Abstract
There is an interest in assessing changes in nasal NO (nNO) levels as an effect marker of upper airways. In this study, we examined methodologic influences on short and long term repeatability of nNO levels assessed by a portable electrochemical analyzer. Nine atopic and eighteen healthy subjects were exposed for 4 h to ethyl acrylate concentration of 0.05 ppm (sham) and mean concentrations of 5 ppm (either constant 5 ppm or variable 0 to 10 ppm). Sampling of nNO was performed by using passive aspiration during both breath-holding (634 ppb) or calm tidal breathing (364 ppb, p < 0.0001). The intra-session (between-session) repeatability in terms of coefficient of variation was 16.4% (18.5%) using the tidal-breathing and 8.6% (13.0%) using the breath-holding method, respectively. Atopic subjects demonstrated a significant increase in nNO (breath-holding mean 16%, tidal-breathing mean 32%) after applying a constant ethyl acrylate concentration (5 ppm). Our findings suggest that the less elaborate tidal-breathing method might be sufficient to detect significant changes at a group level. Given a lower coefficient of variation of breath-holding we assume there is an advantage of that approach at an individual level. Further research is needed to validate the usefulness of nNO in the evaluation of irritative, non-allergic responses.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Atopic subjects
- Breath holding
- Chemosensory challenge
- Ethyl acrylate
- Inflammation
- Methodological approach
- Nasal nitric oxide
- Tidal breathing
- Upper airway
1 Introduction
Sensations of odor and upper airway irritation are cited health effect in indoor air and occupational environments and have gained relevance for the setting of exposure limits (Brüning et al. 2014). Besides symptom complains, response to a chemosensory irritant can also be evaluated by examining local signs of nasal mucosal irritation (Arts et al. 2006). Concerning ethyl acrylate, a trigeminal intranasal perceptions and signs of nose irritation could be provoked by challenge with a concentration of 5 ppm (Hoffmeyer et al. 2016). Objective measures of upper airway irritation include rhinomanometry or acoustic rhinometry to measure functional changes such as irritation-induced congestion, measuring the volume of mucus secretion or the presence of inflammatory mediators in nasal secretion (Raulf et al. 2017; Dalton 2003). The evaluation of mediators in different matrices is a promising way to get information on inflammation at target level in a noninvasive manner (Quirce et al. 2010).
Concerning the lower airways, NO in exhaled breath (FeNO) is a validated noninvasive marker of inflammation, especially when associated with the activation of eosinophils (Dweik et al. 2011). NO is also produced in the nasal epithelium or can be a result of diffusion from communicating cavities, i.e., paranasal sinuses (Maniscalco et al. 2016). There is a continuous and high basal activity of NO synthase resulting in a 100-fold higher nasal NO (nNO) compared to NO produced in the lower respiratory tract (Lundberg et al. 1995). A variation in inducible NO synthase expressed under basal conditions in the human nasal mucosa has been reported (Furukawa et al. 1996). Anatomical means, such as sinus ostiae diameter and volume, may influence the NO output (Palm et al. 2000).
Metabolic or dietary factors, ambient NO concentration, and human biorhythm have been all suggested to be responsible for the variability of nNO level (Gehring et al. 2009). A diurnal variation in nNO with low levels in the morning, a plateau during the day, and decreasing levels in the evening has been reported (Dressel et al. 2008; Palm et al. 2000). NO in the upper respiratory tract associates with the patient’s condition. It often increases in allergic rhinitis and decreases after treatment with nasal glucocorticoids (Kharitonov et al. 1997). A dramatic decrease in nNO has been shown in primary ciliary dyskinesia (Wodehouse et al. 2003). So far, with the exception of ciliary disorders, detection of nNO is not a routine diagnostic method (Antosova et al. 2017).
Nevertheless, there is an interest in assessing changes in nNO as an effect marker for inflammatory processes in the upper airways either in short term after a specific challenge or in longitudinal studies. Low variability and good repeatability in these settings are critical in distinguishing changes due to biological responses from methodological variations. Several issues related to the measurement and reproducibility of nNO have been identified and methodological recommendations have been developed (Horváth et al. 2017). Besides a setup for active single-breath nasal exhalation (FnNO) similar to orally exhaled FeNO (Palm et al. 2000), consensus recommendations have suggested methods of passive aspiration via one nostril (ATS/ERS 2005). Using nasal aspiration, the highest nNO values have been found in the methods with an elevated velum as during breath-holding or while exhaling against a resistance (de Winter-de Groot and van der Ent 2009). A recent suggestion is that calm breathing through the mouth while avoiding any kind of breathing through the nose creates two distinct, independent compartments (oropharynx and nasopharynx) that are not connected to each other (Gelardi et al. 2016).
