Abstract
Urticaria is characterized by the cutaneous presence of wheals (hives), angioedema or both. Acute and chronic urticaria are distinguished based on a duration of less or more than 6 weeks. Chronic urticaria can be further classified into a spontaneous form and several inducible types triggered by specific external stimuli. Lifetime prevalence of urticaria may be up to 20%, with the acute form being way more common than the chronic one. Exacerbating factors (e.g. infections, drugs, food) and immune system alterations have been investigated as main triggers of mast cell activation, which in turn leads to increased vascular permeability and extravasation of inflammatory cells. While diagnostic workup is focused upon history taking, several emerging biomarkers correlate with severity and/or prognosis of the disease and can be necessary to differentiate chronic spontaneous urticaria from other disorders, such as vasculitis and autoinflammatory diseases. Treatment of acute urticaria is based upon H1 antihistamines and short courses of steroids. While H1 antihistamines are also used in chronic spontaneous urticaria, omalizumab is the standard of care in patients who are unresponsive to these. Recently, several new drugs have entered clinical trials to offer a therapeutic possibility for patients unresponsive to omalizumab. Numerous target molecules, such as mediators of mast cells activation, are under investigation. Amongst these, new anti-IgE therapies and possibly IL-5 pathway blockade seem to have reached enough data to move to advanced clinical trials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute urticaria
- Angioedema
- Biologics
- Biomarkers
- Chronic spontaneous urticaria
- Ligelizumab
- Mast cells
- Omalizumab
- Urticaria
- Vasculitis
1 Definition
Urticaria is a common clinical condition characterized by the cutaneous appearance of wheals (hives), angioedema or both (Zuberbier et al. 2018).
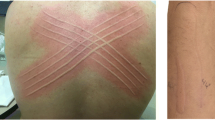
Urticarial wheals are defined by three features: a central swelling of variable dimensions surrounded by an erythematous border; an intense sensation of itching or burning; a short-lived appearance with the skin returning to normal in less than 24 h (Zuberbier et al. 2018). Wheals are mainly due to vasodilation in superficial dermal causing swelling of tissues, local oedema and concurrent sensory neural activation which is associated to pruritus (Antia et al. 2018).
Angioedema is characterized by sudden swelling of the lower dermis and subcutis or mucous membranes (with occasional erythema), sporadic presence of pain and a slower resolution in comparison to wheals (up to 72 h) (Zuberbier et al. 2018). Angioedema is also caused by vasodilation, plasma extravasation and neural stimulation, but contrary to urticaria these phenomena occur in deeper layers of the skin (reticular dermis and subcutaneous tissue) (Antia et al. 2018) (Fig. 1).
2 Classification
Urticaria can have a wide spectrum of presentations and subtypes, which can typically coexist in the same patient. Acute urticaria (AU) is distinguished from chronic urticaria (CU) based upon its duration, with a defining period of less than 6 weeks (compared to a duration longer than 6 weeks for CU, Table 1A). In acute cases of urticaria, anaphylaxis should be excluded by evaluating for signs of respiratory, gastrointestinal, neurologic upset or hemodynamic instability (Antia et al. 2018). The presence of a clear external trigger (e.g. insect sting, food ingestion) or a suggestive past medical history should equally be red flags alarming the physician to consider urticaria as a possible feature of anaphylaxis (Estelle Simons Ledit 2013).
While most cases of acute urticaria remain idiopathic after history taking, the most common associated factors are respiratory infections (40% of cases), drug reactions (9,2%) and food reactions (0.9%) (Antia et al. 2018; Zuberbier et al. 1996).
Chronic urticaria can be divided into chronic inducible urticaria (CIndU) and chronic spontaneous urticaria (CSU). The subdivision is based upon the clinical reproducibility of wheals following exposure to a direct stimulus (e.g. cold, pressure, water, etc.) or absence of such trigger (Zuberbier et al. 2018). While CSU is considered as a single clinical entity, although multiple pathogenetic subsets may be present, CIndU is further split into several conditions based in the specific trigger mechanisms as presented in Table 1B. Behind the appearance of a minor disorder, chronic urticaria carries an important burden of disease for affected patients and their family members (Zuberbier et al. 2018). A reduction in objective well-being and subjective health has been observed in patients suffering from chronic spontaneous urticaria, especially when the condition is refractory to treatment or just partially responsive (Maurer et al. 2017a). Along with an impaired quality of life, chronic spontaneous urticaria is also associated with a considerable impairment in work productivity, which can be a result of the bothersome itching sensation and aesthetic disfigurement (in severe cases) (Maurer et al. 2017b; Maurer et al. 2016).
Rarely, urticaria can be the initial presentation of a systemic autoimmune, hematologic, autoinflammatory or neoplastic disorder (Zuberbier et al. 2018). Therefore, the presence of atypical associated features should prompt the clinician towards a more extensive diagnostic approach.
3 Epidemiology and Genetics
Surveys about the lifetime prevalence of any form of urticaria yielded results ranging from less than 1% to more than 20%, depending on the age of patients, the methods of the study and its location (Antia et al. 2018; Weller et al. 2010). Both AU and CU have been more commonly observed in females, with an apparent ratio of 2 females for each male (Antia et al. 2018; Sánchez-Borges et al. 2015a; Amsler et al. 2014; Lapi et al. 2016; Kalogeromitros et al. 2011; Magen et al. 2013). Apparently, the gender gap in prevalence seems to narrow among the elderly, the children and when considering some forms of CIndU (Cassano et al. 2016; Ban et al. 2014).
AU lifetime prevalence was reported in Europe with estimations going from 12% to 24%, qualifying therefore as a very common condition (Konstantinou et al. 2011; Pogorelov et al. 2016). As only a minority of patients with AU progress to the chronic form of disease, the prevalence of the latter condition is clearly lower. A study from the US estimated the 1-year prevalence of CU to be as low as 0.08%, while data from Europe reported a prevalence for the same period ranging between 0.38% and 0.8% (Lapi et al. 2016; Pogorelov et al. 2016; Zazzali et al. 2012).
In Spain, chronic urticaria was observed more commonly in patients between 25 and 55 years old (Gaig et al. 2004). In contrast, a recent Italian study observed a higher incidence of CSU among patients younger than 20 years and those older than 50 years old (Kalogeromitros et al. 2011). Other studies actually observed a lower incidence of all forms of urticaria in children, with the prevalence of any type of urticaria ranging between 3.4% and 5.4% (Antia et al. 2018; Dilek et al. 2016; Kjaer et al. 2008).
Urticaria is clearly a disorder with no Mendelian inheritance. Nevertheless, several HLA class II alleles were associated with CSU according to the results of a study conducted in 1999 on British patients (O’Donnell et al. 1999). A subsequent Italian study observed a higher prevalence of the disease among family members of patients, suggesting a familial inheritance. Later, additional HLA alleles (class I and class II) were also associated with the disease (Asero 2002; Coban et al. 2008).
4 Pathogenesis
Disclosing the pathophysiology of urticaria appears to be the principal need to bring successful treatment in a clinical setting. Nowadays, it is widely accepted the thesis involving mast cells as the principal mediators of damage. The wheal is microscopically characterized by a pro-inflammatory microenvironment generated by the degranulation of activated mast cells which determine the release of a great amount of soluble molecules such as histamine, leukotrienes, prostaglandins (PGs) and others cytokines.
This pathological niche presents high amount of mediators that have the ability to shape the infiltrate enhancing the activity of the homed cells leading to the perpetration of the damage. The analysis of soluble proteins from lesional skin suggests a mixed Th1/Th2 response by the presence of high amount of interleukin IL-4, IL-5, IL-33, IL-25 and thymic stromal lymphopoietin (TSLP) as well as interferon-gamma (IFNγ) (Caproni et al. 2005; ELIAS et al. 1986; Kay et al. 2015).
These inflammatory factors determine the presence of a perivascular aggregation of immune cells mainly composed by mast cells, lymphocytes, monocytes, neutrophils, eosinophils (Vonakis and Saini 2008) and basophils (Ying et al. 2002); moreover, markers of vascular leakage and angiogenesis were found to be highly expressed on the surface of endothelium of the affected vessels (Kay et al. 2014).
4.1 Acute Urticaria
Considering the acute form of urticaria, the pathophysiology has been less extensively studied with respect to the abnormalities occurring in the chronic condition. Despite its higher frequency in population, AU presents a short duration of disease as well as effective treatments. Precipitating factors have been described in less than 50% of cases. The most common one is the presence of a concomitant viral infection, followed by drugs, food and insect sting reactions (Zuberbier et al. 1996; Kulthanan et al. 2008). Along with viruses, several reports described association with bacterial infections such as cystitis and tonsillitis (Wedi et al. 2009), instead, consumption of raw fish and exposure to Anisakis simplex nematodes, was highlighted only in some studies (Wedi et al. 2009; Del Pozo et al. 1997).
Scientific evidence identifies the same pathological alterations in both forms. An abnormal release of histamine, platelet-activating factor and cytokines by activated mast cells leads to vasodilation, increased vascular permeability and stimulation of sensory nerve endings in the affected skin.1 Furthermore, mediators released from mast cells may act as chemo-attractants for cells of the immune system, such as eosinophils, neutrophils and lymphocytes. As a consequence, the affected dermis becomes inflamed (Radonjic-Hoesli et al. 2018).
4.2 Chronic Urticaria
The chronic form, defined as a spontaneous or induced occurrence of wheals, is considered to be caused by a persistent derangement in functioning of mast cells. The inappropriate and uncontrolled degranulation of those determines the clinical manifestation with the appearance of the short-lived typical lesions.
Even if the pathogenesis of CIndU is clearly dependent upon definite extrinsic factors which activate and enhance mast cells degranulation (Tables 1A and 1B), the pathogenesis of the spontaneous form remains elusive. Because of the peculiar features and not well understood role of mast cells in the human organism, several elements have been analysed tempting to define cellular and molecular pathways responsible of CSU. Recently, the alteration in mast cells activation has been mostly attributed to complex mechanisms of autoimmunity involving various component of the immune system besides to complement cascade and coagulation.
4.2.1 Cellular Alterations
Within skin lesions, a mixed perivascular pro-inflammatory infiltrate can be observed. Attracted by chemokines which are mainly released by mast cells, eosinophils, monocytes, basophils and T helper cells penetrate the skin layers occupying the perivascular space. Interestingly, a peculiar mixed Th1 and Th2 responses seems to be present, with a concurrent increase of both spectra main mediators (Radonjic-Hoesli et al. 2018; Kay et al. 2015; Ying et al. 2002).
