Abstract
Asthma is one of the worldwide respiratory health problem that affect children and adult. Current treatment strategies such as conventional and allergen immunotherapy still fall behind. Mesenchymal stem cells (MSCs) have wide regenerative capacity and immunoregulatory activity with their wide range of secretions and contact dependent manner. In this review, we focus on the current treatment strategies for asthma and MSCs as a new therapeutic tool.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
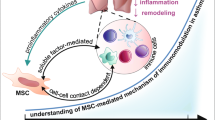
Stem cells have special concern due to their capability to help in regenerative medicine. Broadly they are classified due to their potency as pluripotent (embryonic and inducible) and multipotent (mesenchymal). Among these, mesenchymal stem cells gain much attention because of their postnatal origin, differentiation capacity, lack of immune activity, and safety for the host. Stem cells have important immunomodulatory effect on autoimmune and allergic diseases.
Asthma is a chronic inflammatory disease and its prevalence has significantly increased with the western life style. Th2 dominant immune response is mainly responsible for pathogenesis. Besides conventional therapies with short- and long-acting beta agonists, inhaled low dose corticosteroids and other anti-IgE/anti-leukotriene therapies, there is an immunotherapy approach with sensitized allergens. Recent studies obviously showed the immunoregulatory effects of Mesenchymal Stem Cells (MSCs) in various autoimmune and atopic disorders.
This review outlines the mechanism of the immunotherapy and immunomodulatory effect of MSCs in allergic disease. Also, the implications of recent studies on immunoregulatory aspects of MSC and dysregulated immune systems are discussed.
2 Asthma and the Immune System
2.1 Overview
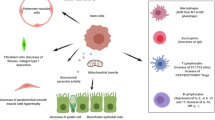
Asthma is a major chronic, non-communicable health problem affecting children and adults (Asamoah et al. 2017). Its prevalence has increased in urbanized regions particularly with westernized lifestyle and it affects more than 300 million people around the world (Papi et al. 2018). It is characterized by dense airway inflammation leading to reversible airflow limitation and lung tissue remodeling (Papadopoulos et al. 2012). Its basic diagnostic symptoms include dyspnea, cough, wheezing and chest tightness (Becker and Abrams 2017).
Asthma is a Th2 skewed disease with increased number of CD4+T cells which produce IL-4 and IL-5 that lead to production of allergen specific IgE and eosinophils, respectively (Brightling et al. 2002). Eosinophilic inflammation is credited to be a substantial contributor to the histopathological inflammatory changes in asthmatic patients (Brightling et al. 2002; Tan et al. 2016). Chronic airway inflammation leads to airway remodeling characterized by basement membrane thickening, goblet cell hyperplasia, smooth muscle proliferation, peribronchial and perivascular inflammatory cell infiltration and mucus plug formation (Akkoc et al. 2001).
Currently there is no radical cure for asthma, but conventional therapeutic approaches control the disease symptomatically. Stepwise pharmacological treatment involves short-acting beta-2 agonist, long acting beta-2 agonists and inhaled low dose corticosteroids alone or with combination depending on the severity of asthma (Parsons et al. 2013; Cates and Karner 2013). Besides these, theophylline, anti-leukotrienes, anticholinergics, and anti-IgE antibodies (Omalizumab) can be used to control severe asthma (Asamoah et al. 2017; Mirra et al. 2018). All of these are symptom relief treatment approaches and do not educate the immune system. Allergen immunotherapy (AIT) is an alternative to regulate the immune response and an effective treatment strategy for allergic diseases (Berings et al. 2017).
2.2 Allergic Disease and the Immune System
The immune system easily differentiates self and non-self and screens harmful pathogens with immune cells in a complex interactive network. Immune tolerance is a well-organized immune mechanism that protects individuals from cancer, autoimmunity, viable pregnancy, graft-versus-host disease (GVHD), rejection of transplanted organs asthma and allergies (Fuchs et al. 2017; Werfel et al. 2016; Chinthrajah et al. 2016). Allergic disease can be classified as allergic rhinitis, allergic asthma, atopic eczema, food allergy and anaphylaxis (Akdis and Akdis 2009; Galli and Tsai 2012; Akdis 2012).