In this study, we examined methodologic influences on short and long term repeatability of nNO levels assessed by a portable electrochemical analyzer. NO sampling was performed by using passive aspiration during breath-holding and calm breathing. The evaluations were done within the frame of an ethyl acrylate challenge study reported recently (Hoffmeyer et al. 2017). Therefore, the atopic status of the subjects and effects of different challenge patterns on nNO changes were also considered.
2 Methods
The study was approved by a local Ethics Committee of the Ruhr University Bochum and was performed in accordance with the Declaration of Helsinki. All study participants gave written informed consent and received financial compensation for their participation.
2.1 Subjects
Twenty-seven healthy subjects were recruited for the study after a medical history was taken. All subjects were healthy never-smokers or had terminated smoking at least half a year before the study and were not taking any medications or nasal decongestants (Table 1). A positive atopic status was assumed in case of specific IgE concentrations to common inhalant allergens ≥0.35 kU/L (sx1 Phadiatop; ThermoFisher Phadia AB; Uppsala, Sweden). No one had an upper or lower airway respiratory disease or infection within 6 weeks prior to the study. No signs of mucosal inflammation or anatomical changes were noted on rhinoscopy. Subjects were informed not to perform any strenuous physical activity within 60 min before the nNO measurements. All asymptomatic subjects serologically identified as being atopic were tested outside their potential allergic risk seasons revealed by prick testing.
2.2 Nasal NO Measurements
The measurements were performed using the nasal application of the hand-held NIOX MINO® system (Circassia, Bad Homburg, Germany) under supervision of the same assistant in accordance with published recommendations (ATS/ERS 2005). NIOX MINO® employs an electrochemical sensor with a measurement range of 5–1700 ppb. Short term repeatability at a flow rate of 5 ml/s was shown to be good with a coefficient of variation of 10% (Marthin and Nielsen 2013).
The subjects were studied in a convenient seated position. The nasal olive application was inserted into one nostril and hold in place by the participant while the contralateral nostril was left open. The aspiration was done at a flow rate of 5 ml/s and automatically stopped after 45 s. Two different aspiration techniques were applied for the measurement. Firstly, subjects performed oral calm breathing during the meausrement aspiration, while avoiding any kind of breathing through the nose. No exhalation resistance was used. Secondly, the measurement was done again during breath-holding while the mouth was closed.
Measurements were done in the frame of a challenge study previously published (Hoffmeyer et al. 2017). Briefly, nNO was measured before (pre) and after (post) challenge with a mean ethyl acrylate concentration of 5 ppm applied in a constant and variable wave-form pattern. The study was randomized and sham-controlled (0.05 ppm ethyl acrylate).
2.3 Statistical Analysis
Coefficient of variation was used to describe the repeatability. It was estimated as a ratio between the within standard deviation (SD) and the mean of individual measurements. The Bland-Altman analysis was performed for comparison of the two methodological approaches and different time points. The effect of nNO was calculated as the percent change after exposure compared to start of exposure [(post-pre)/pre*100%] and reported as ΔnNO. Differences after exposure to 5 ppm were compared with those after sham exposure using a paired t-test or Wilcoxon matched-pairs signed-rank test as appropriate, with a significance level of α = 0.05. Data were expressed as means ±SD or medians with interquartile range (IQR, 25th; 75th percentile). Data were analyzed and visualized by GraphPad Prism v7.03 for Windows (San Diego, CA).
3 Results
3.1 Comparison of nNO Measurements
In the 27 subjects studied, approved nNO measurements were obtained at all occasions with both methodological approaches. At every session (sham, constant, and variable ethyl acrylate concentration) pre- and post-challenge measurements were performed. Therefore, 162 pairs of values could be used for the methodologic comparison. The assessment of between-session repeatability for each method could be based on 81 (27 triplets) respective pre-challenge results.
