Abstract
The processing of proglucagon in intestinal L cells results in the formation of glucagon, GLP-1, and GLP-2. The GLP-1 molecule becomes active through the effect of proconvertase 1, and it is inactivated by dipeptidyl peptidase IV (DPP-IV), so that the half-life of endogenous GLP-1 is 2–3 min. GLP-1 stimulates insulin secretion from β cells in the islets of Langerhans. Human studies show that infusion of GLP-1 results in slowing of gastric emptying and increased fasting and postprandial gastric volumes. Retardation of gastric emptying reduces postprandial glycemia. Exendin-4 is a peptide agonist of the GLP-1 receptor that promotes insulin secretion. Chemical modifications of exendin-4 and GLP-1 molecules have been accomplished to prolong the half-life of GLP-1 agonists or analogs. This chapter reviews the effects of GLP-1-related drugs used in treatment of diabetes or obesity on gastric motor functions, chiefly gastric emptying. The literature shows that diverse methods have been used to measure effects of the GLP-1-related drugs on gastric emptying, with most studies using the acetaminophen absorption test which essentially measures gastric emptying of liquids during the first hour and capacity to absorb the drug over 4–6 h, expressed as AUC. The most valid measurements by scintigraphy (solids or liquids) and acetaminophen absorption at 30 or 60 min show that GLP-1-related drugs used in diabetes or obesity retard gastric emptying, and this is associated with reduced glycemia and variable effects on food intake and appetite. GLP-1 agonists and analogs are integral to the management of patients with type 2 diabetes mellitus and obesity. The effects on gastric emptying are reduced with long-acting preparations or long-term use of short-acting preparations as a result of tachyphylaxis. The dual agonists targeting GLP-1 and another receptor (GIP) do not retard gastric emptying, based on reports to date. In summary, GLP-1 agonists and analogs are integral to the management of patients with type 2 diabetes mellitus and obesity, and their effects are mediated, at least in part, by retardation of gastric emptying.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Secretions from the gastrointestinal tract include hormones and peptides that provide feedback to control gastric function and to stimulate the secretion of insulin from the β cells of the islets of Langerhans in the pancreas. This feedback regulation is referred to as a system of “brakes.” The ileal brake is the most recognized and results from feedback regulation of stomach and jejunal function by ileal products such as peptide YY, neurotensin and oxyntomodulin. However, proximal to the ileum, several products of enteroendocrine cells result in inhibitory effects on gastric motor functions that alter gastric reservoir function and induce antral motility and pyloric contractility, leading to retardation of gastric emptying and thereby reducing the rate of delivery of nutrients and their absorption. The upper gastrointestinal hormones and transmitters include cholecystokinin, glucose-stimulated insulinotropic peptide, glucagon and glucagon-like peptide 1 (GLP-1).
GLP-1 analogs or receptor agonists are established treatments for patients with type 2 diabetes mellitus (T2DM) and obesity. Effects of GLP-1 analogs or receptor agonists on gastric emptying are relevant for at least three reasons: first, because the delay in gastric emptying may reduce postprandial glycemia; second, because delay in gastric emptying may reduce kilocalorie intake, providing beneficial effects in obesity; and third, because delay in gastric emptying may cause symptoms that result in the need to slow the increments in doses of these medications. Over the past two decades, there has been increased understanding of the effects of this class of compounds, including the differentiation between the individual medications, as well as probable differences between the effects of short-acting compared to long-acting formulations of the same chemical entity.
This chapter reviews the effects of GLP-1 and its analogs or agonists on gastric physiology in T2DM and obesity. In addition, given the recent introduction of medications with dual effects on GLP-1 and targets of other hormones, the current state of literature is reviewed for changes in gastric functions in anticipation of further applications of dual agonists.
2 Synthesis, Actions, and Degradation of Glucagon-Like Peptide 1
GLP-1 is cleaved from proglucagon, which is expressed in the gut, pancreas, and brain. The processing of proglucagon in intestinal L cells results in the formation of glucagon (a glucose-regulatory hormone), GLP-1, and GLP-2 (an intestinal growth factor). The GLP-1 molecule becomes an active molecule through the effect of proconvertase 1, and it is inactivated by the cleaving of two amino acids at its N terminal by the enzyme, dipeptidyl peptidase IV (DPP-IV) (Moller 2001). There are two equipotent bioactive forms of GLP-1, GLP-1 (17–36) and GLP-1 (17–37), both of which are rapidly inactivated in the circulation by DPP-IV, rendering GLP-1 half-life a mere 2–3 min (Ritzel et al. 1995).
GLP-1 co-localizes in the distal intestine with oxyntomodulin and PYY. The GLP-1 receptor is expressed in the gut, pancreas, brainstem, hypothalamus, and vagal afferent nerves. Ingested nutrients, especially fats and carbohydrates, stimulate GLP-1 secretion, either indirectly through duodenally activated neurohormonal mechanisms or by direct contact of nutrients within the distal intestine (Cummings and Overduin 2007).
GLP-1 actions include activation of the ileal brake, delay in gastric emptying, increase in glucose-dependent insulin release, decrease in glucagon secretion, and increase in pancreatic β cell growth. Studies employing the specific GLP-1 receptor antagonist, exendin-9-39, show that endogenously released GLP-1 controls fasting plasma glucagon, stimulates insulin, and influences mechanisms controlling gastric emptying in humans (Deane et al. 2010a; Schirra and Göke 2005). The reduced glucagon and increased insulin secretion result in diminished postprandial glucose (Drucker 2003). GLP-1 decreases food intake, possibly via vagal and possibly via direct central pathways, mediated specifically by GLP-1 receptors (Cummings and Overduin 2007). Thus, reduction in spontaneous energy intake was demonstrated using an ad libitum meal in healthy, normal weight volunteers (Flint et al. 1998).
3 Structures and Formulations of GLP-1 Agonists and Analogs
GLP-1 receptor agonists can be modified from the active fragment of the human GLP-1 (7–36) or derived from exendin-4, a GLP-1 receptor agonist originally isolated from the venom of the Gila monster. Homology with human GLP-1 varies across all GLP-1 receptor agonists, but all can replicate the effects of the human peptide in vivo. For example, the exendin-4 derivative, exenatide, shows just 53% amino acid sequence homology with human GLP-1, but binds to the human GLP-1 receptor with affinity equivalent to that of human GLP-1 (Holst 2019). It is thought that, because of this low homology, exenatide would be associated with the most antibodies among the GLP-1 receptor agonists available for clinical use (Garber 2011). Exenatide weekly formulations are more immunogenic than twice daily formulations (Tibble et al. 2013). Those with higher antibody titers were observed to have high incidence of injection site reactions (Fineman et al. 2012).
