Abstract
The clinical challenge on surface engineering of medical devices to prevent microorganisms adhesion and biofilm formation, has become an essential aspect for medical implants. Antibacterial properties of Graphene Oxide (GO) have been demonstrated across a broad spectrum of bacteria, and the different mechanisms of action with which this nanomaterial interacts with the microbial surface have been elucidated in detail. Innovative protective coatings based on graphene film and hydrogel could represent an innovative solution for the prevention of nosocomial pathogens colonization on implantable device. This brief review mainly focuses on the applications of graphene in nanomedicine with a particular deepening on the antibacterial properties of GO and GO-based nanomaterials. In order to evaluate the possible future applications of GO as an anti-biofilm coating material for medical devices, studies on the ability of graphene coated surface to prevent microbial adhesion are also discussed. A concise review on in vitro toxicity and in vivo safety is also presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Health care-associated infections (HAI) represent a major public health threat in hospital setting. According to World Health Organisation (WHO), the definition of HAI is: “Health care-associated infections, or “nosocomial” and “hospital” infections, affect patients in a hospital or other health-care facility, and are not present or incubating at the time of admission. They also include infections acquired by patients in the hospital or facility but appearing after discharge and occupational infections among staff” (Boev and Kiss 2016). They are considered the sixth leading cause of death, with an incidence from 4% to 10% (Cloutier et al. 2015). Medical devices, such as catheters, cardiac pacemakers, joint prosthesis, prosthetic heart valves and dentures, represent one of principal source of nosocomial infection (Percival et al. 2015; Sabir et al. 2017; Boisvert et al. 2016). Microorganisms are able to adhere to biotic and abiotic surfaces, producing biofilm, composed of cells embedded in a self-produced matrix of extra-cellular polymeric substances (EPS). The main components of EPS are proteins, polysaccharides and extracellular DNA (Del Pozo et al. 2018; Ciofu et al. 2017). The biofilm formation proceeds through distinct stages, in the last stage the cells detach from the matrix and disseminate. Biofilm allows microorganisms to survive long period on the surfaces as they are in a starvation state, with low nutrient need (Kumar et al. 2017); therefore, the cells in biofilm are more resistant to the host immune response and to antimicrobial therapies making these infections hard to be treated, as antibiotics usually act against planktonic cells that are actively reproducing.
The main microorganisms isolated from medical devices health-care associated infections are gram-positive and gram-negative bacteria, such as Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumanii and yeasts, particularly, Candida species (Sohail et al. 2018; Jain et al. 2019; Alcántar-Curiel et al. 2018; Touil et al. 2018; Percival et al. 2015; Sabir et al. 2017).
Research focuses on the development of new coating material that can prevent the adhesion and subsequent biofilm formation of bacteria and yeast on medical devices (Francolini et al. 2017, Cyphert and von Recum 2017, Swartjes et al. 2015). In recent years, the use of nanotechnologies has become an interesting approach to prevent: the use of metal or carbon nanoparticles appears to be simple, safe, not expensive and it is possible to overcome the problem of the antibiotic resistance (Polívková et al. 2017; Karahan 2018). Several antimicrobial nanoparticles have been identified, like iron oxide, zinc oxide, copper oxide but till now only silver nanoparticle (AgNps) is currently under clinical trial for evaluation as antimicrobials (Bao et al. 2011; Allahverdiyev et al. 2011; Srividya et al. 2017; Karahan 2018). Therefore, side-effects, such as AgNps accumulation in the tissue and subsequent inflammation that can be toxic for human body, have been reported (Karahan 2018).
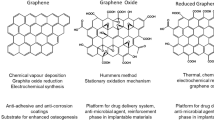
Among the carbon nanomaterial, graphene is a two-dimensional ultra-thin nanomaterial composed only of hybridized-sp2 carbon atoms, arranged in a hexagonal structure. Andrej Gejm and Konstantin Novosëlov, from the University of Manchester, synthetized graphene for the first time in 2004 (Novoselov et al. 2004). It is the first two-dimensional crystalline material ever produced. This material presents several physical properties: stretchability, electrical conductivity, huge surface area and high thermal conductivity (Palmieri et al. 2016; Karahan 2018; Compton and Nguyen 2010).
Since the synthesis, several derivatives have been studied, such as Graphene Oxide (GO) and reduced GO (rGO). Particularly, GO is a precursor of large-scale synthesis of graphene and it is prepared by the oxidation of graphite, making a single monomolecular layer of graphite heavily oxygenated, bearing hydroxyl and epoxide functional groups on their basal planes, in addition to carbonyl and carboxyl groups located at the sheet edges. The presence of these functional groups makes GO sheets strongly hydrophilic (Zhu 2010; Dreyer 2010).
Numerous are the potential applications of GO in biomedicine, such as biological and molecular imaging, drug/gene delivery, cancer therapy, tissue scaffold and antibacterial agent (Yousefi et al. 2017; Depana et al. 2011; Yao et al. 2012; Xia et al. 2019; Pulingam et al. 2019).
In this report, we provide a review of the studies conducted on the antimicrobial activity of GO, especially on the ability to inhibit the microorganism adhesion and biofilm formation on solid phase graphene oxide.
2 GO Antimicrobial Properties
The antimicrobial activity of GO has been widely reported, even if most of the studies focus on the activity of GO in solution while only few reported the activity of GO as coating material in solid phase (Zou 2016; Yousefi et al. 2017; Krishnamoorthy 2012).
Three main GO antimicrobial activities (Mangadlao 2015), are reported and represented in Fig. 1:
-
1.
GO sheets sharp edges can physically interfere with microorganism by cutting the membrane with subsequent intracellular leakage of cytoplasmic constituent and death of the microorganism; this mechanism is called nano-knife or nano-blade effect (Akhavan and Ghaderi 2010; Pham et al. 2015; Castrillón 2015);
-
2.
