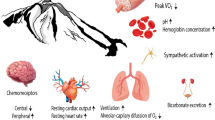
Abstract
Long-term exposure to high altitude causes adaptive changes in several blood biochemical markers along with a marked body mass reduction involving both the lean and fat components. The aim of this study was to evaluate the impact of extended physical strain, due to extensive trekking at high altitude, on body composition, selected biomarkers in the blood, and the protective role of a high-protein diet in muscle dysfunction. We found that physical strain at high altitude caused a significant reduction in body mass and body fat, with a concomitant increase in the cross-sectional area of thigh muscles and an unchanged total lean body mass. Further, we found reductions in plasma leptin and homocysteine, while myoglobin, insulin, and C-reactive protein significantly increased. Creatine kinase, lactate dehydrogenase, and leptin normalized per body fat were unchanged. These findings demonstrate that high-altitude hypoxia, involving extended physical effort, has an impact on muscle function and body composition, facilitating sarcopenia and affecting body mass and fat distribution. It also activates pro-inflammatory metabolic pathways in response to muscular distress. These changes can be mitigated by a provision of a high-protein diet.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The body responds to high-altitude hypoxia with molecular, cellular, and systemic adaptations (West 2012; Bosco et al. 2003; Cerretelli and Di Prampero 1987; Pugh 1962) across cardiovascular (Kylhammar and Rådegran 2017; Luks et al. 2017; Verratti et al. 2015), respiratory (Beretta et al. 2017), musculoskeletal (Bosco et al. 2010; Doria et al. 2011), hormonal (Verratti et al. 2017; Pelliccione et al. 2011), reproductive (Verratti et al. 2011), and metabolic (Boos et al. 2013; San et al. 2013) organ systems. Exercise performance and muscle damage and regeneration in the hypoxic condition have been extensively studied (Sumi et al. 2018; Mancinelli et al. 2016). However, the underlying mechanisms of muscle dysfunction and the significance of enhanced serum creatine kinase content following physical exercise are unsettled (Baird et al. 2012). A combination of hypoxia and physical strain may induce an inflammatory response, which is reflected by enhanced serum C-reactive protein (Li and Fang 2004; Hartmann et al. 2000). Hypoxia also affects oxidative metabolism, stimulating the process of oxygen transport and storage to ensuring adequate tissue oxygenation (Verratti et al. 2009; Di Giulio et al. 2006).
It is known that long-term exposure to high altitude causes a marked reduction in body mass, which involves both the lean and fat components (Cacciani et al. 2008; Rose et al. 1988). Exposure to hypobaric hypoxia changes the attitude toward eating by decreasing appetite and food intake (Westerterp-Plantenga et al. 1999), leading to a negative energy balance (Richalet 2010). Another cause of reduced appetite is a negative feedback system between adipocytes and the hypothalamus, which increases leptin content and energy expenditure (Paoli et al. 2014, 2015). Studies show that leptin alters the neuroendocrine regulation, inducing a loss of appetite and increased energy expenditure which lead to weight loss (Palmer and Clegg 2014; Petousi et al. 2014; Vats et al. 2007; Tschop et al. 1998). Decreased protein intake, associated with physical stress, is a central anabolic stimulus for muscles, which is conducive to sarcopenia (Yanai 2015).
In view of the phenomena above outlined, the present study seeks to define metabolic and endocrine adaptations to high-altitude hypoxia, associated with physical strain due to extensive trekking, in an attempt to get insight into the protective role of a high-protein diet on the sarcopenia related to the extended exposure to high altitude.
2 Methods
2.1 Manaslu Expedition Profile
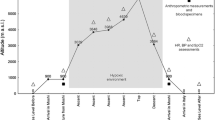
This study describes aspects of medical research performed during the Manaslu Himalaya expedition that has addressed a wide range of physiological issues connected with the fertility, metabolism, muscular changes, and physical performance in subjects exposed to chronic high-altitude hypoxia (Verratti et al. 2017; Doria et al. 2011; Pelliccione et al. 2011; Verratti et al. 2011; Mariggiò et al. 2010). There were seven healthy male climbers, members of the expedition, mean age 39 ± 15 years and height 1.72 ± 0.10 m, who were the subjects in the study framework presented herein.