Strong evidence in support of the autoimmune pathogenesis of CSU is certainly the documented presence of autoreactive CD4+ T cells targeting FcεRIα in subjects with the disease. In a recent study, these lymphocytes were detected in more than a quarter of investigated patients, while they were completely absent in controls. They were associated with a Th1 phenotype, with secretion of IFN-γ (Auyeung et al. 2016).
4.2.1.1 Mast Cells
Mast cells are derived from bone marrow CD34+, CD117+ (Kit), CD13+ pluripotent progenitor cells that mature under the local environment of the tissues into which they migrate (Kirshenbaum and Metcalfe 2006; Bandara et al. 2015). Studies focusing on the content of their granules led to identify two different subtypes which present different interaction with the other cells of immune system as well as a dissimilar localization in the human body. In particular, mast cells which are tryptase-positive but chymase-negative (MCT) (Irani and Schwartz 1989) which contain complete scroll, are located at mucosal tissues, such as the intestine, lung and nose, are T-lymphocyte dependent and are increased in number in allergic disease (Irani and Schwartz 1989; Otsuka et al. 1995). The other subtype is defined to be constituted by both tryptase and chymase-positive granules (MCTC) which appear to form grating or lattice structure (Irani and Schwartz 1989). They are found primarily in the skin and gastrointestinal submucosa and are independent of lymphocytes (Smith et al. 1995).
The role of mast cells in CSU is consensually acknowledged. However, it’s still controversial whether they are increased in numbers in patients with the disease. While some studies supported this hypothesis, others found no association (Saini and Kaplan 2018). Only a few studies that analysed the presence of mast cells in the lesional skin distinguished between MCT and MCTC. One of these, in particular, highlighted how there is no difference between the number of MCTC between CSU patients and heathy ones; however, the presence of the subtype MCT varies significantly (Bradding et al. 1995).
Evidence for mast cell degranulation come firstly from the measurement of total serum tryptase levels, which appear to be slightly elevated in subjects with CSU compared to both healthy and atopic subjects even if still within the normal range (Ferrer et al. 2010). According to some evidence, a functional defect in these cells activation may lead to increased histamine release in CSU (Bédard et al. 1986; Jacques et al. 1992). This alternative hypothesis attributes the chronic evolution of acute urticaria to a defect in mast cells intracellular signalling, rather than autoimmune disturbances (Bracken et al. 2019). Findings in support of this assumption rely on release tests which demonstrate, by the use of 48/80-induced histamine responses via skin chambers, how mast cells coming from CSU patients appear to be more prone to degranulate (Bédard et al. 1986; Jacques et al. 1992).
The molecular basis of the altered response appears to lies in the structure of FcεR1, which contains immunoreceptor tyrosine-based activation motifs (ITAMs). After phosphorylation, ITAMs allow the activation of spleen tyrosine kinase (SYK) and downstream pathways which eventually elicit the degranulation of mast cells (Bracken et al. 2019). Mast cells from CSU patients, when cultured in vitro, were observed to release significantly higher levels of histamine than their counterparts from healthy controls.
Activated mast cells were shown to express significantly higher levels of SYK during the same experiment (Saini et al. 2009). Interestingly, a subsequent study did not found SYK to be upregulated from anti-FcεRI autoantibodies. This implies that the upregulation of SYK observed in a subset of CSU patients depends upon causes other than autoimmunity or, more accurately, other than autoantibodies (MacGlashan 2019).
The presence of activated mast cells has profound effects on local microenvironment. Some recent evidence points the attention on the ability of these cells to produce high amount of neuropeptides, in particular NGF (Aloe et al. 1994; Peters et al. 2011), which is markedly elevated in subject affected by allergic diseases and CSU (Bonini et al. 1996). NGF is a member of neurotrophin, has pivotal roles for the development and survival of nociceptive neurons but also is involved in chronic inflammation maintaining and promoting the proliferation and activity of lymphocytes, neutrophils, eosinophils and mast cells (Minnone et al. 2017).
In CSU has been demonstrated how NGF is able to act stimulate the proliferation of mast cells besides act as a chemotactic factor. This evidence could be at the base for a pathological loop in which the activated mast cells through the stimulation of sensory nerves could enhance the immune infiltrate supported by inflamed nerves (Fig. 2) (Minnone et al. 2017).
Possible mechanisms of mast cell activation in urticaria
Extrinsic factors (green section). Several extrinsic elements can exacerbate urticaria via mast cells activation. Infections and drugs are identified as one of the most frequent precipitating factors. Some evidence demonstrate how emotional stress has been strictly linked to a worsening of skin symptoms instead, foods and food additives have been implicated mainly as a consequence of food allergy. CindU are well-known examples of triggered urticaria
Humoral alteration (yellows section). The main pathogenic mechanisms actually identified and under validation are: Autoimmunity. IgG autoantibodies (IgG) target IgE-receptors (FcεR1) on mast cells (MC). This leads to dimerization of receptors and mast cell activation, with consequent degranulation (DMC). Concurrently, formation of complement fragments (C5a) and binding to C5a receptor (C5aR) on mast cells enhances the process. Autoallergy. IgE antibodies (IgE) targeting human proteins such as interleukin 24 (IL-24) are present in serum. These bind to FcεR1 via their Fc region, leading to allergic reactions against “autoallergens”. Intracellular signalling defects. An abnormal activation of spleen tyrosine kinase (SYK) in mast cells of patients with chronic urticaria leads to increased intracellular signalling and consequent degranulation without external triggers
Degranulation (red section). Mast cells degranulation could be facilitated by intrinsic cell defects which diminishing the activation threshold lead to an enhanced exocytosis. The release of histamine, tryptase, PGD2 and NGF determine an increase in vascular permeability causing the extravasation of several inflammatory cells. Perivascular mixed infiltrate interacts with tissue mast cells generating a pathological loop which worsen and maintain a pro-urticaria microenvironment. The same auto stimulatory condition could be occurring with free nerve endings by secretion of NGF
4.2.1.2 Basophils
Basophils are circulating granulocytes which can express IgE receptors, release histamine and produce several cytokines, such as IL-4, IL-13 and others, in response to IgE receptor activation (Saini and Kaplan 2018; Schroeder 2009; Raap et al. 2017). Blood basophils in patients with CSU were characterized by multiple abnormalities. Along with a reduction in their number (basopenia), reduced histamine release in response to anti-IgE (basophils hyporesponsiveness) and basophil infiltration of skin lesions have been observed (Vonakis and Saini 2008; Saini and Kaplan 2018; Rorsman 1962). Several alterations in the function and number of basophils have been observed in CSU patients suggesting a recruiting from the bloodstream into urticarial skin lesions (Grattan et al. 2003).
Based on the response to IgE-receptor-mediated histamine degranulation, it is possible to identify two different subsets of basophils in CSU patients which are confirmed by a study monitoring CD63 induction after IgE-receptor activation (Rauber et al. 2017). Improvements in both basopenia and basophil IgE-receptor abnormalities are seen in natural remission of CSU and point to basophils as contributor to disease (Eckman et al. 2008). Even if the pathways involved in the processes which recruit basophils to skin lesions are not yet elucidated, some evidence focused the attention on the prostaglandin D2 (PGD2) pathway and the chemoattractant receptor homologous molecule expressed on the Th2 cell (CRTH2) (Oliver et al. 2016).
Moreover, data from omalizumab phase III trials in CSU shows a correlation between the clinical response and that improvement in basopenia (Saini et al. 2017).
Recent finding observed increased IL-31 in skin as well as serum levels of CSU patient (Raap et al. 2010). This molecule is considered to be the major player inducing pruritus in skin diseases such as atopic dermatitis but also determine a powerful chemotactic action on basophils recruitment. This indicates that IL-31 plays a role in the orchestration and accumulation of basophils in inflammatory skin diseases; furthermore, it has been widely demonstrated how basophils are the main cellular sources of IL-31 in skin lesions of CSU patients (Raap et al. 2017). The inhibition of this pathological loop could be thus of therapeutic interest in controlling itch-related symptoms as well as inducing the remission of disease (Fig. 2).
4.2.2 Humoral Alterations
The initial study which provided strength to this hypothesis was the development and testing of the autologous serum skin test (ASST) (Grattan et al. 1986). More than three decades ago, few patients with chronic spontaneous urticaria were drawn blood; serum was separated from it and reinjected intradermally with the result to develop wheals when exposed (Grattan et al. 1986). Clearly, this seemed to imply the presence of an intrinsic serum factor triggering urticaria; however, this finding was later doubted when evidence came out that a substantial percentage of healthy controls also developed positive ASST reactions (Taskapan et al. 2008).
Thyroid autoimmunity, especially Hashimoto’s thyroiditis, has also been associated with CSU for a long time (Levy et al. 2003; Nuzzo et al. 2011). This seemed to provide evidence of a common autoimmune mechanism or predisposition. According to recent evidence, which emerged from a European multicentric study (the PURIST study) (Schoepke et al. 2019), a subset of patients with purely autoimmune pathogenesis may exist, although its relative weight appears minor. Defined three criteria as a red-flag of derangements in immune function, only 8% of the patients studied fulfilled all the conditions, even if this group had higher activity of the disease (UAS 7), lower IgE levels and higher anti-TPO IgG levels.
Besides the association with thyroid autoimmunity, a high frequency of antinuclear antibodies (ANA) has also been observed among these patients (Viswanathan et al. 2012). However, their meaning in CSU is poorly understood and, as ANA are well known to occur in a considerable fraction of the healthy population, they bear no practical use in CSU (Viswanathan et al. 2012).
On the contrary, antibodies specific to CSU and relevant to its pathogenesis are those targeting FcεR1. These were described for the first time in a group of 26 patients with CSU. It was hypothesized that they could act similarly to anti TSHr-antibodies in Graves disease, leading to the activation, rather than the neutralization, of their target (Hide et al. 1993). Specifically, these IgG antibodies bind to FcεRI on mast cells, leading to their activation and degranulation. This mechanism alone, due to activating autoantibodies, seemed to explain up to one quarter of cases of CSU (as anti- FcεR1 IgGs are prevalent in about 25% of patients) (Niimi et al. 1996).
Fascinatingly, in a small subset of patients, anti-IgE autoantibodies were also observed (Niimi et al. 1996). This may sound puzzling to those who are well acquainted with the therapeutic effectiveness of anti-IgE monoclonal antibodies (eg. Omalizumab) in CSU. However, a recent study conducted on asthma patients demonstrated that naturally occurring anti-IgE IgGs are quite frequent and not necessarily inhibitory (Chan et al. 2014). Some of these actually lead to activation of basophils, a finding which may also be coherent with their presence in CSU patients.