GO can induce oxidative stress, this phenomenon is caused by a chemical reduction of graphene sheets by bacteria (Gurunathan 2012; Salas et al. 2010; Liu et al. 2011)
-
3.
GO can wrap and isolate microorganisms from the environment so that they cannot find nutrition, stopping proliferation; this mechanism has been mainly observed in solution phase rather than in GO coated surfaces (Palmieri et al. 2017; Perreault et al. 2015).
It has been reported how different solvents, the method used to prepare the graphene oxide, the size, the dispersion state and other factors influence the antimicrobial activity of GO.
In a study published in 2017, Palmieri et al. investigated the activity of GO in different solvents, such as sodium chloride (NaCl), magnesium chloride (MgCl2), ultra-pure water and Phosphate-buffered saline (PBS) and in the growth media LB (Luria Bertani broth). GO was used at concentrations ranging from 3 up to 200 μg/mL. After GO incubation with Escherichia coli and Staphylococcus aureus the antimicrobial effect of GO was assessed by the colony counting method. This work clearly shows how the solution and the different GO concentrations affect the antibacterial properties: in water, for example, GO is highly stable, presenting a homogenous solution and the main mechanism is nano-blade, cutting the bacteria membranes. In the solution containing electrolytes, the GO quickly aggregates, and the main antibacterial effect is the bacteria wrapping. Interestingly, when GO is dispersed in nutrient media such as LB, it creates aggregates that cannot be resuspended by vortex. The antimicrobial effect was visible in a concentration-dependent manner after 5 h incubation but disappeared after 24 h, when the number of bacteria saturated the GO available in solution (Palmieri et al. 2017). In addition to the antimicrobial properties that GO possess in solution, GO has also been extensively studied for its ability to inhibit microbial adhesion and biofilm formation.
3 Antimicrobial Activity of GO-Coated Surfaces
GO material can be used to coat surfaces, to obtain anti-adhesion and anti-biofilm activity either alone or in combination with other substances.
Different methods have been used to prepared GO-based coating, such as spin coating and electrospinning, vacuum filtration, electroless plating, phase inversion, electrophoretic deposition, hydrogel self-assembly/crosslinking, wet chemical reduction, solvent evaporation and solutions casting (Hu et al. 2010; Duan et al. 2015; Liu et al. 2014; Kumar et al. 2016; Carpio et al. 2012; Nine et al. 2015).
Preliminary studies based on GO-coated surfaces were conducted in 2010. Hu and colleagues created GO and rGO paper by vacuum filtration and tested it against E. coli. They either measured the metabolic activities of E.coli in the presence of GO nano-sheets via a luciferase-based ATP assay and the viability, counting the colony forming unit. Data collected demonstrated a decreased metabolic activity and viability of the bacteria after the interaction with GO paper (almost 85% of viability loss after 2 h incubation using 85 μg/ml GO) while the rGO paper was less effective against bacteria (Hu et al. 2010). The authors further analysed the biocompatibility of GO. Trasmission Electron Microscopy (TEM) analysis suggests that GO can be internalized within A549 cells via endocytosis. The biocompatibility assays showed that GO nanosheets at 20 g/mL exhibited no cytotoxicity to A549 cells after 2 h incubation and a slight decrease in cell viability after 24 h. GO nanosheets at higher concentration (85 g/mL) displayed an increased cytotoxicity (50%) within 24 h.
Another study (Akhavan and Ghaderi 2010), focuses on Go and rGO surfaces formed by electrophoretic deposition on stainless steel substrates. A mechanism of membrane disruption has been reported also in this case, but rGO appeared to be more active than GO. The better antibacterial activity of the rGO was motivated by its more sharpened and charged edges with a consequent major bacterial cell membrane damage caused by direct contact. The authors claim that the Gram-negative E.coli, characterized by an outer membrane was more resistant to membrane impairment caused by the rGO than the Gram-positive S. aureus. In 2015, Perrault et al. explored the different antimicrobial mechanism of GO nano-sheet both in suspension and on GO-coated surfaces, analysing the impact of nano-sheet size on antimicrobial activity. They demonstrated that, on GO-coated surfaces, the higher antimicrobial activity is exploited on GO-smaller sheets size, through a mechanism of bacterial cells oxidation, especially when sizes of 0.10 and 0.01 μm2 are tested, obtaining 50% and 30% of cell viability, respectively. The main antibacterial effect is due to the bacterial cell oxidation, confirmed by the author by measuring the percentage of glutathione (GSH) oxidation that increases when the sheets size is decreasing. In suspension, the higher antimicrobial activity was obtained using larger GO sheets: in particular, the number of viable bacteria is 0.5% when 0,65 μm2 sheets size are used. The GO sheet area inhibited bacterial development by a cell entrapment mechanism, so the largest sheets are more able to wrap the bacteria (Perreault et al. 2015). A paper, published in 2017, highlights the importance of the orientation of the GO sheets for its antimicrobial activity. The antibacterial activity of GO films with different alignments of the sheets was measured using E. coli as a model. Bacteria were deposited for 3 h on the HEMA-GO (Polyhydroxyethylmethacrylate-GO) film. The vertically aligned sharp edges have enhanced antibacterial activity, showing decreased cell viability (56%) compared to random aligned (75.3%) and planar aligned sheets (81.8%) (Lu 2017). Authors showed that either a mechanism of membrane perturbation and oxidative stress contributed to the anti-bacterial activity of the film.
Another way to obtain GO coated surfaces is to mix with hydrogel to obtain a uniform and stable antimicrobial surface. In a paper published in Papi et al. 2016, Papi et al. described a hydrogel made of GO and agar and its activity against S. aureus, E. coli, and the yeast Candida albicans. Particularly, they used the laser induced super cavitation technology to reproduce on the surface of the GO-hydrogel the natural antimicrobial pattern of the Cancer Pagurus. This formulation showed anti-microbial activity due to the GO blade effect already described and the anti-adhesive pattern reproduced on the surface of the GO hydrogel (Papi et al. 2016).
