Abstract
The occurrence of a second lung tumor after surgical removal of lung cancer usually indicates a lung cancer metastasis, but sometimes a new lesion proves to be a new primary lung cancer, i.e., metachronous lung cancer. The goal of the present study was to conduct a clinical evaluation of patients with metachronous lung cancer and lung cancer metastasis, and to compare the early and distant outcomes of surgical treatment in both cancer types. There were 26 age-matched patients with lung cancer metastases and 23 patients with metachronous lung cancers, who underwent a second lung cancer resection. We evaluated the histological type of a resected cancer, the extent of thoracosurgery, the frequency of early postoperative complications, and the probability of 5-year survival after the second operation. The findings were that metachronous lung cancer was adenocarcinoma in 52% of patients, with a different histopathological pattern from that of the primary lung cancer in 74% of patients. In both cancer groups, mechanical resections were the most common surgery type (76% of all cases), with anatomical resections such as segmentectomy, lobectomy, or pneumectomy being much rarer conducted. The incidence of early postoperative complications in metachronous lung cancer and lung cancer metastasis (30% vs. 31%, respectively) and the probability of 5-year survival after resection of either cancer tumor (60.7% vs. 50.9%, respectively) were comparable. In conclusion, patients undergoing primary lung cancer surgery require a long-term follow-up due to the risk of metastatic or metachronous lung cancer. The likelihood of metachronous lung cancer and pulmonary lung cancer metastases, the incidence of postoperative complications, and the probability of 5-year survival after resection of metachronous lung cancer or lung cancer metastasis are similar.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Histopathology
- Lung cancer
- Metachronous cancer
- Metastasis
- Non-small cell lung cancer
- Survival
- Thoracic surgery
- Tumor
1 Introduction
The appearance of another neoplastic focus after resection of non-small cell lung cancer (NSCLC) is an important clinical problem and requires a differentiation between lung cancer metastases and a second primary lung cancer, i.e., metachronous lung cancer. The classic diagnostic criteria, based on clinical and histological data, enabling the distinguishing between the lung cancer metastasis and metachronous lung cancer have been established by Martini and Melamed (1975) and are so far used in practice. Metachronous lung cancer most often has a different histological structure than primary lung cancer or, in case of a similar structure, metachronous lung cancer diagnostic criteria include the occurrence at least 2 years following primary lung cancer, in situ development, localization in another lobe, lack of the same path of spreading, i.e., no tumor lesions in lymph nodes, and no other extrapulmonary metastases.
Surgical treatment differs in scope in lung cancer metastasis and metachronous lung cancer. In case of primary or metachronous lung cancer, anatomic resection with removal of three layers of lymph nodes of the pulmonary hilum and mediastinum is necessary (Gamliel 2016; Maniwa and Kodama 2016; Riquet et al. 2016; Hytych et al. 2013; Asamura et al. 1999; Riquet et al. 1994; Naruke et al. 1988). On the other hand, wedge resection is most often employed in case of lung cancer metastasis, followed only by sampling of potentially affected lymph nodes (Sihag and Muniappan 2016). In general, resection of a second pulmonary neoplasm, irrespective of its histopathological origin, presents an enhanced risk of postoperative complications and may worsen the long-term outcome.
The goal of the present study was to conduct a clinical evaluation of patients with metachronous lung cancer and with lung cancer metastasis, and to compare the early and distant outcomes of surgical treatment in both lung cancer entities.
2 Methods
The study was approved by the Ethics Committee of Wroclaw Medical University in Poland and it was conducted in accord with the principles of the Declaration of Helsinki for Human Research of the World Medical Association. The research material for the study consisted of 49 thoracosurgical patients, a random sample chosen from 6162 patients suffering from primary lung cancer metastasis or a second metachronous lung cancer, operated on in the years 2001–2015 in the Lower Silesian Center of Lung Diseases in the city of Wroclaw, Poland. There were 26 patients (53%) with lung cancer metastases and 23 patients (47%) with metachronous lung cancers. Gender and age of patients, and the stage of lung cancer were similar in both groups. Adenocarcinoma was the most common cancer type in both groups. A few more patients with lung cancer metastasis had more than one metastasis. The detailed figures are given in Table 1.
