Abstract
Hypercoagulability and altered lipid metabolism, which are observed in normal pregnancy, can be enhanced in diabetes mellitus. The aim of the study was to evaluate the influence of glycemic control on coagulation and lipid metabolism in women with pregestational (PGDM) and gestational (GDM) diabetes treated with insulin. There were 50 patients with PGDM and 101 patients with GDM enrolled into the study. Serum lipid and coagulation parameters were assessed at 18–22, 25–28, and 31–34 weeks of pregnancy and were compared within the diabetic groups with reference to the effectiveness of glycemia control. We found that poor glycemic control was associated with shortened activated partial thromboplastin time (APTT) and increased activity of antithrombin III (ATIII) in both diabetic groups and with a higher plasminogen activator inhibitor (PAI-1) content level in the GDM group. Poorly controlled PGDM was associated with higher levels of total cholesterol and high-density cholesterol (HDL) in the second trimester and triglycerides in the third trimester. In patients with poorly controlled GDM, a higher concentration of HDL was observed in third trimester, whereas a higher triglyceride level was found in both second and third trimesters. Positive correlations between total cholesterol and APTT and between triglyceride and APTT and ATIII were found in the poorly controlled PGDM group. We conclude that poor glycemic control of diabetic pregnancy impacts both lipid metabolism and the blood coagulation system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Increased levels of coagulation factors, enhanced thrombin generation, and suppression of fibrinolysis are commonly found in women with uncomplicated pregnancy (Akinci et al. 2008; Comeglio et al. 1996). As pregnancy advances, changes in lipid metabolism become significant. The content of cholesterol increases by 30–60% and reaches the highest values at about 32 weeks of pregnancy. Phospholipids, especially lecithin, sphingomyelin, and cephalin, free fatty acids, triglycerides (TG), and high-density (HDL), low-density (LDL), and VLDL lipoprotein fractions, also increase (Grimes and Wild 2018). Maternal hyperlipoproteinemia observed during pregnancy helps the fetus adapt to an unfavorable diabetic environment.
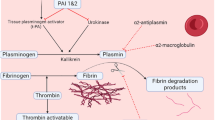
Diabetes mellitus enhances activation of platelets and clotting factors (Grandl and Wolfrum 2018). Plasma coagulation activation markers, such as prothrombin activation fragment 1 + 2 and thrombin-antithrombin complexes, are elevated along with other clotting factors including fibrinogen, kallikrein, von Willebrand factor, and factors VII, VIII, XI, and XII. The fibrinolytic system is relatively inhibited in diabetes due to an increase in plasminogen activator inhibitor (PAI-1) content and the presence of abnormal clot structures that are more resistant to degradation (Kamgar et al. 2006; Vaughan 2005). Peripheral blood platelets are hyperactive in diabetes, which manifests in platelet aggregation in response to the action of platelet agonists, increased platelet contractile force, and the presence of platelet-release products (Carr 2001).
The relationship between lipid metabolism and coagulation cascade is based on the “lipid hypothesis” or “Grutzbalg hypothesis” (Yee et al. 2001). Hypertriglyceridemia is associated with factor VII activation and thus stimulation of the extrinsic coagulation pathway, which takes place through the action of a contact system. The activation of the contact system, in turn, stimulates the intrinsic coagulation pathway by acting on factors XI and IX. Factor XII undergoes self-activation owing to the contact surface created by saturated long-chain fatty acids (Lyons and Basu 2012).
Passive diffusion of glucose into endothelial cells can lead to increases in intracellular glucose concentrations, which increases oxidative stress arising from the degradation of glucose metabolites. Likewise, advanced glycosylation end products (AGE), which result from intracellular hyperglycemia, are involved with vascular damage. Further, protein glycation promotes macro- and microvascular damage. Lastly, it has been reported that hyperglycemia leads to a hypercoagulable state (Brownlee 2005; Carr 2001).