3.1 Inhibition of Microbial Adhesion and Biofilm Formation on GO-Coated Surface
As already described, biofilm related infections represent a crucial point in clinical setting (Singhai 2012).
Anti-biofilm activity of graphene oxide has been described in the last few years. Di Giulio et al., investigated the anti-biofilm activity of GO against the principal wounds pathogens: P. aeruginosa, S. aureus and C. albicans (Di Giulio et al. 2018). They analysed the effect of GO in the inhibition biofilm formation and in the ability to interfere with mature biofilm. For the evaluation of the inhibition, microorganisms were put in contact with 50 mg/L of GO in 96 wells-flat bottom plate for 24 h and the biofilm biomass was quantified, reflecting a significant reduction in the wells treated with GO for the microorganisms under study; the greatest reduction was observed for S.aureus. GO appears to be able to act also in mature biofilm, possibly penetrating in the extra-cellular matrix of biofilm; indeed, author revealed a reduction of the biomass after the exposure of the mature biofilm to 50 mg/L of GO and a detachment of the cells from the biofilm. An interesting study was conducted by Zhang et al. 2018. The effect of GO on P.aeruginosa biofilm was evaluated after short (4 h) and long-term (7 days) exposure. GO at 3 different concentrations (10,20,40 mg/L) was used to evaluate the inhibition of biofilm formation. The bacteria were put in suspension with the different GO concentrations and then incubated in a 96-wells plate to evaluate the biofilm production. A biofilm biomass reduction, more evident at 40 mg/L concentration, was observed. The authors therefore decided to analyse the effect obtained after 7 days of exposure (20 mg/L GO), no differences compared to the control have been detected in biomass production, suggesting that GO may not have long-term effect. The effect of GO on quorum sensing (QS) of P.aeruginosa, implicated in the biofilm formation of this bacteria, have been therefore explored. No impact on the expression of QS-related genes has been detected after either short and long-term exposure but GO affects biofilm formation by absorbing QS signals or related products (such as proteases) in the short term exposure but the altered biofilm formation appears to be restored during the long-term treatment, as the level of proteases and other products increase and any-change detected in the short term exposure are not present after 7 days, indicating the need to analyse the effect on the biofilm in long period with further experiments.
Song et al. tried to determine the anti-biofilm mechanism of graphene oxide, analysing the GO activity against E.coli and Bacillus subtilis biofilm formation. Bacteria were inoculated in 24-wells plate using different concentrations of GO (0, 10, 20, 40, 80, 160 mg/L) for 48 h and the biofilm biomass quantified by crystal violet staining. They found that at low GO concentration (10 mg/ml) the biofilm formation was enhanced compared to the control, while at 20 and 40 mg/ml of GO no significant differences have been detected; the biofilm biomass decreased substantially when high GO concentration (160 mg/ml) was tested. The authors speculated that, at low GO concentration, the cell not in contact with GO might utilize the released cytoplasmatic materials as nutrients for growth, promoting the biofilm formation. The amount of oxidised GSH was furthermore quantified. About 25% of GSH was oxidized in the presence of 10 mg/L GO, and the loss of oxidation increased with the increasing GO concentrations, indicating the oxidative stress one of the anti-biofilm mechanism of GO (Song et al. 2018).
Few studies reported the activity of GO coated surface against microorganism adhesion and biofilm formation. In 2017, Thampi and collaborators deposited GO on polycarbonate urethane (PCU) membrane by a simple method of electrospraying, creating a thin layer of GO surrounding the membrane (GOPCU). PCU is a widely used material for medical devices. They measured the adhesion of the Gram-positive S. aureus and the Gram-negative Pseudomonas aeruginosa to PCU and GOPCU surfaces, detecting, by plate count of viable bacterial colony, a reduction of adhesion of 85.5% and 63.5%, respectively. Authors generically attribute this antibacterial activity to various factors such as size (without actually giving precise information about it), or formation of reactive oxygen species (ROS) by surface functional groups, as already described by Perrault in 2015. GOPCU appears to be biocompatibility, as the hemocompatibility assay conducted showed no adhesion or aggregation of platelets on GOPCU and proliferation assay on fibroblast mammalian cells showed a good percentage of survival (Thampi et al. 2017).
In a paper published in 2017 (Yadav et al. 2017), GO coated surfaces where prepared using two different methods, the traditional Hummer’s method and an improved method that improves the efficiency of the oxidation process providing a greater amount of hydrophilic oxidized graphene material. This result was achieved by excluding the NaNO3 and increasing the amount of KMnO4 regularly used for the traditional Hummer’s method.
These two methods of graphene deposition, resulting in two different surfaces that differ for their nano-sheets size, morphology and exposition of functional groups. They examined the rate of biofilm formation of the bacteria E. coli and S. aureus either by crystal violet assay and microscopy imaging, revealing that both the GO formulations inhibit cell adhesion and biofilm formation.
The GO concentration and the different methods of deposition impact on the ability to inhibit biofilm formation in Gram-positive and Gram-negative bacteria, maybe due to the different cell wall, resulting in different interaction between the microorganism and the surface (Yadav et al. 2017). To explore the physical and chemical mechanism underlying the anti-bacterial activity of the GO surfaces tested, the oxidation of GSH was evaluated. The percentage loss of glutathione increased with the increase of GO concentration that led to ROS mediated oxidative stress.