There were several successive stages of the experimental procedure performed at variable altitude. The high-altitude stage was carried out in Nepal. After arriving in Kathmandu (1,300 m above sea level), the subjects were taken to the Buri Gandaki valley for the initiation of procedures. From there, an acclimatization trek was carried out along the Buri (Budhi) Gandaki River for 13 days, arriving at the Manaslu base camp at 5,000 m. Once at the camp, the subjects stayed there for 22 days without oxygen enrichment. During this time, they climbed Camp 1 (5,900 m) and then to Camp 2 (6,400 m) without oxygen. Finally, they took an 8-day trek back from the base camp to Kathmandu via an exceptionally difficult Larke Pass (5,135 m), covering in all about 450 km by the end of the expedition (Fig. 1).
2.2 Anthropometry and Body Composition
The anthropometric characteristics such as body weight (BW), body mass index (BMI), waist circumference (WC), body fat (BF%), total lean body mass, and the thigh cross-sectional area (CSA) were obtained before and after the expedition in the Exercise Physiology Laboratory of Chieti-Pescara University in Italy, located at the altitude of 110 m above sea level. BF% and total lean body mass were estimated from the measurement of the subcutaneous skinfold thickness (Durnin and Womersley 1974). CSA was calculated from the equation:
CSA = (4.68 × thigh circumference in cm) − (2.09 × anterior thigh skinfold in mm) − 80.99 derived from the multiple regression analysis performed in a study of Housh et al. (1995) for estimation of thigh muscle CSA. Thigh circumference was measured approximately at a third of the trochanter-patella distance. These measurements were based on the previously elaborated methods available in the literature.
2.3 Blood Biomarkers
Venous blood samples were drawn at sea level before (SLB) and 2 days after (SLA) returning to Kathmandu, which ended the expedition. Sampling was performed at 8 am, after fasting for 12 h. The serum was collected for the measurement of biomarkers, and it was frozen in liquid nitrogen until use. The samples were immediately placed on ice and centrifuged for 10 min at 3000 rpm. The following biomarkers were measured leptin, insulin, C-reactive protein, homocysteine, and creatine kinase, myoglobin, and lactate dehydrogenase. Blood analysis was performed with the ADVIA CentaurⓇ CP Immunoassay System (Siemens Healthcare, Milan, Italy) in the Laboratory of Clinical Pathology of Teramo Hospital in Italy.
2.4 Dietary Intervention
The subjects did not follow any individualized dietary program before the expedition, except the instruction of a dietary intake of 2,000 kcal/day, with the following macronutrients content: carbohydrates 49%, proteins 25%, and lipids 26%, which was based on the software WinFood®-Medimatica-Italy. During the expedition, the subjects were instructed to consume about 3,600 kcal/day consisting of carbohydrates 50%, protein 30%, and fat 20% (Table 1).
2.5 Statistical Elaboration
Data were presented as means ±SD. Data distribution was checked with the Kolmogorov-Smirnov test. Differences between the baseline measurements of indices and those after the expedition were compared with a two-tailed paired t-test or Wilcoxon’s test. A p-value <0.05 defined statistically significant changes. The analysis was performed with a commercial Statistica v8.0 package for Windows (StatSoft; Tulsa, OK).
3 Results
We found that chronic physical strain at high altitude caused substantial reductions in body mass and fat components assessed, with a simultaneous increase in CSA (Table 2). A total lean body mass remained unchanged.
Plasma level of leptin decreased by 38% after the stay at high altitude compared with the baseline level before the expedition, from 37.2 ± 11.8 to 23.0 ± 10.1 ng/mL, respectively, p < 0.05. This effect lost significance after normalization for FM due to a large spread of data, although a decreasing trend in leptin was there, from 1.8 ± 1.0 to 1.3 ± 0.5 ng/mL/kg of FM. Likewise, homocysteine decreased by 39%, from 4.74 ± 1.52 before to 2.89 ± 0.57 μmol/L after the expedition, p < 0.03 (Fig. 2).
In contradistinction, myoglobin, insulin, and C-reactive protein showed increased significantly by 29%, 49%, and 26%, from 16.9 ± 3.4 to 21.8 ± 2.4 ng/mL, from 5.3 ± 2.1 to 7.9 ± 2.1 mcU/mL, and from 0.8 ± 0.2 to 1.0 ± 0.3 mg/dL, respectively, p < 0.05 for all (Fig. 3). Creatine kinase and lactate dehydrogenase decreased from 138.1 ± 60.9 to 117.1 ± 43.0 U/L and from 68.4 ± 40.8 to 47.7 ± 27.1 U/L; the decreases failed to reach statistical significance.