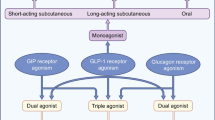
Given the short half-life of endogenous GLP-1, multiple strategies were used to prolong the duration of action of GLP-1 receptor agonists and, thereby, reduce the need for frequent injections. These strategies include: first, changes in the amino acid sequence of GLP-1 to increase resistance to inactivation by DPP-IV, such as lixisenatide, which is mostly homologous with but slightly modified from exenatide, permitting it to be administered only once instead of twice daily; second, binding to albumin, either covalently (e.g., albiglutide and semaglutide) or noncovalently (e.g., liraglutide), or binding to immunoglobulin G (e.g., dulaglutide), all of which limit renal elimination (Fig. 1). A poly-microsphere preparation allowed exenatide to be continuously released from weekly injections (Gentilella et al. 2019).
Structure and properties of glucagon-like peptide 1 receptor agonists. (Reproduced from Gentilella et al. 2019)
4 Effects of GLP-1 on Gastric Functions in Health
Gastrointestinal release of peptides during and after meals has played a critical role in the homeostatic mechanisms regulating caloric intake (Gibbs et al. 1973). GLP-1 has been recognized for a multitude of regulatory functions in humans: secretion of insulin, inhibition of glucagon release, and delay in the emptying of gastric contents into the duodenum. This final feature is referred to as the “ileal brake”, a critical inhibitory control mechanism that modulates food consumption and digestive function in health (Read et al. 1984).
GLP-1 secretion is stimulated through a complex cascade of signaling which involves entry of chime into the intestine, release of nesfatin-1 which stimulates CCK secretion, rise in bile salts from gallbladder emptying, and binding of Takeda P protein couple receptor 5 (TGR5) on the basolateral surface of enteric endocrine L cells (Ramesh et al. 2016; Bronden et al. 2018). This endogenous 30-amino acid peptide acts until it is soon degraded within 5 min by DPP-IV (Steinert et al. 2016).
The inhibition of gastrointestinal motility by GLP-1 is mediated through the GLP-1 receptor at the level of the myenteric neurons and downstream signaling of nitregic and cyclic adenosine monophosphate-dependent mechanisms, inhibiting vagal activity (Halim et al. 2018). This results in reduced phasic contractions of the stomach, as well as delay in gastric emptying and diminished gastric acid secretion (Schirra et al. 2002; Imeryüz et al. 1997). GLP-1 also increases fasting and postprandial gastric volumes (Delgado-Aros et al. 2002). These mechanisms require intact vagal innervation; thus, the increased postprandial accommodation induced by GLP-1 is reduced in patients with diabetes and cardiovagal neuropathy (Delgado-Aros et al. 2003). The effects of GLP-1 on gastric functions are confirmed by the reported effects of a GLP-1 receptor antagonist, which increased antral motility and inhibited pyloric tone (Schirra et al. 2006).
The effects of GLP-1 and its analogs are impacted by physiologic principles. First, increases in circulating levels of GLP-1 occur in the fed rather than the fasting state, and they impact the cholinergic mechanisms pertaining to the postprandial upper gastrointestinal motor function, in contrast to the fasting state (Schirra et al. 2009). Second, intragastric calories stimulated far more robust GLP-1 excursion than did intraduodenal infusion (Steinert et al. 2012); however, intraduodenal fat and carbohydrate infusion and absorption stimulate GLP-1 in rats (Lu et al. 2007) and GLP-1 and GIP in humans (Deane et al. 2010a). Third, the effect of GLP-1 to modulate energy intake was augmented substantially by the presence of protein in the stomach (Degen et al. 2006).
These effects of endogenous GLP-1 on gastric motor functions have been co-opted for management of disease states, which will be explored in the next sections.
5 Methodological Assessment of Gastric Emptying
The gold standard for assessing gastric emptying in humans is nuclear scintigraphy (Odunsi and Camilleri 2009); however, this has seldom been implemented in the study of gastric motor function in the context of GLP-1 and GLP-1 receptor agonists or analogs. A few studies have employed gastric emptying breath tests using stable isotopes, which have reasonable correlation with the gold standard of scintigraphy (Szarka and Camilleri 2009; Szarka et al. 2008). The vast majority of studies of gastric emptying and GLP-1 agonists use the paracetamol/acetaminophen absorption test (which will hereafter for simplicity be referred to as acetaminophen absorption test).
This chapter outlines many such studies, but it is worthwhile mentioning a few key limitations of such an assay. First, acetaminophen is typically administered as a liquid such that, even when given with a solid meal, it more closely follows the exponential pattern of gastric emptying of liquids rather than solids, which are governed by a distinct gastric emptying profile and mechanism (that is, initial retention while solids are triturated to a small particle size before emptying at a relatively constant rate). Second, gastric emptying was calculated by acetaminophen absorption at the end of a 4-, 5-, or 6-h appraisal, at which time much of the acetaminophen will have had the opportunity to be absorbed, consequently missing the potential impact of gastric emptying to the plasma acetaminophen profile. Such studies that express acetaminophen area under the curve (AUC) over 4–6 h undervalue the impact on gastric emptying. Some studies have avoided this potential limitation by assessing acetaminophen AUC from 0 to 1 h, which provides a more precise assessment of gastric emptying. Overall, the literature supports the need to avoid the acetaminophen absorption test to estimate gastric emptying due to the limitations mentioned and to use nuclear scintigraphy which is an accurate, safe, and reproducible method of assessing gastric emptying of solids and liquids.
6 Mechanism of Impairment of GI Motor Function by GLP-1 Agonists
The degree of slowing of gastric emptying by GLP-1 analogs or receptor agonists is dependent on the baseline rates of gastric emptying; thus, the induced delay in gastric emptying is more substantial in those with more rapid baseline gastric emptying (Linnebjerg et al. 2008). In contrast, when baseline emptying is already delayed, there is far less of an effect on gastric emptying from these agents (Marathe et al. 2011). The slowing of gastric emptying induced by GLP-1 agonists is dose dependent (Meier 2012). Delay in gastric emptying, as well as effects on appetite, have not been observed with medications that inhibit the enzyme that breaks down GLP-1 (DPP-IV) (Vella et al. 2007, 2008; Stevens et al. 2012). This is likely due to the lower levels of endogenous GLP-1 activity achieved with the DPP-IV inhibitors compared to the actions of GLP-1 analogs or receptor agonists.
