Abstract
This study seeks to determine the pathogens in respiratory specimens and blood serum obtained from children who present with community acquired pneumonia (CAP) diagnosed on the basis of clinical and radiological evidence. The study group consisted of 46 hospitalized children aged 1–11 years. The material for research consisted of pharyngeal swabs and samples of blood serum. One hundred and thirty eight pharyngeal swabs were examined for the presence of C. pneumoniae antigen, C. pneumoniae DNA, and for typical pathogens. C. pneumoniae DNA was detected in pharyngeal swabs with nested PCR. Classical microbiological culture was used for detection of typical bacteria. ELISA test were used for detection anti-C. pneumoniae and anti-M. pneumoniae antibodies in the serum. C. pneumoniae DNA was identified in 10.9% of children. Positive culture for typical pathogens was observed in 8.7% of children. Specific anti-C. pneumoniae IgM antibodies were found in 8.7% of children, and IgG and IgA antibodies in 1 child each. Specific anti-M. pneumoniae IgG antibodies were found in 13.1% of children and IgM antibodies in 1 child. We conclude that the underlying bacterial etiology of CAP is rather rarely conclusively confirmed in children. Nonetheless, determining the etiology of CAP is essential for the choice of treatment to optimize the use and effectiveness of antimicrobials and to avoid adverse effect. Due to considerable variations in the power of detection of the type of atypical bacteria causing CAP, the search for the optimum diagnostic methods continues.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Atypical bacteria
- Children
- Chlamydophila pneumoniae
- Community acquired pneumonia
- Culture
- DNA
- Mycoplasma pneumoniae
- PCR
- Pharyngeal swabs
- Respiratory specimens
1 Introduction
Pneumonia is one of the leading infectious causes of morbidity worldwide. Lower respiratory tract infections are one of the leading causes of death worldwide in infants and children. Pneumonia is defined as the occurrence of symptoms indicating an acute infection (fever, chills, and leukocytosis) with the involvement of the alveolar regions of lungs, leading to auscultatory changes, cough, tachypnea, and hypoxia. Pneumonia is usually confirmed by the visualization of lung densities in radiological images (Mandell 2015). In children, however, X-ray images may be difficult to interpret due to uncertain radiological criteria of diagnosis and subjective variations in the radiologists’ assessment. Typical bacteria, exemplified by Streptococcus pneumoniae, are the principal contagion of community-acquired pneumonia (CAP) in children. Recent studies show that atypical pathogens are also important causes of lower respiratory tract infections resulting in mild to life threatening illness (Basarab et al. 2014). The most common atypical pathogens include Mycoplasma pneumoniae (M. pneumoniae), Legionella pneumophila (L. pneumophila), Chlamydophila pneumoniae (C. pneumoniae), and respiratory viruses (Cillóniz et al. 2016; Zhou et al. 2013). These pathogens cause acute respiratory infections of varying severity. M. pneumoniae infects both upper and lower respiratory tract and evokes both endemics and epidemics among children and adults worldwide (Arnold et al. 2007). The bacteria adhere to the host’s respiratory epithelium and may be recovered from the nose, pharyngeal, trachea, and sputum of an individual with an active infection. M. pneumoniae is usually associated with mild to moderate pneumonia, but it often causes extrapulmonary manifestations such as encephalitis, optic neuritis, acute psychosis, cranial nerve palsies, or aseptic meningitis (Spuesens et al. 2013; Narita 2010).
