the isolated vesicles are produced by the cells of interest. For instance, culture medium supplemented with fetal bovine serum (FBS) might contain an abundance of Exos. In order to overcome this problem, one should either use another supplementary ingredient such as bovine serum albumin (BSA) or the FBS should be centrifuged at a high speed before use (Mithoefer et al. 2005).
Current isolation techniques depend on size differences between EVs or specific surface markers. Methodologies that include ultracentrifugation, density-gradient centrifugation, ultrafiltration, precipitation, immunoisolation, and chromatography have been used to isolate Exos (Lim et al. 2019) and will be explained in detail.
Ultracentrifugation is the most commonly used approach for isolation and refining of Exos. The particles form pellets after centrifuging at different speeds. A low g-force centrifugation (e.g., 500 for 5–10 min) is performed for separation of intact cells and cellular debris followed by high g-forces (e.g., 100,000 g for 1–2 h) to isolate the Exos. The ultracentrifugation procedure depends on the g-force, rotor type, clearing factor (k-factor for a rotor describes its pelleting efficiency), and viscosity of the solution (Chu et al. 2018).
Precipitation is another widely used method to detect lipid vesicles that involves polymer solutions. The polymer solution is prepared at an optimized salt concentration and low temperature to reduce vesicle solubility. Subsequently, after low speed centrifugation, the pellet is resuspended in phosphate-buffered saline (PBS) or another appropriate solvent for analysis. This procedure may be contaminated by proteins such as albumin and immunoglobulin (Lim et al. 2019).
Ultrafiltration is one of the size-based Exos isolation techniques. A semi-permeable membrane is used for isolation purposes depending on the particle size and different molecular weights. For instance, hollow fiber bioreactor filtration is an appropriate, practical method for Exos isolation which follows this mechanism (Rim and Kim 2016).
Density-gradient ultracentrifugation is a powerful isolation method utilized to separate and isolate different sub-cellular components by a linear sucrose gradient (e.g., sucrose, Ficoll). The centrifugation takes place at ~100,000 × g for ~16 h. The Exos are then located in the density region between 1.10 and 1.18 g mL−1 and the proteins are pelleted at the bottom of the tube. Size-exclusion chromatography (SEC) is a chromatographic technique that acts according to molecular size and can be used to isolate Exos from proteins.
In SEC, larger particles (e.g., Exos) elute faster with the mobile phase and small analytes (e.g., proteins) remain in the stationary phase. Consequently, the Exos can be separated by retrieving the eluted fraction at a definite time (Contreras-Naranjo et al. 2017; Chiriaco et al. 2018). In addition to size-based approaches, other techniques use immunoaffinity-based approaches to isolate Exos. Exos contain numerous specific membrane proteins such as CD63, CD81, CD82, CD9, Alix, annexin, EpCAM, and Rab5 on their surfaces that can be coupled with their corresponding antibodies. Predominantly, the antibodies could be fixed on different types of materials such as magnetic beads, chromatography matrices, plates, and microfluidic devices. Magnetic beads and magnetic nanowires are the most common matrices used in flow cytometry cell sorting. However, this technique is not feasible for isolation of Exos from large quantities of biological samples (Liu and Su 2019).
Most importantly, characterization of Exos is another challenge in exosome mediated therapies. Current methods used to characterize Exos are based on the analysis of specific Exos parameters, which include size, surface markers, protein analysis, and nucleic acid content. Although techniques like transmission electron microscopy (TEM), scanning electron microscopy (SEM), and atomic force microscopy (AFM) are widely used for morphological investigation of individual Exos, other approaches are needed to determine their concentration and size range distribution. Nanoparticle analysis apparatuses like dynamic light scattering (DLS) and nanoparticle tracking analysis (NTA) have been used to quantify the distribution of Exos, while NTA is more applicable for Exos concentration.
Traditional immunoassays that include enzyme-linked immunosorbent assays (ELISA), Western blot, total protein analysis (bicinchoninic acid [BCA], Bradford assays), and flow cytometry are also used to characterize the surface marker and protein content of Exos. Other novel nano-based techniques such as resistive pulse sensing (RPS), surface plasmon resonance (SPR)-based nanosensors, and nano-deterministic lateral displacement (DLD) have been used to isolate and detect Exos (Rim and Kim 2016). TEM is the best technique for determining particle size distribution and shape. TEM images of EVs are normally seen as round and saucer/cup shaped particles (Rim and Kim 2016). The same mechanism is true for SEM, except the electron beam is reflected from the sample. DLS is the standard technique used to measure size concentration of nanoparticles such as Exos by light scattered from particles under Brownian motion in a liquid suspension (Hubbard 1996).