In another study, GO was used to coat PVDF (Polyvinylidene fluoride) membrane by vacuum-filtration. Also in this study, the microorganisms taken as models are the Gram-positive S. aureus and the Gram-negative E. coli. Bacteria were incubated in contact with the surface for 2, 4 and 6 h. The bactericidal and anti-adhesive effect was evaluated by colony forming unit quantification, live/dead assay and the biofilm formation rate quantified by crystal violet assay (Farid et al. 2018) revealing low rate of survival and cell growth on GO surfaces. The bactericidal effect increases with the increasing time of incubation. Crystal violet quantification revealed inhibition of biofilm formation. The anti-biofilm activity displayed+ has been attributed by the authors to physiochemical properties of the surface: GO membrane has a hydrophilic surface compared to the PVDF membrane leading to a minor adsorption of substances released from the bacteria after exposure with GO that can represent nutrient source for bacteria. Moreover, the exposure of carboxylic functional groups of the surface determines a negatively-charge GO surface that can inhibit the initial adhesion of negative-charged S.aureus and E.coli due to electrostatic repulsion.
Biofilm was further monitored under continuous flow condition over 48 h. The lower biofilm formation formed on GO coated PVDF membrane compared to pristine PVDF was related mainly to the formation of a “wrinkled surface with sharp exposed nano-sheet edges which serve as nano-blades that strongly impair the cell’s outer membranes by piercing or laceration. The thickness of biofilm formation on GO coated membrane was 56.5 μm in contrast to the thickness of the biofilm developed on the virgin PVDF membrane, 110,3 μm. Moreover, the stability of the biofilm was evaluated washing the membrane after measuring the thickness, that revealed to be high on the untreated membrane (106,2) and low on the GO treated membrane (4,8 μm).
The authors investigated whether the mechanical disruption or the chemical oxidation could be the mechanism responsible for GO bactericidal effect. To examine the physical destruction of the membranes, the intra-cytoplasmic material released upon membrane destruction has been quantified and the cell integrity analysed by TEM. Loss of membrane integrity and release of intracellular components confirmed the physical destruction of GO surfaces after interaction with GO. The GO-induced oxidative stress has been confirmed by measuring the increased intracellular level of ROS in E.coli and S.aureus following contact with GO surfaces. Authors speculate that the intracellular ROS induction is mainly attributed to the presence of oxygen-containing functional groups on the surface of GO (Farid et al. 2018).
3.2 GO Functionalization
GO can be combined with other substances either to enhance its antimicrobial activity or to improve the stability of the coating. Different studies evaluated the antimicrobial properties of GO mixed with nanoparticles, such as silver (Xie et al. 2017), zinc oxide (Jones et al. 2008; Chen et al. 2016), gold (Hussain et al. 2014), titanium dioxin (He et al. 2013) or with other polymers, such as chitosan (Konwar et al. 2016; Chen et al. 2013) or antibacterial substances like lysozyme (Bera et al. 2018) or curcumin (Bugli et al. 2018).
Silver nanoparticle is one of the most studied nanoparticles as it presents good features, such as broad antimicrobial activity, high resistance to oxidation and high-thermal conductivity (Yousefi et al. 2017, De Faria et al. 2014). In Zhao et al. 2018 Zhao et al., synthesize a polyethyleneimine (PEI)-modified and AgNP-decorated GO nanocomposite (GO–PEI–Ag) with a size of around 5 nm that displayed antimicrobial activity against Gram-negative E.coli, Acinetobacter baumanii, Shigella sonnei, the Gram-positive bacteria S. aureus and the fungi Aspergillus fumigatus and C. albicans. This composition can kill the adhered bacteria resulting in a complete biofilm formation inhibition. Cell membrane disruption and intra-cytoplasmic leakage appear to be the main inhibition mechanism. Moreover, this formulation showed long-term stability compared to GO-Ag and biocompatibility as HeLa cells treated for 8hs and 24 h using 10, 20 and 50 μg/mL of GO−PEI − Ag were 86.1, 81.4, and 80.2% of viability, respectively, for the 8 h group and 80.0, 77.9, and 72.0%, respectively, for the 24 h group (Zhao et al. 2018).
GO can be also combined with antibacterial substances that can enhance its efficacy in killing and inhibiting bacteria adhesion, such as lysozyme, one example is the work of Duan et al. They prepared a GO-lysozyme surface resulting in a effective activity against Escherichia coli (Duan et al. 2015).
In 2016, an antimicrobial film composed by chitosan-iron oxide coated GO has been realized; specifically, iron oxide coated GO nanomaterial was prepared by a modified co-precipitation method and, in a second step, chitosan hydrogel was incorporated thought hydrogen bonding and electrostatic interaction. The antimicrobial activity was compared to the individually synthesized chitosan GO and chitosan iron oxide hydrogel against methicillin-resistant S. aureus (MSRA), S. aureus, E. coli and Candida albicans; authors noticed an improved activity of chitosan-GO nanocomposite respect to the individual former type of films. This mixed GO appeared also to be biocompatible as the haemolysis test conducted on human erythrocytes displayed low to mild haemolytic activity (0,37%) and, moreover, the cytotoxicity assay on L929 fibroblast cell line showed a much higher viability (80–93%) compared to the negative control. (Konwar et al. 2016).
Curcumin is a yellow–orange polyphenol compound that possesses a wide range of pharmacological activities due to its anti-inflammatory, anticarcinogenic, and anti-infectious properties. The major issue in curcumin-based therapies is the poor solubility of this hydrophobic compound and the cytotoxicity at high doses. Bugli et al. (2018) load the curcumin on the surface area of GO and demonstrated its effectiveness against Staphylococcus aureus MRSA. Curcumin (CU) is a hydrophobic compound, it is able to spontaneously absorb on GO surface by π-π stacking interactions, without the need of chemical agents, leading to the creation of a ‘green’ and safe antimicrobial solution. GO and curcumin showed a synergic effect as MIC of GO alone ranges from 2.35 to 18.75 μg/ml, MIC of curcumin alone against MRSA ranges from 125 to 256 μg/ml while the MIC of the GO-CU combination are between 1.06 and 2.8 μg/ml. Furthermore, this formulation has no cytotoxic effects on eukaryotic cells.
