Abstract
Historically, the sympathetic nervous system (SNS) has been mostly associated with the ‘fight or flight’ response and the regulation of cardiovascular function. However, evidence over the past 30 years suggests that SNS may also influence the function of immune cells. In this review we describe the basic research being done in the area of SNS regulation of immune function. Further, we show that the SNS-immune interplay during circadian rhythm may modulate the robustness of the inflammatory response, critical for survival during periods of increased activity. Finally, new concepts of a close relationship between these systems in the pathogenesis of hypertension are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Primary (bone marrow and thymus) and secondary (spleen and lymph nodes) lymphoid organs are abundantly innervated by autonomic, mostly sympathetic efferent fibers. Norepinephrine (NE), a sympathetic nervous system (SNS) neurotransmitter, is released into the lymphoid tissue and modulates the function of immune cells. Bellinger et al. (2008) have mentioned the following criteria of neuroimmune transmission: (1) the presence of noradrenergic fibers in lymphoid organs, (2) the release of NE from noradrenergic terminals in these organs, and (3) the expression of adrenoceptors on lymphoid cells capable to respond functionally to the stimulation. Therefore, sympathetic innervation of lymphoid organs meets the criteria for neurotransmission, with immune cells as target cells.
2 Physiological Immune-Sympathetic Nervous System Interplay
2.1 Bone Marrow
Activation of β-adrenoceptors stimulates the proliferation and differentiation of hematopoietic/mesenchymal stem cells. Isoproterenol, a β-adrenergic agonist, added to murine bone marrow cell culture increases cellular proliferation and granulopoiesis in a dose-dependent manner (Felten et al. 1996), while the addition of a β-antagonist to cultured human bone marrow cells diminishes cellular proliferation and decreases the number of granulocyte-macrophage colony forming units (GM-CFU) (Dresch et al. 1981). Activation of α-adrenoceptors suppresses myelopoiesis and augments lymphopoiesis. Prazosin, an α1-adrenergic antagonist, increases GM-CFU formation and accelerates myelopoiesis while it reduces the differentiation of precursor cells into thymocytes and splenic T and B cells in vitro (Maestroni and Conti 1994; Maestroni 1995).
Both α- and β-adrenoceptor antagonists provide protection against radiation-induced cell death. However, the protective effects of these antagonists are time-dependent. β-adrenoceptor antagonists are protective when administered prior to irradiation, while α-adrenoceptor antagonists are more effective after radiation exposure (Byron 1972; Byron and Fox 1969; Lipski 1980). It seems that the expression of α- and β-adrenoceptors on immune cells may change over the time; β-adrenoceptor expression predominates in the early phases of bone marrow stem cell activation, and α-adrenoceptor expression increases at later stages (Dresch et al. 1981). In animals exposed to stress, corticosteroids enhance, while catecholamines suppress, T cell accumulation in the bone marrow, suggesting that these two groups of stress mediators may act, at least in part, in opposition to each other (Sudo et al. 1997).
2.2 Thymus
The regulation of thymic function by the SNS was extensively explored in the 1970s and 1980s. These early studies highlighted β-adrenoceptor dependent enhancement of thymocyte differentiation and suppression of thymocyte proliferation in the presence of intact NE innervation (Singh and Owen 1975; Singh and Owen 1976; Singh 1985). An in vivo exposure to isoproterenol reduces thymic weight and thymocyte number in mice (Durant 1986). Similarly, catecholamines or isoproterenol decrease concanavalin A- or lipopolysaccharide-induced proliferation of murine thymocytes (Cook-Mills et al. 1995). However, thymocyte proliferation increases after treatment with epinephrine, isoproterenol, and NE. Morgan et al. (1984) reported that stimulation of β2-adrenoceptors can elevate the level of intracellular cAMP, while stimulation of α-adrenoceptor by NE increases intracellular Ca2+. Elevated cAMP production induces cell apoptosis (McConkey et al. 1990). Thymus weight and tissue cell count decrease and the number of peripheral proliferating T cells are reduced following chemical sympathectomy, whereas apoptosis increases (Kendall and al-Shawaf 1991). Sympathetic modulation of thymocyte development and maturation has been reviewed in detail elsewhere (Bellinger et al. 2008).