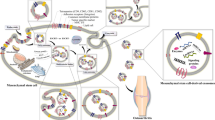
NTA is a novel method that estimates particle sizes that range from 10 to 1000 nm based on the Brownian motion of nanoparticle in liquids (Hangody and Fules 2003). Various Exos surface marker proteins are characterized by flow cytometry, although Western Blotting is a more common approach for surface marker protein analysis. RPS has a detection limit of 100 nm–100 μm to measure particle size and concentration. The principle is based on the difference in electrical resistance of two nanoparticle filled and non-filled cells in a weak electrolyte. The two cells are connected with a nano constriction (Rim and Kim 2016; Szatanek et al. 2017). SPR is based on incident light stimulation of oscillating electrons at the boundary between positive and negative permittivity material. This technique has been optimized into a nano-based device, the nano-plasmonic exosome (nPLEX), in order to characterize Exos. Extraordinary optical transmission (EOT) in periodic nanoholes is the basis of nPLEX. The mechanism is based on a spectral change during the binding of an Exos to nanoholes coated with affinity ligands for various Exos protein markers in the nanopore optical transmittance. Finally, nano-DLD is a continuous process used in microfluidic devices that use pillar array gradients with a critical cutoff diameter defined in their geometry. Therefore, DLD is used to isolate or detect parasites, bacteria, Exos, blood cells, and circulating tumor cells in the blood. (Rim and Kim 2016; Rana et al. 2018; Smith et al. 2018). Thorough characterization of EVs is the first major step to identify and preserve therapeutic components. The long-term safety of paracrine secretomes need more investigations. Next, we intend to focus on particular applications of Exos as drugs or drug delivery systems in articular cartilage, OA and RA in clinical settings (Fig. 1).
3.2 Clinical Applications of Stem Cell-Derived Exosomes (Exos) in Cartilage Defects
The present studies related to cartilage and/or OA repair using EVs are limited to experimental animal models of inflammation and OA. EVs have been shown to reduce inflammation and enhance hyaline-like cartilage formation in vitro and in vivo. For instance, Zhang et al. considered the effect of weekly intra-articular injections of human embryonic MSC-derived Exos in rat models with osteochondral defects. The results showed enhanced appearance and histological scores compared with PBS-treated defects. Interestingly, complete restoration of cartilage and subchondral bone was observed in the Exos-treated defects after 12 weeks (Zhang et al. 2016b). A related study found that Exos derived from embryonic MSCs have successful therapeutic effects on OA by balancing the synthesis and degradation of ECM cartilage (Wang et al. 2017b). According to the majority of scientists, the therapeutic effects of MSCs are largely dependent upon the form of secretory vesicles (Manferdini et al. 2013). Cosenza et al. assessed the function of Exos or microparticles (MPs) in OA. They found that MPs and Exos had similar in vitro chondroprotective and anti-inflammatory activities, and protected mice from OA progression in vivo. Their data indicated that the key therapeutic effects of BmMSCs were addressed by either Exos or MPs. (Cosenza et al. 2017). Vonk et al. demonstrated that Exos harvested from BmMSCs restored OA cartilage by reducing inflammatory responses and stimulating osteoarthritic chondrocytes to secret ECM (Vonk et al. 2018).
Although promising results were reported in small animals, to date, few studies have examined the effects of EVs in large animals and in clinical settings. However, with longer follow-up periods need to confirm the presence of repaired cartilage or a reduction in OA progression. In addition, it has been shown that Exos have biological functions like the cells from which they are derived and there are no unwanted effects like immunogenicity or tumorigenesis with the use of Exos (Xin et al. 2014; Burger et al. 2015). For instance, MSCs isolated from synovial fluid can potentially repair cartilage; however, the use of these cells have limitations such as immunogenicity. In this regard, Tao et al. compared the therapeutic effect of two types of Exos released from synovial derived MSCs (SMSC-Exos) and SMSCs that overexpress miR-140-5p (SMSC-140-Exos) in a rat OA model (Tao et al. 2017). MiR-140-5p plays an important role in MSC chondrogenic differentiation as well as cartilage homeostasis and development (Miyaki et al. 2009). In vitro and in vivo evaluations have shown that SMSC-140 can enhance the in vitro proliferation and migration of articular chondrocytes. Furthermore, relative to SMSC-Exos, SMSC-140-Exos substantially prevented OA in an OA rat model (Tao et al. 2017). Thus, Exos from gene-manipulated cells show remarkable therapeutic ability for use in clinical settings.