Hyperglycemia leads to the degeneration of endothelial cells and neurons triggering the following main metabolic pathways: polyol pathway, late protein glycation, activation of protein kinase C, and the hexosamine pathway (Bornfeldt and Tabas 2011; Moreno and Fuster 2004). The last two pathways increase the PAI-1 content, which promotes inhibition of fibrinolysis and enhances coagulation. Oxidative stress, developed in the course of hyperglycemia, specifically impairs endothelial functions, which associates with prothrombotic propensity (Guerin-Dubourg et al. 2017; Brownlee 2005). On the other hand, hypercoagulability and altered lipid metabolism, which are the features of normal pregnancy, can exaggerate in the presence of diabetes (Grimes and Wild 2018; Cerneca et al. 1997). In the face of the intertwined relationships among pregnancy, diabetes, oxidative stress, and the blood coagulation cascade above outlined, we set out to examine in this study the influence of glycemic control on the coagulation and lipid metabolism in women with pregestational (PGDM) and gestational (GDM) diabetes treated with insulin.
2 Methods
The PGDM group consisted of 50 patients with pregestational diabetes, which included 40 patients with White class B-D (PGDM B-D group) and 10 patients with RF class (PGDM RF group). The GDM group consisted of 101 patients, diagnosed with an oral glucose tolerance test (75 g glucose load), carried out between 24th and 28th gestational week, who required treatment with diet and insulin.
Glucose content was measured 5 times a day, and it was averaged for the 2nd and 3rd trimester of pregnancy. Glycated hemoglobin (HbA1c) and fructosamine contents were measured at 6-week intervals, and they also were averaged for the 2nd and 3rd of pregnancy. The groups were divided according to effectiveness of glycemic control. Criteria for good glycemic control were as follows: mean fasting blood glucose <95 mg/dL, mean HbA1c level < 6.0%, and mean fructosamine concentration < 280 mg/dL. Distribution of patients in the groups is presented in Table 1. Clinical data, obtained from medical records, are presented in Table 2.
In the 2nd trimester, blood samples were taken twice in PGDM patients (between the 18th–22nd and 25th–28th pregnancy week) and once in GDM patients (between the 25th and 28th pregnancy weeks). In the 3rd trimester, blood samples were collected from all patients between the 31st and 34th pregnancy weeks. The following blood indices were determined to evaluate coagulation and fibrinolytic activation: number of platelets, prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen concentration, antithrombin III (ATIII) activity, globulin clot lysis time, and plasminogen activator inhibitor (PAI-1) activity. The activities of plasma ATIII and PAI-1 were determined with a coagulometric method (Bio-Ksel Sp. z o.o., Grudziądz, Poland) and with a Spectrolyse PAI-1 kit (American Diagnostica Inc., Greenwich, CT), respectively. Total cholesterol (CH-T), LDL, HDL, and TG contents were the lipid indices measured in the blood. The assessment of carbohydrate metabolism also included the mean fasting glycemia, the mean postprandial glycemia, and the diurnal glycemia in consecutive trimesters of pregnancy.
Data were presented as means ±SD. Differences between the two groups were assessed with student’s t-test after the confirmation of normality of data distribution. Spearman’s correlation coefficient was used to establish the relationship between parameters. A p-value <0.05 defined statistically significant differences.
3 Results
Glycemic control had a significant effect on the results of coagulation tests in women with PGDM and GDM in both the second and third trimesters of pregnancy (Table 3). The patients whose glycemia was poorly controlled had a shorter mean APTT and a greater activity of ATIII. In addition, patients with poorly controlled GDM presented a higher mean PAI-1 activity in both trimesters. Glycemic control had inappreciable effects on the other indices measured such as the platelet count, fibrinogen content, PT, and TT in either trimester of pregnancy in both PGDM and GDM patients.