Diagnostic tests performed in all patients prior to surgery included the following: bronchofiberoscopy, chest X-ray, thoracic computed tomography, and abdominal ultrasonography. Since 2007, also positron emission tomography (PET) was performed. Mediastinoscopy was performed in case of the possible involvement of mediastinal lymph nodes, i.e., their enlargement of more than 10 mm or grouping into packages seen in the imaging scans. Since 2008, mediastinoscopy was replaced with the needle biopsy of mediastinal nodes under the endobronchial ultrasonography (EBUS) control. Surgical treatment was abandoned in case of mediastinal lymph node or remote metastases.
The surgical procedures employed are listed in Table 2. In both groups, mechanical wedge, tangent, and laser resections were the most common – in 76% of all cases. Anatomical resections consisting of segmentectomy or lobectomy were rarer – in 16% of all cases, and pneumectomy was conducted only in individual cases. The examples are illustrated in Figs. 1 and 2. The resection failed to be radical in two patients treated for lung cancer metastasis. Lymphadenectomy was performed with similar frequency in both groups of patients; in 43% cases in total (Table 2).
(a) Anatomical resection: postoperative loge with Satynski clamp (yellow star) closing the bronchus stump is seen in the left-hand part of the photograph. Anatomical resection usually involves removal of the lymph nodes of groups 11, 10, and the mediastinal nodes of group 7 (b) Resected lung lobe with cancer foci (green arrow); atelectatic neighboring lung parenchyma (yellow arrow)
Continuous data were presented as means ±SD or medians, as indicated, and discrete data as counts and percentages. The Mann-Whitney U was used to assess differences between the two independent groups of patients and the Chi-squared test to compare features between the groups such as histopathological changes, cancer stages, and surgical treatments. The Kaplan-Meier estimator was used to assess the probability of patient survival, and the difference between the two survival curves was assessed with the Mantel-Cox test. A Cox regression analysis also was performed to determine the difference in patient survival with respect to clinical and pathological data. A p-value < 0.05 defined the statistically significant differences. Commercial StatSoft v1.3 (Statsoft, Cracow, Poland) and GraphPad Prism v5.0 (La Jola, CA) statistical packages were used for all data analysis.
3 Results
Among 49 patients who underwent the second resection of a lung cancer, metachronous cancer was diagnosed in 23 (47%) of patients. A histopathological examination revealed a different cellular organization of cancer tissue, compared with primary lung cancer, in 17 patients (77%). In the remaining six patients metachronous cancer was histologically the same as the primary tumor. However, since the metachronous cancer appeared after more than 2 years from the detection and surgery of the primary tumor, it was considered metachronous. The detailed data are presented in Table 3.
The median time elapsing from the resection of a primary tumor to lung cancer metastasis resection was 24.5 months and it was significantly shorter than that elapsing from the resection of a primary tumor to metachronous lung cancer resection, which was 49 months (p < 0.05). The early results of surgical treatment in patients treated for both lung cancer metastasis and metachronous lung cancer were similar (Table 4). The incidence of postoperative complications was noted in 31% patients with lung cancer metastasis and 30% patients with metachronous lung cancer.
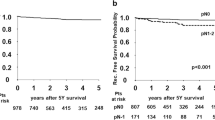
The analysis of long-term surgery results showed that the probability of the 5-year survival rate in patients with lung cancer metastasis (50.9%) and those with metachronous lung cancer (60.7%) was similar. The survival results are displayed in Fig. 3. Concerning the prognostic factors in patients with metachronous lung cancer after surgical treatment we failed to demonstrate any effect of such factors as tumor size or its localization in the lung, age of patient, or co-morbidities on the survival rate (Table 5).
4 Discussion
The major finding of this study was that a second operation of lung cancer concerned metachronous lung cancer in 47% of cases, i.e., in about one half of operations occurring after surgical resection of the primary lung cancer; the other half being due to lung metastases of the primary cancer. Metachronous lung cancer was observed mostly in men and appeared, on average, 49 months after the first surgery. The majority of metachronous cancers were adenocarcinomas, and their histological pattern usually was different from that present in the primary lung cancer. Metachronous lung cancer was subject to mechanical resection in most cases. The outcome of surgical treatment of metachronous lung cancers was akin to that of lung cancer metastases, with a similar rate of complication (30% and 31%, respectively) and the similar probability of the 5-year survival rate (60.7% and 50.9%, respectively).