Along with humoral autoimmunity, a role for humoral “autoallergy” has been postulated in CSU. Autoallergy refers to the presence of IgE antibodies against human proteins, which would be capable of inducing mast cells degranulation without extrinsic triggers. The first autoallergen to be identified in CSU was thyroid peroxidase (TPO). Anti-TPO IgEs were shown to be significantly more prevalent in patients than in controls (Altrichter et al. 2011). Subsequently, interleukin 24 (IL-24) was identified as a more sensitive and specific autoallergen for CSU (Schmetzer et al. 2018). Anti-IL-24 IgEs were additionally shown to be able to bind mast cells and lead to histamine release, which is a needed condition to prove their pathogenicity (Schmetzer et al. 2018).
Despite that, some difficulties arise when trying to develop a coherent model of pathogenesis for autoallergens and their antibodies. In fact, TPO is not present in the skin and IL-24, which is present in the epidermis, is not known to be found in the dermis as well (Poindexter et al. 2009). As the epidermis is normal in CSU, a definite role of autoallergy can’t be ultimately proven (Fig. 2) (Kaplan 2019).
4.2.2.1 Complement
Whereas autoantibodies constitute the humoral arm of the adaptive immune system, the complement system provides innate immunity with an equally effective means of molecular defence. Unfortunately, when complement derails from its normal function, it may compound damage in a dysregulated immune system.
Soon after the discovery of autoantibodies targeting IgE receptors, it was found that a subset of these was able to activate mast cells only in vivo. This was hypothesized to be due to the exclusive in vivo presence of complement and its fragments, which could bind to antibodies and complement receptors (C5aR.) on mast cells (Fiebiger et al. 1995).
Purified C5a fragments were later tested for their effects upon histamine release in the presence of anti-IgE receptor IgGs. C5a was associated with a significant increase in histamine release from mast cells, when compared to complement-depleted serum (Ferrer et al. 1999). It was therefore suggested that IgGs cross binding FcεRI on mast cells could have a stronger degranulating effect in the presence of higher amounts of activated complement fragments (Fig. 2) (Kikuchi and Kaplan 2002).
4.2.2.2 Coagulation
Although the coagulation cascade is not directly linked with the immune system, its relevance in the pathogenesis of CSU has been supported by several studies (Asero et al. 2007). After initial findings of an increased activation of the coagulation, use of anticoagulant drugs was considered as a valid adjuvant in the treatment for CSU (although no increase in the risk of thrombosis was observed in these patients) (Cugno et al. 2010).
The increased activation of fibrin and other coagulation factors may be the result of vascular damage taking place in the affected skin. The same group of researchers also showed a prompt decrease in levels of D-Dimer (a degradation product of fibrin) in patients with a clinical response after being treated with either cyclosporine or omalizumab (Asero 2015; Asero et al. 2017).
4.2.3 Extrinsic Precipitating Factors
As it is the case for AU, the chronic spontaneous form has also been associated with several extrinsic factors. Among these, infections of viral, parasitic, bacterial or fungal aetiology have all been implicated in causing chronic spontaneous urticaria or its exacerbations (Antia et al. 2018). Helicobacter pylori was long believed to play a causative role in a significant subset of patients.
A study highlighted how the eradication of the bacterium had provided significantly beneficial effects to patients with chronic spontaneous urticaria, with a complete or partial remission of symptoms (Di Campli et al. 1998). Unfortunately, results were not replicated by subsequent studies, which didn’t found any association with H. pylori, nor any improvement of CSU eradication therapy (Curth et al. 2015; Kohli et al. 2018).
Several foods and food additives have been implicated and extensively studied in the pathogenesis of chronic spontaneous urticaria (Antia et al. 2018). Patients may sometimes indicate certain foods as the cause of their symptoms and consequently develop avoidance mechanisms when choosing what to eat. However, studies which investigated the presence of urticarial symptoms after oral food challenge only observed them in a small minority of patients with such claims (Hsu and Li 2012; Chung et al. 2016).
Drugs can exacerbate urticaria through different mechanisms. ACE inhibitors are well known to cause angioedema by causing an increase in the amounts of bradykinin (Scalese and Reinaker 2016). Nonsteroidal anti-inflammatory drugs (NSAIDs) may also induce urticaria, either through an allergic (IgE-mediated) reaction or through the inhibition of COX-1 and increased production of leukotrienes (Antia et al. 2018).
Emotional stress has also been associated with chronic spontaneous urticaria. Patients suffering from the disorder suffer from higher rates of depression, anxiety and somatoform disorders with respect to the general population. However, it’s unclear whether this is a consequence of coping with the disorder or a possible predisposing factor (Fig. 2) (Uguz et al. 2008).
5 Diagnosis
According to the latest guidelines AU is to be considered as a self-limiting disorder, which requires no investigation unless a couple of specific features are present. Namely, these are the presence of a type I food allergy (IgE-mediated) or of another eliciting factor such as NSAIDs (or other drugs) (Zuberbier et al. 2018). In these cases, the patient should be counselled towards allergy tests and avoidance of re-exposure to proven precipitating factors.
In CU, the workup is more extensive. The most relevant component of the investigation is the history taking, which, if conducted properly, usually spares the physician and the patients time-consuming, costly and often improper laboratory testing.
5.1 Diagnostic Workup
A summary of the crucial questions in history taking and their diagnostic role has been included in Table 2, still based upon the latest international recommendations (Zuberbier et al. 2018).
After conducting history taking, physical examination should be performed. Along with a general survey of the patient, attention should be focused on features of the skin lesions (when present at the time of the visit). Eventually, each form of CIndU should be confirmed with the appropriate testing (see Tables 1A and 1B).
CSU wheals may have variable appearance, but they should always blanch with pressure and leave no trace after 24 h. In patients with typical features of CSU, lab testing should be restricted to a differential blood count, ESR and CRP. In patients with atypical features (e.g. wheals not resolving within 24 h, presence of angioedema only) or other associated symptoms, a more extensive workup may be appropriate. Testing for Helicobacter pylori, functional auto-antibodies presence (e.g. ASST), thyroid autoimmunity, allergy tests, vasculitis (e.g. skin biopsy) or other systemic disorders may be warranted depending upon the history (Zuberbier et al. 2018).
5.2 Biomarkers of Diagnosis and Severity
Biomarkers are clinical features (clinical biomarkers) or molecules present in serum (molecular biomarkers) which are able to offer some diagnostic, therapeutic or prognostic information. They clearly depend upon the disease pathogenesis and are one of the most important fields of clinical research in many conditions, including CSU. Clinical biomarkers for diagnosis, stratification of disease and treatment response have been observed in CSU. These include age and gender (Folci et al. 2018). Being female has been associated with a more prolonged clinical course and a worse quality of life, while an association of older age and longer duration of disease has also been established (Gregoriou et al. 2009; Hiragun et al. 2013).
The presence of angioedema is particularly taken in consideration because of a strict correlation to a less favorable prognosis (Toubi et al. 2004; Champion et al. 1969). Exacerbations occurring with the use of aspirin or non-steroidal anti-inflammatory drugs (NSAID) have been widely described as being related to a more severe and chronic disease (Sánchez-Borges et al. 2015b; Shin et al. 2015).
For what concerns molecular biomarkers, a considerable number is present, even if no one of them has yet gained the clinical practice. C reactive protein (CRP) is certainly one of the most important and reproducible. High CRP levels were demonstrated to be associated with higher activity of disease and quality of life impairment (Kolkhir et al. 2017; Kolkhir et al. 2018). Another molecule with marked inflammatory activity, interleukin 6 (IL-6), was also found to be increased (Kasperska-Zajac et al. 2015). Furthermore, Vitamin D, well-known because of its crucial role in bone metabolism, less because of its immunomodulatory activity, has been implicated in the pathogenesis of chronic urticaria. Apparently, depletion in Vitamin D is associated with an increased disease severity. As a consequence, it may be an effective marker with potential therapeutic implications (Vitamin D supplementation) (Piemonti et al. 2000; Holick and Vitamin 2007; Woo et al. 2015).
As it was previously described, D-Dimer levels were long thought to be an effective marker of response to treatment, especially when high before administration (Takeda et al. 2011). However, recent evidence contradicts this view and actually confirms the validity of D-dimer only as a marker of CSU severity.
In order to evaluate patients who are less likely to respond to Omalizumab promptly, due to the presence of anti-IgE receptor IgGs, the basophil histamine release assay (BHRA) may be used. BHRA evaluates the presence of IgGs targeting FcεRI in serum. In patients who test positive, omalizumab will take time to be effective. This is due to the need for all FcεRI on mast cells to be uncovered from IgE (which occurs immediately) and slowly decay (over several weeks) (Grattan et al. 1986; Folci et al. 2018; Metz et al. 2017; Saini and MacGlashan 2012).
Other biomarkers, such as interleukin 18 (IL-18) and matrix metalloproteinase-9 (MMP-9), have also been considered. IL-18 may be involved in the recruitment of eosinophils in skin affected from urticaria, while MMP-9 may be involved in the migration of immune cells by remodelling tissue extracellular matrix (Novick et al. 2001; Wang et al. 2001; Ram et al. 2006; Song et al. 2013; Belleguic et al. 2002). However, results are far from being conclusive and they will need validation from other groups.
Eventually, IL-24 should be mentioned once again. If a standardized assay (e.g. ELISA testing) for the quantification of anti-IL-24 IgE were developed, it would appear more feasible to start considering autoallergy in the clinical setting. Also in this case, although results are still preliminary, they seem to be quite promising (Table 3).
5.3 Differential Diagnosis
A broad spectrum of disorders is included in the differential diagnosis of chronic urticaria, with several of these conditions being rare but severe systemic disorders (Radonjic-Hoesli et al. 2018). The clinician should be particularly careful when urticaria is associated with other signs and symptoms or presents with atypical features (Radonjic-Hoesli et al. 2018). When wheals last for longer than 24 h, lesions are burning more than itching and don’t disappear after digital pressure or the patient suffers from a systemic autoimmune disorder (such as systemic lupus erythematosus or primary Sjogren’s syndrome), urticarial vasculitis (UV) may be present. Several other autoimmune, autoinflammatory and hematologic disorders may likewise present with urticaria (a summary of the most relevant conditions with their distinctive features has been included in Table 4).
6 Treatment
While treatment of acute urticaria has remained roughly the same in the last decades, the approach to management of chronic urticaria has been revolutionized by the development of biologic drugs. Omalizumab is now the recommended drug in patients with CSU refractory to antihistamines treatment. Soon enough, a new generation of biologic and target drugs promises to further reduce the fraction of patients who does not respond fully to available therapies.