Chondrocytes are the only resident cells in cartilage tissue; evidence has shown that apoptosis of chondrocytes can be a major cause for initiation and progression of OA. Qi et al. have reported that MSC-Exos can inhibit chondrocyte apoptosis and improve their viability under inflammatory conditions (Qi et al. 2019). Therefore, mounting evidence suggests that MSC-Exos could be a beneficial, effective tool in a novel cell-free approach for OA treatment.
Some scientist believe that EVs derived from specific tissues can imitate the niche or microenvironment of the cells by stimulating the tissue-inductive mediators due to the presence of tissue-related factors (mRNA and proteins) that play an important role in local induction of tissue regeneration (Becerra et al. 2011). For instance, chondrocytes are the main cells in cartilage tissue that maintain the cartilage microstructure (Leyh et al. 2014; Zhao et al. 2017; Ahmed et al. 2007). The effect of chondrocyte-derived Exos (CC-Exos), as a stimulator of chondrogenesis in subcutaneous environments, was investigated by Chen et al. for successful ectopic cartilage regeneration compared to Exos derived from BmMSCs (BmMSC-Exos) (Chen et al. 2018). The cartilage generated in the presence of CC-Exos was associated with minimal hypertrophy and angiogenesis, whereas hypertrophy was evident in the presence of BMSC-Exos. They concluded that CC-Exos could imitate the chondrogenesis niche in the subcutaneous environment.
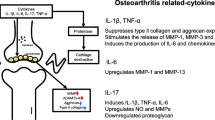
The results of studies indicated the important role of MSC paracrine factors in tissue regeneration, and researchers reported that the condition media of ADSCs exhibited anti-inflammatory properties in OA chondrocytes (Ratajczak et al. 2014; Platas et al. 2013). Tofiño-Vian et al. investigated the chondroprotective function of EVs isolated from hADSCs on OA chondrocytes. MVs and Exos reduced the levels of inflammatory factors such as TNF-a, IL-6, PGE2, and NO in OA chondrocytes stimulated by IL-1β. In OA chondrocytes, EVs reduced the release of matrix metalloproteinase (MMP) activity and MMP-13 expression, but significantly enhanced anti-inflammatory cytokine IL-10 and collagen II expressions (Tofino-Vian et al. 2018).
Despite numerous therapeutic properties of Exos as alternative cell-free therapies for cartilage regeneration, the method of its administration is the main challenge for EVs. Injections are the most common administration route for EVs; however, this is not effective for treatment of cartilage disorders because of rapid leakage from the defect site. On the other hand, constant supervision of EVs at the injury site is a promising method for cartilage repair. Loading Exos into the hydrogel is an appropriate technique to stabilize them into the defect site. Exos have been encapsulated into the photo-induced imine crosslinking (PIC) hydrogel glue in an attempt to prepare an Exos-complex hydrogel tissue patch. The researchers demonstrated that this tissue patch could be easily integrated with the native cartilage tissue and the Exos were effectively maintained at the defect site. This tissue patch, as a novel cell-free material, has been proposed for the comprehensive repair of tissues and organs (Liu et al. 2017).
Under normal conditions, chondrocytes have a dynamic balance of anabolic and catabolic activity that depends on glycolysis activity and is required to provide basic energy (Mobasheri et al. 2017). In OA, chondrocytes lose their metabolic flexibility properties, which results in decreased cellular mitochondrial biogenesis and increased mitochondrial DNA damage (Mobasheri et al. 2017; Luo et al. 2015). Currently, evidence suggests that Exos play an important role in intercellular mitochondrial communication. Exos contents may include the mitochondrial genome or the entire mitochondria (Singh et al. 2017). Chen et al. investigated the effect of MSC-derived Exos on mitochondrial homeostasis. They fabricated a 3D printed scaffold made of ECM, GelMA, and Exos. They found that this construct promoted chondrocyte migration into the defect site and sustainably released Exos. The study results indicated that damages were caused by mitochondrial dysfunction; oxidative stress in the degraded cartilage could be recovered by MSC-Exos through mitochondrial related proteins (Chen et al. 2019).