Martini and Melamed (1975) criteria were adopted in the present study for distinguishing between metachronous lung cancer and lung cancer metastasis, including the time lapse of at least 2 years between the resection of a primary lung cancer and the appearance of metachronous lung cancer. These criteria are commonly used, although they are sometimes subject to critical evaluation and modification. For example, some studies have adopted the criterion of at least a 4-year disease-free time after primary lung cancer resection, which enables the diagnosis of metachronous lung cancer (Ha et al. 2015). In the present study, the mean time from resection of primary lung cancer to resection of metachronous lung cancer amounted to 4.6 ±2.1 years. Currently, the classical criteria for the diagnosis of metachronous lung cancer provided by Martini and Melamed (1975) are more often replaced by an extended imaging, histological, genetic, and molecular diagnostics (Stiles 2017; Liu et al. 2016). The differentiation of metachronous lung cancer from lung cancer metastasis, when both have the same histopathological cancer tissue structure, can be assisted with comparative genomic hybridization and somatic mutation testing (Arai et al. 2012; Girard et al. 2010; Moffat-Bruce et al. 2010; Wang et al. 2009). Genetic studies, however, have a limited value due to the possibility of different mutations in multiple tumors in the same patient. Such tests also are seldom employed since they are not commonly available and pricey.
The risk of metachronous lung cancer development in patients after NSCLC resection is 1–2% per patient per year (Johnson 1998; Johnson et al. 1997). The literature demonstrates that the incidence of metachronous lung cancer among patients operated on due to primary lung cancer is about 5% (Ishigaki et al. 2013; Vansteenkiste et al. 2013). In Poland, the incidence of multiple cancers, most commonly a second lung cancer, has also been reported at 5% in patients with lung cancer (Romaszko et al. 2016). In the present study, however, this risk appeared at just 0.4%, which may have been due to erratic and insufficient patient attendance to follow-up examinations after the surgery.
In our opinion, greater attention should be paid to the results of a histopathological examination of metachronous lung cancer. In the present study, adenocarcinoma was the most common histological metachronous cancer type, found in 57% of patients. Similar data on the adenocarcinoma prevalence among metachronous lung cancers are provided by other authors (Yang et al. 2014; Hamaji et al. 2013; Zuin et al. 2013).
The recommended method of surgical treatment of metachronous lung cancer is an anatomical resection with removal of regional lymph nodes (Wen et al. 2016; Zuin et al. 2013). In the present study, lymph nodes were removed in 48% of metachronous lung cancer cases. A low percentage of lymphadenectomy was often caused by a misleading treatment of metachronous lung cancer as lung cancer metastases. The decision on the extent of resection was made on the basis of an ad-hoc intra-operative inspection of a resected tumor; the inspection that usually is capable of providing only the information on the tumor’s neoplastic character. The anatomical resection was performed in just 17% of cases metachronous lung cancer. In the present study, no patient passed away in the perioperative period. In literature, perioperative mortality associated with metachronous lung cancer resection ranges from 1.4% (Yang et al. 2014) to 2.5% (Zuin et al. 2013). We found other postoperative complications following metachronous lung cancer surgery in about one third of patients, as described also by other authors who noted the perioperative occurrence of complications ranging from 19% (Zuin et al. 2013) to 34.3% (Yang et al. 2014).
The probability of 5-year survival in the patients of the present study treated for metachronous lung cancer was evaluated as 60.7%. Almost the identical 5-year survival rate of 60.8% has been shown in a study of Hamaji et al. (2013). A higher survival rate of 69.5% has been shown in a most recent study of Zhao et al. (2017). In that study, however, only were the patients examined in whom metachronous lung cancer was of adenocarcinoma type. In other studies, the 5-year survival rate after surgical treatment of metachronous lung cancer has been calculated at a somehow lower level. Yang et al. (2014) have demonstrated a 54.5% survival rate, whereas Koezuka et al. (2015) have found it at 56.5%. Zuin et al. (2013) have demonstrated a 42% survival rate in 121 patients with metachronous lung cancer diagnosed according to Martini and Melamed’s (1975) criteria. The 2014 meta-analysis of nine studies demonstrates that the 5-year survival rate after surgery of a second primary NSCLC is 46% (Hamaji et al. 2015). There is a clear relationship between the 5-year survival rate and the stage of metachronous lung cancer (Koezuka et al. 2015), or the extent of surgery: from 57% in patients with lobectomy to 36% in patients who undergo segmentectomy or wedge resection (Zuin et al 2013). One of the prognostically adverse factors seems the size of metachronous lung cancer being resected (Hamaji et al. 2013). In the present study, however, tumor size was not a predicting factor for the 5-year survival rate.