2.3 Lymphocytes
Human and murine T naïve, Th1, and CD8+ lymphocytes express β2-adrenoceptors (Fuchs et al. 1988; Radojcic et al. 1991; Swanson et al. 2001). In contrast, Th2 clones do not express β2-adrenoceptors (Sanders et al. 1997; Kohm et al. 2002). In vitro, adrenergic agonists can modulate all phases of the immune response (induction, proliferation, and effector function). Stimulation of β-adrenoceptors inhibits mitogen- and anti-CD3 antibody-induced T cell proliferation and diminishes naïve T cell differentiation into Th1 cells (Swanson et al. 2001; Sanders et al. 1997; Ramer-Quinn et al. 1997). However, under Th1-promoting culture conditions, β-adrenoceptor stimulation drives Th1 effector cell development from naïve T cells. These effector Th1 cells produce higher amounts of IFN-γ after re-stimulation (Swanson et al. 2001). Under Th2-promoting culture conditions, naïve T cells exposed to NE transform into Th2 cells, but adrenergic agents have no effect on IL-4, IL-5, or IL-10 production (Swanson et al. 2001; Sanders et al. 1997; Ramer-Quinn et al. 1997). Taken together, these in vitro data suggest that NE stimulation of β2-adrenoceptor plays a role in the development of Th1 polarized cell-mediated immunity (Felten et al. 1996).
B lymphocytes express β2-adrenoceptors, and their stimulation elevates the intracellular concentration of cAMP, similarly to that seen in T cells (Fuchs et al. 1988; Bishopric et al. 1980). The enhancement or inhibition of B cell proliferation through β2-adrenoceptors depends on the type of mitogen used. Receptor stimulation or elevation of intracellular cAMP inhibits lipopolysaccharide-induced B cell proliferation (Diamantstein and Ulmer 1975; Vischer 1976) and the production of immunoglobulins (Ig) (Cohen and Rothstein 1989; Whisler et al. 1992). In contrast, activation of β2-adrenoceptors and increased cAMP enhance ionomycin-induced B cell proliferation (Cohen and Rothstein 1989; Whisler et al. 1992). NE administration and β2-adrenoceptor stimulation enhance IgM production in murine spleen cells cultured with a Th1 cell-dependent antigen. This effect is blocked by the addition of a β-adrenoceptor antagonist (Sanders and Munson 1984a, b).
2.4 Macrophages
α2 and β2-adrenoceptors are expressed on normal macrophages. Functional effects of stimulation of these adrenoceptors depend on the receptor subtype. β2-adrenoceptor stimulation suppresses macrophage activity, whereas α2-adrenoceptor stimulation enhances it (Bellinger et al. 2008).
2.5 Circadian Rhythm
Circadian rhythms generated by the central biological clock of the SNS regulate the majority of physiological and behavioral conditions, including locomotor activity and sleep-awake cycles, as well as autonomic, endocrine, and immune functions. In rodents, the highest SNS input to the spleen is provided during the day. Granzyme-B, perforin, INF-γ, and INF-α are expressed in natural killer (NK) cells in a rhythmic manner, with the highest levels during the dark period (a period of activity for rodents) (Arjona and Sarkar 2005). In vitro studies suggest that NE and other β-agonists suppress the mRNA expression of granzyme-B, perforin, and INF-γ (Dokur et al. 2004). Further, splenic denervation increases TNF-α expression under various experimental conditions (Molina 2001; Kees et al. 2003; Meltzer et al. 2004). The rhythmic pattern of NK cell function may have evolved for the benefit of having them readily available during periods of activity, when injuries and infections are more likely to occur. Circadian disruption or desynchronization between the central and peripheral systems could alter rhythms of sympathetic NE input to the spleen, thereby compromising the cytotoxic activity of NK cells. It is possible that compromised NK cell function resulting from altered release of the mediators outlined above could play a role in metastatic tumor growth or other diseases (Logan et al. 2011).