6.1 Acute Urticaria
Emergency physicians and general practitioners are often involved in the management of acute urticaria. Being described as a self-limiting condition, its treatment was not discussed by the most recent guidelines on urticaria (nor the previous ones), where it was just suggested that possible provoking factors should be avoided and symptomatic relief should be warranted (Zuberbier et al. 2018; Zuberbier et al. 2014). Previous literature on this topic is equally sparse. A short course of prednisone was proven to be effective in obtaining a quicker response in one trial, while in a nationwide survey conducted in the US, physicians reported the use of histamine (H1) antagonists and prednisone in affected children (Pollack and Romano 1995; Beno et al. 2007). In a recent review written by a worldwide expert on allergy and urticaria, it’s suggested that H1 antihistamines are given up to four times a day, with an additional short course of steroids when severity requires it (eg. 40 mg of prednisone for 3 days, tapered by 5 mg/day or stopped without tapering) (Kaplan 2019). Second generation H1 antihistamines (eg. cetirizine, levocetirizine, loratadine) are recommended over first generation ones due to improved safety profile, especially a lower incidence of drowsiness (which can still be present, especially when more than one tablet is taken daily) (Snidvongs et al. 2017).
6.2 Chronic Urticaria
The treatment of chronic urticaria depends on the presence of specific trigger factors, as it occurs in CIndU, or the absence of such, as in CSU. Importantly, the physician should acknowledge the difficulties experienced by the patient coping with a long-lasting disorder with no curative treatment. In order to provide the most effective solution without exposing patients to unnecessary risks, a stepwise approach should be followed. Corticosteroids should generally be withdrawn from therapy due to their profile of severe side effects in chronic use.
6.2.1 Chronic Inducible Urticaria
The management of CIndU clearly involves the avoidance of physical stimuli, although this is rarely possible. While it may be feasible to substitute latex products with other equivalent ones in the case of a contact urticaria, it may be more complex to avoid sunlight, exposure to cold or to water. In the case of delayed pressure urticaria, it’s important to make the patient aware that by using wider handles for heavy bags the surface in contact with the skin is increased and, as a consequence, the pressure exerted is reduced (Zuberbier et al. 2018). Second generation H1 antihistamines have been successfully used in acquired cold urticaria, where four daily dosage (i.e. 20 mg desloratadine daily) was associated with improved clinical course with respect to lower dosages and placebo (Siebenhaar et al. 2009).
In those forms of inducible urticaria which are responsive to antihistamines, these are clearly the indicated therapy as long as needed. However, delayed pressure urticaria is unresponsive to these (Kaplan 2019). For these and other patients refractory to antihistamines, omalizumab has proven effective and safe in several studies (Metz et al. 2014; Quintero et al. 2017; Maurer et al. 2018). For the few patients who don’t respond to omalizumab, no effective pharmacological treatment is available. Patients would clearly respond to steroids but the side effects of a prolonged treatment would almost certainly offset the benefits (Kaplan 2019).
6.2.2 Chronic Spontaneous Urticaria
Management of CSU is a careful process characterized by an add-on strategy to achieve the best control of disease, if not a complete remission.
6.2.2.1 Identification and Avoidance of Aggravating Factors
When reported in the course of history taking, drugs suspected of exacerbating CSU should be withdrawn from therapy and substituted with others, if possible. Other factors which have been associated with CSU are infections, such as H. pylori colonization. As previously described, the association between H. pylori and CSU has not been proven conclusively. However, when colonization is found, eradication therapy should be pursued, also because of the association of H. pylori with several other disorders (e.g. gastric cancer) (Zuberbier et al. 2018; Curth et al. 2015; Kohli et al. 2018). Reduction in emotional stress and avoidance of certain foods may also provide some degree of benefit, although this is questionable and not strongly supported by available research.
6.2.2.2 Pharmacological Management: A Stepwise Approach
The pharmacological management of CSU begins, as with all types of urticaria, with second generation, H1 antihistaminergic drugs. These are preferred over first generation H1 antihistamines because of their lower propensity to cause drowsiness. According to the most recent guidelines on treatment, these drugs are roughly equivalent, and patients may choose any of them (e.g. cetirizine, levocetirizine, desloratadine, ebastine, fexofenadine). However, the use of multiple antihistamines at the same time is not recommended (Zuberbier et al. 2018). In many patients, standard dosage is not effective and it may need to be escalated up to four times in order to warrant remission. Although this approach is also supported by urticaria guidelines, it may pose the patient at a higher risk of sleepiness and is not necessarily compliant to all countries prescribing regulation.
When high doses of regularly administered antihistamines are not effective, omalizumab should be prescribed as an add-on to therapy. Omalizumab is a humanized monoclonal IgG immunoglobulin which targets IgE and prevents their binding to FcεRI (Tonacci et al. 2017). With time, uncovered FcεRI on basophils and mast cells undergo a process of slow decay and reduction in number (Saini and MacGlashan 2012). Omalizumab was administered for the first time in patients with CSU in a small study carried out in 2008 (Kaplan et al. 2008). The effectiveness and safety of the drug was later confirmed by one phase two and three phase three trials (Saini et al. 2011; Maurer et al. 2013; Kaplan et al. 2013; Saini et al. 2015).
These trials brought evidence that doses of 150 mg and 300 mg of omalizumab monthly were both effective, although the 300 mg dose was clearly more effective than the lower one. About 40% of patients reached complete remission and more than half (around 60%) obtained partial remission (calculated with UAS7 score lower than 6) (Kaplan 2019). It also emerged how patients often relapsed when the drug was discontinued, which didn’t seem to affect the course of the disease in the long term. Interestingly, omalizumab also proved to be effective in patients suffering from idiopathic nonhistaminergic acquired angioedema (InH-AAE) in a preliminary case series. This led the investigators to hypothesize that InH-AAE may actually be an IgE-mediated disease, sharing the pathogenesis with CSU (Brunetta et al. 2018).
In patients who are refractory to omalizumab, along with reconsideration of alternative diagnoses from CSU, it is recommended that ciclosporin A is added to treatment. Ciclosporin is an immunosuppressant drug whose main mechanism is the inhibition of calcineurin in T cells. However, it has long been known to impede histamine release in mast cells and basophils (Cirillo et al. 1990; Stellato et al. 1992). Ciclosporin has been proven to be an effective drug for the treatment of CSU by two randomized controlled clinical trials, however, with low number of patients treated, and with short periods of treatment and observation after the interruption of therapy (Grattan et al. 2000; Vena et al. 2006).
Moreover, its use is limited by the severe side effects of hypertension and renal injury, which are dose-dependent and require monthly controls (Kulthanan et al. 2018). A daily ciclosporin dose of 3–3.5 mg/kg has obtained a success rate of 70–80%, while higher doses (4–4.5 mg/kg) are usually avoided because of higher risks of toxicity (Kaplan 2019). A recent metanalysis established the efficacy of cyclosporin with respect to placebo along with its mixed safety profile, with the common occurrence of side effects requiring interruption of treatment or reduction of the dosage (Kulthanan et al. 2018).
In patients in whom antihistamines, omalizumab and cyclosporin failed, a number of other medications have been used with variable success. These include leukotriene receptor antagonists, mycophenolate mofetil, methotrexate and hydroxicloroquine (Rutkowski and Grattan 2017). Guidelines do not discard completely this off-label approaches, which may be useful in individual and well-selected patients, but they instruct the physician to adopt a certain degree of caution due to the very low level of evidence which supports them (Zuberbier et al. 2018).
6.2.2.3 Biomarkers of Response to Treatment
Nowadays predicting the efficacy of a therapeutical scheme before prescribing the drug, seems pivotal to reach remission, improve the health of the patients and avoid waste. Several studies tried to identify and cluster groups of patients by clinical efficacy to a defined drug; nonetheless, foreseeing the response remains an unmet need in CSU treatment. In the table below are listed the main biomarkers described by the recent evidence (Table 5).
6.3 Investigational Therapies
While existing pharmacological measures are effective in the majority of patients, they do not offer a long-lasting remission of symptoms after discontinuation of treatment. Moreover, they are not focused upon pathogenetic subsets within the disorder, but rather tackle CSU in a standardized manner.
On the contrary, the emerging approach of tailored treatment aims at providing the appropriate drug for each patient. This has become feasible due to an improved knowledge of CSU pathogenesis and biomarkers of disease. Currently, several drugs are under clinical and preclinical development for chronic urticaria.
6.3.1 Anti-IgE Humanized Monoclonal Antibodies
Of the drugs currently under investigation, the following two are certainly those with the greatest expectations in CSU. These are the anti-IgE humanized monoclonal antibodies quilizumab and ligelizumab.
Quilizumab targets membrane bound IgEs on IgE-switched B lymphocytes and plasma cells. It was found to reduce levels of total and specific IgEs in serum in patients with asthma and CSU (Harris et al. 2016a). However, in two randomized trials conducted in asthma and CSU, it was unable to provide any clinical benefit compared respectively to standard therapy and placebo (Harris et al. 2016a, b).
Ligelizumab mechanism of action is instead closer to omalizumab, as it equally binds soluble IgEs (Kocatürk et al. 2017). However, ligelizumab appears to be six to nine-fold more powerful than omalizumab. Furthermore, it shows a more prolonged suppression of IgE levels in serum (Kocatürk et al. 2017). This may be due to a different functional profile of the two drugs (Gasser et al. 2020). Indeed, not only does ligelizumab neutralize serum IgEs with increased affinity, but it also appears to inhibit Ige production (Gasser et al. 2020). According to a recent study, ligelizumab may be able to downregulate IgE production by binding the CD23:IgE complex on the surface of B-cells, a feature which has not been shown with omalizumab (Gasser et al. 2020).
In a phase IIb randomized controlled trial which was recently published, 382 patients were either administered omalizumab, ligelizumab or placebo with varying doses and the response on weekly urticaria activity (with a focus on complete control of hives) was compared (Maurer et al. 2019). The primary endpoint of the study (complete control of hives) was assessed after 12 weeks of treatment. The study successfully determined a dose-response curve for ligelizumab and a statistically significant improvement in symptoms control with respect to omalizumab (complete hives response in 51% of patients treated with 72 mg ligelizumab with respect to 26% of patients who received 300 mg omalizumab) (Maurer et al. 2019). As the authors of the trial observed, the low percentage of patients who responded to omalizumab is puzzling and inconsistent with previous literature (Maurer et al. 2019). This has been interpreted as a consequence of patients selections criteria. Dose-limiting side effects were not observed, with generally no patients reporting anaphylaxis (Maurer et al. 2019).