In the present study, the 5-year survival rate after surgery for metachronous lung cancer was inappreciably greater than that for lung cancer metastases. In contrast, the 2015 meta-analysis that included 1,796 patients in 22 studies has demonstrated that the overall survival of patients with multiple primary lung cancers, both metachronous and synchronous tumors were taken into consideration, is longer than that in patients operated on due to intra-pulmonary lung cancer metastases; relative risk of 2.66 with 95%CI of 1.30–5.44, p < 0.01 (Jiang et al. 2015).
5 Conclusions
Among lung tumors arising after resection of the primary lung cancer, the likelihood of metachronous lung cancer is akin to that of pulmonary lung cancer metastasis. Surgically resected metachronous lung cancer is in most cases of adenocarcinoma type, and the histopathological pattern usually differs from that of the primary lung cancer. Patients who undergo primary lung cancer surgery require a long-term follow-up due to the risk of lung cancer metastasis or metachronous lung cancer. The incidence of early postoperative complications and the probability of 5-year survival after metachronous lung cancer and lung cancer metastasis resection are similar.
References
Arai J, Tsuchiya T, Oikawa M, Mochinaga K, Hayashi T, Yoshiura K, Tsukamoto K, Yamasaki N, Matsumoto K, Miyazaki T, Nagayasu T (2012) Clinical and molecular analysis of synchronous double lung cancers. Lung Cancer 77:281–287
Asamura H, Nakayama H, Kondo H, Tsuchiya R, Naruke T (1999) Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 117:1102–1111
Edge SB, Compton CC (2010) The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474
Gamliel Z (2016) Mediastinal staging in non-small cell lung cancer. Surg Oncol Clin N Am 25:493–502
Girard N, Deshpande C, Azzoli CG, Rusch VW, Travis WD, Ladanyi M, Pao W (2010) Use of epidermal growth factor receptor/Kirsten rat sarcoma 2 viral oncogene homolog mutation testing to define clonal relationships among multiple lung adenocarcinomas: comparison with clinical guidelines. Chest 137(1):46–52
Ha D, Choi H, Chevalier C, Zell K, Wang XF, Mazzone PJ (2015) Survival in patients with metachronous second primary lung cancer. Ann Am Thorac Soc 12(1):79–84
Hamaji M, Allen MS, Cassivi SD, Deschamps C, Nichols FC, Wigle DA, Shen KR (2013) Surgical treatment of metachronous second primary lung cancer after complete resection of non-small cell lung cancer. J Thorac Cardiovasc Surg 145:683–690
Hamaji M, Ali SO, Burt BM (2015) A meta-analysis of resected metachronous second non-small cell lung cancer. Ann Thorac Surg 99:1470–1478
Hytych V, Taskova A, Horazdovsky P, Konopa Z, Demes R, Cermak J, Vrabcova A, Hoferka P, Pohnan R (2013) Importance of systemic mediastinal lymphadenectomy in exact staging of bronchogenic carcinoma. Bratisl Lek Listy 114:569–572
Ishigaki T, Yoshimasu T, Oura S, Ota F, Nakamura R, Hirai Y, Okamura Y (2013) Surgical treatment for metachronous second primary lung cancer after radical resection of primary lung cancer. Ann Thorac Cardiovasc Surg 19:341–344
Jiang L, He J, Shi X, Shen J, Liang W, Yang C, He J (2015) Prognosis of synchronous and metachronous multiple primary lung cancers: systematic review and meta-analysis. Lung Cancer 87:303–310
Johnson BE (1998) Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 90:1335–1345
Johnson BE, Cortazar P, Chute JP (1997) Second lung cancers in patients successfully treated for lung cancer. Semin Oncol 24:492–499