Allergic asthma is basically defined as a type 2 immune response-associated disease. The type 2 dominant immune response is characterized by increased number of CD4 + Th2 cells, IL-4-high and IL-5-high immune activity with enhanced allergen specific IgE, eosinophil and mast cells. This leads to airway hyperresponsiveness with increased airway eosinophilia. Consequently, eosinophilic inflammation responsible for pathophysiological changes causes remodeling seen in asthmatics (Eiwegger and Akdis 2011) (Fig. 1).
2.3 Allergen Immunotherapy
Allergen immunotherapy seems like the only therapeutic approach for allergic conditions and respiratory allergies. This kind of therapy involves delivery of sensitized allergens to patients with gradually increasing amounts of dose for subcutaneous immunotherapy (SCIT), sublingual immunotherapy (SLIT), or oral immunotherapy. Regarding this approach, the allergen content, administration route, and duration of administration are important for the safety of therapy and immunoregulatory stimulation (Matricardi et al. 2019). The underlying mechanism of AIT involves shifting the T cell immune response from Th2 to regulatory type. T regulatory cells remain at the center of AIT with their anti-inflammatory cytokine production nature like interleukin 10 (IL-10) and transforming growth factor-beta (TGF-β). Among these IL-10 is responsible for downregulation of allergen specific immunoglobulin E (IgE) antibody production and upregulation of immunoglobulin G4 (IgG4) which are called blocking antibodies (Frew 2010). Also, Treg cells act on mast cells, eosinophils and T cells to reduce the release of proinflammatory cytokines. Further AIT prevents localization and functions of mast cells, basophils and eosinophils in the local sensitized tissues, such as bronchial mucosa (Cox et al. 2011a; Moote et al. 2018) (Fig. 2).
Beside AITs safe clinical outcomes due to the nature of the antigen as natural allergens, some systemic adverse reactions (SARs) are monitored during therapy (Cox et al. 2011b).
3 Mesenchymal Stem Cells
3.1 Overview
MSCs are non-specialized multipotent cells that have self-renewal and differentiation capacity into diverse cell types such as adipose, chondrocyte and osteocyte (Al-Nbaheen et al. 2013). The International Society of Cellular Therapy (ISCT) stated the minimum criteria for MSCs as plastic-adherent in standard culture, expressing special cell surface markers such as CD73, CD90 and CD105 while negative for CD14, CD34, CD45, CD19, CD11b, CD79a, and HLA-DR, and differentiation capability into adipocyte, osteocyte, or chondrocytes in vitro (Dominici et al. 2006).
Various sources of MSCs are described as including adipose tissue, umbilical cord blood, Wharton’s jelly, the placenta, bone marrow and dental tissue (Sueblinvong et al. 2008; Gronthos et al. 2000). Because of minor ethical apprehensions, ease of attaining them in tissue and isolation, suppression of inflammation and role in immunomodulation, they are a promising therapeutic approach for several autoimmune disorders (Ogulur et al. 2014a; Yu et al. 2010).
3.2 Immunomodulation and MSC
The immune system is a communicating network of cells and molecules with the competency to protect the host from a broad range of pathogens, while distinguishing self and non-self-tolerance. Once the immune system loses its capability to discriminate self-cells and tissues from others, immune-tolerance-related diseases arise such as cancer, autoimmunity and allergy (Fuchs et al. 2017; Palomares et al. 2014).
MSCs are specialized cells that regulate the immune system and control inflammatory disease-related immune reactions. Mainly MSCs express low- to moderate-intensity levels of human leukocyte antigen (HLA) and major histocompatibility complex class I (MHC-I) while they lack MHC-II and costimulatory molecules such as CD80, CD86, CD40 and CD40L (Le Blanc and Ringden 2007; De Miguel et al. 2012).
Conversation and regulation of the immune system is provided by cytokines. T cell subsets are signatures with different tendencies toward cytokine secretions. Regarding their cytokine profile, they control or direct the immune system to autoimmunity or allergic disease. Inflammation is also modulated via cytokines and mainly pro-inflammatory cytokines (TNF-α, IL-1-α, IL-6) increase in that state.