In another study, Wu et al. demonstrated that infrapatellar fat pad (IPFP) MSC-derived Exos (MSCIPFP-Exos) could inhibit the apoptosis of cartilage, balance the anabolic and catabolic processes, and protect cartilage from OA. They recommended that this mechanism could be correlated with the miR100-5p-mediated inhibition of the mTOR-autophagy pathway (Wu et al. 2019). Taken together, Exos could be considered as a new treatment for cartilage injuries (Fig. 2).
3.3 Limitations, Future Trends, and Concluding Remarks
Recently, cell-free regenerative medicine, which is based on the unique ability of EVs derived from stem cells, is a promising new candidate therapy (Pang et al. 2020). Although numerous studies have demonstrated the tremendous ability of EVs isolated from stem cells to improve treatments of various diseases (Reza-Zaldivar et al. 2018; Yu et al. 2020; Ferrara et al. 2018), the use of EVs in cartilage regeneration and OA pathogenesis is still in its early stage. Consequently, EV therapy for large animal models is essential before clinical trials can be conducted (Cheng and Schorey 2013; Yang et al. 2017; Toghraie et al. 2011). Due to the complexity of the cartilage structure, regeneration of focal defects more than 3 cm must be treated with a combination of EVs and other appropriate matrices under dynamic conditions (Brittberg et al. 2003). Therefore, researchers should address the following: how to use EVs; determine the biological properties of different types of EVs; the optimal dose of EVs in relation to different sizes of cartilage lesions; stability of EVs at the defect site; and determine the role of EVs in homeostasis and pathogenesis of joints.
A major challenge for the combination of novel biomaterials and EVs is to discover the optimal EV dose. Further experimentations should be designed in order for these stem cell-derived EVs to become available for clinical settings. Although phenomenal progress has been made in understanding the Exos cargo’s biological properties, future studies must also concentrate on the challenges of obtaining regulatory approval and their future translation into clinical platforms.
In summary, the most important challenges of clinical applications for EVs to be recognized are the pharmacodynamics and biological distribution of the injected Exos. Although homing of the Exos to soft organs such as the lungs, liver or spleen have been reported a few minutes after the injection, more thorough investigations of the pharmacokinetics, metabolism, and biological dosage should be conducted for safety. These investigations will take time before Exos can be used in clinical applications (Table 1).
Abbreviations
- ACI:
-
Autologous chondrocyte implantation
- ADMSCs:
-
Adipose-derived MSCs
- AFM:
-
Atomic force microscopy
- DLS:
-
Dynamic light scattering
- ECM:
-
Extracellular matrix
- ELISA:
-
Enzyme-linked immunosorbent assays
- EVs:
-
Extracellular vesicles
- GFP:
-
Green fluorescent protein
- GMP:
-
Good manufacturing practice
- HA:
-
Hyaluronic acid
- IPFP:
-
Infrapatellar fat pad
- iPSCs :
-
Induced pluripotent stem cells
- MACI:
-
Matrix-induced autologous chondrocyte implantation
- MMP :
-
Matrix metalloproteinase
- MSCs:
-
Mesenchymal stem cell
- MVs:
-
Microvesicles
- NPCs:
-
Nucleus pulposus cells
- NTA:
-
Nanoparticle tracking analysis
- OA:
-
Osteoarthritis
- PCs:
-
Progenitor cells
- PRP:
-
Platelet-rich plasma
- SEM:
-
Scanning electron microscopy
- STR:
-
Short tandem repeat
- TEM:
-
Transmission electron microscopy
- TFF:
-
Tangential flow filtration
References
Ahmed N et al (2007) Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem 20(5):665–678
Asik M et al (2008) The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy 24(11):1214–1220
Bang OY, Kim EH (2019) Mesenchymal stem cell-derived extracellular vesicle therapy for stroke: challenges and progress. Front Neurol 10:211
Becerra J et al (2011) The stem cell niche should be a key issue for cell therapy in regenerative medicine. Stem Cell Rev Rep 7(2):248–255
Bexkens R et al (2017) Clinical outcome after arthroscopic debridement and microfracture for osteochondritis dissecans of the capitellum. Am J Sports Med 45(10):2312–2318
Bhosale AM, Richardson JB (2008) Articular cartilage: structure, injuries and review of management. Br Med Bull 87:77–95
Blonda M et al (2018) New insights into immune cell-derived extracellular vesicles in multiple sclerosis. Front Neurol 9:604
Bobick BE et al (2009) Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today 87(4):351–371
Bouwmeester SJ et al (1997) Long-term results of rib perichondrial grafts for repair of cartilage defects in the human knee. Int Orthop 21(5):313–317