Poorly controlled glycemia hampered lipid metabolism in pregnancy. In the PGDM group with hyperglycemia, CH-T was elevated in both trimesters, whereas the levels of HDL and TG were elevated in the 2nd or 3rd trimester in either group, respectively. In the GDM group, poor glycemic control was associated with a higher HDL content in the 3rd and a higher TG content in both the 2nd and 3rd trimester. There were no significant differences in CH-T content in this group (Table 4).
The PGDM group with poor glycemic control was selected to evaluate the correlation between lipid and coagulation indices. The analysis excluded the GDM patients, as the duration of diabetes in this group was considered too short to expect a consolidation of changes in both lipid and coagulation metabolism. Both CH-T and TG were associated with APPT, and TG was associated with ATIII activity. A particularly pronounced relationship was noticed between TG content and APTT (r = 0.35; p < 0.01) (Table 5).
4 Discussion
Pregnancy acts to increase the propensity for coagulation and impairs fibrinolysis, which is a manifestation of the adaptive mechanism to prevent excessive bleeding from the placental site during labor and postpartum (O’Riordan and Higgins 2003). Likewise, diabetes creates a hypercoagulable state due to increased activation of platelets and prothrombotic coagulation factors, accompanied with a decrease in fibrinolysis (Gorar et al. 2016; Alzahrani and Ajjan 2010). Among the proposed mechanisms of the prothrombotic influence of hyperglycemia, there are oxidative stress, along with its direct effect on gene transcription of coagulation factors, loss of the endothelial glycocalyx layer shielding the coagulation factors, and a direct glycation of coagulation factors, which alters their activity (Lemkes et al. 2010). Expectedly, co-occurrence of both conditions could potentiate these effects.
The major finding of this study was a significantly lower APTT, the essential measure of coagulability, in pregestational and gestational diabetes in patients whose hyperglycemia was poorly controlled. These results are consistent with the findings of Gorar et al. (2016) in gestational diabetes. However, literature data are contentious. van Wersch et al. (1990) have examined female and male patients with insulin-dependent diabetes and have noticed a prolongation of APTT in subjects with lower HbA1 values.
Hyperglycemia reduces the action of antithrombin III, a natural factor X inhibitor (Erem et al. 2005), which decreases the formation of thrombin-antithrombin complexes, and thus also thrombin overactivity, and enhances propensity for hypercoagulability. There are data that demonstrate either a positive (Griffin et al. 2001) or negative (Ceriello 1993) relationship between HbA1 and ATIII activity. In this study, ATIII activity was significantly higher in both 2nd and 3rd trimesters of pregnancy in PGDM and GDM patients with unsatisfactory glycemic control. This result is consistent with the observations of Donders et al. (1993) who have found a positive correlation between the content of glycated hemoglobin and ATIII activity in patients with diabetes. Such a relationship may be a manifestation of a defense mechanism against endovascular coagulation or a reflection of increased ATIII release from damaged endothelium. ATIII activity may also be reduced due to nonenzymatic glycation (Ceriello 1993). Decreased antithrombin activity has been noticed in nonpregnant patients with insulin-dependent diabetes, irrespective of the incidence of vascular complications, in a study of Leurs et al. (1997). There are no data in the literature evaluating this aspect in pregnant women.
In pregnancy, a decrease in fibrinolysis and enhancement of coagulation also occur due to an increase in PAI-1 and PAI-2 that are synthesized by the endothelium and placenta, respectively. High plasma levels of PAI-1 have been shown in normal pregnancy (Cerneca et al. 1997). Kvasnicka et al. (1996) have reported elevated PAI-1 content also in gestational diabetes. Hyperexpression of the PAI-1 antigen is present in nonpregnant patients with type 1 and type 2 diabetes (Vaughan 2005). Poorly controlled diabetes can decrease fibrinolysis by reducing the nonenzymatic glycosylated susceptibility to plasmin digestion. Untreated fibrin deposits accumulate in tissues and contribute to the development of diabetic complications. The available knowledge can hardly enable the presentation of a unified and unambiguous view on the function of fibrinolysis in patients with diabetes. Both a decrease (Kearney et al. 2017; Carr 2001) and no change (Gorar et al. 2016; Bellart et al. 1998) in fibrinolytic activity have been noticed. The discrepancy may result from inhomogeneity of patients investigated concerning the type and duration of diabetes, the effectiveness of glycemic control, and the coexistence of complications. In our study, PAI-1 activity was significantly enhanced in the condition of poor glycemic control in GDM patients only. This finding is consistent with those of Kvasnicka et al. (1996) in GDM and Erem et al. (2005) in non-insulin-dependent diabetes.