4 Discussion
Surprisingly, considering the reduction of FM in the climbers, it is interesting to note that insulin level increased. This increase could be secondary to FM reduction since the energy expenditure was not adequately compensated by the caloric intake during the trek. The FM reduction may have stimulated the release of a liposynthetic hormone, such as insulin, in an attempt to balance off a loss of fat. Larsen et al. (1997) have shown that healthy subjects exposed to hypobaric hypoxia at high altitude have hyperinsulinemia that persists for 1 week but reverts with the time of adaptation to low oxygen pressure. The literature suggests that a decrease in BW is generally associated with a decrease of leptin, while the opposite is true for BW excess (Benso et al. 2007; Broglio et al. 2006). In line with that notion, in this study we found a significant decrease in leptin content of about 38% compared with the baseline level before the expedition. A consistent decreasing trend of about 28% remained when leptin content was normalized for FM, even though it lost significance due to a large data spread.
In this study we found an increase in C-reactive protein, a marker of inflammation, while lactate dehydrogenase was unchanged. In addition, creatine kinase was unchanged, although we would have expected an increase in its content. The creatine kinase-related manifestation of muscle damage due to intensive trekking strain could possibly be masked by a short half-life of creatine kinase, amounting to about 1 day and a half. Creatine kinase increases in the first 12 h after onset of rhabdomyolysis, peaks within 3 days, returning to the baseline level in 3–5 days (Zhang 2012). Concerning homocysteine, the amino acid appreciably decreased by about 39%. Since a high-protein diet, in and by itself, does not alter fasting plasma total homocysteine content (Verhoef et al. 2005), it seems a reasonable assumption that a decrease in homocystein would reflect the exposure to chronic hypoxia. Bailey et al. (2000) have reported a decrease in homocysteine after hypoxic as opposed to normoxic physical training. An increase in the myoglobin level we demonstrate in this study is a normal adaptive response to training at high altitude. However, a persisting myoglobin enhancement may be a foretelling sign of muscle damage and dysfunction. This finding is consistent with the data reported by Nedergaard et al. (2013) and Hoppeler and Vogt (2001). In addition, high level of myoglobin is found in the populations living at high altitude, which probably belongs to the compensatory mechanisms of chronic exposure to hypoxic environment (Moore et al. 2002). A diet rich in calories, with a 30% protein content, has been highlighted as a fundamental nutritional intervention in sarcopenia (Woo 2018). On the other side, an increase in CSA of lower limbs with unchanged total lean body mass, we noticed in this study, is difficult to explain. We believe it might likely be the edema-forming redistribution of body fluids during chronic physical strain at high altitude. Trekkers are known to suffer from a moderate lower extremities edema that reverts on return to a lower altitude (Hultgren 1978).
In conclusion, the results of this study demonstrate that long strenuous trekking at high altitude is conducive to inflammatory response and exerts pro-cachexic and pro-sarcopenic effects. Degradation of muscle proteins, if sustained, may be a presage of muscular failure. These changes, in all likelihood, reflect the effects of exposure to chronic hypobaric hypoxia. A high caloric intake, in general, and a high protein diet, in particular, may at least partially counteract such untoward effects of high altitude hypoxia.
References
Bailey DM, Davies B, Baker J (2000) Training in hypoxia: modulation of metabolic and cardiovascular risk factors in men. Med Sci Sports Exerc 32(6):1058–1066
Baird MF, Graham SM, Baker JS, Bickerstaff GF (2012) Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J Nutr Metab 2012:960363
Benso A, Broglio F, Aimaretti G, Lucatello B, Lanfranco F, Ghigo E, Grottoli S (2007) Endocrine and metabolic responses to extreme altitude and physical exercise in climbers. Eur J Endocrinol 157(6):733–740