GO-CU nanocomposite was investigated by the same group (Palmieri et al. 2018) for its ability to inhibit biofilm formation of the fungus C. albicans, often isolated from medical devices related infections. While GO seems to not have effect in the adhesion of Candida cells on GO-coated plastic discs, GO-CU shows a partial inhibition of adhesion and poor biofilm formation of C.albicans. Furthermore, PEG (polyethylene glycol) was used to coat GO discs either alone or with curcumin. The GO-CU-PEG nanocomposite showed anti-adhesion property and the ability to act as drug release surface: CU is released in the media when the coating is in ddH2O showing anti-fungal effect.
Frigols and collaborators recently presented a zinc alginate graphene-oxide film (Frigols et al. 2019) with strong anti-bacterial activity against S. aureus and S. epidermidis enhanced by the release of Zn2+ in the solution. Although the antibacterial properties of this innovative material can only be attributed to the presence of Zn2+, GO stabilizes and promotes long-term ion release, confirming the potential drug-release technology of the graphene oxide-based coating.
Table 1 shows some studies focusing on the antimicrobial properties of surfaces covered with graphene with different coating methods and types of functionalization. Anti-biofilm activity tested microbial species and biocompatibility are also revised in the table.
4 Go-Based Antibacterial Materials Safety Profile in Mammals
With the perspective to promote the clinical use of GO, it is fundamental to evaluate the short- and long-term cytotoxicity profiles of GO towards eukaryotic cells. Despite the benefits that GO possesses for antibacterial applications, such as high surface area and exceptional solubility in aqueous environments, its biocompatibility is at the moment controversial. In the biological environment, GO-based nanomaterials with their peculiar physico-chemical surface are straight exposed to direct contact with cells and biological macromolecules. In recent years several publications (Zhang et al. 2016; Ou et al. 2016; Wang et al. 2011) have deepened the potential toxic effects of GO in mammalian cells and animals. Zhang B. and co-workers (Zhang et al. 2016) highlighted a widespread toxic effect of GO in mammalian systems due to cell membrane disruption and lysosomal and mitochondria dysfunction with massive ROS generation. GO toxicity was closely linked to three different parameters: GO concentration, lateral size and surface functionalization. These factors are, not surprisingly, the same ones that influence the antibacterial properties of GO (Ou et al. 2016).
4.1 GO Concentration
GO dose-dependent toxicity in cells lines and animals has been demonstrated in recent years by numerous studies. The main toxic effects result in cell apoptosis, liver and kidney lesion and lung fibrosis. Wang et al. (Wang et al. 2011) analyzed GO cytotoxic effects on human dermal fibroblast cells (HDF). In this study, different concentration of GO, ranging from 10 to 100 μg/ml were exposed to HDF for 24 h. Significant increase in cytotoxicity was observed at 50 μg/ml with decreased cell survival rate and increased apoptosis. GO sheets that enter the cells were found in the cytoplasm and very few within the nucleus. The internalized GO was mainly distributed inside the lysosomes and mitochondria and the size of GO sheets was between 100 and 200 nm. Another study from Lammel and co-workers (Lammel et al. 2013) showed that the exposure of HepG2 cells to both GO and carboxylated graphene nanoplatelets (CXYG) displayed a dose and time dependent increase in ROS production. The predominating size distribution of GO and CXYG determined by means of dynamic light scattering was 385 and 1,110 nm respectively. Both graphene-based nanomaterials penetrate into the cell by impairing the membrane phospholipid bilayer. These data were recently confirmed by a study from Duan and collaborators (Duan et al. 2017) in which they demonstrated that graphene sheets, both pristine and GO with a lateral sizes ranging from 200 nm to 700 nm, caused the formation of pores in the cytoplasmic membranes of A549 and Raw264.7 cells, consequently reducing cell viability. Other studies confirm the fact that the amount of cellular uptake increases with the exposure time and increasing dose. (Wang et al. 2011, 2015; Lee et al. 2017). In in vivo studies, dose and surface modification are considered the main parameters that modulate graphene-based nanomaterials bio-distribution and toxicity. One of the most basic pre-clinical in vivo studies demonstrated that GO, with an average size of 200 nm, if injected via tail-vein in mice at a concentration of 0.25 mg/kg does not affect lifetime, while a high GO dose of 0.4 mg/kg showed chronic toxicity (Wang et al. 2011).
4.2 GO Lateral Size
GO lateral size may influence its cellular uptake and bio-distributions in mammalian organs.
Gurunathan and collaborators (Gurunathan et al. 2019) profiled a significant size and dose dependent toxicity of different sized GO nanosheets with an average size of 100 and 20 nm on two different germ cell lines. Smaller flakes resulted more cytotoxic in a dose depending manner showing higher cellular internalization with loss of cell viability and cell proliferation. A study from Ma et al. (2015) highlighted that large GO flakes showed evident binding with plasma membrane and minor phagocytosis, eliciting robust activation of NF-kB pathway while small GO sheets were highlighted much more internalized into cell cytoplasm. Liao and coworkers investigated the effects of graphene on human erythrocytes and shown that 350 nm-sized graphene could induce strong hemolysis compared to 3 μm-sized graphene sheets (Liao 2015). Drastic membrane disruption by nanoscale sized graphene could be related to the strong electrostatic interactions between the graphene surface and the lipid bilayer of the erythrocyte membrane. On the other hand, low toxicity of microscale sized graphene sheets may be attributed to their lower overall surface areas.