It is estimated that for every 1000 infants and children in North America and Europe, 35–40 will be affected by CAP. Viral pathogens are the most common cause of CAP in children younger than 2 years of age, accounting for 80% of cases (Bradley et al. 2011). The prevalence of individual pathogens varies from country to country, which may have to do with seasonal and geographical differences. An investigation of children’s CAP etiology is hampered by a low yield of blood cultures, lack of satisfactory sputum specimens, and hesitancy to perform pulmonary aspiration or bronchoalveolar lavage (Song et al. 2015). Intracellular pathogens are difficult to culture and isolate. Diagnostic microbiological assays consists of serology, antigen detection, and molecular methods such as polymerase chain reaction (PCR). Currently, the essential issue is what would be the best microbiological method and the optimum specimen for diagnosing CAP caused by these pathogens (She et al. 2010). Concerning C. pneumoniae infection, serology remains the diagnostic method of choice, although it cannot always identify current infection and is limited by tests’ sensitivity and specificity (Kumar and Hammerschlag 2007). It is difficult to clinically distinguish M. pneumoniae from C. pneumoniae infections, making the laboratory tests essential for the pathogen identification. Serological detection, although commonly used, is complicated by false negative results in the early acute phase of infection and the difficulty in obtaining convalescent serum after short hospital stays of 1 week or less. In the serological retrospective diagnostics for Chlamydophila sp. or Mycoplasma sp., it is necessary to take blood samples 2–3 weeks apart to detect an increase in IgG levels during a symptomatic period and then after treatment completion (Villegas et al. 2010). Another disadvantage of serological tests for atypical bacteria is variable sensitivity and insufficient repeatability of results (Miyashita et al. 2013; Kishimoto et al. 2009). This applies particularly to the diagnosis of Chlamydophila sp., for which serological tests are not recommended.
The prevalence of C. pneumoniae and M. pneumoniae varies widely in previous studies on CAP. PCR has been found to be highly sensitive and specific in the diagnostics of acute M. pneumoniae and C. pneumoniae infections (Chen et al. 2013). Despite its rapidity, sensitivity and specificity, one of the major issues with PCR is that it cannot distinguish between colonizing and actual pathogens. Clinical judgment should to be exercised whenever PCR is used (Padalko et al. 2013). Infections with these atypical pathogens do not respond to beta-lactams, which raises concern due to possible serious complications. Macrolides and quinolones remain the best empirical treatment due to good antimicrobial activity and high intracellular concentrations. Although antibiotic resistance concerning these pathogens does not currently represent a clinical problem in the majority of settings, recent studies from Asia and some European countries report the isolation of M. pneumoniae strains resistant to macrolides, suggesting a need to monitor these pathogens (Principi and Esposito 2013; Li et al. 2009). Therefore, the aim of this study was to investigate the presence of a variety of pathogens in respiratory specimens and sera obtained from children who present with pneumonia based on clinical and radiological evidence.
2 Methods
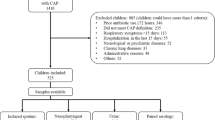
The study included 46 patients (16 girls, 30 boys) with pneumonia, aged from 1 to 11 years (mean 4.6 ± 3.2 years). The children were hospitalized in the First Department and Clinic of Pediatrics, Allergology and Cardiology of Wroclaw Medical University in Poland. Clinical and epidemiological characteristics of the study group are presented in Table 1.
The diagnosis of pneumonia was based on physical symptoms such as wheezing, tachypnea, chest retractions, abnormal auscultatory findings, and on radiologic evidence of pneumonia consisting of the presence of abnormal inflammatory densities in lung parenchyma. Specimens for microbiological investigation consisted of pharyngeal swabs and blood serum, taken before the onset of treatment. Three pharyngeal swabs and 4 ml of blood were taken from each child. Blood samples for clots were collected by venipuncture in vacuum tubes, without the addition of anticoagulants, and were stored at −20 °C until further use. Antibodies against C. pneumoniae (IgM, IgG, and IgA) were detected in serum samples using the enzyme-linked immunosorbent assay (ELISA) (Vircell SL; Granada, Spain). Antibodies against M. pneumoniae (IgG and IgM) were detected using another ELISA test (Euroimmun; Medizinische Labordiagnostika AG, Germany). Samples with both IgM and IgG or IgM antibody alone were considered positive for the ongoing M. pneumoniae infection. Samples in which only IgG was detected were classified as signifying a past infection.
The presence of C. pneumoniae antigen was determined in the pharyngeal swabs using indirect immunofluorescence test (Chlamydia Cel PN-IFT Kit; Cellabs Pty Ltd., Sydney, Australia). This test detects fluorescent elementary bodies using two types of antibodies: anti-chlamydial mouse monoclonal antibodies that bind to the antigen present in the test material and goat anti-mouse antibodies conjugated to fluorescein isothiocyanate (FITC), which provide a color reaction with the C. pneumoniae antigen.