Koezuka S, Hata Y, Otsuka H, Makino T, Tochigi N, Shibuya K, Iyoda A (2015) Metachronous second primary lung cancer surgically treated five years or more after the initial surgery. Mol Clin Oncol 3:1025–1028
Liu Y, Zhang J, Li L, Yin G, Zhang J, Zheng S, Cheung H et al (2016) Genomic heterogeneity of multiple synchronous lung cancer. Nat Commun 7:13200
Maniwa T, Kodama K (2016) Has lobe-specific nodal dissection for early-stage non-small lung cancer already become standard treatment? J Thorac Dis 8:2407–2410
Martini N, Melamed MR (1975) Multiple primary lung cancers. J Thorac Cardiovasc Surg 70:606–612
Moffatt-Bruce SD, Ross P, Leon ME, He G, Finkelstein SD, Vaida AM, Iwenofu OH, Frankel WL, Hitchcock CL (2010) Comparative mutational profiling in the assessment of lung lesions: should it be the standard of care? Ann Thorac Surg 90:388–396
Naruke T, Goya T, Tsuchiya R, Suemasu K (1988) The importance of surgery to non-small cell carcinoma of lung with mediastinal lymph node metastasis. Ann Thorac Surg 46:603–610
Riquet M, Manach D, Dupont P, Dujon A, Hidden G, Debesse B (1994) Anatomic basis of lymphatic spread of lung carcinoma to the mediastinum: anatomo-clinical correlations. Surg Radiol Anat 16:229–238
Riquet M, Pricopi C, Arame A, Le Pimpec BF (2016) From anatomy to lung cancer: questioning lobe-specific mediastinal lymphadenectomy reliability. J Thorac Dis 8:2387–2390
Romaszko A, Świetlik E, Doboszyńska A, Szpruch P, Luks J (2016) Lung cancer and multiple neoplasms: a retrospective analysis. Adv Exp Med Biol 911:53–58
Sihag S, Muniappan A (2016) Lymph node dissection and pulmonary metastasectomy. Thorac Surg Clin 26:315–323
Stiles BM (2017) Say goodbye to martini and Melamed: genomic classification of multiple synchronous lung cancer. J Thorac Dis 9:E87–E88
Vansteenkiste J, De Ruysscher D, Eberhardt WE, Lim E, Senan S, Felip E, Peters E, ESMO Guidelines Working Group (2013) Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):vi89–vi98
Wang X, Wang M, MacLennan GT, Abdul-Karim FW, Eble JN, Jones TD, Olobatuyi F, Eisenberg R, Cummings OW, Zhang S, Lopez-Beltran A, Montironi R, Zheng S, Lin H, Davidson DD, Cheng L (2009) Evidence for common clonal origin of multifocal lung cancers. J Natl Cancer Inst 101:560–570
Wen CT, Fu JY, Wu CF, Hsieh MJ, Liu YH, Wu YC, Tsai YH, Wu CY (2016) Survival impact of locoregional metachronous malignancy in survival of lung cancer patients who received curative treatment. J Thorac Dis 8:1139–1148
Yang J, Liu M, Fan J, Song N, He WX, Yang YL, Xia Y, Jiang GN (2014) Surgical treatment of metachronous second primary lung cancer. Ann Thorac Surg 98:1192–1198
Zhao H, Yang H, Han K, Xu J, Yao F, Zhao Y, Fan L, Gu H, Shen Z (2017) Clinical outcomes of patients with metachronous second primary lung adenocarcinomas. Onco Targets Ther 10:295–302
Zuin A, Andriolo LG, Marulli G, Schiavon M, Nicotra S, Calabrese F, Romanello P, Rea F (2013) Is lobectomy really more effective than sublobar resection in the surgical treatment of second primary lung cancer? Eur J Cardiothorac Surg 44:e120
Acknowledgements
Funded by the statutory budget of Wroclaw Medical University.
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Rzechonek, A. et al. (2017). Metachronous Lung Cancer: Clinical Characteristics and Effects of Surgical Treatment. In: Pokorski, M. (eds) Current Concepts in Medical Research and Practice. Advances in Experimental Medicine and Biology(), vol 1039. Springer, Cham. https://doi.org/10.1007/5584_2017_82
Download citation
DOI: https://doi.org/10.1007/5584_2017_82
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74149-9
Online ISBN: 978-3-319-74150-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)