7 Effect of GLP-1 Receptor Agonism in Disease State: Diabetes Mellitus
While much has been published on GLP-1 receptor agonism enhancement in islet cell function (Bunck et al. 2009), the physiologic underpinnings driving improvement in diabetes appear more complex. Indeed, postprandial serum glucose levels were correlated with the degree of slowing of gastric emptying (Linnebjerg et al. 2008; Little et al. 2006; Lorenz et al. 2013; Deane et al. 2010b), and modulation of postprandial serum glucose more closely associated with delay in gastric emptying than it did increase in levels of insulin in the setting of exogenous GLP-1 administration, underscoring the importance of gastric motor functions in homeostatic mechanisms (Little et al. 2006; Willms et al. 1996; Nauck et al. 1997). In addition, when gastric emptying was accelerated by administration of erythromycin, GLP-1 was less able to modulate postprandial serum glucose levels (Meier et al. 2005). This mechanism may also explain why infusion of exogenous GLP-1 improved satiety scores and reduced meal intake in patients with diabetes (Gutzwiller et al. 1999).
The following text and Table 1 summarize key findings of the studies that evaluated gastric emptying in subjects with diabetes mellitus who were exposed to GLP-1 analogs or receptor agonists. The effects of GLP-1 analogs and receptor agonists have ushered in a more “personalized” approach for the management of both type 1 (Marathe et al. 2018) and type 2 diabetes (Holst et al. 2016). Since postprandial glycemic excursions predominate in patients with HbA1c <8% (Monnier et al. 2003), the use of the “short-acting” GLP-1 receptor agonists, exenatide twice daily and lixisenatide alone or in combination with basal insulin, has been proposed as a method to diminish postprandial glycemic excursions, predominantly by slowing gastric emptying (Holst et al. 2016).
Dulaglutide
Dulaglutide is a long-acting GLP-1 receptor agonist. One study of dulaglutide at multiple subcutaneous (SC) doses (0.05, 0.3, 1, 3, and 5 mg once weekly) in subjects with T2DM revealed a decrease in acetaminophen AUC0-12h after 5 weeks of treatment (Barrington et al. 2011). Given the protracted assessment of acetaminophen absorption, the clinical significance of this AUC is not clear. It is possible, as has been seen with other long-acting GLP-1 receptor agonists, that the effect on gastric emptying is minimal to non-existent.
Exenatide
Several studies have evaluated the effect of exenatide in short- or long-acting formulations on gastric emptying in subjects with T2DM. Delayed gastric emptying by the gold standard assessment—nuclear scintigraphy of a solid meal—was observed after 5 days’ of either 5 μg or 10 μg SC exenatide, twice daily, compared to placebo (Linnebjerg et al. 2008). Four studies evaluated exenatide using acetaminophen absorption. These revealed delayed gastric emptying over a 6-h meal after an intravenous infusion of exenatide (roughly equivalent to one-half the peak concentration of a 5 μg SC dose exenatide) compared to placebo (Cervera et al. 2008); after 4 days of infusion of 0.05 μg/kg and 0.10 μg/kg compared to placebo (Kolterman et al. 2005); after 2 weeks of 10 μg SC, twice daily compared to placebo (DeFronzo et al. 2008); and after 14 weeks of exenatide, 10 μg SC, twice daily, compared to placebo (Drucker et al. 2008). Delay in gastric emptying based on a plasma acetaminophen absorption test was not observed with the once-weekly long-acting formulation compared to baseline (Drucker et al. 2008). However, exenatide QW substantially slowed gastric emptying measured scintigraphically and this relates to the reduction in postprandial glucose (Jones et al. 2020).
Liraglutide
Assessments of liraglutide’s effects on gastric emptying in subjects with T2DM have primarily used acetaminophen absorption. After eight doses of 6 μg/kg SC daily liraglutide, no difference in gastric emptying was observed, based on acetaminophen AUC0-4h (Degn et al. 2004). Using a similar treatment duration of 1 week and acetaminophen AUC0-5h, delayed gastric emptying was observed with 1.2 mg liraglutide daily, but not with 0.6 mg or 1.8 mg daily. However, when acetaminophen AUC0-1h was instead used in the same study, gastric emptying was delayed for both 1.2 mg daily and 1.8 mg daily (Dejgaard et al. 2016). When longer treatment duration was studied, liraglutide, 1.8 mg SC daily, was shown to delay gastric emptying at 3 and 24 weeks, also based on acetaminophen AUC0-4h (Flint et al. 2011). Similarly, after 4 weeks of treatment with liraglutide, gastric emptying was delayed with liraglutide, 1.8 mg SC daily, compared to placebo, based on acetaminophen AUC0-1h (Horowitz et al. 2012).
Finally, one study examined gastric emptying effects of liraglutide in subjects with T2DM using gastric transit time of capsule endoscopy. While overall gastric emptying time was not changed from baseline after liraglutide use, when subjects were stratified by presence or absence of diabetic neuropathy, gastric transit time was significantly increased compared to baseline for those without diabetic neuropathy after liraglutide; whereas, those with diabetic neuropathy saw no significant delay in gastric emptying from liraglutide (Nakatani et al. 2017). While there are challenges in interpreting gastric emptying profiles of a solid meal and a capsule, this finding nevertheless illustrates the role of vagal mechanisms in the delay of gastric emptying induced by GLP-1 agonism.
Overall, the reported differences in effects of exenatide and liraglutide on gastric emptying may be more likely related to differences in methods of measurement rather than biological differences, given relatively minor structural differences between the two molecules, as well as the common mechanism of action of binding to the same G-protein-coupled, 7-transmembrane domain GLP-1 receptor. Details of each study are summarized in Table 1.
Lixisenatide
Lixisenatide is a relatively more novel, short-acting, once-daily SC GLP-1 receptor agonist. Despite its short half-life (3 h), it is nonetheless administered once daily, most likely due to its ability to delay gastric emptying (Lorenz et al. 2013; Horowitz et al. 2013). Lixisenatide’s effect on gastric emptying was appraised with nuclear scintigraphy using a liquid meal and a scan over 3 h. While this method could not calculate T1/2 because T1/2 exceeded 3 h in the majority of both healthy and diabetic participants of this study, gastric emptying rate (in kcal/min) for the first 2 h was observed to be delayed in both healthy participants and those with T2DM exposed to lixisenatide compared to placebo (Jones et al. 2019).