Referring to all 162 measurement pairs, the nNO level for tidal-breathing [364 (IQR 259; 497) ppb] was significantly lower than that for the breath-holding (reference) [634 (IQR 499; 771) ppb] (p < 0.0001). The Bland-Altman analysis of distance for the two methods revealed a mean nNO difference of 243 ppb (50%). The SD of the difference was 191 ppb (51%) yielding the limits of agreement of −131 ppb (−18%) and 616 ppb (121%). The Bland-Altman plot revealing two subgroups is shown in Fig. 1. One subgroup of subjects demonstrated a small difference between nNO results for the tidal-breathing and breath-holding methods. As shown in Fig. 1, the difference was lower than 15% in 40 comparisons and the methods could be considered equally. Thirty-seven of these equal results for the tidal-breathing and breath-holding methods could be attributed to seven out of the 27 subjects.
Bland-Altman plot of agreement between nasal NO (nNO) measured during breath-holding (BH) and tidal-breathing (TB) for all time points (n = 162). The continuous line represents the mean difference and the dashed lines represent the ±2SD for the differences. The shaded area indicates the values that could be considered equalin both methods of measurement
3.2 Repeatability of nNO Due to Method Applied
3.2.1 Intra-session Repeatability
The condition with sham exposure was used to examine the intra-session repeatability of nNO, as individual variation (n = 27) between baseline and post-challenge measurement. The coefficient of variation was 16.4% (95%CI 11.7–21.1) using the tidal-breathing and 8.6% (95%CI 6.0–11.2) using the breath-holding method (Table 2). In addition, the Bland-Altman analysis revealed a bias of 3.9% and 0.6% between first and second measurement for the tidal-breathing and the breath-holding method, respectively (data not shown). Overall, nNO levels were not different at baseline (9 am) and post-challenge (1 pm).
3.2.2 Between-Session Repeatability
At all three sessions, significantly lower nNO levels for the tidal-breathing compared to the breath- holding method were observed at baseline (for each session p < 0.0001). Referring to one particular method, no significant difference in the baseline nNO level could be determined between the three sessions (tidal-breathing method; p = 0.249, breath-holding method; p = 0.452). The individual between-session repeatability in terms of the coefficient of variation was 13.0% (95%CI 9.4–16.7) for the breath-holding and 18.5% (95%CI 12.4–24.5) for the tidal-breathing method (Table 2).
3.2.3 nNO Levels After Ethyl Acrylate Challenge
The two challenge conditions (constant, variable) resulted in an equivalent total amount of ethyl acrylate delivered during 4 h. The effects were also assessed following the sham exposure. Table 3 summarizes the results for these three exposure conditions and the two measurement modes. No changes in nNO could be observed either after sham or variable ethyl acrylate exposure regardless of the measurement technique. In contrast, nNO increased after applying a constant pattern. Despite differences in the baseline levels, the increase was significant for both measurement modes (breathhold; p = 0.035, tidal breathing; p = 0.030).
Next, results were stratified by the atopic status using sx1 ≥ 0.35 kU/L as a cut-off value. Accordingly, nine subjects were classified as atopic. Baseline, pre-exposure nNO did not differ between atopic and non-atopic subjects before sham, variable, or constant ethyl acrylate challenge, regardless of the measurement technique used (data not shown).
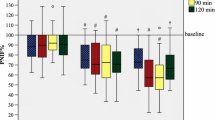
Changes in nNO adjusted for the sham condition (net-response) for both methodological approaches are illustrated in Fig. 2. In non-atopic subjects, no significant net-effect on nNO could be observed after either constant or variable ethyl acrylate exposure, regardless of the measurement technique. Atopic subjects demonstrated a significant increase in nNO (breath-holding mean 16%, tidal-breathing method mean 32%) after applying a constant ethyl acrylate concentration (5 ppm). Differences in net-ΔnNO in atopics compared to non-atopics were significant using breath-holding (p = 0.043). In case of the tidal-breathing technique, differences due to the atopic status could also be suggested only after applying a constant ethyl acrylate exposure pattern (p = 0.067).
Net responses of nNO after ethyl acrylate challenge (mean 5 ppm) using the breath-holding (a) and tidal-breathing method (b). Ethyl acrylate was applied either in a constant (5 ppm, open symbols) or variable pattern (0–10 ppm, solid symbols). Results are adjusted for sham exposure and stratified according to non-atopics (n = 9, circle) and atopics (n = 18, rhombus)
4 Discussion
Reproducibility of clinical tests is an important consideration in research protocols. It is to stress that variability is the result of different influences including analytical impact, technical factors of collection, and intra-subject variability (Antosova et al. 2017). In this study we could demonstrate a better short- and long-term repeatability using the breath-holding than the tidal-breathing technique for nNO measurement. In a challenge setting and referring to comparisons based on groups, the tidal-breathing technique demonstrated sufficient performance in detecting nNO changes.