Circadian migration of leukocytes into tissues is also regulated by signals from the SNS; the peak of this recruitment occurs in rodents at night. Circadian hematopoietic cell recruitment is synchronized by the molecular clock via sympathetic nerves, modulated through β- adrenoceptor oscillations, the expression of endothelial cell adhesion molecules, and the chemokine release by endothelial cells (Scheiermann et al. 2012). Moreover, diurnal mechanisms stabilizing neutrophil numbers may interfere with the course of inflammatory cardiovascular diseases (Coller 2005). Circadian rhythms modulate the robustness of the inflammatory response, critical for survival during periods of increased activity (Scheiermann et al. 2012). In humans, hypersensitivity to inflammatory stimuli associated with increased SNS tone may have a detrimental effect, potentially linked to a higher incidence of acute vascular events in the morning (Boudreau et al. 2012; Reavey et al. 2013).
3 Sympathetic-Immune-CNS Interaction Concept of Hypertension
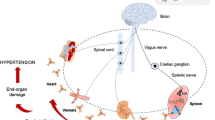
It is widely accepted that the pathophysiology of hypertension includes autonomic nervous system dysfunction, as well as immune system involvement (Guzik et al. 2007; Zubcevic et al. 2011; de Kloet et al. 2013; Santisteban et al. 2013). However, the close relationship between these systems in the pathogenesis of hypertension and in particular the role of the brain in this interaction is still evolving. Two aspects will be discussed in depth: the effect of inflammatory cytokines on the paraventricular nucleus and subfornical organ (cardiovascular control centers in the brain), and the potential role of the bone marrow in hypertension.
3.1 Effect of Inflammatory Cytokines on the Paraventricular Nucleus and Subfornical Organ
Inflammatory cytokines such as TNF-α and IL-1β activate cyclooxygenase-2 (Cox-2) in perivascular macrophages of the blood-brain barrier. Cox-2 catalyzes the production of prostaglandin E2 which enters the brain and stimulates paraventricular nucleus neurons (PVN) which, in turn, regulate adrenocorticotropic hormone release and increase sympathetic drive (Felder et al. 2009; Felder 2010). Further, direct microinjection of TNF-α and IL-1β to PVN increases blood pressure and sympathetic outflow and enhances the cardiac sympathetic afferent reflex (Shi et al. 2011). Intracerebroventricular administration of IL-10 decreases TNF-α, IL-1α, prostaglandin E2, and Cox-2 levels in the PVN and attenuates sympathoexcitation (Yu et al. 2007). Chronic angiotensin II-induced hypertension is associated with increased expression of inflammatory cytokines and microglial activation in PVN (Shi et al. 2011; Colombari et al. 2010). Minocycline, an antibiotic that can cross the blood-brain barrier, inhibits microglial activation, attenuates angiotensin II-induced high blood pressure, decreases the amount of PVN inflammatory cytokines, and mitigates cardiac hypertrophy (Shi et al. 2011). Most likely, the subfornical organ, a forebrain circumventricular organ that lacks a blood-brain barrier, plays a major role and is a predominant site in the brain at which circulating proinflammatory cytokines act to elicit cardiovascular and sympathetic responses. Intracarotid injection of TNF-α or IL-1β significantly increases mean blood pressure, heart rate, and renal sympathetic nerve activity in rats with an intact sunfornical organ, while these excitatory responses are significantly attenuated in sunfornical organ-lesioned rats (Wei et al. 2013).