Using a 13C-sodium-octanoic acid-containing solid meal with breath test over 4 h, lixisenatide was further observed to delay gastric emptying after 4 weeks at a dose of 20 μg daily, compared to placebo (Lorenz et al. 2013). Lixisenatide, 20 μg daily, after 8 weeks was also shown to delay gastric emptying by the same type of breath test. Because the gastric emptying delay was so profound, particularly compared to the liraglutide doses studied in the same trial, the face values of the gastric emptying times present challenges in interpretation (Meier et al. 2015), especially in view of the documented differences in estimated time to half gastric emptying using different mathematical formulas (Odunsi et al. 2009). Nevertheless, these findings do align with delay in gastric emptying by acetaminophen AUC0-1h seen after a single dose of lixisenatide (5, 10, or 20 μg) compared to placebo in healthy participants (Becker et al. 2015).
8 Effects on Gastric Emptying with GLP-1 Receptor Agonists in Obesity
The secretion of GLP-1 in obesity has been reported to be reduced in some studies (Carr et al. 2010; Verdich et al. 2001; Adam and Westerterp-Plantenga 2005), although the results are inconsistent, as documented in a comprehensive review of the literature (Steinert et al. 2017). As outlined throughout this chapter, GLP-1 receptor agonism has a multitude of effects which may be useful to exploit for management of obesity. Indeed, several trials have investigated its role in weight management (le Roux et al. 2017; Pi-Sunyer et al. 2015). One such trial of 3.0 mg daily liraglutide observed a weight loss of 8.4 ± 7.3 kg compared to 2.8 ± 6.5 kg in the placebo arm (p < 0.001) after 56 weeks of intervention (Pi-Sunyer et al. 2015). It is likely that a contribution to weight loss results from appetite mediation by delay in gastric emptying, which has been observed in both human and animal models (Szayna et al. 2000).
The use of GLP-1 receptor agonism in obesity may be partly related to effects on gastric motor function. While the gastric motor functions in obesity are heterogeneous, a substantial portion of patients has accelerated gastric emptying (Acosta et al. 2015a, b), which may provide an opportunity for “personalized” treatment with a medication that delays gastric emptying. Infusion of exogenous GLP-1 in subjects with obesity, resulted in reduced hunger and calorie intake, and these measures correlated with the degree of gastric emptying delay, measured by the acetaminophen absorption test (Flint et al. 2001; Näslund et al. 1999). Nevertheless, weight loss associated with GLP-1 receptor agonism may be independent of gastric motor changes, and there was similar weight loss with liraglutide and exenatide, despite the differences in gastric emptying (Holst 2013). Weight loss with GLP-1 receptor agonists was not related to adverse gastrointestinal effects (which are largely driven by delays in gastric emptying) in several reports (Nauck et al. 2009; Buse et al. 2004; DeFronzo et al. 2005; Garber et al. 2011; Russell-Jones et al. 2009; Zinman et al. 2007). Another confounder is the fact that there appears to be tachyphylaxis in the retardation of gastric emptying from 5 to 16 weeks of liraglutide treatment, even though, at both times, the gastric emptying delay was significantly correlated with the degree of weight loss (le Roux et al. 2017). Table 1 summarizes key findings in gastric motor functions in the studies that evaluated gastric emptying in subjects with obesity exposed to GLP-1 receptor agonists.
Given the focus of this chapter on gastric effects of GLP-1 and its analogs and receptor agonists, the central mechanisms will not be extensively discussed. GLP-1 receptors are expressed throughout the central nervous system (Vrang and Larsen 2010), particularly the hypothalamus and brainstem, and they play a role in regulation of appetite (Holst 2013), as well as blood glucose (Alvarez et al. 2005), independent of gastrointestinal effects of GLP-1 and its analogs and receptor agonists. Nevertheless, peripheral stimuli have been shown to interact with central GLP-1 mechanisms to induce weight loss in preclinical studies. For example, gastric body or fundus distention activated GLP-1 containing neurons in the nucleus of the solitary tract (NTS) of rats (Vrang et al. 2003) and this decreased food intake, an effect that was reversed with exendin-9-39, a GLP-1 receptor antagonist, administered directly into the fourth ventricle (Hayes et al. 2009). Apart from the GLP-1 effects on appetite, which appear to have a significant central component, there is evidence that GLP-1 inhibits gastric emptying through mechanisms that involve vagal afferents (Imeryüz et al. 1997), as well as inhibition of central parasympathetic outflow (Wettergren et al. 1998).
Exenatide
Exenatide’s effects on gastric emptying have been examined in obesity (without concomitant type 2 diabetes mellitus) in one study that measured gastric emptying of solids by nuclear scintigraphy. All subjects in that study had accelerated gastric emptying at baseline. Gastric emptying was delayed compared to placebo after 30 days of exenatide, 5 μg SC twice daily, based on both 1-h gastric emptying and time to half gastric emptying (Acosta et al. 2015a).
Liraglutide
One study of liraglutide, 3.0 mg SC daily, showed delayed gastric emptying compared to placebo at both 5 and 16 weeks, measured by nuclear scintigraphy of a solid meal (Halawi et al. 2017). Notably, the delay in gastric emptying was less at 16 weeks compared to 5 weeks, consistent with the tachyphylaxis of GLP-1 agonism on gastric emptying described later in this chapter. Using acetaminophen AUC0-5h, a separate study showed no difference between liraglutide, 1.8 mg or 3.0 mg SC daily, after 5 weeks; however, when acetaminophen AUC0-1h was used, both doses showed delayed gastric emptying compared with placebo (van Can et al. 2014). This provides a fitting example of the shortcomings of the assessment of gastric emptying based on a prolonged acetaminophen absorption test and how this can be potentially mitigated by testing the absorption over the first hour.
Semaglutide
Semaglutide is a long-acting GLP-1 receptor agonist. Despite our understanding of the diminished effects on gastric emptying induced by long-acting GLP-1 agonists (see below), one study did observe a significant reduction in acetaminophen AUC0-1h (but not acetaminophen AUC0-5h) with 1.0 mg weekly SC semaglutide compared to placebo after 12 weeks of intervention, suggesting that semaglutide may delay gastric emptying (Hjerpsted et al. 2018).