4.3 GO Surface Functionalization
Recent perspective strategies for future biological applications of GO-based nanomaterials include several surface functionalization strategies to improve their safety profile. GO owns a strong hydrophobic surface that allow the formation of a protein corona in biological environment, thus minimizing the physical interactions between GO and cell membranes and reducing cytotoxicity (Hu et al. 2011). This study evaluated the effect of fetal bovine serum (FBS), a common component in cell culture medium, on GO cytotoxicity. At 1% concentrations of FBS, GO showed concentration-dependent cytotoxicity. Interestingly, the cytotoxicity of GO was much lower at 10% FBS, the concentration usually employed in cell culture medium.
The influences of surface charge have also been highlighted in GO-induced toxicities because the electrostatic repulsion between GO and non-phagocytes plays an important role in particle internalization (Ou et al. 2016). Also, in in vivo studies, bio-distribution of graphene nanomaterials is influenced by surface functionalization which in turn modulates their toxicological profiles. (Kanakia 2014). In a study by Yang et al. (Yang et al. 2011), radiolabeled PEG functionalized GO and rGO were administrated intragastrically to female BALB/c mice and bio-distribution. After 4 h of injection, was mainly observed in stomach and intestine, disappearing 1-day post feeding. The same study highlighted the bio-distribution of the same PEG functionalized GO and rGO after intra peritoneal administration. Conversely to oral administration, intraperitoneal injection resulted in high accumulation of graphene nanoparticles on liver and spleen, still evident 30 days after administration.
5 Conclusion
Preventing the bacterial colonization of biomedical devices is the key for limiting the spread of hospital-acquired infections. Antibacterial coatings have become a very active field of research, strongly stimulated by the urgent and increasing need to identify innovative strategies instead of using traditional antibiotics. This review focuses on the antibacterial properties of GO, analyzing the different mechanisms of action exerted to impair bacteria integrity. The antibacterial mechanisms of graphene oxide have still to be completely understood, but most of the current findings and advances support its antibacterial efficacy. Most of the studies agree on the importance of precise physicochemical parameters that most influence the GO bacteriolytic and bacteriostatic properties, like the GO size, the GO concentration and the quantity of hydroxyl groups on the surface. These characteristics impact on the different interaction of GO with the microbial surface: cutting bacteria membranes by the sharp edges, inducing oxidative stress and wrapping bacteria between large GO sheets. Several studies reported in this article focus on the functionalization of the GO with different compounds, some with known antimicrobial activities but not usable individually for intrinsic toxicity (Xie et al. 2017), others highly hydrophobic that can be delivered by GO nano sheets (Bugli et al. 2018). It is now largely accepted that bacteria can attach to solid substrates, in sessile structured communities called biofilms, where they can persist for long periods, acting as a pathogens reservoir. In this regard, the prospect of exploiting the antimicrobial properties of GO to coat medical devices and prevent microbial adhesion represents a challenge of great impact on human health. Key topic of this brief review focused on GO-coated surface ability to interfere with microbial adhesion. Data on this subject are still rather limited and deriving from different methods of GO deposition on as many different surfaces. Overall, the results are promising and demonstrate an effective capacity of GO-coated surfaces to interfere with microbial adhesion and biofilm formation. Plastics are the most widespread material used for medical devices construction, for weight, cost, and performance purposes. To translate results obtained from such a considerable number of studies, it is mandatory to standardize the best method of covering specific materials used for medical devices (e.g. polyethylene, polypropylene, polyvinyl chloride, polycarbonate), accurately assessing the stability and biocompatibility. Further, the present review offers a concise overview of research on biosafety of GO towards mammals, both in vitro and in vivo. Toxicity of GO and graphene family nanomaterials is deeply reviewed in current literature, but the research is still in its infancy and it is hard to conclude the potential risks to human health associated with the use of such innovative nanomaterials. As an extraordinary material with remarkable and unique properties, we are convinced that graphene will have a significant impact on many aspects of our life, ranging from general biotech up to biomedical applications, like “anti-biofilm devices”. The enormous increase in research and development of graphene will replace most of the materials on the market so far.
References
Akhavan O, Ghaderi E (2010) Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4:5731–5736
Alcántar-Curiel MD et al (2018) Association of antibiotic resistance, cell adherence, and biofilm production with the endemicity of nosocomial Klebsiella pneumoniae. Biomed Res Int 2018:7012958
Allahverdiyev AM et al (2011) Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev Anti-Infect Ther 9:1035–1052
An X et al (2013) Graphene oxide reinforced polylactic acid/polyurethane antibacterial composites. J Nanomater 2013:18
Bao Q, Zhang D, Qi P (2011) Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection. J Colloid Interface Sci 360:463–470
Bera S et al (2018) Molecular features of interaction involving hen egg white lysozyme immobilized on graphene oxide and the effect on activity. Int J Biol Macromol 120:2390–2398
Boev C, Kiss E (2016) Hospital-acquired infections: current trends and prevention. Crit Care Nurs Clin N Am 29(1):0899-5885/16