6.3.2 Blockade of IL-1
Due to the efficacy of IL-1 blockade in autoinflammatory syndromes such as CAPS and Schnitzler’s syndrome, along with the evidence of effect in some patients with physical urticarias, anti-IL-1 drugs have also been investigated in CSU (Bodar et al. 2009; Krause et al. 2012; Lenormand and Lipsker 2012). A phase II trial was conducted on the efficacy of canakinumab compared to placebo by the University of Zurich but results haven’t been published yet (although the study was scheduled to end several years ago) (Fig. 3).
Investigational therapies in CSU
Each segment represents a distinct pathway involved in the activation of mast cells (centre). Molecular mechanisms are represented on the outer part of the figure, as well as drugs studied to block them (Grey circle: cellular membrane; BTK: Bruton’s tyrosine kinase; SYK: spleen tyrosine kinase)
6.3.3 DARPins
A different and novel group of drugs is that of designed ankyrin repeat proteins (DARPins). These are small molecules, engineered to mimic antibodies binding capabilities, which can be administered orally (Kocatürk and Zuberbier 2018). Two of these were able to inhibit the binding of IgEs to their mast cells receptors and consequently block their activation (Kim et al. 2012). Unfortunately, they haven’t reached yet a clinical stage of experimentation (Fig. 3).
6.3.4 SYK Inhibitors
In mast cells, spleen tyrosine kinase (SYK) plays an important role in intracellular signalling. It leads to a cascade of events ultimately causing release of histamine, prostaglandins and other mediators. The development of a topical formulation of a SYK inhibitor (GSK2646264) is currently being studied clinically in healthy controls and patients with CSU (Fig. 3) (Ramirez Molina et al. 2019; Barker et al. 2018).
6.3.5 BTK Inhibitors
A mechanistically similar target of future therapies may be Bruton’s tyrosine kinase. Involved in B cell signalling and relevant to the production of IgEs, the inhibition of BTK yielded encouraging results in a study investigating the efficacy of oral ibrutinib (a BTK inhibitor) on mast cells and basophils activation (Dispenza et al. 2018; Herman et al. 2018). A phase II trial is now being conducted on patients with CSU for a different oral BTK inhibitor, fenebrutinib, in order to understand its safety and efficacy (Fig. 3).
6.3.6 Anti-Prostaglandin
CRTH2, a PGD2 receptor, was observed to be over-expressed by eosinophils in CSU patients (Yahara et al. 2010). An oral antagonist of CRTH2, AZD1981, was therefore studied in patients with CSU and H1-antihistamines refractoriness (Oliver et al. 2019). Interestingly, the short trial (4 weeks, placebo-controlled) showed benefit especially in terms of reduced itching, rather than hives extension. The drug was well tolerated and, at least in some aspects, more effective than placebo. Although not yet investigated in CSU, fevipiprant, a drug acting similarly to AZD1981 (a CRTH2 antagonist), has been extensively studied in asthma with important preliminary success, especially in severe eosinophilic asthma (Fig. 3) (White et al. 2018).
6.3.7 Anti-Complement Fragments
As described by several studies, complement binding plays a crucial role in enhancing the activating effects of IgG autoantibodies targeting mast cells IgE receptors (Fiebiger et al. 1995). In particular, C5 fragments have been postulated to bind mast cells C5a receptors leading to their activation (Kikuchi and Kaplan 2002). Eculizumab is a monoclonal antibody targeting C5, which has proven effective in paroxysmal nocturnal hemoglobinuria and atypical hemolytic-uremic syndrome. It would be of great interest to evaluate the safety and efficacy of eculizumab in CSU (Kocatürk et al. 2017). Besides, a molecule targeting C5a receptor was recently developed and tested with preliminary success in patients with granulomatosis with polyangiitis (previously known as Wegener’s granulomatosis, a vasculitis part of the group of ANCA-associated vasculitides) (Jayne et al. 2017; Tesar and Hruskova 2018). This molecule, whose name is avacopan, should equally be studied in CSU, where it may provide an additional clinical benefit in patients refractory to other treatments (Fig. 3).
6.3.8 Anti-IL-5 and IL-5 Receptors
Eventually, monoclonal antibodies targeting interleukin 5 and its receptor (respectively mepolizumab and benralizumab) may be effective in CSU, at least according to those who place eosinophils at the centre of urticarial pathogenesis (Kocatürk et al. 2017). This theory has gained popularity after a case was reported in which mepolizumab caused complete remission of CSU refractory to other treatments in a patient with concurrent severe uncontrolled asthma (Magerl et al. 2018). Currently, a phase I study of mepolizumab is undergoing in patients with CSU (Fig. 3) (Kocatürk and Zuberbier 2018). Recently, a non-randomized trial on 12 patients was published which evaluated the response to benralizumab in patients unresponsive to antihistamines (Bernstein et al. 2020). Patients were first administered placebo and subsequently three monthly doses of benralizumab (30 mg). The primary endpoint was the change in UAS7 after 20 weeks, which was found to be highly statistically significant (−15.7 points, 95% CI: −6.6 to −24.8, p < 0.001) (Bernstein et al. 2020). However, due to the small size of the patient group and the absence of randomization or even a control group treated with standard of care (omalizumab), it is difficult to infer any reliable information on benralizumab efficacy from this preliminary data.
6.3.9 Anti Sialic Acid-Binding Immunoglobulin-like Lectins (Siglecs)
The expression of Sialic acid-binding immunoglobulin-like lectins (Siglecs), a family receptors member of type I lectin, on human leukocytes is well demonstrated. The function of Siglecs in the human immune system is various, with inhibitory and activatory function depending on the cells expressing them (Varchetta et al. 2012). In particular, Siglec-8 is selectively expressed on human eosinophils, basophils and mast cells (Crocker et al. 2007). Its activation brings eosinophils to apoptosis and mast cells to inhibition of response (Nutku et al. 2003). For this reason, an anti Siglec-8 receptor was synthetized, AK002. Treatment of healthy subjects showed a rapidly depletion in blood eosinophils even after the single dose (Rasmussen et al. 2018). An open-label, phase 2a, pilot study showed how almost 92% of CSU patients naïve to omalizumab and 36% omalizumab-refractory obtained a complete remission (Fig. 3).
7 Prognosis
More than 80% of cases of acute urticaria don’t progress beyond 6 weeks (Antia et al. 2018; Zuberbier et al. 1996; Aoki et al. 1994). In a study conducted on children, the rate of acute urticaria becoming recurrent or turning into chronic urticaria was estimated at about 30% (Mortureux et al. 1998). For what concerns the duration of chronic urticaria, different studies have provided variable results. A rate of 3-year remission of 32%, a 1-year remission rate of 47% and a 55% 1-year remission rate were all described by different authors (Champion et al. 1969; Quaranta et al. 1989; Kozel et al. 2001). 5-year remission rates have been observed to be 29% in adults and 67.7% in children (van der Valk et al. 2002; Chansakulporn et al. 2014).
In order to assess severity of disease and quality of life in chronic spontaneous urticaria, several tools have been developed. Among these, Chronic Urticaria Quality of Life Questionnaire (CU-Q20L) focuses on health-related quality of life, instead, the Urticaria Severity Score (USS) evaluates quality of life and need for medications. The most used in the clinical setting is the urticaria activity score-7 (UAS-7), which evaluates severity of the disease (Antia et al. 2018) by the calculation of a weekly score based upon daily itching (0–3 points) and the number of wheals (0–3 points) (Mlynek et al. 2013; Mathias et al. 2010). When the total weekly score sums up to less than 7, disease is well controlled. With higher scores, disease is progressively less controlled.
Studies performed on patients with CSU, in order to assess their quality of life and satisfaction with treatment, have invariably brought forward worrying results. From an online survey conducted in Germany, it emerged that approximately half of the more than 17,000 participants felt their symptoms were uncontrolled (Maurer et al. 2016). A subsequent observational study, conducted on patients with CSU who had persistence of symptoms despite treatment, demonstrated that more than half of the patients had moderate-to-severe disease activity based upon the UAS-7 (Maurer et al. 2017b). Unfortunately, according to another recent study, it appears that a large percentage of patients is undertreated due to lack of adherence to guidelines. In a cohort of patients with CSU refractory to antihistamines, only a minority was informed about the possibility of omalizumab treatment (Maurer et al. 2017a).
Eventually, it should be kept in mind how CSU, although not a life-threatening condition, is a chronic disorder carrying an important burden on patients health and quality of life. While treatment is not always effective, it may still elicit adverse reactions. The ineffectiveness of treatment may be due to the standardized “one-size-fits-all” stepwise approach which is adopted in CSU. Indeed, the possibility of adopting customized therapies for individual patients is not presently available in CSU.
However, the implementation of a laboratory diagnostics (a set of molecular biomarkers) capable of distinguishing subsets of patients with different mechanisms of disease is the contemporary goal of the most respected investigators. Concurrently, an impressive number of novel medications are being tested for future use in CSU. Some of these, due to their specific targets, may only be used in specific cases. All things considered, it seems to be a matter of time before the revolution of precision medicine fully takes place in the setting of urticaria.
Abbreviations
- AAC:
-
area above the curve
- ACEi:
-
Angiotensin converting enzyme inhibitors
- ANA:
-
anti-nuclear antibodies
- AOSD:
-
Adult onset Still’s disease
- ASST:
-
autologous serum skin test
- AU:
-
Acute urticaria
- BHRA:
-
basophil histamine release assay
- BTK:
-
Bruton’s tyrosine kinase
- C5aR:
-
Complement 5a receptor
- CAPS:
-
Cryopirin-associated periodic syndromes
- CIndU:
-
Chronic Inducible Urticaria
- COX-1:
-
cyclooxygenase 1
- CRP:
-
c-reactive protein
- CRTH2:
-
chemoattractant receptor homologous molecule expressed on the Th2 cell
- CSU:
-
Chronic Spontaneous Urticaria
- CU:
-
Chronic urticaria
- CU-Q20L:
-
Chronic Urticaria Quality of Life
- DARPins:
-
designed ankyrin repeat proteins
- ESR:
-
erythrocyte sedimentation rate
- F1 + 2:
-
Prothrombin fragment 1 + 2
- FcεRI:
-
high-affinity IgE receptor
- FcεRIα:
-
high-affinity IgE receptor alpha chain
- HLA:
-
Human Leukocyte Antigen
- HUV:
-
hypocomplementemic urticarial vasculitis
- IFNγ:
-
interferon gamma
- IL-1:
-
interleukin 1
- IL-18:
-
interleukin 18
- IL-24:
-
interleukin 24
- IL-25:
-
interleukin 25
- IL-33:
-
interleukin 33
- IL-4:
-
interleukin 4
- IL-5:
-
interleukin 5
- IL-6:
-
interleukin 6
- Il-6sR:
-
interleukin 6 soluble receptor
- InH-AAE:
-
idiopathic nonhistaminergic acquired angioedema
- ITAMs:
-
immunoreceptor tyrosine-based activation motifs
- LCN2:
-
serum Lipocalin-2
- MCT:
-
tryptase-positive chymase-negative mast cells
- MCTC:
-
tryptase-positive chymase-positive mast cells
- MMP-9:
-
matrix metalloproteinase-9
- MPV:
-
mean platelet volume
- NGF:
-
nerve growth factor
- NSAID:
-
non-steroidal anti-inflammatory drug
- PG:
-
Prostaglandins.