The actual concentration of NO could be assessed either by chemiluminescence or by electrochemical means. The hand-held electrochemical analyzer NIOX MINO used in this study is a reliable, simple to handle analytic tool that demonstrates the NO values in good agreement with those obtained by stationary chemiluminescence systems (Montella et al. 2011; Maniscalco et al. 2008). Nasal application of the analyzer employs an aspiration time of 45 s, a length shown to be sufficient to obtain measurements at breath-hold within a steady NO plateau (Kharitonov et al. 2005). However, even short interruptions during sampling result in measurement error and require repeated attempts.
Reservoir and continuous techniques for nNO measurement can be distinguished. The current recommended method is the aspiration at a constant flow rate from one naris with gas entrained via the other naris or orally (ATS/ERS 2005). An overview of reported normal nNO levels suggests an inverse flow-dependence with higher NO levels being related to lower sampling airflow rates (Antosova et al. 2017; Bartley et al. 1999). In the present study, air was continuously sampled through an olive application tightly inserted inside one nostril at a flow rate of 5 ml/s (i.e., 300 ml/min) as recommended (ATS/ERS 2005). At a flow-rate of 250 ml/min, mean nNO levels between 651 and 1,197 ppb were reported (Ferguson and Eccles 1997) which could be confirmed by our results.
The particular sampling mode is another major influential matter. Nasal aspiration can be performed during breath-holding, tidal-breathing, or oral exhalation against a resistance. Lowest values are reported during quiet exhalation and methods in which there is a turbulence of nasal flow as in tidal-breathing. Highest values are reported in the methods with an elevated velum as during breath-holding or while exhaling against a resistance (de Winter-de Groot and van der Ent 2009). Recently, it has been suggested that calm breathing through the mouth while avoiding any kind of breathing through the nose creates two distinct, independent compartments (oropharynx and nasopharynx) that are not connected to each other. Applying this technique, our results are similar to a mean nNO level of 427 ppb reported by Gelardi et al. (2016) in 32 healthy nonsmoking subjects of the mean age of 30 years. Antosova et al. (2017), using the same approach, have also reported an average nNO value of 379.6 ± 170.4 ppb in the right and 401.6 ± 207.8 in the left nostril (n = 141, mean age 25 years).
Overall, in this study we demonstrate a lower mean nNO level using the technique of tidal-breathing compared to breath-holding. An explanation might be the air with a low NO concentration from the lower respiratory tract diluting the higher concentration in the nasal area. However, during breath-holding the velum keeps elevated, which closes off the nasal passages as demonstrated by a simultaneously assessment of CO2 (Bartley et al. 1999). In some subjects similarly high nNO levels are detected using both techniques. This finding suggests that the velum may also remain elevated while breathing calmly through the mouth.
Breath-holding improves the reproducibility of nNO measurement. At a flow rate of 500 ml/min and aspiration during breath-holding, the reproducibility of 6.6% at a single point in time, in terms of coefficient of variation, has been demonstrated (Bartley et al. 1999). For three consecutive measurements during breath-holding at a flow rate of 300 ml/min a lower mean intra-subject coefficients of variation of 3.8% has been reported for breath-holding compared to 9.9% using tidal-breathing (de Winter-de Groot and van der Ent 2009). Kharitonov et al. (2005) have demonstrated by applying the breath-holding technique a repeatability of two and three consecutive measurements of 6.2% and 7.7%, respectively, in healthy adults with nNO mean values of about 870 ppb.