3.2 Potential Role of Bone Marrow in Hypertension
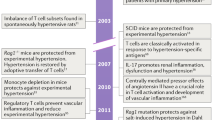
Although the bone marrow is known as a primary lymphoid organ, according to some authors it has a potential to serve as a secondary immune organ and may be involved in systemic T cell-mediated immunity. It has been demonstrated that the bone marrow may harbor memory T cells and, importantly, may be a site for the initiation of T cell activation (Feuerer et al. 2003). A critical role of T cells in hypertension has been discussed elsewhere (Guzik et al. 2007; Zubcevic et al. 2011; de Kloet et al. 2013; Santisteban et al. 2013). The bone marrow is also the primary source of endothelial progenitor cells (Asahara et al. 1999) which play a particularly important role in endothelial repair in arterial injury following inflammatory or hypertensive stimuli (Rabelink et al. 2004; Hristov et al. 2007; Zhu et al. 2013). Acute inflammatory stimuli trigger endothelial progenitor cell mobilization, while chronic inflammation can have the opposite effect, decreasing the number of circulating endothelial progenitor cells (Andreou et al. 2006). As in other vascular beds, NE acts as a vasoconstrictor in the bone marrow and plays an important role in controlling blood flow (Feitelson et al. 2002). It has been proposed that hypertension, which presents with increased circulating NE, is responsible for extensive vasoconstriction in bone marrow, creating a hypoxic environment. Consequently, hypertension could negatively modulate stem and endothelial progenitor cells and enhance local inflammatory responses, i.e., T cell activation and cytokine production (Santisteban et al. 2013). The last hypothesis, however, has to be verified experimentally.
4 Conclusions
Current evidence suggests that the SNS plays an important role in both activating and limiting immune and inflammatory responses. This, in turn, encourage thinking that manipulation of the SNS may be useful in regulation of immune reactivity, including the inflammatory reactions in hypertension and other cardiovascular diseases. However, therapeutic manipulation will become possible only when our knowledge about the mechanisms responsible for the fine-tuning of the immune system by the SNS is significantly increased. Another important issue for all cardiovascular diseases, including hypertension, is how the disease itself influences sympathetic activity. Finally, activation of the autonomic system in the brain and periphery is quite frequently in opposition to each other, which makes therapeutic interventions even more complex and difficult.
Undoubtedly, it is necessary to develop new technologies for manipulating the SNS in time and space, in conjunction with activation of other neuroendocrine hormone systems. Some of such technologies are already being investigated in clinical trials. Of particular interest would be to investigate the full impact of new modalities used to treat resistant hypertension, such as renal innervation, carotid glomectomy or baroreflex activation therapy, on immune system. The assessment of the immune system should become a standard analysis when searching for new methods of the SNS modulation.
References
Andreou I, Tousoulis D, Tentolouris C, Antoniades C, Stefanadis C (2006) Potential role of endothelial progenitor cells in the pathophysiology of heart failure: clinical implications and perspectives. Atherosclerosis 189:247–254
Arjona A, Sarkar DK (2005) Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J Immunol 174:7618–7624
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85:221–228
Bellinger DL, Millar BA, Perez S, Carter J, Wood C, Thyaga-Rajan S, Molinaro C, Lubahn C, Lorton D (2008) Sympathetic modulation of immunity: relevance to disease. Cell Immunol 252:27–56
Bishopric NH, Cohen HJ, Lefkowitz RJ (1980) Beta adrenergic receptors in lymphocyte subpopulations. J Allergy Clin Immunol 65:29–33