9 Short- vs. Long-Acting GLP-1 Receptor Agonists and Gastric Emptying and Tachyphylaxis
Nauck and colleagues demonstrated that gastric emptying of liquid meals in healthy participants, assessed by double-sampling dye dilution technique over 4 h, was delayed with administration of exogenous GLP-1; they also observed that deceleration of gastric emptying was subject to tachyphylaxis during ingestion of a second meal (Nauck et al. 2011). This loss of the delay in gastric emptying was associated with a statistically significant increase in postprandial glycemia during the second meal. Given this time frame, it is postulated that this tachyphylaxis phenomenon is driven more by the response of the vagal nerve function rather than by GLP-1 receptor downregulation or desensitization. Umapathysivam and colleagues observed similar tachyphylaxis in the delay of gastric emptying from prolonged or intermittent GLP-1 agonism compared to short-acting GLP-1 agonism (Umapathysivam et al. 2014).
There are multiple examples of tachyphylaxis in prolonged GLP-1 agonism. For instance, delay in gastric emptying was observed with short-acting exenatide, but not with the once-weekly, long-acting formulation, compared to placebo (Drucker et al. 2008). In addition, in a separate study, the delay in gastric emptying compared to baseline from 16 weeks of liraglutide (a short-acting GLP-1 receptor agonist) was less substantial than that of 5 weeks of liraglutide (Halawi et al. 2017).
Thus, delay in gastric emptying appears to be more characteristic of short-acting GLP-1 receptor agonists than long-acting GLP-1 receptor agonists (Madsbad 2016; Uccellatore et al. 2015). Tachyphylactic effects on delayed gastric emptying have not been observed with short-acting GLP-1 receptor agonists (Linnebjerg et al. 2008; Drucker et al. 2008; Flint et al. 2011), and may explain the decreased burden of upper gastrointestinal symptoms such as nausea and vomiting observed with long-acting formulations of GLP-1 agonists (Trujillo and Nuffer 2014). On the other hand, it is likely that long-acting formulations, such as albiglutide, dulaglutide, and exenatide long-acting release, improve glycemic control through restoration of balance between insulin and glucagon, rather than robust delays in gastric emptying (Meier 2012).
10 Effects of GLP-1 Agonist on Pharmacokinetics and Pharmacodynamics of Other Medications
An important consideration when prescribing medications in patients with diabetes and/or obesity is the potential of polypharmacy pharmacokinetics. Given the delays in gastric emptying observed with GLP-1 receptor agonists, there are hypothesized effects on other commonly prescribed medications for this demographic. For example, exenatide has been observed to have variable effects on several medications. In healthy volunteers, exenatide did not change steady concentration of digoxin, but it did cause a 17% decrease in mean plasma digoxin and a delay in time to reach steady state (Kothare et al. 2005). Similarly, exenatide was associated with decreased mean lovastatin plasma concentration AUC and time to maximum plasma concentration, although this did not affect 30-week changes in lipid profile (Kothare et al. 2007). Pharmacodynamics of warfarin in healthy volunteers (Soon et al. 2006) or lisinopril in subjects with mild-to-moderate hypertension (Linnebjerg et al. 2009) were not substantially affected by exenatide. Semaglutide was not observed to derange AUC plasma concentrations for lisinopril, warfarin, and digoxin, although the AUC was increased by 32% for metformin, and this may be of limited clinical concern, given the wide therapeutic index of metformin (Bækdal et al. 2019).
11 Variations in GLP-1 Receptor and Responses to GLP-1 Agonists
The minor A allele of GLP-1R (rs6923761) is associated with greater delay in time to half gastric emptying in response to liraglutide and exenatide. These studies provide data to plan pharmacogenetics testing of the hypothesis that GLP-1R influences weight loss in response to GLP-1R agonists (Chedid et al. 2018). The significance of target receptor genetic variation requires further study with other GLP-1 agonists.
12 Oral Semaglutide
Given that most commercially available GLP-1 receptor agonists have been studied in intravenous or subcutaneous injection formulations, oral semaglutide warrants specific mention. The adverse effects from semaglutide are similar to those of other GLP-1 receptor agonists, namely, nausea and vomiting, and these mirror the safety profile of once-weekly injectable semaglutide (Davies et al. 2017). The gastrointestinal side effects appear most consistently with the 14 mg dose, suggestive of a dose-limiting gastrointestinal side effect profile. Overall, there was a small increase in discontinuations compared to other active drug treatment arms in clinical trials, including liraglutide (le Roux et al. 2017). This underscores the importance of understanding the gastrointestinal related adverse effects from GLP-1 receptor agonists, as well as the potential for therapeutic choice when GLP-1 agents are being considered for treatment of obesity. Given these side effects, it is recommended that dose escalation of oral semaglutide be carried out over 4 weeks or longer (Davies et al. 2017).
13 Effects of Combined GLP-1 and Other Hormone Agonism on Gastric Motor Functions
The evolving landscape of pharmacologic therapy has now incorporated combination therapies with efficacy of GLP-1 receptors and receptors of other hormones, including glucose dependent insulinotropic polypeptide (GIP).
GIP is released from intestinal K cells and, like GLP-1, the release of GIP is triggered by ingestion of nutrients and its activity is modulated by degradation by DPP-IV (Diakogiannaki et al. 2012; Vilsbøll et al. 2006). Dual infusions of GIP and GLP-1 receptor antagonists in healthy participants showed that the combination infusion not only caused poor postprandial glycemic control (compared with placebo and either infusion alone), but also that the combination antagonists accelerated gastric emptying, although perhaps not notably more than the GLP-1 receptor infusion alone (Gasbjerg et al. 2019). In a randomized, cross-over study of overweight or obese subjects, co-infusion of GIP and GLP-1 did not enhance the energy intake or appetite modulating effects of GLP-1 monotherapy, suggesting that GIP likely has little role in altering gastric motor functions, particularly gastric emptying (Gasbjerg et al. 2019). This finding is supported by preliminary data in patients with T2DM, subjected to gastric emptying of a standardized liquid meal (Mathiesen et al. 2019), as well as a phase 1 study of tirzepatide (a novel combination GIP and GLP-1 receptor agonist) using the acetaminophen absorption test (Urva et al. 2019). While many studies of these novel combination agents cite upper gastrointestinal symptoms of nausea and vomiting as relatively frequent and dose-dependent adverse effects from these medications (Coskun et al. 2018; Schmitt et al. 2017; Frias et al. 2018), it is unlikely that these result from a synergistic effect of GLP-1 and GIP on delay in gastric emptying.