To detect C. pneumoniae DNA, pharyngeal swabs were used. The positive results of nested PCRs were confirmed by positive and negative DNA controls of C. pneumoniae. The main outer membrane protein genes (ompA) of C. pneumoniae were chosen as the amplification target for PCR, 147 bases pair product. The amplified products were visualized by standard agarose electrophoresis in 2% gel stained with ethidium bromide. In the samples containing C. pneumoniae DNA as well as in the positive control, a 147-bp band was detected. Nested PCR is generally more sensitive than is single-step PCR due to a two-step amplification and the use of two sets of primers.
3 Results
The most common bacterial etiological factors were atypical pathogens, which were found with varying frequency depending on the detection method used. Specific antibodies against C. pneumoniae in the IgM class were detected in 4 (8.7%) children, in the IgG and IgA class in 1 (2.2%) child each. C. pneumoniae antigen was detected in pharyngeal swabs in 11 (23.9%) children. C. pneumoniae DNA was detected in pharyngeal swabs in 5 (10.9%) children. Concomitant occurrence of C. pneumoniae antigen in indirect immunofluorescence test and genetic material in PCR was observed in 2 children. Likewise, concomitant occurrence of C. pneumoniae antigen in pharyngeal swabs and specific antibodies against C. pneumoniae in IgM class in the serum was found in 2 children. There was no case of the concomitant occurrence of C. pneumoniae antigen and both IgG and IgA antibodies. Finally, concomitant occurrence of C. pneumoniae DNA and IgM antibodies was found in 1 patient. Antibodies against M. pneumoniae in the IgM class were found in 1 (2.2%) child and in the IgG class in 6 (13.1%) children. Classical microbiological cultures showed the presence of pathogenic bacteria in 4 out of the 46 (8.7%) children. In 3 cases, Staphylococcus aureus was detected and in 1 case Pseudomonas aeruginosa (Table 2).
4 Discussion
CAP in children occurs most often below 5 years of age with an annual incidence between 36 and 46 cases per 1000, which clearly decreases in older children, amounting to approximately 16 cases per year per 1000. According to a large retrospective Finnish study, most children under 5 years of age with CAP and about half of those over 5 years of age are treated in a hospital. In highly developed countries, mortality due to CAP in children under 15 years of age was approximately 0.1 per 1000 cases at the beginning of the 1990s and it mainly concerned children with the accompanying chronic diseases (Principi and Esposito 2011).
Worldwide, three atypical pneumonia agents such as M. pneumoniae, C. pneumoniae, and L. pneumophila are each responsible for 10–30% of pneumonia in children. Determining the etiology of pneumonia raises many difficulties due to low efficacy of routine diagnostic methods and colonization of the respiratory tract by potentially pathogenic bacteria whose identification is not necessarily tantamount to their being the etiological factor of pneumonia. In addition, it may be hard to obtain diagnostic material from young children, such as sputum or lung biopsy specimens, which would enable a verifiable determination of the etiology. According to the literature, atypical pathogens, especially M. pneumoniae and C. pneumoniae, could be considered co-infective agents in severe pneumonia, significantly enhancing the severity of pneumonia. The study of Huong et al. (2014) have demonstrated that mixed typical bacterial-viral infections occur in less than one half of cases. In that study, 722 children, hospitalized with community acquired pneumonia, were recruited. Atypical pathogens were detected in 215 children. M. pneumoniae was detected in 190/215 cases (88.4%), and in 181/190 (95.3%) cases the detection was done by PCR, in 148/190 (77.9%) cases by ELISA-IgM, and in 139/190 (73.2%) cases by both PCR and ELISA. C. pneumoniae was detected in 13/190 (6.8%) cases, and in 7/13 (5.4%) cases the detection was done by PCR.