Further, we demonstrate that the short- and long-term repeatability are influenced by the method used for nNO measurements. We used the Bland and Altman analysis and evaluated repeatability in terms of coefficient of variation. The variance of the measurements repeated within 4 weeks remained stable indicating the absence of external factors influencing it. Our findings are consistent with the results reported previously. Within-day repeatability of 13.4% and between-day repeatability (1 week later) of 11.8% have been demonstrated at a flow rate of 500 ml/min and aspiration during breath-holding (Bartley et al. 1999). A higher within-day than between-day variability might indicate a systematic diurnal variation. The Bland and Altman analysis also reveals good short- (t = 0–1 h) and long-term (t = 0–24 h) reproducibility for breath-holding at a flow rate of 300 ml/min and is recommended for standardized measurements of nasal NO (de Winter-de Groot and van der Ent 2009). Boot et al. (2007) have reported the reproducibility of nNO levels for 1 day (coefficient of variation 16.5%), up to 7 days (coefficient of variation 21.5%), and 21 days (38.3%) in patients with allergic rhinitis. In that study, nNO was aspirated during exhalation against a resistance at a flow rate of 300 ml/min. The authors suggest that a decreasing reproducibility over time might be due to subclinical seasonal influences. Repeatability data for day-to-day (coefficient of variation 8.1% morning, 17.5% afternoon), week-to-week (coefficient of variation 12.3%), and seasonal comparisons (21.0%) have been revealed when measuring nNO by application of the flow exhalation technique at a flow rate of 100 ml/min, using a chemiluminescence analyzer (Stark et al. 2007).
So far, there are few data on nNO as an effect marker in follow-up, intervention, or challenge trials. With respect to a possible underlying biorhythm of nNO levels, it is important to consider the time of measurement for repeatable measurements during challenge or longitudinal studies (Dressel et al. 2008; Palm et al. 2000). In order to avoid misinterpretation of time dependent variations, our measurements during the sessions were performed at the same hours. Moreover, control sham conditions were regularly included in our challenge studies. Ambient nNO levels were controlled during exposure with ethyl acrylate. Therefore, any impact of ambient NO on nNO levels as by other authors (Gehring et al. 2009) could be excluded in our study. Overall, the present findings suggest no underlying biorhythm during a 4-h-long exposure.
We observed similar baseline nNO levels in atopic and non-atopic subjects. In the subjects suffering from allergic rhinitis, nNO in the off-allergen-season has been shown not to be significantly different from controls (Henriksen et al. 1999). An association between the nNO level and the presence and type of sensitization is described in another study in which nNO increased with the number of perennial allergens to which subjects were IgE-sensitized (Krantz et al. 2014).
Concerning the usefulness of nNO in terms of a biomarker of inflammation, lower nNO levels are seen when patients with allergic rhinitis were treated with topic steroids compared to non-treated ones (Kharitonov et al. 1997). Also, increasing nNO and fractional nasal (FnNO) are reported under the influence of a specific allergen challenge in subjects with allergic rhinitis and mold problems, respectively (Boot et al. 2007). However, after Aspergillus fumigatus and placebo inhalation, FnNO profiles are almost identical (Stark et al. 2005). Taking the effects of a sham challenge into account, we found in the present study a net increase in nNO after constant exposure to 5 ppm ethyl acrylate in atopic subjects. Nasal NO levels have also been shown to fall after a specific challenge (Serrano et al. 2012). A decline in nNO may be caused by acute inflammation-induced nasal congestion, edema, and secretions, leading to a reduction in NO-rich air passage through the paranasal ostia. Recently, we have found that after a constant challenge with 5 ppm of ethyl acrylate, complaints and signs of nose irritation were in a range of weak-to-moderate (Hoffmeyer et al. 2016). Thus, we assume that no severe nasal congestion affecting nNO levels should be considered in this study.
5 Conclusions
Significant increases in nNO could be demonstrated in response to ethyl acrylate challenge with both passive aspiration during breath-holding or calm tidal breathing. The increases were consistent in that nearly all subjects demonstrated a positive net-change and the mean change exceeded the respective methodological coefficients of variation. The findings, restricted to atopic subjects, are in line with our recent study concerning changes in fractional exhaled NO (FeNO) after a constant ethyl acrylate challenge (Hoffmeyer et al. 2017). Our findings suggest that nNO might be a useful effect marker and that the less elaborate tidal-breathing method is sufficient to detect significant changes at a group level. Further research is needed to validate the usefulness of nNO in the evaluation of irritative, non-allergic responses. Given a lower coefficient of variation of breath-holding we assume the advantage of that approach at an individual level.