MSCs have a high capacity to modulate immune responses. Basically, they suppress pathologic T cell proliferation and regulate the balance of Th1 (autoimmunity related)/Th2(allergic disease related). In addition to this they modulate the T regulatory cells (Tregs), regulate antibody secretion profile of B cells and antigen presentation of dendritic cells (Le Blanc et al. 2003; Saldanha-Araujo et al. 2011). The innate and adaptive immune system is also regulated by MSCs originating other immunomodulatory molecules such as interleukin-10 (IL-10), TGF-β, indoleamine 2,3-dioxygenase (IDO), and nitric oxide (NO) (Del Fattore et al. 2015; Kyurkchiev et al. 2014; Castro-Manrreza 2016).
Our previous studies indicate the immunoregulatory properties of MSCs from different sources on different dysregulated-immune system related diseases (Ulusoy et al. 2015; Duman et al. 2019; Cerman et al. 2016). One of these showed human dental follicle MSC treatment for a MuSK-associated experimental autoimmune myasthenia gravis (EAMG) model led to the outcome of downregulated anti-MUSK IgG1, IgG2 and IgG3 and prevention of IgG3 and Complement 3 (C3) deposition into the neuromuscular junction (NMJ) in vivo. This regulation was established by suppression of proinflammatory IL-6 and IL-12. These immunoregulatory outcomes also improved clinical grades in the model (Ulusoy et al. 2015). Recent studies also showed that rat bone marrow derived MSCs suppress inflammation by downregulating TNF-α, fibrosis and enhancing NK cells in rat liver in a rat hepatic fibrosis model. Also, IFN-γ, TNF-α and IL-1α are downregulated and IL-10 levels are upregulated in the periphery (Duman et al. 2019).
4 MSCs and Asthma
4.1 Overview
Asthma is characterized by chronic airway inflammation related to dominant Th2 immune response and infiltration of eosinophils and mast cells within small airways (Lai et al. 2009). Several therapeutic approaches are designed to suppress allergic inflammation in acute and chronic murine models. Among them most promising was an immunotherapy approach with an allergen which upregulates Treg cell and downregulates Th2 type immune response in acute and chronic asthma models (Akkoc et al. 2008a, b, 2010, 2011; Eifan et al. 2010; Keles et al. 2011; Townley et al. 2004; Yazi et al. 2008). Allergen-specific immunotherapy is a basic and approved treatment option for allergic diseases. Also, it was shown previously that adjuvants such as Mycobacterium vaccae induce T reg cells while reversing Th2 immune deviation in murine models (Akkoc et al. 2018).
Recent studies address MSC’s as an encouraging therapeutic approach for curing allergic and autoimmune disorders. Studies were carried out on murine models and in-vitro human studies.
4.2 Murine Models
Close models for type-I allergic disease are important to reflect bronchial asthma that resembles the main features of the human allergic disorder. Depending the severity of disease, acute and chronic models were developed. The acute asthma model is characterized by high levels of allergen-specific IgE production, bronchial hypersensitive reactions to allergens and methacholine provocation test, and cellular infiltrate in proximal airways (Herz et al. 2004). Changes are more dramatic in chronic asthma models. Airway remodeling is mostly seen in chronic models with goblet cell hyperplasia, thickening of smooth, muscle and basement membrane. These dramatic changes also reach the distal small airways (Akkoc et al. 2001). Recent meta-analyses of MSC transplantation in asthmatic models have collected studies (Zhang and He 2019). Really various ways of administrating (intravenous and intratracheal) MSC’s successfully downregulates airway inflammation and airway remodeling in acute and chronic asthma models. Our results demonstrated that allogeneic pluripotent stem cells also control allergic acute inflammation, reverse airway cell infiltration in proximal airways, downregulate eosinophil accumulation in Broncho alveolar lavage and allergen specific IgE levels in serum. Further while IL-10 levels increased, IL-4 levels were downregulated in lung cell suspensions (Ogulur et al. 2014b). These results successfully reveal the therapeutic ability of stem cells in acute and chronic asthma models.
4.3 Experimental Human Studies
Stem cell application to patients needs time regarding ethical issues and some unknown side effects that may be seen during long term follow up. Allogeneic MSC’s were successfully used without serious adverse reactions. Also encouraging results are seen in those studies.