Beretta E, Lanfranconi F, Grasso GS, Bartesaghi M, Alemayehu HK, Pratali L, Catuzzo B, Giardini G, Miserocchi G (2017) Air blood barrier phenotype correlates with alveolo–capillary O2 equilibration in hypobaric hypoxia. Respir Physiol Neurobiol 246:53–58
Boos CJ, Hodkinson PD, Mellor A, Green NP, Bradley D, Greaves K, Woods DR (2013) The effects of prolonged acute hypobaric hypoxia on novel measures of biventricular performance. Echocardiography 30(5):534–541
Bosco G, Ionadi A, Panico S, Faralli F, Gagliardi R, Data P, Mortola JP (2003) Effects of hypoxia on the circadian patterns in men. High Alt Med Biol 4(3):305–318
Bosco G, Verratti V, Fanò G (2010) Performances in extreme environments: effects of hyper/hypobarism and hypogravity on skeletal muscle. Eur J Translat Myol 20(3):83–90
Broglio F, Prodam F, Riganti F, Muccioli G, Ghigo E (2006) Ghrelin: from somatotrope secretion to new perspectives in the regulation of peripheral metabolic functions. Front Horm Res 35:102–114
Cacciani N, Paoli A, Reggiani C, Patruno M (2008) Hypoxia: the third wheel between nerve and muscle. Neurol Res 30(2):149–154
di Cerretelli P, Prampero PE (1987) Gas exchange at exercise. In: Farhi LE, Tenney SM (eds) Handbook of physiology. the respiratory system IV. American Physiological Society, Bethesda, pp 555–632
Di Giulio C, Bianchi G, Cacchio M, Artese L, Piccirilli M, Verratti V, Valerio R, Iturriaga R (2006) Neuroglobin, a new oxygen binding protein is present in the carotid body and increases after chronic intermittent hypoxia. Adv Exp Med Biol 580:15–19
Doria C, Toniolo L, Verratti V, Cancellara P, Pietrangelo T, Marconi V, Paoli A, Pogliaghi S, Fanò G, Reggiani C, Capelli C (2011) Improved VO2 uptake kinetics and shift in muscle fiber type in high–altitude trekkers. J Appl Physiol 111(6):1597–1605
Durnin JV, Womersley J (1974) Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 32:77–97
Hartmann G, Tschöp M, Fischer R, Bidlingmaier C, Riepl R, Tschöp K, Hautmann H, Endres S, Toepfer M (2000) High altitude increases circulating interleukin–6, interleukin–1 receptor antagonist and C–reactive protein. Cytokine 12:246–252
Hoppeler H, Vogt M (2001) Muscle tissue adaptations to hypoxia. J Exp Biol 204(Pt 18):3133–3139
Housh DJ, Housh TJ, Weir JP, Weir LL, Johnson GO, Stout JR (1995) Anthropometric estimation of thigh muscle cross–sectional area. Med Sci Sports Exerc 27(5):784–791
Hultgren HN (1978) High–altitude edema. JAMA 239(21):2239
Kylhammar D, Rådegran G (2017) The principal pathways involved in the in vivo modulation of hypoxic pulmonary vasoconstriction, pulmonary arterial remodelling and pulmonary hypertension. Acta Physiol (Oxf) 219(4):728–756
Larsen JJ, Hansen JM, Olsen NV, Galbo H, Dela F (1997) The effect of altitude hypoxia on glucose homeostasis in men. J Physiol 504(1):241–249
Li JJ, Fang CH (2004) C-reactive protein is not only an inflammatory marker but also a direct cause of cardiovascular diseases. Med Hypotheses 62(4):499–506
Luks AM, Levett D, Martin DS, Goss CH, Mitchell K, Fernandez BO, Feelisch M, Grocott MP, Swenson ER, Investigators CXE (2017) Changes in acute pulmonary vascular responsiveness to hypoxia during a progressive ascent to high altitude (5300 m). Exp Physiol 102(6):711–724
Mancinelli R, Di Filippo ES, Verratti V, Fulle S, Toniolo L, Reggiani C, Pietrangelo T (2016) The regenerative potential of female skeletal muscle upon hypobaric hypoxic exposure. Front Physiol 7:303
Mariggiò MA, Falone S, Morabito C, Guarnieri S, Mirabilio A, Pilla R, Bucciarelli T, Verratti V, Amicarelli F (2010) Peripheral blood lymphocytes: a model for monitoring physiological adaptation to high altitude. High Alt Med Biol 11(4):333–342
Moore LG, Zamudio S, Zhuang J, Droma T, Shohet RV (2002) Analysis of the myoglobin gene in Tibetans living at high altitude. High Alt Med Biol 3(1):39–47
Nedergaard A, Karsdal MA, Sun S, Henriksen K (2013) Serological muscle loss biomarkers: an overview of current concepts and future possibilities. J Cachexia Sarcopenia Muscle 4(1):1–17
Palmer BF, Clegg DJ (2014) Ascent to altitude as a weight loss method: the good and bad of hypoxia inducible factor activation. Obesity (Silver Spring) 22(2):311–317
Paoli A, Bianco A, Damiani E, Bosco G (2014) Ketogenic diet in neuromuscular and neurodegenerative diseases. Biomed Res Int 2014(474296):1