Boisvert AA et al (2016) Microbial biofilms in pulmonary and critical care diseases. Ann Am Thorac Soc 3(9):1615–1623
Bugli F et al (2018) Curcumin-loaded graphene oxide flakes as an effective antibacterial system againstmethicillin-resistant Staphylococcus aureus. Interface Focus 8:20170059
Carpio IE et al (2012) Toxicity of a polymer–graphene oxidecomposite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 4:4746–4756
Castrillón RV (2015) Interaction of graphene oxide with bacterial cell membranes: insights from force spectroscopy. Environ Sci Technol Lett 2(4):112–117
Chen Y et al (2013) Graphene oxide–chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J Mater Chem 1:1992–2001
Chen J et al (2016) Osteogenic activity and antibacterial effect of zinc oxide/carboxylated graphene oxide nanocomposites: preparation and in vitro evaluation. Colloids Surf B Biointerfaces 147:397–407
Ciofu O et al (2017) Antibiotic treatment of biofilm infections. APMIS 125(4):304–319
Cloutier M, Mantovani D, Rosei F (2015) Antibacterial coatings: challenges, perspectives, and opportunities. Trends Biotechnol 33(11):637–652
Compton OC, Nguyen ST (2010) Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based material. Small 6:711
Cyphert EL, von Recum HA (2017) Emerging technologies for long-term antimicrobial device coatings: advantages and limitations. Exp Biol Med (Maywood) 242(8):788–798
De Faria AF et al (2014) Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf B Biointerfaces 113:115–124
Del Pozo JL et al (2018) Biofilm-related disease. Expert Rev Anti-Infect Ther 16(1):51–65
Depana D, Shahb J, Misraa RDK (2011) Controlled release of drug from folate-decorated and graphene mediated drug delivery system: synthesis, loading efficiency, and drug release response. Mater Sci Eng 31:1305–1312
Di Giulio M et al (2018) Antimicrobial and Antibiofilm efficacy of graphene oxide against chronic wound microorganisms. Antimicrob Agents Chemother 62(7):e00547–e00518
Dreyer DR (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240
Duan L et al (2015) Graphene immobilized enzyme/polyethersulfone mixedmatrix membrane: enhanced antibacterial, permeable and mechanical properties. Appl Surf Sci 355:436–445
Duan G et al (2017) Graphene-induced pore formation on cell membranes. Sci Rep 7:42767
Farid MU, Guo J, Kyoungjin A (2018) Bacterial inactivation and in situ monitoring of biofilm development on graphene oxide membrane using optical coherence tomography. J Membr Sci 564:22–34
Francolini I, Vuotto C, Piozzi A, Donelli G (2017) Antifouling and antimicrobial biomaterials: an overview. APMIS 125(4):392–417
Frigols B et al (2019) Graphene oxide in zinc alginate films: antibacterial activity, cytotoxicity, zinc release, water sorption/diffusion, wettability and opacity. PLoS One 14(3):e0212819
Gurunathan JW (2012) Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomedicine 7:5901–5914
Gurunathan S, Kang M-H, Jeyaraj M, Kim J-H (2019) Differential cytotoxicity of different sizes of graphene oxide nanoparticles in leydig (TM3) and sertoli (TM4) cells. Nanomaterials 9(2):139
He W et al (2013) Photocatalytic and antibacterial properties of Au-TiO2 nanocomposite on monolayer graphene: from experiment to theory. J Appl Phys 114:204701
Hu W et al (2010) Graphene-based antibacterial paper. ACS Nano 4:4317–4323
Hu W et al (2011) Protein corona-mediated mitigation of cytotoxicityof graphene oxide. ACS Nano 5:3693–3700
Hussain N et al (2014) Reduced graphene oxide nanosheets decorated with Au nanoparticles as an effective bactericide: investigation of biocompatibility and leakage of sugars and proteins. ChemPlusChem 79:1774–1784
Jain M et al (2019) Phenotypic and molecular characterization of Acinetobacter baumannii isolates causing lower respiratory infections among ICU patients. Microb Pathog 128:75–81
Janković A et al (2015) Graphene-based antibacterial composite coatings electrodeposited on titanium for biomedical applications. Prog Org Coat 83:1–10
Jones N et al (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279:71–76
Kanakia S (2014) Dose ranging, expanded acutetoxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations. Biomaterials 35:7022–7031
Karahan KE (2018) Graphene materials in antimicrobial nanomedicine: status and future perspectives. Adv Healthc Mater 7(1701406):1–18
Konwar A et al (2016) Chitosan-iron oxide coated graphene oxide nanocomposite hydrogel: a robust and soft antimicrobial biofilm. Appl Mater Surfaces 8(32):20625–20634
Krishnamoorthy K (2012) Investigation of the antibacterial activity of graphene oxide nanosheets. Sci Adv Mater 4:1111–1117
Kumar S et al (2016) Engineering a multi-biofunctional composite using poly(ethylenimine) decorated graphene oxide for bone tissue regeneration. Nanoscale 8:6820–6836
Kumar A et al (2017) Biofilms: survival and defence strategy for pathogens. Int J Med Microbiol 307(8):481–489
Lammel T et al (2013) Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part Fibre Toxicol 10:27
Lee JK et al (2017) The role of surface functionalization on the pulmonary inflammogenicity and translocation into mediastinal lymph nodes of graphene nanoplatelets in rats. Arch Toxicol 91:1–10
Liao KH (2015) Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl Mater Interfaces 3:2607–2615
Liu S et al (2011) Antibacterial activity of graphite, graphite oxide, graphene oxide and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5:6971–6980
Liu Y et al (2014) Facile preparation and characterization of poly (vinyl alcohol)/chitosan/graphene oxidebiocomposite nanofibers. J Ind Eng Chem 20:4415–4420
Lu X, Feng X, Werber JR, Chu C, Zucker I, Kim JH, Osuji CO, Elimelech M (2017) Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc Natl Acad Sci USA 114(46):E9793–E9801. https://doi.org/10.1073/pnas.1710996114. Epub 2017 Oct 26. PubMed PMID: 29078354; PubMed Central PMCID:PMC5699062
Ma J et al (2015) Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano 9:10498–10515