Importantly the immunomodulatory properties of MSCs are accredited for allergic disease and autoimmune disorders. The most expected results for MSC in allergic disease are downregulation of Th2 type cytokines, allergen specific T cell proliferation and detrimental memory T cells, and upregulation of Treg cells and naive T cells. Genç et al. compared the in vitro immunomodulatory effect of dental follicle MSC (DF-MSC) on mononuclear cells of asthmatic patients and patients that completed 3 years of allergen specific immunotherapy (Genc et al. 2018). DF-MSCs properly suppressed proliferative responses of CD4+ T cells, IL-4 and GATA-3 expression. The outstanding results of this study are downregulation of effector and effector memory CD4+ T cells, and downregulation of IDO and costimulatory pathway in antigen presentation as in immunotherapy group. Another study revealed that IFN-γ pre-treated DF-MSCs enhanced Treg cells andIL10 levels (Genc et al. 2019). Both studies showed the immunomodulatory effect of DF-MSCs on Derp-1+ allergic polymorphonuclear cell of patients in vitro.
5 Conclusion and Perspective
Recent studies of MSC administration in murine models of asthma provide valuable information concerning the safe application in vivo with lack of immunogenicity and adverse effects. MSC applications may be intravenous, intranasal or intratracheal. The source of MSCs is an important manner. In murine models of asthma, MSCs are derived from bone marrow, adipose tissue or umbilical cord. All sources provided safe results with in vivo experiments.
In humans, recent developments in relation to the clinical and cellular/molecular mechanisms of AIT aim at enhancing clinical and immunologic tolerance, decreasing side effects, and increasing efficacy. Hypoallergenic recombinant allergen and allergoid vaccines and use of probiotics, vitamins and biological agent supplements to support AIT are expected to enhance efficacy. Many novel developments in molecular mechanisms that affect early desensitization, T- and B- cell tolerance, specific antibody regulation, and induction of IgG4 and several key molecules that can act as biomarkers are continuously being developed. As new technologies and novel strategies emerge, we are in need of more research into the mechanisms, biomarker discovery, and disease phenotyping for AIT. There will always be take-home messages for other immune tolerance-related conditions, such as autoimmunity, organ transplantation, chronic infections, cancer, and recurrent abortion.
Abbreviations
- MSCs:
-
Mesenchymal Stem Cells
- AIT:
-
Allergen immunotherapy
- GVHD:
-
Graft versus host disease
- SCIT:
-
Subcutaneous immunotherapy
- SLIT:
-
Sublingual immunotherapy
- TGF-β:
-
Transforming growth factor beta
- SARSs:
-
Systemic adverse reactions
- HLA:
-
Human leukocyte antigen
- MHC:
-
Major histocompatibility complex
- IDO:
-
Indoleamine 2,3-dioxygenase
- NO:
-
Nitric oxide
- EAMG:
-
Experimental autoimmune myasthenia gravis
References
Akdis CA (2012) Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med 18:736–749
Akdis M, Akdis CA (2009) Therapeutic manipulation of immune tolerance in allergic disease. Nat Rev Drug Discov 8:645–660
Akkoc T, Tolunay S, Barlan I, Basaran M (2001) Airway remodeling and serum total immunoglobulin E (IgE) levels in a murine model of asthma. J Asthma 38:585–591
Akkoc T, Eifan AO, Aydogan M, Ozkara S, Bahceciler NN, Barlan IB (2008a) Transfer of T cells from intranasal ovalbumin-immunized mice ameliorates allergic response in ova-sensitized recipient mice. Allergy Asthma Proc 29:411–416
Akkoc T, Eifan AO, Ozdemir C, Yazi D, Yesil O, Bahceciler NN, Barlan IB (2008b) Mycobacterium vaccae immunization to OVA sensitized pregnant BALB/c mice suppressed placental and postnatal IL-5 and inducing IFN-gamma secretion. Immunopharmacol Immunotoxicol 30:1–11
Akkoc T, Aydogan M, Yildiz A, Karakoc-Aydiner E, Eifan A, Keles S, Akin M, Kavuncuoglu S, Bahceciler NN, Barlan IB (2010) Neonatal BCG vaccination induces IL-10 production by CD4+ CD25+ T cells. Pediatr Allergy Immunol 21:1059–1063
Akkoc T, Akdis M, Akdis CA (2011) Update in the mechanisms of allergen-specific immunotheraphy. Allergy Asthma Immunol Res 3:11–20