M. pneumoniae, C. pneumoniae, and L. pneumophila belong to intracellular bacteria that are common causes of CAP. These bacteria cannot be easily or at all cultured in typical media and respond to macrolide treatment rather than beta-lactam antibiotics. There are considerable and changeable regional variations in the reported incidence of CAP induced by the intracellular bacteria, which may be due to diagnostics difficulties. The ELISA-based serological detection of C. pneumoniae-related IgA, IgG, and IgM antibodies often yields false-positive and false-negative results. In addition, the evaluation of paired serum samples taken at 4–8-week intervals is usually necessary for the diagnosis of C. pneumoniae infection. Thus, serology provides a rather late, retrospective diagnosis. Recently, detection of C. pneumoniae using PCR has been introduced into clinical practice. However, the diagnosis is often confounded by the nasopharyngeal carriage or colonization of the upper respiratory tract, or a low grade chronic infection by C. pneumoniae (Gaydos 2013). One reason for the difficulties in detection of C. pneumoniae may be differences in specimen sampling and detection procedures. Another lies in the lack of standardized diagnostic microbiological testing procedures for routine use.
Herrera et al. (2016) have carried out a study in 49 children hospitalized for radiologically confirmed lung inflammation. The age of the children varied from 1 month to 17 years. The specimens examined included nasopharyngeal swabs, sputum, and serum. The IgM and IgG anti-M. pneumoniae and IgG anti-C. pneumoniae antibodies were detected in the sera. Blood samples were collected again after 4 weeks to capture the eventual increase in the antibody titres. Twenty one samples were positive for M. pneumoniae and 6 samples were positive for C. pneumoniae. Six serologically positive samples, 2 for M. pneumoniae and 4 for C. pneumoniae were also detected in the control group without symptoms of CAP. Multiplex PCR (mPCR) provided a different number of positive results. M. pneumoniae was detected in 25 specimens; 7 samples of nasopharyngeal swabs and 18 samples of induced sputum. No amplification was observed concerning C. pneumoniae. The authors compared the mPCR results with the amplification results obtained with the use of commercial PCR kits. The latter provided positive results for M. pneumoniae in just 13 of the children, i.e., about one half of those positively tested with mPCR. The lack of amplification concerning C. pneumoniae was confirmed. Those authors conclude that serology, in some aspects, may provide more positive results of infection with intracellular bacteria than PCR techniques. They also find that induced sputum is superior to nasopharyngeal swabs as material for PCR testing that, overall, is characterized by lower sensitivity than is serology. Nonetheless, nucleic acid amplification techniques are currently considered faster, more sensitive, and more specific than cultures or serology. However, the risk of contamination and difficulties in interpreting positive results as a disease or just bacteria colonization are the main limitations.
Chen et al. (2015) have investigated 1204 children with CAP, using an indirect immunofluorescence technique of Pneumoslide IgM (Vircell-slide; Granada, Spain) that makes it possible to simultaneously diagnose 9 viral and atypical bacterial pathogens in serum samples. Those authors reported the presence of 624 (51.8%) positive findings. The most predominant pathogen was M. pneumoniae, with a positive percentage of 40.8%. The pathogens ranked in second to fourth place were influenza B virus, parainfluenza 1, 2, 3 virus, and respiratory syncytial virus, with the positive percentage of 7.1%, 4.8%, and 3.3%, respectively. C. pneumoniae was found in 4 (0.33%) children.
Kumar et al. (2016) have examined 100 children, aged from 2 months to 12 years, hospitalized for pneumonia. The specimens consisted of nasopharyngeal aspirates for the analysis of C. pneumoniae by a PCR method and serum for the detection of IgM and IgG anti-C. pneumoniae by an ELISA test (Calbiotech Inc., CA). Serological evidence of C. pneumoniae infection was overall found in 12 (12.0%) children, with specific IgM antibodies in acute phase of the disease detected in 10 (83.3%) children, and specific IgG antibodies in both acute and paired sera in 2 children (16.7%). C. pneumoniae DNA was detected in 5 (5.0%) patients; 4 of which corresponded to the serology proven C. pneumoniae infection. Thus, nested PCR and ELISA detected C. pneumoniae altogether in 13 (13.0%) children.
The findings of the present study interlace well other previous studied above outlined pointing to considerable variations in the detection of type and in frequency of atypical bacteria causing CAP. We found a rather dismal, less than 25% detection rate of a pathogen in the children population consisting of 46 children with symptoms compliant with the possibility of CAP. In case of M. pneumoniae, nested PCR and serology tests were on par in terms of the ability to identify the pathogen. For C. pneumoniae, the diagnostic method used seemed of a greater meaning, as the immunofluorescent test identified the pathogen in twice as many cases as the serology tests. We failed to find any conclusive difference concerning the specimen used for the identification, be it pharyngeal swabs or blood serum. However, the interpretation of the findings was hampered by the paucity of the identified pathogenetic causes of infection.