There is also a GLP-1 and glucagon receptor dual agonist which results in clinically meaningful reductions in blood glucose, appetite and body weight in obese or overweight individuals with type 2 diabetes mellitus, as well as increase in treatment-emergent gastrointestinal disorders (Ambery et al. 2018). Another GLP-1 and glucagon dual agonist is cotagutide, which enhances insulin release and delays gastric emptying (Parker et al. 2019).
14 Conclusion
GLP-1 agonists and analogs are integral to the management of patients with type 2 diabetes mellitus and obesity. Overall, it appears that their effects are mediated at least in part by retardation of gastric emptying, although the effects on gastric emptying are reduced with long-acting preparations or long-term use of short-acting preparations as a result of tachyphylaxis.
References
Acosta A, Camilleri M, Burton D, O’Neill J, Eckert D, Carlson P et al (2015a) Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiol Rep 3(11):e12610
Acosta A, Camilleri M, Shin A, Vazquez-Roque MI, Iturrino J, Burton D et al (2015b) Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology 148(3):537–546.e4
Adam TCM, Westerterp-Plantenga MS (2005) Glucagon-like peptide-1 release and satiety after a nutrient challenge in normal-weight and obese subjects. Br J Nutr 93(6):845–851
Alvarez E, Martínez MD, Roncero I, Chowen JA, García-Cuartero B, Gispert JD et al (2005) The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem 92(4):798–806
Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L et al (2018) MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet 391(10140):2607–2618
Bækdal TA, Borregaard J, Hansen CW, Thomsen M, Anderson TW (2019) Effect of Oral Semaglutide on the pharmacokinetics of lisinopril, warfarin, digoxin, and metformin in healthy subjects. Clin Pharmacokinet 58(9):1193–1203
Barrington P, Chien JY, Showalter HDH, Schneck K, Cui S, Tibaldi F et al (2011) A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab 13(5):426–433
Becker RHA, Stechl J, Steinstraesser A, Golor G, Pellissier F (2015) Lixisenatide reduces postprandial hyperglycaemia via gastrostatic and insulinotropic effects. Diabetes Metab Res Rev 31(6):610–618
Bronden A, Alber A, Rohde U, Gasbjerg LS, Rehfeld JF, Holst JJ et al (2018) The bile acid-sequestering resin sevelamer eliminates the acute GLP-1 stimulatory effect of endogenously released bile acids in patients with type 2 diabetes. Diabetes Obes Metab 20(2):362–369
Bunck MC, Diamant M, Cornér A, Eliasson B, Malloy JL, Shaginian RM et al (2009) One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 32(5):762–768
Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD et al (2004) Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 27(11):2628–2635
Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ et al (2010) Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 95(2):872–878
Cervera A, Wajcberg E, Sriwijitkamol A, Fernandez M, Zuo P, Triplitt C et al (2008) Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 294(5):E846–E852
Chedid V, Vijayvargiya P, Carlson P, Van Malderen K, Acosta A, Zinsmeister A et al (2018) Allelic variant in the glucagon-like peptide 1 receptor gene associated with greater effect of liraglutide and exenatide on gastric emptying: a pilot pharmacogenetics study. Neurogastroenterol Motil 30(7):e13313-e
Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB et al (2018) LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab 18:3–14
Cummings DE, Overduin J (2007) Gastrointestinal regulation of food intake. J Clin Invest 117(1):13–23
Davies M, Pieber TR, Hartoft-Nielsen M-L, Hansen OKH, Jabbour S, Rosenstock J (2017) Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA 318(15):1460–1470
Deane AM, Nguyen NQ, Stevens JE, Fraser RJL, Holloway RH, Besanko LK et al (2010a) Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab 95(1):215–221
Deane AM, Chapman MJ, Fraser RJL, Summers MJ, Zaknic AV, Storey JP et al (2010b) Effects of exogenous glucagon-like peptide-1 on gastric emptying and glucose absorption in the critically ill: relationship to glycemia. Crit Care Med 38(5):1261–1269
DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD (2005) Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 28(5):1092–1100
DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L (2008) Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 24(10):2943–2952
Degen L, Oesch S, Matzinger D, Drewe J, Knupp M, Zimmerli F et al (2006) Effects of a preload on reduction of food intake by GLP-1 in healthy subjects. Digestion 74(2):78–84
Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V et al (2004) One week’s treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 53(5):1187–1194
Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U et al (2016) Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 4(3):221–232
Delgado-Aros S, Kim D-Y, Burton DD, Thomforde GM, Stephens D, Brinkmann BH et al (2002) Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol 282(3):G424–GG31
Delgado-Aros S, Vella A, Camilleri M, Low PA, Burton DD, Thomforde GM et al (2003) Effects of glucagon-like peptide-1 and feeding on gastric volumes in diabetes mellitus with cardio-vagal dysfunction. Neurogastroenterol Motil 15(4):435–443
Diakogiannaki E, Gribble FM, Reimann F (2012) Nutrient detection by incretin hormone secreting cells. Physiol Behav 106(3):387–393
Drucker DJ (2003) Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 17(2):161–171
Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D et al (2008) Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372(9645):1240–1250
Fineman MS, Mace KF, Diamant M, Darsow T, Cirincione BB, Booker Porter TK et al (2012) Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes Metab 14(6):546–554
Flint A, Raben A, Astrup A, Holst JJ (1998) Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101(3):515–520
Flint A, Raben A, Ersbøll AK, Holst JJ, Astrup A (2001) The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord 25(6):781–792
Flint A, Kapitza C, Hindsberger C, Zdravkovic M (2011) The once-daily human glucagon-like peptide-1 (GLP-1) analog liraglutide improves postprandial glucose levels in type 2 diabetes patients. Adv Ther 28(3):213–226
Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C et al (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392(10160):2180–2193
Garber AJ (2011) Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care 34(Suppl 2):S279–SS84
Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B et al (2011) Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab 13(4):348–356