A decrease in fibrinolysis often associates with disorders of lipid metabolism and diabetes (Latron et al. 1991). Also, a relationship between PAI-1 content and serum CH-T and TG has been demonstrated. However, no effect of insulin has been noticed on PAI-1 in diabetic patients, irrespective of the presence of vascular complications. Konieczynska et al. (2014) have shown that PAI-1 content is unaffected by glycemic control and diabetes duration in patients with non-insulin-dependent diabetes. In contradistinction, in the present study, we showed that poor glycemic control increased PAI-1 activity in GDM patients. It seems that hyperglycemia and hypoinsulinemia are responsible for abnormal lipid metabolism (Ritchie et al. 2017; Briguori et al. 2004). In patients with insulin deficiency, reduced lipoprotein lipase activity impedes the removal of triglyceride-rich lipoproteins from circulation. Cholesterol is essential for normal fetal development, as it is essential for the formation of cell membranes. In the present study, patients with unsatisfactorily controlled PGDM had a higher level of CH-T, and those with GDM had a higher level of TG in the 2nd and 3rd trimesters of pregnancy. That is consistent with the studies in which glycemic control, assessed by HbA1c, correlated with the contents of CH-T, LDL, and triglycerides (Koukkou et al. 1996). We also found that patients with poorly controlled PGDM had a significantly higher mean HDL content in the 2nd trimester. However, diabetic-induced changes in HDL are less clear. In the DCCT study, a lower HDL level and a higher TG level, associated with the effectiveness of diabetes control, assessed from the value of HbA1c, were found in young women with insulin-dependent diabetes (DCCT Research Group 1992).
Hypertriglyceridemia is associated with high levels of prothrombin, fibrinogen, and factors VII, VIII, IX, X, and PAI-1 (Griffin et al. 2001). In the present study, however, we noticed positive associations only between ATIII activity and a shortened APTT and hypertriglyceridemia in patients with poorly controlled PGDM. In a study of Erem et al. (2005), ATIII activity was associated with plasma CH-T and TG. Although the plasma lipids associate with the severity of diabetes mellitus, the level of glycemia, above which disorders of lipid metabolism appear, is unknown (Kalaria et al. 2016). The exact mechanisms that underlie the influence of hyperlipidemia on fibrinolytic activity and in particular on PAI-1 activity are still elusive (Morelli et al. 2017). Any factors that change the balance between thrombin generation and fibrinolytic activity may lead to the formation of thrombosis or bleeding complications.
The regulation of hemostasis and thrombosis involves numerous plasma factors that contribute to procoagulant and anticoagulant pathways. Lipids and hyperglycemia are among such factors. Procoagulant lipids/lipoproteins include triglyceride-rich particles and oxidized low-density lipoprotein (LDL) in plasma which can accelerate the activation of prothrombin and factors VII and X. The potentially anticoagulant lipids and lipoproteins, including HDL, enhance the inactivation of factor Va (Griffin et al. 2001). The procoagulant and anticoagulant lipoproteins in the plasma are a viable part of the regulatory system of thrombin generation.
In conclusion, poor glycemic control adversely affects lipid metabolism and coagulation process in pregnancies complicated by diabetes. Hampered lipid metabolism intensifies the prothrombotic propensity and decreases fibrinolytic activity.