There are considerable variations in the epidemiology of atypical pneumonia pathogens reported in the literature, which might be explicable by different age of populations studied and by geographical and season differences. There are also inconsistencies in the availability and type of microbiological investigations used, all of which may add to the possible underestimation of the burden of contagions inducing CAP (Padalko et al. 2013; Burillo and Bouza 2010).
In conclusion, although the understanding of the etiology of CAP has improved, the infection still remains a significant cause of morbidity and mortality. There is a need for better diagnostic tools that would enable a rapid identification of pathogens and for potential markers of bacterial resistance in order to use antimicrobials as expeditiously and judiciously as possible. Many patients with CAP do not have an etiologic pathogen promptly enough identified after admission to the hospital or indeed at all. The use of empirical antibiotics has removed the emphasis on the diagnostics of CAP, but this may be changing. The importance of rationalizing the antibiotic use and the public health implications for some of the atypical pathogens, e.g., the plausible role of C. penumoniae in sclerosis multiplex or coronary artery disease (Conklin et al. 2013; Cunha 2006), are drivers for the development and emergence of rapid diagnostic tests.
References
Arnold FW, Summersgill JT, Lajoie AS, Peyrani P, Marrie TJ, Rossi P, Blasi F, Fernandez P, File TM Jr, Rello J, Menendez R, Marzoratti L, Luna CM, Ramirez JA, Community-Acquired Pneumonia Organization (CAPO) Investigators (2007) A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med 175(10):1086–1093
Basarab M, Macrae MB, Curtis CM (2014) Atypical pneumonia. Curr Opin Pulm Med 20(3):247–251
Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, GH MC Jr, Moore MR, St Peter SD, Stockwell JA, Swanson JT Pediatric Infectious Diseases Society and the Infectious Diseases Society of America (2011) The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 53(7):e25–e76
Burillo A, Bouza E (2010) Chlamydophila pneumoniae. Infect Dis Clin N Am 24(1):61–71
Chen Z, Ji W, Wang Y, Yan Y, Zhu H, Shao X, Xu J (2013) Epidemiology and associations with climatic conditions of Mycoplasma pneumoniae and Chlamydophila pneumoniae infections among Chinese children hospitalized with acute respiratory infections. Ital J Pediatr 39:34
Chen K, Jia R, Li L, Yang C, Shi Y (2015) The aetiology of community associated pneumonia in children in Nanjing, China and aetiological patterns associated with age and season. BMC Public Health 15:113
Cillóniz C, Torres A, Niederman M, van der Eerden M, Chalmers J, Welte T, Blasi F (2016) Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med 42(9):1374–1386
Conklin L, Adjemian J, Loo J, Mandal S, Davis C, Parks S, Parsons T, McDonough B, Partida J, Thurman K, Diaz MH, Benitez A, Pondo T, Whitney CG, Winchell JM, Kendig N, Van Beneden C (2013) Investigation of a Chlamydia pneumoniae outbreak in a federal correctional facility in Texas. Clin Infect Dis 57(5):639–647
Cunha BA (2006) The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect 12(Suppl 3):12–24
Gaydos CA (2013) What is the role of newer molecular tests in the management of CAP? Infect Dis Clin N Am 27(1):49–69
Herrera M, Aguilar YA, Rueda ZV, Muskus C, Vélez LA (2016) Comparison of serological methods with PCR-based methods for the diagnosis of community-acquired pneumonia caused by atypical bacteria. J Negat Results Biomed 15:3
Huong Ple T, Hien PT, Lan NT, Binh TQ, Tuan DM, Anh DD (2014) First report on prevalence and risk factors of severe atypical pneumonia in Vietnamese children aged 1-15 years. BMC Public Health 14:1304
Kishimoto T, Ando S, Numazaki K, Ouchi K, Yamazaki T, Nakahama C (2009) Assay of Chlamydia pneumoniae-specific IgM antibodies by ELISA method-reduction of non-specific reaction and resetting of serological criteria by measuring IgM antibodies. Jpn J Infect Dis 62(4):260–264