Gasbjerg LS, Helsted MM, Hartmann B, Jensen MH, Gabe MBN, Sparre-Ulrich AH et al (2019) Separate and combined glucometabolic effects of endogenous glucose-dependent Insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes 68(5):906–917
Gentilella R, Pechtner V, Corcos A, Consoli A (2019) Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev 35(1):e3070-e
Gibbs J, Young RC, Smith GP (1973) Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84(3):488–495
Gutzwiller JP, Drewe J, Göke B, Schmidt H, Rohrer B, Lareida J et al (1999) Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Phys 276(5):R1541–R15R4
Halawi H, Khemani D, Eckert D, O’Neill J, Kadouh H, Grothe K et al (2017) Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol 2(12):890–899
Halim MA, Degerblad M, Sundbom M, Karlbom U, Holst JJ, Webb D-L et al (2018) Glucagon-like peptide-1 inhibits prandial gastrointestinal motility through myenteric neuronal mechanisms in humans. J Clin Endocrinol Metab 103(2):575–585
Hayes MR, Bradley L, Grill HJ (2009) Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150(6):2654–2659
Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, Blundell J (2018) Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes Metab 20(3):610–619
Holst JJ (2013) Incretin hormones and the satiation signal. Int J Obes 37(9):1161–1168
Holst JJ (2019) From the incretin concept and the discovery of GLP-1 to today’s diabetes therapy. Front Endocrinol (Lausanne) 10:260
Holst JJ, Gribble F, Horowitz M, Rayner CK (2016) Roles of the gut in glucose homeostasis. Diabetes Care 39(6):884–892
Horowitz M, Flint A, Jones KL, Hindsberger C, Rasmussen MF, Kapitza C et al (2012) Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract 97(2):258–266
Horowitz M, Rayner CK, Jones KL (2013) Mechanisms and clinical efficacy of lixisenatide for the management of type 2 diabetes. Adv Ther 30(2):81–101
Imeryüz N, Yeğen BC, Bozkurt A, Coşkun T, Villanueva-Peñacarrillo ML, Ulusoy NB (1997) Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Phys 273(4):G920–G9G7
Jones KL, Rigda RS, Buttfield MDM, Hatzinikolas S, Pham HT, Marathe CS et al (2019) Effects of lixisenatide on postprandial blood pressure, gastric emptying and glycaemia in healthy people and people with type 2 diabetes. Diabetes Obes Metab 21(5):1158–1167
Jones KL, Huynh LQ, Hatzinikolas S, Rigda RS, Phillips LK, Pham HT et al (2020) Exenatide once weekly slows gastric emptying of solids and liquids in healthy, overweight, subjects under steady-state concentrations. Diabetes Obes Metab. https://doi.org/10.1111/dom.13956
Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS et al (2005) Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 62(2):173–181
Kothare PA, Soon DKW, Linnebjerg H, Park S, Chan C, Yeo A et al (2005) Effect of exenatide on the steady-state pharmacokinetics of digoxin. J Clin Pharmacol 45(9):1032–1037
Kothare PA, Linnebjerg H, Skrivanek Z, Reddy S, Mace K, Pena A et al (2007) Exenatide effects on statin pharmacokinetics and lipid response. Int J Clin Pharmacol Ther 45(2):114–120
le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L et al (2017) 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 389(10077):1399–1409
Linnebjerg H, Park S, Kothare PA, Trautmann ME, Mace K, Fineman M et al (2008) Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 151(1–3):123–129
Linnebjerg H, Kothare P, Park S, Mace K, Mitchell M (2009) The effect of exenatide on lisinopril pharmacodynamics and pharmacokinetics in patients with hypertension. Int J Clin Pharmacol Ther 47(11):651–658
Little TJ, Pilichiewicz AN, Russo A, Phillips L, Jones KL, Nauck MA et al (2006) Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: relationships with postprandial glycemic and insulinemic responses. J Clin Endocrinol Metab 91(5):1916–1923
Lorenz M, Pfeiffer C, Steinstrasser A, Becker RH, Rutten H, Ruus P et al (2013) Effects of lixisenatide once daily on gastric emptying in type 2 diabetes--relationship to postprandial glycemia. Regul Pept 185:1–8
Lu WJ, Yang Q, Sun W, Woods SC, D’Alessio D, Tso P (2007) The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am J Physiol Gastrointest Liver Physiol 293(5):G963–GG71
Madsbad S (2016) Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab 18(4):317–332
Marathe CS, Rayner CK, Jones KL, Horowitz M (2011) Effects of GLP-1 and incretin-based therapies on gastrointestinal motor function. Exp Diabetes Res 2011:279530
Marathe CS, Rayner CK, Wu T, Jones KL, Horowitz M (2018) Gastric emptying and the personalized management of type 1 diabetes. J Clin Endocrinol Metab 103(9):3503–3506
Mathiesen DS, Bagger JI, Bergmann NC, Lund A, Christensen MB, Vilsbøll T et al (2019) The effects of dual GLP-1/GIP receptor agonism on glucagon secretion-a review. Int J Mol Sci 20(17):4092
Meier JJ (2012) GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 8(12):728–742
Meier JJ, Kemmeries G, Holst JJ, Nauck MA (2005) Erythromycin antagonizes the deceleration of gastric emptying by glucagon-like peptide 1 and unmasks its insulinotropic effect in healthy subjects. Diabetes 54(7):2212–2218
Meier JJ, Rosenstock J, Hincelin-Mery A, Roy-Duval C, Delfolie A, Coester HV et al (2015) Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on Optimized insulin glargine with or without metformin: a randomized, open-label trial. Diabetes Care 38(7):1263–1273
Moller DE (2001) New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414(6865):821–827
Monnier L, Lapinski H, Colette C (2003) Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 26(3):881–885
Nakatani Y, Maeda M, Matsumura M, Shimizu R, Banba N, Aso Y et al (2017) Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab 43(5):430–437
Näslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ et al (1999) Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord 23(3):304–311
Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R et al (1997) Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Phys 273(5):E981–E9E8
Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH et al (2009) Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 32(1):84–90
Nauck MA, Kemmeries G, Holst JJ, Meier JJ (2011) Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 60(5):1561–1565
Odunsi ST, Camilleri M (2009) Selected interventions in nuclear medicine: gastrointestinal motor functions. Semin Nucl Med 39(3):186–194
Odunsi ST, Camilleri M, Szarka LA, Zinsmeister AR (2009) Optimizing analysis of stable isotope breath tests to estimate gastric emptying of solids. Neurogastroenterol Motil 21(7):706–e38