References
Akinci B, Demir T, Saygili S, Yener S, Alacacioglu I, Saygili F, Bayraktar F, Yesil S (2008) Gestational diabetes has no additional effect on plasma thrombin-activatable fibrinolysis inhibitor antigen levels beyond pregnancy. Diabetes Res Clin Pract 81(1):93–96
Alzahrani SH, Ajjan RA (2010) Coagulation and fibrinolysis in diabetes. Diab Vasc Dis Res 7(4):260–273
Bellart J, Gilabert R, Fontcuberta J, Carreras E, Miralles RM, Cabero L (1998) Coagulation and fibrinolysis parameters in normal pregnancy and in gestational diabetes. Am J Perinatol 15(8):479–486
Bornfeldt KE, Tabas I (2011) Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab 14(5):575–585
Briguori C, Condorelli G, Airoldi F, Mikhail GW, Ricciardelli B, Colombo A (2004) Impact of glycaemic and lipid control on outcome after percutaneous coronary interventions in diabetic patients. Heart 90(12):1481–1482
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54(6):1615–1625
Carr ME (2001) Diabetes mellitus: a hypercoagulable state. J Diabet Complicat 15(1):44–54
Ceriello A (1993) Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia 36(11):1119–1125
Cerneca F, Ricci G, Simeone R, Malisano M, Alberico S, Guaschino S (1997) Coagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysis. Eur J Obstet Gynecol Reprod Biol 73(1):31–36
Comeglio P, Fedi S, Liotta AA, Cellai AP, Chiarantini E, Prisco D, Mecacci F, Parretti E, Mello G, Abbate R (1996) Blood clotting activation during normal pregnancy. Thromb Res 84(3):199–202
DCCT Research Group (1992) Lipid and lipoprotein levels in patients with IDDM diabetes control and complication. Trial experience. Diabetes Care 15(7):886–894
Donders SH, Lustermans FA, van Wersch JW (1993) Glycometabolic control, lipids, and coagulation parameters in patients with non-insulin-dependent diabetes mellitus. Int J Clin Lab Res 23(3):155–159
Erem C, Hacihasanoğlu A, Celik S, Ovali E, Ersöz HO, Ukinç K, Deger O, Telatar M (2005) Coagulation and fibrinolysis parameters in type 2 diabetic patients with and without diabetic vascular complications. Med Princ Pract 14(1):22–30
Gorar S, Alioglu B, Ademoglu E, Uyar S, Bekdemir H, Candan Z, Saglam B, Koc G, Culha C, Aral Y (2016) Is there a tendency for thrombosis in gestational diabetes mellitus? J Lab Physicians 8(2):101–105
Grandl G, Wolfrum C (2018) Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin Immunopathol 40(2):215–224
Griffin JH, Fernández JA, Deguchi H (2001) Plasma lipoproteins, hemostasis and thrombosis. Thromb Haemost 86(1):386–394
Grimes SB, Wild R (2018) Effect of pregnancy on lipid metabolism and lipoprotein levels. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP (eds) Endotext [Internet]. MDText.com, Inc., South Dartmouth. ;2000-
Guerin-Dubourg A, Cournot M, Planesse C, Debussche X, Meilhac O, Rondeau P, Bourdon E (2017) Association between fluorescent advanced glycation end-products and vascular complications in type 2 diabetic patients. Biomed Res Int 2017:7989180
Kalaria TR, Sirajwala HB, Gohel MG (2016) Serum fructosamine, serum glycated albumin and serum glycated β-lipoprotein in type 2 diabetes mellitus patients with and without microvascular complications. J Diabetes Metab Disord 15:53
Kamgar M, Nobakhthaghighi N, Shamshirsaz AA, Estacio RO, McFann KK, Schrier RW (2006) Impaired fibrinolytic activity in type II diabetes: correlation with urinary albumin excretion and progression of renal disease. Kidney Int 69(10):1899–1903