- PGD2:
-
prostaglandin D2
- sgp130:
-
soluble glycoprotein 130
- Siglec:
-
Sialic acid-binding immunoglobulin-like lectin
- SYK:
-
spleen tyrosine kinase
- Th1:
-
T helper 1
- Th2:
-
T helper 2
- TPO:
-
thyroid peroxidase
- TSH:
-
thyroid stimulating hormone
- TSLP:
-
thymic stromal lymphopoietin
- UAS7:
-
weekly urticaria activity score
- USS:
-
Urticaria Severity Score (USS)
- UV:
-
urticarial vasculitis
References
Abajian M, Schoepke N, Altrichter S, Zuberbier T, Maurer M (2014) Physical Urticarias and cholinergic Urticaria. Immunol Allergy Clin N Am 34:73–88
Aloe L, Skaper SD, Leon A, Levi-Montalcini R (1994) Nerve growth factor and autoimmune diseases. Autoimmunity 19:141–150
Altrichter S et al (2011) IgE mediated autoallergy against thyroid peroxidase – a novel Pathomechanism of chronic spontaneous Urticaria? PLoS One 6:e14794
Amsler E, Soria A, Vial-Dupuy A (2014) What do we learn from a cohort of 219 French patients with chronic urticaria? Eur J Dermatol 24:700–701
Antia C, Baquerizo K, Korman A, Bernstein JA, Alikhan A (2018) Urticaria: a comprehensive review. J Am Acad Dermatol 79:599–614
Aoki T, Kojima M, Horiko T (1994) Acute Urticaria: history and natural course of 50 cases. J Dermatol 21:73–77
Asero R (2002) Chronic idiopathic urticaria: a family study. Ann Allergy Asthma Immunol 89:195–196
Asero R (2015) Plasma D-dimer levels and clinical response to ciclosporin in severe chronic spontaneous urticaria. J Allergy Clin Immunol 135:1401–1403
Asero R et al (2007) Activation of the tissue factor pathway of blood coagulation in patients with chronic urticaria. J Allergy Clin Immunol 119:705–710
Asero R, Marzano AV, Ferrucci S, Cugno M (2017) D-dimer plasma levels parallel the clinical response to Omalizumab in patients with severe chronic spontaneous Urticaria. Int Arch Allergy Immunol 172:40–44
Auyeung P, Mittag D, Hodgkin PD, Harrison LC (2016) Autoreactive T cells in chronic spontaneous urticaria target the IgE Fc receptor Iα subunit. J Allergy Clin Immunol 138:761–768.e4
Ban G-Y et al (2014) Clinical features of elderly chronic urticaria. Korean J Intern Med 29:800
Bandara G, Metcalfe DD, Kirshenbaum AS (2015) Growth of human mast cells from bone marrow and peripheral blood-derived CD34(+) pluripotent hematopoietic cells. Methods Mol Biol 1220:155–162
Barker MD et al (2018) Discovery of potent and selective Spleen Tyrosine Kinase inhibitors for the topical treatment of inflammatory skin disease. Bioorg Med Chem Lett 28:3458–3462
Baroni A, Faccenda F, Russo T, Teresa R, Piccolo V (2012) Figurate paraneoplastic urticaria and prostate cancer. Ann Dermatol 24:366–367
Bech R, Kibsgaard L, Vestergaard C (2018) Comorbidities and treatment strategies in bullous pemphigoid: an appraisal of the existing Litterature. Front Med 5:238
Bédard PM, Brunet C, Pelletier G, Hébert J (1986) Increased compound 48/80 induced local histamine release from nonlesional skin of patients with chronic urticaria. J Allergy Clin Immunol 78:1121–1125
Belleguic C et al (2002) Increased release of matrix metalloproteinase-9 in the plasma of acute severe asthmatic patients. Clin Exp Allergy 32:217–223
Beno SM, Nadel FM, Alessandrini EA (2007) A Survey of Emergency Department Management of Acute Urticaria in Children. Pediatr Emerg Care 23:862–868
Bernstein JA et al (2020) Benralizumab for chronic spontaneous Urticaria. N Engl J Med 383:1389–1391
Bodar EJ, Simon A, de Visser M, van der Meer JWM (2009) Complete remission of severe idiopathic cold urticaria on interleukin-1 receptor antagonist (anakinra). Neth J Med 67:302–305
Bonini S et al (1996) Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci 93:10955–10960
Boyden SE et al (2016) Vibratory Urticaria Associated with a Missense Variant in ADGRE2. N Engl J Med 374:656–663
Bracken SJ, Abraham S, MacLeod AS (2019) Autoimmune theories of chronic spontaneous Urticaria. Front Immunol 10:627
Bradding P, Okayama Y, Howarth PH, Church MK, Holgate ST (1995) Heterogeneity of human mast cells based on cytokine content. J Immunol 155:297–307
Brunetta E et al (2018) Omalizumab for idiopathic nonhistaminergic angioedema: evidence for efficacy in 2 patients. Case Rep Immunol 2018:8067610
Caproni M et al (2005) Chronic idiopathic urticaria: infiltrating cells and related cytokines in autologous serum-induced wheals. Clin Immunol 114:284–292
Cassano N, Colombo D, Bellia G, Zagni E, Vena GA (2016) Gender-related differences in chronic urticaria. G Ital Dermatol Venereol 151:544–552
Champion RH, Roberts SO, Carpenter RG, Roger JH (1969) Urticaria and angio-oedema. A review of 554 patients. Br J Dermatol 81:588–597
Chan Y-C et al (2014) ‘Auto-anti-IgE’: naturally occurring IgG anti-IgE antibodies may inhibit allergen-induced basophil activation. J Allergy Clin Immunol 134:1394–1401.e4
Chansakulporn S et al (2014) The natural history of chronic urticaria in childhood: a prospective study. J Am Acad Dermatol 71:663–668
Chung BY, Cho YS, Kim HO, Park CW (2016) Food allergy in Korean patients with chronic Urticaria. Ann Dermatol 28:562–568
Cirillo R et al (1990) Cyclosporin A rapidly inhibits mediator release from human basophils presumably by interacting with cyclophilin. J Immunol 144:3891–3897
Coban M et al (2008) HLA class I and class II genotyping in patients with chronic Urticaria. Int Arch Allergy Immunol 147:135–139
Crocker PR, Paulson JC, Varki A (2007) Siglecs and their roles in the immune system. Nat Rev Immunol 7:255–266
Cugno M, Marzano AV, Asero R, Tedeschi A (2010) Activation of blood coagulation in chronic urticaria: pathophysiological and clinical implications. Intern Emerg Med 5:97–101
Curth H-M et al (2015) Effects of helicobacter pylori eradication in chronic spontaneous Urticaria: results from a retrospective cohort study. Am J Clin Dermatol 16:553–558
de Koning HD, Bodar EJ, van der Meer JWM, Simon A (2007) Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment. Semin Arthritis Rheum 37:137–148
Del Pozo MD et al (1997) Anisakis simplex, a relevant etiologic factor in acute urticaria. Allergy 52:576–579
Di Campli C et al (1998) Beneficial effects of helicobacter pylori eradication on idiopathic chronic urticaria. Dig Dis Sci 43:1226–1229
Dice J (2004) Physical urticaria. Immunol Allergy Clin N Am 24:225–246
Dilek F et al (2016) Plasma levels of matrix Metalloproteinase-9 in children with chronic spontaneous Urticaria. Allergy Asthma Immunol Res 8:522
Dispenza MC, Pongracic JA, Singh AM, Bochner BS (2018) Short-term ibrutinib therapy suppresses skin test responses and eliminates IgE-mediated basophil activation in adults with peanut or tree nut allergy. J Allergy Clin Immunol 141:1914–1916.e7
Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS (2008) Basophil phenotypes in chronic idiopathic Urticaria in relation to disease activity and autoantibodies. J Invest Dermatol 128:1956–1963
Elias J, Boss E, Kaplan A (1986) Studies of the cellular infiltrate of chronic idiopathic urticaria: prominence of T-lymphocytes, monocytes, and mast cells. J Allergy Clin Immunol 78:914–918
Estelle Simons Ledit RF Ardusso Vesselin Dimov Motohiro Ebisawa Yehia M El-Gamal Richard F Lockey Mario Sanchez-Borges Gian Enrico Senna Aziz Sheikh Bernard Y Thong Margitta Worm FR, Rosario D, Estelle Simons FR (2013) World allergy organization anaphylaxis guidelines: 2013 update of the evidence base. Int Arch Allergy Immunol 162:193–204
Feliciani C et al (2015) Management of bullous pemphigoid: the European dermatology forum consensus in collaboration with the European academy of dermatology and venereology. Br J Dermatol 172:867–877
Ferrer M, Nakazawa K, Kaplan AP (1999) Complement dependence of histamine release in chronic urticaria☆☆☆. J Allergy Clin Immunol 104:169–172
Ferrer M et al (2010) Serum total tryptase levels are increased in patients with active chronic urticaria. Clin Exp Allergy 40:1760–1766
Fiebiger E et al (1995) Serum IgG autoantibodies directed against the alpha chain of Fc epsilon RI: a selective marker and pathogenetic factor for a distinct subset of chronic urticaria patients? J Clin Invest 96:2606–2612
Folci M, Heffler E, Canonica GW, Furlan R, Brunetta E (2018) Cutting edge: biomarkers for chronic spontaneous Urticaria. J Immunol Res 2018:5615109
Gaig P, Olona M, Muñoz Lejarazu D, Caballero MT, Domínguez FJ, Echechipia S, García Abujeta JL, Gonzalo MA, Lleonart R, Martínez Cócera C, Rodríguez A, Ferrer M (2004) Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol 14:214–220
Gasser P et al (2020) The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat Commun 11:1–14
Grattan CE, Wallington TB, Warin RP, Kennedy CT, Bradfield JW (1986) A serological mediator in chronic idiopathic urticaria–a clinical, immunological and histological evaluation. Br J Dermatol 114:583–590
Grattan CEH et al (2000) Randomized double-blind study of cyclosporin in chronic ‘idiopathic’ urticaria. Br J Dermatol 143:365–372
Grattan CEH, Dawn G, Gibbs S, Francis DM (2003) Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin Exp Allergy 33:337–341
Gregoriou S et al (2009) Etiologic aspects and prognostic factors of patients with chronic urticaria: nonrandomized, prospective, descriptive study. J Cutan Med Surg 13:198–203
Harris JM et al (2016a) A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respir Res 17:29
Harris JM et al (2016b) A randomized trial of quilizumab in adults with refractory chronic spontaneous urticaria. J Allergy Clin Immunol 138:1730–1732
Herman AE et al (2018) Safety, pharmacokinetics, and pharmacodynamics in healthy volunteers treated with GDC-0853, a selective reversible Bruton’s tyrosine kinase inhibitor. Clin Pharmacol Ther 103:1020–1028