Bouwmeester P et al (1999) Histological and biochemical evaluation of perichondrial transplants in human articular cartilage defects. J Orthop Res 17(6):843–849
Brittberg M et al (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331(14):889–895
Brittberg M et al (1996) Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res 326:270–283
Brittberg M et al (2003) Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am 85A(Suppl 3):109–115
Brittberg M et al (2016) Cartilage repair in the degenerative ageing knee. Acta Orthop 87(Sup 363):26–38
Brouwer RW et al (2014) Osteotomy for treating knee osteoarthritis. Cochrane Database Syst Rev 12:CD004019
Buckwalter JA (2002) Articular cartilage injuries. Clin Orthop Relat Res 402:21–37
Burger D et al (2015) Human endothelial colony-forming cells protect against acute kidney injury: role of exosomes. Am J Pathol 185(8):2309–2323
Carranza-Bencano A et al (1999) Comparative study of the reconstruction of articular cartilage defects with free costal perichondrial grafts and free tibial periosteal grafts: an experimental study on rabbits. Calcif Tissue Int 65(5):402–407
Chen Y et al (2018) Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res Ther 9(1):318
Chen P et al (2019) Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 9(9):2439–2459
Cheng Y, Schorey JS (2013) Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur J Immunol 43(12):3279–3290
Cheng A et al (2014) Cartilage repair using human embryonic stem cell-derived chondroprogenitors. Stem Cells Transl Med 3(11):1287–1294
Chiang ER et al (2016) Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS One 11(2):e0149835
Chijimatsu R et al (2017) Characterization of mesenchymal stem cell-like cells derived from human iPSCs via neural crest development and their application for osteochondral repair. Stem Cells Int 2017:1960965
Chiriaco MS et al (2018) Lab-on-chip for exosomes and microvesicles detection and characterization. Sensors (Basel) 18(10):3175
Chu CR et al (2018) Minimally manipulated bone marrow concentrate compared with microfracture treatment of full-thickness chondral defects: a one-year study in an equine model. J Bone Joint Surg Am 100(2):138–146
Colao IL et al (2018) Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med 24(3):242–256
Contreras-Naranjo JC, Wu HJ, Ugaz VM (2017) Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip 17(21):3558–3577
Corso G et al (2017) Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci Rep 7(1):11561
Cosenza S et al (2017) Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep 7(1):16214
de Windt TS et al (2017) Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells 35(1):256–264
Di Rocco G, Baldari S, Toietta G (2016) Towards therapeutic delivery of extracellular vesicles: strategies for in vivo tracking and biodistribution analysis. Stem Cells Int 2016:5029619
Duchamp de Lageneste O et al (2018) Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun 9(1):773
Ebrahimi A, Hosseini SA, Rahim F (2014) Immunosuppressive therapy in allograft transplantation: from novel insights and strategies to tolerance and challenges. Cent Eur J Immunol 39(3):400–409
Eckstein F et al (1996) Determination of knee joint cartilage thickness using three-dimensional magnetic resonance chondro-crassometry (3D MR-CCM). Magn Reson Med 36(2):256–265
Ferrara D et al (2018) Role of extracellular vesicles in amyotrophic lateral sclerosis. Front Neurosci 12:574
Fickert S et al (2012) One-year clinical and radiological results of a prospective, investigator-initiated trial examining a novel, purely autologous 3-dimensional autologous chondrocyte transplantation product in the knee. Cartilage 3(1):27–42
Filardo G et al (2015) Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc 23(9):2459–2474
Fisher JN et al (2017) The application of stem cells from different tissues to cartilage repair. Stem Cells Int 2017:2761678
Galieva LR et al (2019) Therapeutic potential of extracellular vesicles for the treatment of nerve disorders. Front Neurosci 13:163
Gimona M et al (2017) Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int J Mol Sci 18(6):1190
Greening DW et al (2015) A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol 1295:179–209
Gupta PK et al (2016) Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel(R)): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther 18(1):301
Hangody L, Fules P (2003) Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am 85-A(Suppl 2):25–32
Hangody L et al (1998) Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics 21(7):751–756
Haraszti RA et al (2018) Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther 26(12):2838–2847
Herberts CA, Kwa MS, Hermsen HP (2011) Risk factors in the development of stem cell therapy. J Transl Med 9:29