Paoli A, Bosco G, Camporesi EM, Mangar D (2015) Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol 6:27
Pelliccione F, Verratti V, D’Angeli A, Micillo A, Doria C, Pezzella A, Iacutone G, Francavilla F, Di Giulio C, Francavilla S (2011) Physical exercise at high altitude is associated with a testicular dysfunction leading to reduced sperm concentration but healthy sperm quality. Fertil Steril 96(1):28–33
Petousi N, Croft QP, Cavalleri GL, Cheng HY, Formenti F, Ishida K, Lunn D, McCormack M, Shianna KV, Talbot NP, Ratcliffe PJ, Robbins PA (2014) Tibetans living at sea level have a hyporesponsive hypoxia-inducible factor system and blunted physiological responses to hypoxia. J Appl Physiol 116(7):893–904
Pugh LG (1962) Physiological and medical aspects of the Himalayan scientific and mountaineering expedition, 1960–61. Br Med J 2(5305):621–627
Richalet JP (2010) Operation Everest III: COMEX ’97. High Alt Med Biol 11(2):121–132
Rose MS, Houston CS, Fulco CS, Coates G, Sutton JR, Cymerman A (1988) Operation Everest. II: nutrition and body composition. J Appl Physiol 65(6):2545–2551
San T, Polat S, Cingi C, Eskiizmir G, Oghan F, Cakir B (2013) Effects of high altitude on sleep and respiratory system and theirs adaptations. ScientificWorldJournal 2013:241569
Sumi D, Kojima C, Goto K (2018) Impact of endurance exercise in hypoxia on muscle damage, inflammatory and performance responses. J Strength Cond Res 32(4):1053–1062
Vats P, Singh VK, Singh SN, Singh SB (2007) High altitude induced anorexia: effect of changes in leptin and oxidative stress levels. Nutr Neurosci 10(5–6):243–249
Verhoef P, van Vliet T, Olthof MR, Katan MB (2005) A high–protein diet increases postprandial but not fasting plasma total homocysteine concentrations: a dietary controlled, crossover trial in healthy volunteers. Am J Clin Nutr 82(3):553–558
Verratti V, Di Giulio C, Bianchi G, Cacchio M, Petruccelli G, Artese L, Lahiri S, Iturriaga R (2009) Neuroglobin in aging carotid bodies. Adv Exp Med Biol 648:191–195
Verratti V, Falone S, Fanò G, Paoli A, Reggiani C, Tenaglia R, Di Giulio C (2011) Effects of hypoxia on nocturnal erection quality: a case report from the Manaslu expedition. J Sex Med 8(8):2386–2390
Verratti V, Falone S, Doria C, Pietrangelo T, Di Giulio C (2015) Kilimanjaro Abruzzo expedition: effects of high–altitude trekking on anthropometric, cardiovascular and blood biochemical parameters. Sport Sci Health 11(3):271–278
Verratti V, Ietta F, Paulesu L, Romagnoli R, Ceccarelli I, Doria C, Fanò Illic G, Di Giulio C, Aloisi AM (2017) Physiological effects of high–altitude trekking on gonadal, thyroid hormones and macrophage migration inhibitory factor (MIF) responses in young lowlander women. Phys Rep 5(20):e13400. https://doi.org/10.14814/phy2.13400
West JB (2012) High–altitude medicine. Am J Respir Crit Care Med 186(12):1229–1237
Westerterp-Plantenga MS, Westerterp KR, Rubbens M, Verwegen CR, Richalet JP, Gardette B (1999) Appetite at “high altitude” [Operation Everest III (Comex-’97)]: a simulated ascent of Mount Everest. J Appl Physiol 87:391–399
Woo J (2018) Nutritional interventions in sarcopenia: where do we stand? Curr Opin Clin Nutr Metab Care 21(1):19–23
Yanai H (2015) Nutrition for sarcopenia. J Clin Med Res 7(12):926–931
Zhang MH (2012) Rhabdomyolosis and its pathogenesis. World J Emerg Med 3(1):11–15
Acknowledgments
Our thanks go to all the porters and Sherpas, whose role was crucial to the success of this scientific project.
Competing Interests
The authors declare no competing interests in relation to this article.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of “G. D’Annunzio” University of Chieti-Pescara in Italy.
Subjects’ Consent
Written informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bosco, G. et al. (2019). Body Composition and Endocrine Adaptations to High-Altitude Trekking in the Himalayas. In: Pokorski, M. (eds) Advancements and Innovations in Health Sciences. Advances in Experimental Medicine and Biology(), vol 1211. Springer, Cham. https://doi.org/10.1007/5584_2019_414
Download citation
DOI: https://doi.org/10.1007/5584_2019_414
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32787-3
Online ISBN: 978-3-030-32788-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)