Kumar S, Hammerschlag MR (2007) Acute respiratory infection due to Chlamydia pneumoniae: current status of diagnostic methods. Clin Infect Dis 44(4):568–576
Kumar S, Saigal SR, Sethi GR, Kumar S (2016) Application of serology and nested polymerase chain reaction for identifying Chlamydophila pneumoniae in community-acquired lower respiratory tract infections in children. Indian J Pathol Microbiol 59(4):499–503
Li X, Atkinson TP, Hagood J, Makris C, Duffy LB, Waites KB (2009) Emerging macrolide resistance in Mycoplasma pneumoniae in children: detection and characterization of resistant isolates. Pediatr Infect Dis J 28(8):693–696
Mandell LA (2015) Community-acquired pneumonia: an overview. Postgrad Med 127(6):607–615
Miyashita N, Akaike H, Teranishi H, Kawai Y, Ouchi K, Kato T, Hayashi T, Okimoto N, Atypical Pathogen Study Group (2013) Chlamydophila pneumoniae serology: cross-reaction with Mycoplasma pneumoniae infection. J Infect Chemother 19(2):256–260
Narita M (2010) Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother 16(3):162–169
Padalko E, Boel A, Lagrou K, Reynders M, China B, Vernelen K (2013) Expert Committee on Infectious Serology. Low yield by molecular detection of Chlamydophila pneumoniae in respiratory samples in Belgium questioning its etiological role in respiratory tract infections. Acta Clin Belg 68(3):166–168
Principi N, Esposito S (2011) Management of severe community-acquired pneumonia of children in developing and developed countries. Thorax 66(9):815–822
Principi N, Esposito S (2013) Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. J Antimicrob Chemother 68(3):506–511
She RC, Thurber A, Hymas WC, Stevenson J, Langer J, Litwin CM, Petti CA (2010) Limited utility of culture for Mycoplasma pneumoniae and Chlamydophila pneumoniae for diagnosis of respiratory tract infections. J Clin Microbiol 48(9):3380–3382
Song Q, Xu BP, Shen KL (2015) Effects of bacterial and viral co-infections of Mycoplasma pneumoniae pneumonia in children: analysis report from Beijing children’s hospital between 2010 and 2014. Int J Clin Exp Med 8(9):15666–15674
Spuesens EB, Fraaij PL, Visser EG, Hoogenboezem T, Hop WC, van Adrichem LN, Weber F, Moll HA, Broekman B, Berger MY, van Rijsoort-Vos T, van Belkum A, Schutten M, Pas SD, Osterhaus AD, Hartwig NG, Vink C, van Rossum AM (2013) Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med 10(5):e1001444
Villegas E, Sorlózano A, Gutiérrez J (2010) Serological diagnosis of Chlamydia pneumoniae infection: limitations and perspectives. J Med Microbiol 59.(Pt 11:1267–1274
Zhou W, Lin F, Teng L, Li H, Hou J, Tong R, Zheng C, Lou Y, Tan W (2013) Prevalence of herpes and respiratory viruses in induced sputum among hospitalized children with non-typical bacterial community-acquired pneumonia. PLoS One 8(11):e79477
Acknowledgments
Supported by grant E090.16.065 from the Medical University in Wroclaw, Poland.
Conflicts of Interest
The authors declare no conflicts of interest in relations to this article.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocols were accepted by the Ethics Committee of the Medical University of Wroclaw, Poland.
Consent
Written informed consent was obtained from all individual participants and/or their legal guardians for participation in the study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jama-Kmiecik, A., Frej-Mądrzak, M., Sarowska, J., Teryks-Wołyniec, D., Skiba, A., Choroszy-Król, I. (2019). Atypical and Typical Bacteria in Children with Community Acquired Pneumonia. In: Pokorski, M. (eds) Advances in Pulmonary Medicine: Research and Innovations. Advances in Experimental Medicine and Biology(), vol 1160. Springer, Cham. https://doi.org/10.1007/5584_2019_377
Download citation
DOI: https://doi.org/10.1007/5584_2019_377
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21098-4
Online ISBN: 978-3-030-21099-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)