Hide M et al (1993) Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic Urticaria. N Engl J Med 328:1599–1604
Hiragun M et al (2013) Prognosis of chronic spontaneous urticaria in 117 patients not controlled by a standard dose of antihistamine. Allergy 68:229–235
Holick M, Vitamin F (2007) D deficiency. N Engl J Med 357:266–281
Hsu M-L, Li L-F (2012) Prevalence of food avoidance and food allergy in Chinese patients with chronic urticaria. Br J Dermatol 166:747–752
Irani AM, Schwartz LB (1989) Mast cell heterogeneity. Clin Exp Allergy 19:143–155
Jachiet M et al (2015) The clinical Spectrum and therapeutic Management of Hypocomplementemic Urticarial Vasculitis: data from a French Nationwide study of fifty-seven patients. Arthritis Rheumatol 67:527–534
Jacques P, Lavoie A, Bédard PM, Brunet C, Hébert J (1992) Chronic idiopathic urticaria: profiles of skin mast cell histamine release during active disease and remission. J Allergy Clin Immunol 89:1139–1143
Jayne DRW et al (2017) Randomized trial of C5a receptor inhibitor Avacopan in ANCA-associated Vasculitis. J Am Soc Nephrol 28:2756–2767
Kalogeromitros D et al (2011) Can internet surveys help us understanding allergic disorders? – results from a large survey in urticaria in Greece. J Eur Acad Dermatol Venereol 25:532–537
Kaplan AP (2019) Treatment of urticaria: a clinical and mechanistic approach. Curr Opin Allergy Clin Immunol 19:387–392
Kaplan AP, Joseph K, Maykut RJ, Geba GP, Zeldin RK (2008) Treatment of chronic autoimmune urticaria with omalizumab. J Allergy Clin Immunol 122:569–573
Kaplan A et al (2013) Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol 132:101–109
Kasi PM, Hieken TJ, Haddad TC (2016) Unilateral arm Urticaria presenting as a paraneoplastic manifestation of Metachronous bilateral breast Cancer. Case Rep Oncol 9:33–38
Kasperska-Zajac A, Grzanka A, Damasiewicz-Bodzek A (2015) IL-6 transsignaling in patients with chronic spontaneous Urticaria. PLoS One 10:e0145751
Kay AB et al (2014) Elevations in vascular markers and eosinophils in chronic spontaneous urticarial weals with low-level persistence in uninvolved skin. Br J Dermatol 171:505–511
Kay AB, Clark P, Maurer M, Ying S (2015) Elevations in T-helper-2-initiating cytokines (interleukin-33, interleukin-25 and thymic stromal lymphopoietin) in lesional skin from chronic spontaneous (‘idiopathic’) urticaria. Br J Dermatol 172:1294–1302
Kikuchi Y, Kaplan AP (2002) A role for C5a in augmenting IgG-dependent histamine release from basophils in chronic urticaria. J Allergy Clin Immunol 109:114–118
Kim B et al (2012) Accelerated disassembly of IgE-receptor complexes by a disruptive macromolecular inhibitor. Nature 491:613–617
Kirshenbaum AS, Metcalfe DD (2006) Growth of human mast cells from bone marrow and peripheral blood-derived CD34+ pluripotent progenitor cells. Methods Mol Biol 315:105–112
Kjaer HF, Eller E, Høst A, Andersen KE, Bindslev-Jensen C (2008) The prevalence of allergic diseases in an unselected group of 6-year-old children. The DARC birth cohort study. Pediatr Allergy Immunol 19:737–745
Kocatürk E, Zuberbier T (2018) New biologics in the treatment of urticaria. Curr Opin Allergy Clin Immunol 18:425–431
Kocatürk E, Maurer M, Metz M, Grattan C (2017) Looking forward to new targeted treatments for chronic spontaneous urticaria. Clin Transl Allergy 7:1
Kohli S et al (2018) Clinicoepidemiologic features of chronic Urticaria in patients with versus without subclinical Helicobacter pylori infection: a cross-sectional study of 150 patients. Int Arch Allergy Immunol 175:114–120
Kolkhir P, André F, Church MK, Maurer M, Metz M (2017) Potential blood biomarkers in chronic spontaneous urticaria. Clin Exp Allergy 47:19–36
Kolkhir P, Altrichter S, Hawro T, Maurer M (2018) C-reactive protein is linked to disease activity, impact, and response to treatment in patients with chronic spontaneous urticaria. Allergy 73:940–948
Konstantinou GN et al (2011) Childhood acute urticaria in northern and southern Europe shows a similar epidemiological pattern and significant meteorological influences. Pediatr Allergy Immunol 22:36–42
Kozel MMA, Mekkes JR, Bossuyt PMM, Bos JD (2001) Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol 45:387–391
Krause K, Metz M, Makris M, Zuberbier T, Maurer M (2012) The role of interleukin-1 in allergy-related disorders. Curr Opin Allergy Clin Immunol 12:477–484
Krause K et al (2019) Association of CCL2 with systemic inflammation in Schnitzler syndrome. Br J Dermatol 180:859–868
Kuemmerle-Deschner JB et al (2017) Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis 76:942–947
Kulthanan K, Chiawsirikajorn Y, Jiamton S (2008) Acute urticaria: etiologies, clinical course and quality of life. Asian Pac J Allergy Immunol 26:1–10
Kulthanan K et al (2018) Cyclosporine for chronic spontaneous Urticaria: a meta-analysis and systematic review. J Allergy Clin Immunol Pract 6:586–599
Lapi F et al (2016) Epidemiology of chronic spontaneous urticaria: results from a nationwide, population-based study in Italy. Br J Dermatol 174:996–1004
Lenormand C, Lipsker D (2012) Efficiency of Interleukin-1 blockade in refractory delayed-pressure Urticaria. Ann Intern Med 157:599
Levy Y, Segal N, Weintrob N, Danon YL (2003) Chronic urticaria: association with thyroid autoimmunity. Arch Dis Child 88:517–519
Lipsker D et al (2001) The Schnitzler syndrome. Four new cases and review of the literature. Medicine (Baltimore) 80:37–44
MacGlashan D (2019) Autoantibodies to IgE and FcεRI and the natural variability of spleen tyrosine kinase expression in basophils. J Allergy Clin Immunol 143:1100–1107.e11
Macri A, Cook C (2019) Urticaria Pigmentosa (cutaneous Mastocytosis). StatPearls. StatPearls Publishing
Magen E, Mishal J, Schlesinger M (2013) Clinical and laboratory features of chronic idiopathic urticaria in the elderly. Int J Dermatol 52:1387–1391
Magerl M et al (2018) Benefit of mepolizumab treatment in a patient with chronic spontaneous urticaria. J Dtsch Dermatol Ges 16:477–478
Mathias SD et al (2010) Development of a daily diary for patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol 105:142–148
Maurer M et al (2013) Omalizumab for the treatment of chronic idiopathic or spontaneous Urticaria. N Engl J Med 368:2340–2341
Maurer M et al (2016) ATTENTUS, a German online survey of patients with chronic urticaria highlighting the burden of disease, unmet needs and real-life clinical practice. Br J Dermatol 174:892–894
Maurer M et al (2017a) H1-antihistamine-refractory chronic spontaneous urticaria: it’s worse than we thought – first results of the multicenter real-life AWARE study. Clin Exp Allergy 47:684–692
Maurer M et al (2017b) The burden of chronic spontaneous urticaria is substantial: real-world evidence from ASSURE-CSU. Allergy 72:2005–2016
Maurer M et al (2018) Omalizumab treatment in patients with chronic inducible urticaria: a systematic review of published evidence. J Allergy Clin Immunol 141:638–649
Maurer M et al (2019) Ligelizumab for chronic spontaneous Urticaria. N Engl J Med 381:1321–1332
Metz M, Ohanyan T, Church MK, Maurer M (2014) Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: a retrospective clinical analysis. J Dermatol Sci 73:57–62
Metz M et al (2017) Clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with a reduction of FcεRI-positive cells in the skin. Theranostics 7:1266–1276
Minnone G, De Benedetti F, Bracci-Laudiero L (2017) NGF and its receptors in the regulation of inflammatory response. Int J Mol Sci 18:1028
Mlynek A et al (2013) A novel, simple, validated and reproducible instrument for assessing provocation threshold levels in patients with symptomatic dermographism. Clin Exp Dermatol 38:360–366
Mortureux P et al (1998) Acute Urticaria in infancy and early childhood. Arch Dermatol 134:319
Niimi N et al (1996) Dermal mast cell activation by autoantibodies against the high affinity IgE receptor in chronic urticaria. J Invest Dermatol 106:1001–1006
Novick D et al (2001) A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine 14:334–342
Nutku E, Aizawa H, Hudson SA, Bochner BS (2003) Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 101:5014–5020
Nuzzo V, Tauchmanova L, Colasanti P, Zuccoli A, Colao A (2011) Idiopathic chronic urticaria and thyroid autoimmunity: experience of a single center. Dermatoendocrinol 3:255–258
O’Donnell E, Havyer R (2014) Breast malignancy masquerading under the cloak of acute urticaria. BMJ Case Rep 2014:bcr2013202494
O’Donnell BF et al (1999) Human leucocyte antigen class II associations in chronic idiopathic urticaria. Br J Dermatol 140:853–858
Oliver ET, Sterba PM, Devine K, Vonakis BM, Saini SS (2016) Altered expression of chemoattractant receptor–homologous molecule expressed on T H 2 cells on blood basophils and eosinophils in patients with chronic spontaneous urticaria. J Allergy Clin Immunol 137:304–306.e1
Oliver ET et al (2019) Effects of an Oral CRTh2 antagonist (AZD1981) on eosinophil activity and symptoms in chronic spontaneous Urticaria. Int Arch Allergy Immunol 179:21–30
Otsuka H, Inaba M, Fujikura T, Kunitomo M (1995) Histochemical and functional characteristics of metachromatic cells in the nasal epithelium in allergic rhinitis: studies of nasal scrapings and their dispersed cells. J Allergy Clin Immunol 96:528–536
Pappalardo E et al (2000) Frequent de novo mutations and exon deletions in the C1inhibitor gene of patients with angioedema. J Allergy Clin Immunol 106:1147–1154
Peters EMJ et al (2011) Nerve growth factor partially recovers inflamed skin from stress-induced worsening in allergic inflammation. J Invest Dermatol 131:735–743