Akkoc T, Genc D, Zibandeh N, Akkoc T (2018) Intranasal ovalbumin immunotherapy with mycobacterial adjuvant promotes regulatory T cell accumulation in lung tissues. Microbiol Immunol 62:531–540
Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, Prigione A, Adjaye J, Kassem M, Aldahmash A (2013) Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev 9:32–43
Asamoah F, Kakourou A, Dhami S, Lau S, Agache I, Muraro A, Roberts G, Akdis C, Bonini M, Cavkaytar O, Flood B, Izuhara K, Jutel M, Kalayci O, Pfaar O, Sheikh A (2017) Allergen immunotherapy for allergic asthma: a systematic overview of systematic reviews. Clin Transl Allergy 7:25
Becker AB, Abrams EM (2017) Asthma guidelines: the global initiative for asthma in relation to national guidelines. Curr Opin Allergy Clin Immunol 17:99–103
Berings M, Karaaslan C, Altunbulakli C, Gevaert P, Akdis M, Bachert C, Akdis CA (2017) Advances and highlights in allergen immunotherapy: on the way to sustained clinical and immunologic tolerance. J Allergy Clin Immunol 140:1250–1267
Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ (2002) TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol 110:899–905
Castro-Manrreza ME (2016) Participation of mesenchymal stem cells in the regulation of immune response and cancer development. Bol Med Hosp Infant Mex 73:380–387
Cates CJ, Karner C (2013) Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database Syst Rev 2013:Cd007313
Cerman E, Akkoc T, Eraslan M, Sahin O, Ozkara S, Vardar Aker F, Subasi C, Karaoz E, Akkoc T (2016) Retinal electrophysiological effects of intravitreal bone marrow derived mesenchymal stem cells in streptozotocin induced diabetic rats. PLoS One 11:e0156495
Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC (2016) Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol 137:984–997
Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI, Blessing-Moore J, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph C, Schuller DE, Spector SL, Tilles S, Wallace D (2011a) Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 127:S1–S55
Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI, Blessing-Moore J (2011b) Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 127:S1–S55
De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F (2012) Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 12:574–591
Del Fattore A, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M, Fierabracci A, Muraca M (2015) Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant 24:2615–2627
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Duman DG, Zibandeh N, Ugurlu MU, Celikel C, Akkoc T, Banzragch M, Genc D, Ozdogan O, Akkoc T (2019) Mesenchymal stem cells suppress hepatic fibrosis accompanied by expanded intrahepatic natural killer cells in rat fibrosis model. Mol Biol Rep 46:2997–3008
Eifan AO, Akkoc T, Yildiz A, Keles S, Ozdemir C, Bahceciler NN, Barlan IB (2010) Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy 40:922–932
Eiwegger T, Akdis CA (2011) IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur J Immunol 41:1535–1538
Frew AJ (2010) Allergen immunotherapy. J Allergy Clin Immunol 125:S306–S313
Fuchs O, Bahmer T, Rabe KF, von Mutius E (2017) Asthma transition from childhood into adulthood. Lancet Respir Med 5:224–234
Galli SJ, Tsai M (2012) IgE and mast cells in allergic disease. Nat Med 18:693–704
Genc D, Zibandeh N, Nain E, Gokalp M, Ozen AO, Goker MK, Akkoc T (2018) Dental follicle mesenchymal stem cells down-regulate Th2-mediated immune response in asthmatic patients mononuclear cells. Clin Exp Allergy 48:663–678
Genc D, Zibandeh N, Nain E, Arig U, Goker K, Aydiner EK, Akkoc T (2019) IFN-gamma stimulation of dental follicle mesenchymal stem cells modulates immune response of CD4(+) T lymphocytes in Der p1(+) asthmatic patients in vitro. Allergol Immunopathol (Madr) 47:467–476
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630
Herz U, Renz H, Wiedermann U (2004) Animal models of type I allergy using recombinant allergens. Methods 32:271–280
Keles S, Karakoc-Aydiner E, Ozen A, Izgi AG, Tevetoglu A, Akkoc T, Bahceciler NN, Barlan I (2011) A novel approach in allergen-specific immunotherapy: combination of sublingual and subcutaneous routes. J Allergy Clin Immunol 128:808-15.e7
Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S (2014) Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 6:552–570
Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S (2009) Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 64:476–483
Le Blanc K, Ringden O (2007) Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med 262:509–525
Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O (2003) Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 57:11–20
Matricardi PM, Dramburg S, Potapova E, Skevaki C, Renz H (2019) Molecular diagnosis for allergen immunotherapy. J Allergy Clin Immunol 143:831–843
Mirra V, Montella S, Santamaria F (2018) Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment. BMC Pediatr 18:73
Moote W, Kim H, Ellis AK (2018) Allergen-specific immunotherapy. Allergy Asthma Clin Immunol 14:53
Ogulur I, Gurhan G, Aksoy A, Duruksu G, Inci C, Filinte D, Kombak FE, Karaoz E, Akkoc T (2014a) Suppressive effect of compact bone-derived mesenchymal stem cells on chronic airway remodeling in murine model of asthma. Int Immunopharmacol 20:101–109
Ogulur I, Gurhan G, Kombak FE, Filinte D, Barlan I, Akkoc T (2014b) Allogeneic pluripotent stem cells suppress airway inflammation in murine model of acute asthma. Int Immunopharmacol 22:31–40
Palomares O, Martin-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, Akdis CA (2014) Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-beta. Genes Immun 15:511–520
Papadopoulos NG, Agache I, Bavbek S, Bilo BM, Braido F, Cardona V, Custovic A et al (2012) Research needs in allergy: an EAACI position paper, in collaboration with EFA. Clin Transl Allergy 2:21
Papi A, Brightling C, Pedersen SE, Reddel HK (2018) Asthma. Lancet 391:783–800
Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, Storms WW, Weiler JM, Cheek FM, Wilson KC, Anderson SD (2013) An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 187:1016–1027
Saldanha-Araujo F, Ferreira FI, Palma PV, Araujo AG, Queiroz RH, Covas DT, Zago MA, Panepucci RA (2011) Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res 7:66–74
Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ (2008) Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med 177:701–711
Tan HT, Sugita K, Akdis CA (2016) Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep 16:70
Townley RG, Barlan IB, Patino C, Vichyanond P, Minervini MC, Simasathien T, Nettagul R, Bahceciler NN, Basdemir D, Akkoc T, Pongprueksa S, Hopp RJ (2004) The effect of BCG vaccine at birth on the development of atopy or allergic disease in young children. Ann Allergy Asthma Immunol 92:350–355
Ulusoy C, Zibandeh N, Yildirim S, Trakas N, Zisimopoulou P, Kucukerden M, Tasli H, Tzartos S, Goker K, Tuzun E, Akkoc T (2015) Dental follicle mesenchymal stem cell administration ameliorates muscle weakness in MuSK-immunized mice. J Neuroinflammation 12:231
Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, Hoetzenecker W, Knol E, Simon HU, Wollenberg A, Bieber T, Lauener R, Schmid-Grendelmeier P, Traidl-Hoffmann C, Akdis CA (2016) Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol 138:336–349
Yazi D, Akkoc T, Yesil O, Ozdemir C, Aydogan M, Koksalan K, Bahceciler NN, Barlan IB (2008) Treatment with Mycobacterium vaccae ameliorates airway histopathology in a murine model of asthma. Allergy Asthma Proc 29:67–73
Yu J, Zheng C, Ren X, Li J, Liu M, Zhang L, Liang L, Du W, Han ZC (2010) Intravenous administration of bone marrow mesenchymal stem cells benefits experimental autoimmune myasthenia gravis mice through an immunomodulatory action. Scand J Immunol 72:242–249
Zhang LB, He M (2019) Effect of mesenchymal stromal (stem) cell (MSC) transplantation in asthmatic animal models: a systematic review and meta-analysis. Pulm Pharmacol Ther 54:39–52
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Akkoc, T. (2019). Mesenchymal Stem Cells in Asthma. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 8. Advances in Experimental Medicine and Biology(), vol 1247. Springer, Cham. https://doi.org/10.1007/5584_2019_460
Download citation
DOI: https://doi.org/10.1007/5584_2019_460
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45892-8
Online ISBN: 978-3-030-45893-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)