Homminga GN et al (1990) Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br 72(6):1003–1007
Huang T, He J (2017) Characterization of extracellular vesicles by size-exclusion high-performance liquid chromatography (HPLC). Methods Mol Biol 1660:191–199
Hubbard MJ (1996) Articular debridement versus washout for degeneration of the medial femoral condyle. A five-year study. J Bone Joint Surg Br 78(2):217–219
Iranifar E et al (2019) Exosomes and microRNAs: new potential therapeutic candidates in Alzheimer disease therapy. J Cell Physiol 234(3):2296–2305
Jiang XC, Gao JQ (2017) Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm 521(1–2):167–175
Jin Y et al (2018) Extracellular vesicles secreted by human adipose-derived stem cells (hASCs) improve survival rate of rats with acute liver failure by releasing lncRNA H19. EBioMedicine 34:231–242
Kim N, Cho SG (2015) New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells 8(1):54–68
Kim DK et al (2013) EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles 2013:2
Ko JY et al (2014) In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 35(11):3571–3581
Kotaka S et al (2017) Magnetic targeted delivery of induced pluripotent stem cells promotes articular cartilage repair. Stem Cells Int 2017:9514719
Kozhemyakina E, Lassar AB, Zelzer E (2015) A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development 142(5):817–831
Kwan AP et al (1991) Macromolecular organization of chicken type X collagen in vitro. J Cell Biol 114(3):597–604
Kwon H et al (2019) Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat Rev Rheumatol 15(9):550–570
La Greca A et al (2018) Extracellular vesicles from pluripotent stem cell-derived mesenchymal stem cells acquire a stromal modulatory proteomic pattern during differentiation. Exp Mol Med 50(9):119
Lee J et al (2015) Early induction of a prechondrogenic population allows efficient generation of stable chondrocytes from human induced pluripotent stem cells. FASEB J 29(8):3399–3410
Lespasio MJ et al (2017) Knee osteoarthritis: a primer. Perm J 21:16–183
Leyh M et al (2014) Osteoarthritic cartilage explants affect extracellular matrix production and composition in cocultured bone marrow-derived mesenchymal stem cells and articular chondrocytes. Stem Cell Res Ther 5(3):77
Li P et al (2017) Progress in exosome isolation techniques. Theranostics 7(3):789–804
Li L et al (2018) Mesenchymal stem cells in combination with hyaluronic acid for articular cartilage defects. Sci Rep 8(1):9900
Li X et al (2019) Challenges and opportunities in exosome research-perspectives from biology, engineering, and cancer therapy. APL Bioeng 3(1):011503
Lim J et al (2019) Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires. J Nanobiotechnol 17(1):1
Liu C, Su C (2019) Design strategies and application progress of therapeutic exosomes. Theranostics 9(4):1015–1028
Liu X et al (2017) Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 9(13):4430–4438
Lo Monaco M et al (2018) Stem cells for cartilage repair: preclinical studies and insights in translational animal models and outcome measures. Stem Cells Int 2018:9079538
Logli AL et al (2019) Osteochondritis dissecans lesions of the capitellum in overhead athletes: a review of current evidence and proposed treatment algorithm. Curr Rev Musculoskelet Med 12(1):1–12
Lu K et al (2017) Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res Ther 8(1):108
Lu L et al (2019) Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther 10(1):143
Lukomska B et al (2019) Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int 2019:9628536
Luo Z et al (2015) Mechano growth factor (MGF) and transforming growth factor (TGF)-beta3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model. Biomaterials 52:463–475
Luo Y et al (2017) The minor collagens in articular cartilage. Protein Cell 8(8):560–572
Luo J et al (2018) Bone marrow mesenchymal stem cells reduce ureteral stricture formation in a rat model via the paracrine effect of extracellular vesicles. J Cell Mol Med 22(9):4449–4459
Manferdini C et al (2013) Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum 65(5):1271–1281
Matsusue Y, Yamamuro T, Hama H (1993) Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy 9(3):318–321
Medvedeva EV et al (2018) Repair of damaged articular cartilage: current approaches and future directions. Int J Mol Sci 19(8):2366
Meyer U et al (2012) Cartilage defect regeneration by ex vivo engineered autologous microtissue – preliminary results. In Vivo 26(2):251–257
Mitchell R et al (2019) Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res Ther 10(1):116
Mithoefer K et al (2005) The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am 87(9):1911–1920