Parker VER, Robertson D, Wang T, Hornigold DC, Petrone M, Cooper AT et al (2019) Efficacy, safety, and mechanistic insights of cotadutide a dual receptor glucagon-like peptide-1 and glucagon agonist. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz047
Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M et al (2015) A randomized, controlled trial of 3.0 mg of Liraglutide in weight management. N Engl J Med 373(1):11–22
Ramesh N, Mortazavi S, Unniappan S (2016) Nesfatin-1 stimulates cholecystokinin and suppresses peptide YY expression and secretion in mice. Biochem Biophys Res Commun 472(1):201–208
Read NW, McFarlane A, Kinsman RI, Bates TE, Blackhall NW, Farrar GB et al (1984) Effect of infusion of nutrient solutions into the ileum on gastrointestinal transit and plasma levels of neurotensin and enteroglucagon. Gastroenterology 86(2):274–280
Ritzel R, Orskov C, Holst JJ, Nauck MA (1995) Pharmacokinetic, insulinotropic, and glucagonostatic properties of GLP-1 [7-36 amide] after subcutaneous injection in healthy volunteers. Dose-response-relationships. Diabetologia 38(6):720–725
Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S et al (2009) Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 52(10):2046–2055
Schirra J, Göke B (2005) The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul Pept 128(2):109–115
Schirra J, Wank U, Arnold R, Göke B, Katschinski M (2002) Effects of glucagon-like peptide-1(7-36)amide on motility and sensation of the proximal stomach in humans. Gut 50(3):341–348
Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ et al (2006) Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 55(2):243–251
Schirra J, Nicolaus M, Woerle HJ, Struckmeier C, Katschinski M, Göke B (2009) GLP-1 regulates gastroduodenal motility involving cholinergic pathways. Neurogastroenterol Motil 21(6):609–e22
Schmitt C, Portron A, Jadidi S, Sarkar N, DiMarchi R (2017) Pharmacodynamics, pharmacokinetics and safety of multiple ascending doses of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 in people with type 2 diabetes mellitus. Diabetes Obes Metab 19(10):1436–1445
Soon D, Kothare PA, Linnebjerg H, Park S, Yuen E, Mace KF et al (2006) Effect of exenatide on the pharmacokinetics and pharmacodynamics of warfarin in healthy Asian men. J Clin Pharmacol 46(10):1179–1187
Steinert RE, Meyer-Gerspach AC, Beglinger C (2012) The role of the stomach in the control of appetite and the secretion of satiation peptides. Am J Physiol Endocrinol Metab 302(6):E666–EE73
Steinert RE, Beglinger C, Langhans W (2016) Intestinal GLP-1 and satiation: from man to rodents and back. Int J Obes 40(2):198–205
Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N (2017) Ghrelin, CCK, GLP-1, and PYY(3-36): secretory controls and physiological roles in eating and Glycemia in health, obesity, and after RYGB. Physiol Rev 97(1):411–463
Stevens JE, Horowitz M, Deacon CF, Nauck M, Rayner CK, Jones KL (2012) The effects of sitagliptin on gastric emptying in healthy humans – a randomised, controlled study. Aliment Pharmacol Ther 36(4):379–390
Szarka LA, Camilleri M (2009) Methods for measurement of gastric motility. Am J Physiol Gastrointest Liver Physiol 296(3):G461–G475
Szarka LA, Camilleri M, Vella A, Burton D, Baxter K, Simonson J et al (2008) A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol 6(6):635–643.e1
Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH et al (2000) Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 141(6):1936–1941
Tibble CA, Cavaiola TS, Henry RR (2013) Longer acting GLP-1 receptor agonists and the potential for improved cardiovascular outcomes: a review of current literature. Expert Rev Endocrinol Metab 8(3):247–259
Trujillo JM, Nuffer W (2014) Albiglutide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Ann Pharmacother 48(11):1494–1501
Uccellatore A, Genovese S, Dicembrini I, Mannucci E, Ceriello A (2015) Comparison review of short-acting and long-acting glucagon-like peptide-1 receptor agonists. Diabetes Ther 6(3):239–256
Umapathysivam MM, Lee MY, Jones KL, Annink CE, Cousins CE, Trahair LG et al (2014) Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide 1 receptor on gastric emptying and glycemia. Diabetes 63(2):785–790
Urva S, Nauck MA, Coskun T, Cui X, Haupt A, Benson C et al (2019) 58-OR: the novel dual GIP and GLP-1 receptor agonist tirzepatide transiently delays gastric emptying similarly to a selective long-acting GLP-1 receptor agonist. Diabetes 68(Supplement 1):58-OR
van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM (2014) Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes 38(6):784–793
Vella A, Bock G, Giesler PD, Burton DB, Serra DB, Saylan ML et al (2007) Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes 56(5):1475–1480
Vella A, Bock G, Giesler PD, Burton DB, Serra DB, Saylan ML et al (2008) The effect of dipeptidyl peptidase-4 inhibition on gastric volume, satiation and enteroendocrine secretion in type 2 diabetes: a double-blind, placebo-controlled crossover study. Clin Endocrinol 69(5):737–744
Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A (2001) The role of postprandial releases of insulin and incretin hormones in meal-induced satiety--effect of obesity and weight reduction. Int J Obes Relat Metab Disord 25(8):1206–1214
Vilsbøll T, Agersø H, Lauritsen T, Deacon CF, Aaboe K, Madsbad S et al (2006) The elimination rates of intact GIP as well as its primary metabolite, GIP 3-42, are similar in type 2 diabetic patients and healthy subjects. Regul Pept 137(3):168–172
Vrang N, Larsen PJ (2010) Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol 92(3):442–462
Vrang N, Phifer CB, Corkern MM, Berthoud H-R (2003) Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 285(2):R470–R4R8
Wettergren A, Wøjdemann M, Holst JJ (1998) Glucagon-like peptide-1 inhibits gastropancreatic function by inhibiting central parasympathetic outflow. Am J Phys 275(5):G984–GG92
Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA (1996) Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab 81(1):327–332
Zinman B, Hoogwerf BJ, Durán García S, Milton DR, Giaconia JM, Kim DD et al (2007) The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 146(7):477–485
Acknowledgements
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Maselli, D.B., Camilleri, M. (2020). Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. In: Islam, M.S. (eds) Diabetes: from Research to Clinical Practice. Advances in Experimental Medicine and Biology(), vol 1307. Springer, Cham. https://doi.org/10.1007/5584_2020_496
Download citation
DOI: https://doi.org/10.1007/5584_2020_496
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51088-6
Online ISBN: 978-3-030-51089-3
eBook Packages: MedicineMedicine (R0)