Piemonti L et al (2000) Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 164:4443–4451
Pogorelov D, Olisova O, Nekrasova T, Kolkhir P (2016) Chronische spontane Urtikaria in Verbindung mit einer primär billiären Cholangitis: ein Fallbericht und Literaturüberblick. J Dtsch Dermatol Ges 14:1134–1136
Poindexter NJ et al (2009) IL-24 is expressed during wound repair and inhibits TGFα-induced migration and proliferation of keratinocytes. Exp Dermatol 19:714–722
Pollack CV, Romano TJ (1995) Outpatient management of Acute Urticaria: the role of prednisone. Ann Emerg Med 26:547–551
Quaranta JH et al (1989) The natural history and response to therapy of chronic urticaria and angioedema. Ann Allergy 62:421–424
Quintero OP, Arrondo AP, Veleiro B (2017) Rapid response to omalizumab in 3 cases of delayed pressure urticaria. J Allergy Clin Immunol Pract 5:179–180
Raap U et al (2010) Increased levels of serum IL-31 in chronic spontaneous urticaria. Exp Dermatol 19:464–466
Raap U et al (2017) Human basophils are a source of – and are differentially activated by – IL-31. Clin Exp Allergy 47:499–508
Radonjic-Hoesli S et al (2018) Urticaria and angioedema: an update on classification and pathogenesis. Clin Rev Allergy Immunol 54:88–101
Ram M, Sherer Y, Shoenfeld Y (2006) Matrix Metalloproteinase-9 and autoimmune diseases. J Clin Immunol 26:299–307
Ramirez Molina C et al (2019) GSK2646264, a spleen tyrosine kinase inhibitor, attenuates the release of histamine in ex vivo human skin. Br J Pharmacol 176:1135–1142
Rasmussen HS, Chang AT, Tomasevic N, Bebbington C (2018) A randomized, double-blind, placebo-controlled, ascending dose phase 1 study of AK002, a novel Siglec-8 selective monoclonal antibody, in healthy subjects. J Allergy Clin Immunol 141:AB403
Rauber MM, Pickert J, Holiangu L, Möbs C, Pfützner W (2017) Functional and phenotypic analysis of basophils allows determining distinct subtypes in patients with chronic urticaria. Allergy 72:1904–1911
Rorsman H (1962) Basophilic leucopenia in different forms of urticaria. Acta Allergol 17:168–184
Rothbaum R, McGee J (2016) Aquagenic urticaria: diagnostic and management challenges. J Asthma Allergy 9:209–213
Rutkowski K, Grattan CEH (2017) How to manage chronic urticaria ‘beyond’ guidelines: a practical algorithm. Clin Exp Allergy 47:710–718
Saini SS, Kaplan AP (2018) Chronic spontaneous Urticaria: the Devil’s itch. J Allergy Clin Immunol Pract 6:1097–1106
Saini SS, MacGlashan DW (2012) Assessing basophil functional measures during monoclonal anti-IgE therapy. J Immunol Methods 383:60–64
Saini SS et al (2009) Cultured peripheral blood mast cells from chronic idiopathic urticaria patients spontaneously degranulate upon IgE sensitization: relationship to expression of Syk and SHIP-2. Clin Immunol 132:342–348
Saini S et al (2011) A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol 128:567–573.e1
Saini SS et al (2015) Efficacy and safety of Omalizumab in patients with chronic idiopathic/spontaneous Urticaria who remain symptomatic on H 1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol 135:67–75
Saini SS et al (2017) Effect of Omalizumab on blood basophil counts in patients with chronic idiopathic/spontaneous Urticaria. J Invest Dermatol 137:958–961
Sánchez-Borges M, Capriles-Hulett A, Caballero-Fonseca F (2015a) Demographic and clinical profiles in patients with acute urticaria. Allergol Immunopathol (Madr) 43:409–415
Sánchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A, González-Aveledo L (2015b) Aspirin-exacerbated cutaneous disease (AECD) is a distinct subphenotype of chronic spontaneous urticaria. J Eur Acad Dermatol Venereol 29:698–701
Santiago-Vázquez M, Barrera-Llaurador J, Carrasquillo OY, Sánchez S (2019) Chronic spontaneous urticaria associated with colon adenocarcinoma: a paraneoplastic manifestation? A case report and review of literature. JAAD Case Rep 5:101–103
Scalese MJ, Reinaker TS (2016) Pharmacologic management of angioedema induced by angiotensin-converting enzyme inhibitors. Am J Health Syst Pharm 73:873–879
Schmetzer O et al (2018) IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J Allergy Clin Immunol 142:876–882
Schoepke N et al (2019) Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria (aiCSU): Results of the PURIST Study. Allergy 74(12):2427–2436
Schroeder JT (2009) Basophils beyond effector cells of allergic inflammation. Adv Immunol 101:123–161
Shin YS, Suh D-H, Yang E-M, Ye Y-M, Park H-S (2015) Serum specific IgE to thyroid peroxidase activates basophils in aspirin intolerant Urticaria. J Korean Med Sci 30:705–709
Siebenhaar F, Degener F, Zuberbier T, Martus P, Maurer M (2009) High-dose desloratadine decreases wheal volume and improves cold provocation thresholds compared with standard-dose treatment in patients with acquired cold urticaria: a randomized, placebo-controlled, crossover study. J Allergy Clin Immunol 123:672–679
Smith C, Kepley C, Schwartz L, Lee T (1995) Mast cell number and phenotype in chronic idiopathic urticaria. J Allergy Clin Immunol 96:360–364
Snidvongs K, Seresirikachorn K, Khattiyawittayakun L, Chitsuthipakorn W (2017) Sedative effects of Levocetirizine: a systematic review and meta-analysis of randomized controlled studies. Drugs 77:175–186
Song J, Wu C, Zhang X, Sorokin LM (2013) In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1 -induced peritonitis. J Immunol 190:401–410
Stellato C et al (1992) Anti-inflammatory effect of Cyclosporin A on human skin mast cells. J Invest Dermatol 98:800–804
Takeda T et al (2011) Increase of coagulation potential in chronic spontaneous urticaria. Allergy 66:428–433
Taskapan O, Kutlu A, Karabudak O (2008) Evaluation of autologous serum skin test results in patients with chronic idiopathic urticaria, allergic/non-allergic asthma or rhinitis and healthy people. Clin Exp Dermatol 33:754–758
Tesar V, Hruskova Z (2018) Avacopan in the treatment of ANCA-associated vasculitis. Expert Opin Investig Drugs 27:491–496
Tonacci A, Billeci L, Pioggia G, Navarra M, Gangemi S (2017) Omalizumab for the treatment of chronic idiopathic Urticaria: systematic review of the literature. Pharmacotherapy 37:464–480
Tosi M (1998) Molecular genetics of C1 inhibitor. Immunobiology 199:358–365
Toubi E et al (2004) Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy 59:869–873
Uguz F, Engin B, Yilmaz E (2008) Axis I and Axis II diagnoses in patients with chronic idiopathic urticaria. J Psychosom Res 64:225–229
van der Valk PGMGM, Moret G, Kiemeney LALMALM (2002) The natural history of chronic urticaria and angioedema in patients visiting a tertiary referral centre. Br J Dermatol 146:110–113
Varchetta S et al (2012) Engagement of Siglec-7 receptor induces a pro-inflammatory response selectively in monocytes. PLoS One 7:e45821
Vena GA, Cassano N, Colombo D, Peruzzi E, Pigatto P (2006) Cyclosporine in chronic idiopathic urticaria: a double-blind, randomized, placebo-controlled trial. J Am Acad Dermatol 55:705–709
Viswanathan RK, Biagtan MJ, Mathur SK (2012) The role of autoimmune testing in chronic idiopathic urticaria. Ann Allergy Asthma Immunol 108:337–341.e1
Vonakis BM, Saini SS (2008) New concepts in chronic urticaria. Curr Opin Immunol 20:709–716
Wang W et al (2001) Interleukin-18 enhances the production of interleukin-8 by eosinophils. Eur J Immunol 31:1010–1016
Wedi B, Raap U, Wieczorek D, Kapp A (2009) Urticaria and infections. Allergy Asthma Clin Immunol 5:10
Weller K et al (2010) Chronische Urtikaria. Hautarzt 61:750–757
White C, Wright A, Brightling C (2018) Fevipiprant in the treatment of asthma. Expert Opin Investig Drugs 27:199–207
Williams JDL et al (2008) Occupational contact urticaria: Australian data. Br J Dermatol 159:125–131
Woo YR, Jung KE, Koo DW, Lee JS (2015) Vitamin D as a marker for disease severity in chronic Urticaria and its possible role in pathogenesis. Ann Dermatol 27:423–430
Yahara H, Satoh T, Miyagishi C, Yokozeki H (2010) Increased expression of CRTH2 on eosinophils in allergic skin diseases. J Eur Acad Dermatol Venereol 24:75–76
Yamaguchi M et al (1992) Preliminary criteria for classification of adult Still’s disease. J Rheumatol 19:424–430
Yamamoto T (2012) Cutaneous manifestations associated with adult-onset Still’s disease: important diagnostic values. Rheumatol Int 32:2233–2237
Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP (2002) TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol 109:694–700
Zazzali JL, Broder MS, Chang E, Chiu MW, Hogan DJ (2012) Cost, utilization, and patterns of medication use associated with chronic idiopathic urticaria. Ann Allergy Asthma Immunol 108:98–102
Zuberbier T, Iffländer J, Semmler C, Henz BM (1996) Acute urticaria: clinical aspects and therapeutic responsiveness. Acta Derm Venereol 76:295–297
Zuberbier T et al (2014) The EAACI/GA2/LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy 69:868–887
Zuberbier T et al (2018) The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 73:1393–1414
Acknowledgments
Pictures edited with BioRender (www.BioRender.com).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Folci, M., Ramponi, G., Brunetta, E. (2020). A Comprehensive Approach to Urticaria: From Clinical Presentation to Modern Biological Treatments Through Pathogenesis. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 12. Advances in Experimental Medicine and Biology(), vol 1326. Springer, Cham. https://doi.org/10.1007/5584_2020_612
Download citation
DOI: https://doi.org/10.1007/5584_2020_612
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-71932-6
Online ISBN: 978-3-030-71933-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)