References
Antosova M, Mokra D, Tonhajzerova I, Mikolka P, Kosutova P, Mestanik M, Pepucha L, Plevkova J, Buday T, Calkovsky V, Bencova A (2017) Nasal nitric oxide in healthy adults - reference values and affecting factors. Physiol Res 66(Suppl 2):247–255
Arts JH, de Heer C, Woutersen RA (2006) Local effects in the respiratory tract: relevance of subjectively measured irritation for setting occupational exposure limits. Int Arch Occup Environ Health 79:283–298
ATS/ERS (2005) American Thoracic Society; European Respiratory Society recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med 71:912–930
Bartley J, Fergusson W, Moody A, Wells AU, Kolbe J (1999) Normal adult values, diurnal variation, and repeatability of nasal nitric oxide measurement. Am J Rhinol 13:401–405
Boot JD, de Kam ML, Mascelli MA, Miller B, van Wijk RG, de Groot H, Cohen AF, Diamant Z (2007) Nasal nitric oxide: longitudinal reproducibility and the effects of a nasal allergen challenge in patients with allergic rhinitis. Allergy 62:378–384
Brüning T, Bartsch R, Bolt HM, Desel H, Drexler H, Gundert-Remy U, Hartwig A, Jäckh R, Leibold E, Pallapies D, Rettenmeier AW, Schlüter G, Stropp G, Sucker K, Triebig G, Westphal G, van Thriel C (2014) Sensory irritation as a basis for setting occupational exposure limits. Arch Toxicol 88:1855–1879
Colantonio D, Brouillette L, Parikh A, Scadding GK (2002) Paradoxical low nasal nitric oxide in nasal polyposis. Clin Exp Allergy 32:698–701
Dalton P (2003) Upper airway irritation, odor perception and health risk due to airborne chemicals. Toxicol Lett 140-141:239–248
De Winter-de Groot KM, van der Ent CK (2009) Measurement of nasal nitric oxide: evaluation of six different sampling methods. Eur J Clin Investig 39:72–77
Dressel H, Bihler A, Jund F, de la Motte D, Nowak D, Jörres RA, Kramer MF (2008) Diurnal variation of nasal nitric oxide levels in healthy subjects. J Investig Allergol Clin Immunol 18:316–317
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR, American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications (2011) An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 184:602–615
Ferguson EA, Eccles R (1997) Changes in nasal nitric oxide concentration associated with symptoms of common cold and treatment with a topical nasal decongestant. Acta Otolaryngol 117:614–617
Furukawa K, Harrison DG, Saleh D, Shennib H, Chagnon FP, Giaid A (1996) Expression of nitric oxide synthase in the human nasal mucosa. Am J Respir Crit Care Med 153:847–850
Gehring U, Oldenwening M, Brunekreef B, Wieringa MH, Kerkhof M, Smit HA, van der Ent CK, De Jongste JC (2009) The impact of ambient NO on online measurements of exhaled and nasal NO: the PIAMA study. Pediatr Allergy Immunol 20:665–672
Gelardi M, Abbattista G, Quaranta VN, Quaranta N, Seccia V, Buttafava S, Frati F, Ciprandi G (2016) Standardization procedure for the nasal nitric oxide measurement method using Niox MINO® and the tidal-breathing technique with velum-closure. J Biol Regul Homeost Agents 30:853–858
Henriksen AH, Sue-Chu M, Holmen TL, Langhammer A, Bjermer L (1999) Exhaled and nasal NO levels in allergic rhinitis: relation to sensitization, pollen season and bronchial hyperresponsiveness. Eur Respir J 13:301–306
Hoffmeyer F, Bünger J, Monsé C, Berresheim H, Jettkant B, Beine A, Brüning T, Sucker K (2016) Clinical effects, exhaled breath condensate pH and exhaled nitric oxide in humans after ethyl acrylate exposure. Adv Exp Med Biol 921:11–20
Hoffmeyer F, Sucker K, Berresheim H, Monsé C, Jettkant B, Beine A, Raulf M, Bünger J, Brüning T (2017) Impact of internal and external factors on EBC-pH and FeNO changes in humans following challenge with ethyl acrylate. Adv Exp Med Biol 1020:7–16