Mithoefer K et al (2012) Evolution and current role of autologous chondrocyte implantation for treatment of articular cartilage defects in the football (soccer) player. Cartilage 3(1 Suppl):31S–36S
Miyaki S et al (2009) MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum 60(9):2723–2730
Mobasheri A et al (2017) The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 13(5):302–311
Mora JC, Przkora R, Cruz-Almeida Y (2018) Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res 11:2189–2196
Muller P, Lemcke H, David R (2018) Stem cell therapy in heart diseases – cell types, mechanisms and improvement strategies. Cell Physiol Biochem 48(6):2607–2655
Musial-Wysocka A, Kot M, Majka M (2019) The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant 28(7):801–812
Nam Y et al (2018) Current therapeutic strategies for stem cell-based cartilage regeneration. Stem Cells Int 2018:8490489
Nasiri N et al (2019) Targeted cell delivery for articular cartilage regeneration and osteoarthritis treatment. Drug Discov Today 24(11):2212–2224
Navabi H et al (2005) Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial. Blood Cells Mol Dis 35(2):149–152
Negoro T et al (2018) Trends in clinical trials for articular cartilage repair by cell therapy. NPJ Regen Med 3:17
Nejadnik H et al (2015) Improved approach for chondrogenic differentiation of human induced pluripotent stem cells. Stem Cell Rev Rep 11(2):242–253
Nicolas C et al (2016) Stem cell therapies for treatment of liver disease. Biomedicine 4(1):2
Nooshabadi VT et al (2018) The extracellular vesicles-derived from mesenchymal stromal cells: a new therapeutic option in regenerative medicine. J Cell Biochem 119(10):8048–8073
Ong SG, Wu JC (2015) Exosomes as potential alternatives to stem cell therapy in mediating cardiac regeneration. Circ Res 117(1):7–9
Onuora S (2016) Regenerative medicine: a nose for cartilage repair. Nat Rev Rheumatol 12(12):691
Ozeki N et al (2016) Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthr Cartil 24(6):1061–1070
Pang B et al (2020) Extracellular vesicles: the next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis. Theranostics 10(5):2309–2326
Pers YM et al (2016) Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase i dose-escalation trial. Stem Cells Transl Med 5(7):847–856
Peterson L et al (2003) Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 85A(Suppl 2):17–24
Platas J et al (2013) Conditioned media from adipose-tissue-derived mesenchymal stem cells downregulate degradative mediators induced by interleukin-1beta in osteoarthritic chondrocytes. Mediat Inflamm 2013:357014
Properzi F, Logozzi M, Fais S (2013) Exosomes: the future of biomarkers in medicine. Biomark Med 7(5):769–778
Qi H et al (2019) Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. In Vitro Cell Dev Biol Anim 55(3):203–210
Qin Set al (1996) Folk medicine of the Qiang nationality. Zhongguo Zhong Yao Za Zhi 21(8):453–456, 509
Qu C et al (2013) Chondrogenic differentiation of human pluripotent stem cells in chondrocyte co-culture. Int J Biochem Cell Biol 45(8):1802–1812
Rana A, Zhang Y, Esfandiari L (2018) Advancements in microfluidic technologies for isolation and early detection of circulating cancer-related biomarkers. Analyst 143(13):2971–2991
Ratajczak MZ et al (2014) New advances in stem cell research: practical implications for regenerative medicine. Pol Arch Med Wewn 124(7–8):417–426
Ren K (2019) Exosomes in perspective: a potential surrogate for stem cell therapy. Odontology 107(3):271–284
Reza-Zaldivar EE et al (2018) Potential effects of MSC-derived exosomes in neuroplasticity in Alzheimer’s disease. Front Cell Neurosci 12:317
Rim KT, Kim SJ (2016) Quantitative analysis of exosomes from murine lung cancer cells by flow cytometry. J Cancer Prev 21(3):194–200
Rota C, Morigi M, Imberti B (2019) Stem cell therapies in kidney diseases: progress and challenges. Int J Mol Sci 20(11):2790
Rovira J et al (2017) Therapeutic application of extracellular vesicles in acute and chronic renal injury. Nefrologia 37(2):126–137
Ryan JA et al (2009) Mechanical compression of articular cartilage induces chondrocyte proliferation and inhibits proteoglycan synthesis by activation of the ERK pathway: implications for tissue engineering and regenerative medicine. J Tissue Eng Regen Med 3(2):107–116
Saito T et al (2015) Hyaline cartilage formation and tumorigenesis of implanted tissues derived from human induced pluripotent stem cells. Biomed Res 36(3):179–186
Satue M et al (2019) Intra-articularly injected mesenchymal stem cells promote cartilage regeneration, but do not permanently engraft in distant organs. Sci Rep 9(1):10153
Schultz W, Gobel D (1999) Articular cartilage regeneration of the knee joint after proximal tibial valgus osteotomy: a prospective study of different intra- and extra-articular operative techniques. Knee Surg Sports Traumatol Arthrosc 7(1):29–36