Kearney K, Tomlinson D, Smith K, Ajjan R (2017) Hypofibrinolysis in diabetes: a therapeutic target for the reduction of cardiovascular risk. Cardiovasc Diabetol 16(1):34
Konieczynska M, Fil K, Bazanek M, Undas A (2014) Prolonged duration of type 2 diabetes is associated with increased thrombin generation, prothrombotic fibrin clot phenotype and impaired fibrinolysis. Thromb Haemost 111(4):685–693
Koukkou E, Watts GF, Lowy C (1996) Serum lipid, lipoprotein and apolipoprotein changes in gestational diabetes mellitus: a cross-sectional and prospective study. J Clin Pathol 49(8):634–637
Kvasnicka J, Bendl J, Zivný J, Umlaufová A, Maslowská H (1996) Changes in hemostasis and fibrinolysis in gestational diabetes. Cas Lek Cesk 135(4):106–110
Latron Y, Chautan M, Anfosso F, Alessi MC, Nalbone G, Lafont H, Juhan-Vague I (1991) Stimulating effect of oxidized low density lipoproteins on plasminogen activator inhibitor-1 synthesis by endothelial cells. Arterioscler Thromb 11(6):1821–1829
Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JC, Hoekstra JB (2010) Hyperglycemia: a prothrombotic factor? J Thromb Haemost 8(8):1663–1669
Leurs PB, van Oerle R, Wolffenbuttel BH, Hamulyak K (1997) Increased tissue factor pathway inhibitor (TFPI) and coagulation in patients with insulin-dependent diabetes mellitus. Thromb Haemost 77(3):472–476
Lyons TJ, Basu A (2012) Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl Res 159(4):303–312
Morelli VM, Lijfering WM, Bos MHA, Rosendaal FR, Cannegieter SC (2017) Lipid levels and risk of venous thrombosis: results from the MEGA-study. Eur J Epidemiol 32(8):669–681
Moreno PR, Fuster V (2004) New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol 44(12):2293–2300
Murthy EK, Pavli-Renar I, Metelko Z (2002) Diabetes and pregnancy. Diabetol Croat 31:131–146
O’Riordan MN, Higgins JR (2003) Haemostasis in normal and abnormal pregnancy. Best Pract Res Clin Obstet Gynaecol 17(3):385–396
Ritchie RH, Zerenturk EJ, Prakoso D, Calkin AC (2017) Lipid metabolism and its implications for type 1 diabetes-associated cardiomyopathy. J Mol Endocrinol 58(4):R225–R240
van Wersch JW, Westerhuis LW, Venekamp WJ (1990) Glycometabolic control and fibrinolysis in diabetic patients. Haemostasis 20(4):241–250
Vaughan DE (2005) PAI-1 and atherothrombosis. J Thromb Haemost 3(8):1879–1883
Yee KO, Ikari Y, Schwartz SM (2001) An update of the Grützbalg hypothesis: the role of thrombosis and coagulation in atherosclerotic progression. Thromb Haemost 85(2):207–217
Conflicts of Interest
The authors declare no conflict of interest in relation to this article.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of Warsaw Medical University in Warsaw, Poland.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Teliga-Czajkowska, J., Sienko, J., Zareba-Szczudlik, J., Malinowska-Polubiec, A., Romejko-Wolniewicz, E., Czajkowski, K. (2019). Influence of Glycemic Control on Coagulation and Lipid Metabolism in Pregnancies Complicated by Pregestational and Gestational Diabetes Mellitus. In: Pokorski, M. (eds) Advances in Biomedicine. Advances in Experimental Medicine and Biology(), vol 1176. Springer, Cham. https://doi.org/10.1007/5584_2019_382
Download citation
DOI: https://doi.org/10.1007/5584_2019_382
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25372-1
Online ISBN: 978-3-030-25373-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)