Mangadlao JD (2015) On the antibacterial mechanism of graphene oxide (GO) Langmuir-Blodgett films. Chem Commun 51:2886–2889
Nine MJ et al (2015) Graphene: a multipurpose material for protective coatings. J Mater Chem 3:12580–12602
Novoselov KS, Geim AH et al (2004) Electric field effect in atomically thin carbon film. Science 306(5696):666–669
Ou L et al (2016) Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Part Fibre Toxicol 13:57
Palmieri V et al (2016) The future development of bacteria fighting medical devices: the role of graphene oxide. Expert Rev Med Devices 13(11):1013–1019
Palmieri V et al (2017) Bacteria meet graphene: modulation of graphene oxide Nanosheet interaction with human pathogens for effective antimicrobial therapy. ACS Biomater Sci Eng 3(4):619–627
Palmieri V et al (2018) Graphene oxide coatings prevent Candida albicans biofilm formation with a controlled release of curcumin-loaded nanocomposites. Nanomedicine 13(22):2867–2879
Papi M et al (2016) Biomimetic antimicrobial cloak by graphene-oxide agar hydrogel. Sci Rep 6(1):12
Parra C et al (2015) A nanomolecular approach to decrease adhesion of biofouling-producing bacteria to graphene-coated material. J Nanobiotechnol 13:82
Percival SL et al (2015) Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. Rev J Med Microbiol 64:323–334
Perreault F et al (2015) Antimicrobial properties of graphene oxide Nanosheets: why size matters. ACS Nano 9(7):7226–7236
Pham VTH et al (2015) Graphene induces formation of pores that kill spherical and rod-shaped Bacteria. ACS Nano 9(8):8458–8467
Polívková M et al (2017) Antimicrobial treatment of polymeric medical devices by silver nanomaterials and related technology. Int J Mol Sci 18:419
Pulingam T et al (2019) Graphene oxide exhibits differential mechanistic action towards gram-positive and gram-negative bacteria. Colloids Surf B Biointerfaces 181:6–15
Sabir N et al (2017) Bacterial biofilm-based catheter-associated urinary tract infections: causative pathogens and antibiotic resistance. Am J Infect Control 45(10):1101–1105
Salas EC, Sun Z, Luttge A, Tour JM (2010) Reduction of graphene oxide via bacterial respiration. ACS Nano 4(8):4852–4856
Santos CM et al (2011) Antimicrobial graphene polymer (PVK-GO) nanocomposite films. Chem Commun 47:8892–8894
Singhai M (2012) A study on device-related infections with special reference to biofilm production and antibiotic resistance. J Global Infect Dis 4:193–198
Sohail M et al (2018) Molecular analysis, biofilm formation, and susceptibility of methicillin-resistant Staphylococcus aureus strains causing community- and health care-associated infections in central venous catheters. Rev Soc Bras Med Trop 51:603–609
Song C et al (2018) Influences of graphene oxide on biofilm formation of gram-negative and gram-positive bacteria. Environ Sci Pollut Res 25:2853–2860
Srividya N, Ghoora MD, Padmanabh PR (2017) Nanotechnology in the agri-food industry. In: Grumezescu AM (ed) Food preservation, vol 6. Academic, London, pp 125–165
Swartjes JJ et al (2015) Current developments in antimicrobial surface coatings for biomedical applications. Curr Med Chem 22(18):2116–2129
Thampi S et al (2017) Differential adhesive and bioactive properties of polymeric surface coated with graphene oxide thin film. ACS Appl Mater Interfaces 9(5):4498–4508
Touil HFZ, Boucherit-Otmani Z, Boucherit K (2018) In vitro activity of antifungal combinations against planktonic and sessile cells of Candida albicans isolated from medical devices in an intensive care department. J Mycol Med 3:414–418
Wang K et al (2011) Biocompatibility of graphene oxide. Nanoscale Res Lett 6(1):8
Wang X et al (2015) Use of a pro-fibrogenic mechanismbased predictive toxicological approach for tiered testing and decision analysis of carbonaceous nanomaterials. ACS Nano 9:3032–3043
Xia M-Y et al (2019) Graphene-based nanomaterials: the promising active agents for antibiotics-independent antibacterial applications. J Control Release 181:6–15
Xie X et al (2017) Synergistic bacteria killing through photodynamic and physical actions of graphene oxide/Ag/collagen coating. ACS Appl Mater Interfaces 9(31):26417–26428
Yadav N et al (2017) Graphene oxide-coated surface: inhibition of bacterial biofilm formation due to specific surface−interface interactions. ACS Omega 2:3070–3082
Yang K, Wan J, Zhang S, Zhang Y, Lee S, Liu Z (2011) In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 5:516–522
Yao J et al (2012) Chemistry, physics and biology of graphene-based nanomaterials: new horizons for sensing, imaging and medicine. J Mater Chem 22:14313–14329
Yousefi M et al (2017) Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater Sci Eng C 74:568–581
Zhang B et al (2016) Interactions of graphene with mammalian cells: molecular mechanisms and biomedical insights. Adv Drug Deliv Rev 105:145–162
Zhang et al (2018) Interference of non-lethal levels of grapheneoxide in biofilm formation and adaptive response of quorum sensing in bacteria. Environ Sci Nano 5:2809–2818
Zhao R et al (2018) Highly stable graphene-based nanocomposite (GO–PEI–ag) with broad-spectrum, long-term antimicrobial activity and antibiofilm effects. ACS Appl Mater Interfaces 10(21):17617–17629
Zhu Y (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22:3906
Zou X (2016) Mechanisms of the antimicrobial activities of graphene materials. J Am Chem Soc 138(7):2064–2077
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cacaci, M., Martini, C., Guarino, C., Torelli, R., Bugli, F., Sanguinetti, M. (2019). Graphene Oxide Coatings as Tools to Prevent Microbial Biofilm Formation on Medical Device. In: Donelli, G. (eds) Advances in Microbiology, Infectious Diseases and Public Health. Advances in Experimental Medicine and Biology(), vol 1282. Springer, Cham. https://doi.org/10.1007/5584_2019_434
Download citation
DOI: https://doi.org/10.1007/5584_2019_434
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53646-6
Online ISBN: 978-3-030-53647-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)