Horváth I, Barnes PJ, Loukides S, Sterk PJ, Högman M, Olin AC, Amann A, Antus B, Baraldi E, Bikov A, Boots AW, Bos LD, Brinkman P, Bucca C, Carpagnano GE, Corradi M, Cristescu S, de Jongste JC, Dinh-Xuan AT, Dompeling E, Fens N, Fowler S, Hohlfeld JM, Holz O, Jöbsis Q, Van De Kant K, Knobel HH, Kostikas K, Lehtimäki L, Lundberg J, Montuschi P, Van Muylem A, Pennazza G, Reinhold P, Ricciardolo FLM, Rosias P, Santonico M, van der Schee MP, van Schooten FJ, Spanevello A, Tonia T, Vink TJ (2017) A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J 49:1600965
Kharitonov SA, Rajakulasingam K, O’Connor B, Durham SR, Barnes PJ (1997) Nasal nitric oxide is increased in patients with asthma and allergic rhinitis and may be modulated by nasal glucocorticoids. J Allergy Clin Immunol 99:58–64
Kharitonov SA, Walker L, Barnes PJ (2005) Repeatability of standardised nasal nitric oxide measurements in healthy and asthmatic adults and children. Respir Med 99:1105–1114
Krantz C, Janson C, Borres MP, Nordvall L, Alving K, Malinovschi A (2014) Nasal nitric oxide is associated with exhaled NO, bronchial responsiveness and poor asthma control. J Breath Res 8(2):026002
Lundberg JO, Farkas-Szallasi T, Weitzberg E, Rinder J, Lidholm J, Anggåard A, Hökfelt T, Lundberg JM, Alving K (1995) High nitric oxide production in human paranasal sinuses. Nat Med 1:370–373
Maniscalco M, de Laurentiis G, Weitzberg E, Lundberg JO, Sofia M (2008) Validation study of nasal nitric oxide measurements using a hand-held electrochemical analyser. Eur J Clin Investig 38:197–200
Maniscalco M, Bianco A, Mazzarella G, Motta A (2016) Recent advances on nitric oxide in the upper airways. Curr Med Chem 23:2736–2745
Marthin JK, Nielsen KG (2013) Hand-held tidal breathing nasal nitric oxide measurement--a promising targeted case-finding tool for the diagnosis of primary ciliary dyskinesia. PLoS One 8(2):e57262
Montella S, Alving K, Maniscalco M, Sofia M, De Stefano S, Raia V, Santamaria F (2011) Measurement of nasal nitric oxide by hand-held and stationary devices. Eur J Clin Investig 41:1063–1070
Palm JP, Graf P, Lundberg JO, Alving K (2000) Characterization of exhaled nitric oxide: introducing a new reproducible method for nasal nitric oxide measurements. Eur Respir J 16:236–241
Quirce S, Lemière C, de Blay F, del Pozo V, Gerth Van Wijk R, Maestrelli P, Pauli G, Pignatti P, Raulf-Heimsoth M, Sastre J, Storaas T, Moscato G (2010) Noninvasive methods for assessment of airway inflammation in occupational settings. Allergy 65:445–458
Raulf M, van Kampen V, Neumann HD, Liebers V, Deckert A, Brüning T, Bünger J, Hoffmeyer F (2017) Airway and blood inflammatory markers in waste collectors. Adv Exp Med Biol 1021:1–14
Serrano CD, Valero A, Bartra J, Roca-Ferrer J, Muñoz-Cano R, Sánchez-López J, Mullol J, Picado C (2012) Nasal and bronchial inflammation after nasal allergen challenge: assessment using noninvasive methods. J Investig Allergol Clin Immunol 22:351–356
Stark HJ, Randell JT, Hirvonen MR, Purokivi MK, Roponen MH, Tukiainen HO (2005) The effects of Aspergillus fumigatus challenge on exhaled and nasal NO levels. Eur Respir J 26:887–893
Stark H, Purokivi M, Kiviranta J, Randell J, Tukiainen H (2007) Short-term and seasonal variations of exhaled and nasal NO in healthy subjects. Respir Med 101:265–271
Wodehouse T, Kharitonov SA, Mackay IS, Barnes PJ, Wilson R, Cole PJ (2003) Nasal nitric oxide measurements for the screening of primary ciliary dyskinesia. Eur Respir J 21:43–47
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Hoffmeyer, F. et al. (2018). Methodological Implications and Repeatability of Nasal Nitric Oxide: Relevance for Challenge Studies. In: Pokorski, M. (eds) Respiratory Ailments in Context. Advances in Experimental Medicine and Biology(), vol 1113. Springer, Cham. https://doi.org/10.1007/5584_2018_166
Download citation
DOI: https://doi.org/10.1007/5584_2018_166
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04024-6
Online ISBN: 978-3-030-04025-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)