Seo Y, Kim HS, Hong IS (2019) Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int 2019:5126156
Shen G (2005) The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res 8(1):11–17
Singh B, Modica-Napolitano JS, Singh KK (2017) Defining the momiome: promiscuous information transfer by mobile mitochondria and the mitochondrial genome. Semin Cancer Biol 47:1–17
Smith GD, Knutsen G, Richardson JB (2005) A clinical review of cartilage repair techniques. J Bone Joint Surg Br 87(4):445–449
Smith JT et al (2018) Integrated nanoscale deterministic lateral displacement arrays for separation of extracellular vesicles from clinically-relevant volumes of biological samples. Lab Chip 18(24):3913–3925
Soler R et al (2016) Final results of a phase I-II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 23(4):647–654
Solheim E et al (2016) Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc 24(5):1587–1593
Steinwachs M (2009) New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy 25(2):208–211
Szatanek R et al (2017) The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci 18(6):1153
Tao SC et al (2017) Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7(1):180–195
Tofino-Vian M et al (2018) Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem 47(1):11–25
Toghraie FS et al (2011) Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in rabbit. Knee 18(2):71–75
Umeda K et al (2012) Human chondrogenic paraxial mesoderm, directed specification and prospective isolation from pluripotent stem cells. Sci Rep 2:455
Vonk LA et al (2018) Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 8(4):906–920
Wang CH, Cherng WJ, Verma S (2008) Drawbacks to stem cell therapy in cardiovascular diseases. Futur Cardiol 4(4):399–408
Wang M et al (2017a) Advances and prospects in stem cells for cartilage regeneration. Stem Cells Int 2017:4130607
Wang Y et al (2017b) Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther 8(1):189
Watson DC et al (2016) Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 105:195–205
Wei Y et al (2012) Chondrogenic differentiation of induced pluripotent stem cells from osteoarthritic chondrocytes in alginate matrix. Eur Cell Mater 23:1–12
Willms E et al (2016) Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep 6:22519
Wu J et al (2019) miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 206:87–100
Xin H, Li Y, Chopp M (2014) Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci 8:377
Xu X et al (2015) Full-thickness cartilage defects are repaired via a microfracture technique and intraarticular injection of the small-molecule compound kartogenin. Arthritis Res Ther 17:20
Yan IK et al (2018) Use of a hollow fiber bioreactor to collect extracellular vesicles from cells in culture. Methods Mol Biol 1740:35–41
Yang VK et al (2017) Circulating exosome microRNA associated with heart failure secondary to myxomatous mitral valve disease in a naturally occurring canine model. J Extracell Vesicles 6(1):1350088
Yoshimura H et al (2007) Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 327(3):449–462
Yu H et al (2020) Potential roles of exosomes in Parkinson’s disease: from pathogenesis, diagnosis, and treatment to prognosis. Front Cell Dev Biol 8:86
Zhang W et al (2016a) Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res 4:15040
Zhang S et al (2016b) Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr Cartil 24(12):2135–2140
Zhang L et al (2018a) Exosomal miRNA profiling to identify nanoparticle phagocytic mechanisms. Small 14(15):e1704008
Zhang Y et al (2018b) Repair of full-thickness articular cartilage defect using stem cell-encapsulated thermogel. Mater Sci Eng C Mater Biol Appl 88:79–87
Zhao X et al (2017) Chondrogenesis by bone marrow-derived mesenchymal stem cells grown in chondrocyte-conditioned medium for auricular reconstruction. J Tissue Eng Regen Med 11(10):2763–2773
Zwolanek D et al (2017) Tracking mesenchymal stem cell contributions to regeneration in an immunocompetent cartilage regeneration model. JCI Insight 2(20):e87322
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Taghiyar, L., Jahangir, S., Khozaei Ravari, M., Shamekhi, M.A., Eslaminejad, M.B. (2021). Cartilage Repair by Mesenchymal Stem Cell-Derived Exosomes: Preclinical and Clinical Trial Update and Perspectives. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 12. Advances in Experimental Medicine and Biology(), vol 1326. Springer, Cham. https://doi.org/10.1007/5584_2021_625
Download citation
DOI: https://doi.org/10.1007/5584_2021_625
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-71932-6
Online ISBN: 978-3-030-71933-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)