Boudreau P, Brouse CJ, Dumont GA, Boivin DB (2012) Sleep-wake and circadian-dependent variation of cardiorespiratory coherence. Conf Proc IEEE Eng Med Biol Soc 2012:3817–3820
Byron JW (1972) Evidence for a -adrenergic receptor initiating DNA synthesis in haemopoietic stem cells. Exp Cell Res 71:228–232
Byron JW, Fox M (1969) Adrenergic receptor blocking agents modifying the radioprotective action of T. A. B. Br J Radiol 42:400
Cohen DP, Rothstein TL (1989) Adenosine 3′,5′-cyclic monophosphate modulates the mitogenic responses of murine B lymphocytes. Cell Immunol 121:113–124
Coller BS (2005) Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler Thromb Vasc Biol 25:658–670
Colombari E, Colombari DS, Li H, Shi P, Dong Y, Jiang N, Raizada MK, Sumners C, Murphy D, Paton JF (2010) Macrophage migration inhibitory factor in the paraventricular nucleus plays a major role in the sympathoexcitatory response to salt. Hypertension 56:956–963
Cook-Mills JM, Cohen RL, Perlman RL, Chambers DA (1995) Inhibition of lymphocyte activation by catecholamines: evidence for a non-classical mechanism of catecholamine action. Immunology 85:544–549
de Kloet AD, Krause EG, Shi PD, Zubcevic J, Raizada MK, Sumners C (2013) Neuroimmune communication in hypertension and obesity: a new therapeutic angle? Pharmacol Ther 138:428–440
Diamantstein T, Ulmer A (1975) The antagonistic action of cyclic GMP and cyclic AMP on proliferation of B and T lymphocytes. Immunology 28:113–119
Dokur M, Boyadjieva N, Sarkar DK (2004) Catecholaminergic control of NK cell cytolytic activity regulatory factors in the spleen. J Neuroimmunol 151:148–157
Dresch C, Minc J, Poirier O, Bouvet D (1981) Effect of beta adrenergic agonists and beta blocking agents on hemopoiesis in human bone marrow. Biomedicine 34:93–98
Durant S (1986) In vivo effects of catecholamines and glucocorticoids on mouse thymic cAMP content and thymolysis. Cell Immunol 102:136–143
Feitelson JB, Kulenovic E, Beck DJ, Harris PD, Passmore JC, Malkani AL, Fleming JT (2002) Endogenous norepinephrine regulates blood flow to the intact rat tibia. J Orthop Res 20:391–396
Felder RB (2010) Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure. Exp Physiol 95:19–25
Felder RB, Yu Y, Zhang ZH, Wei SG (2009) Pharmacological treatment for heart failure: a view from the brain. Clin Pharmacol Ther 86:216–220
Felten DL, Gibson-Berry K, Wu JH (1996) Innervation of bone marrow by tyrosine hydroxylase-immunoreactive nerve fibers and hemopoiesis-modulating activity of a β-adrenergic agonist in mouse. Mol Biol Hematopoiesis 5:627–636
Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, Hämmerling GJ, Kyewski B, Hamann A, Umansky V, Schirrmacher V (2003) Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med 9:1151–1157
Fuchs BA, Albright JW, Albright JF (1988) Beta-adrenergic receptors on murine lymphocytes: density varies with cell maturity and lymphocyte subtype and is decreased after antigen administration. Cell Immunol 114:231–245
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204:2449–2460
Hristov M, Zernecke A, Liehn EA, Weber C (2007) Regulation of endothelial progenitor cell homing after arterial injury. Thromb Haemost 98:274–277
Kees MG, Pongratz G, Kees F, Schölmerich J, Straub RH (2003) Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J Neuroimmunol 145:77–85
Kendall MD, al-Shawaf AA (1991) Innervation of the rat thymus gland. Brain Behav Immun 5:9–28
Kohm AP, Mozaffarian A, Sanders VM (2002) B cell receptor- and beta 2-adrenergic receptor-induced regulation of B7-2 (CD86) expression in B cells. J Immunol 168:6314–6322
Lipski S (1980) Effect of beta-adrenergic stimulation by isoprenaline on proliferation and differentiation of mouse bone marrow cells in vivo. Pol J Pharmacol Pharm 32:281–287
Logan RW, Arjona A, Sarkar DK (2011) Role of sympathetic nervous system in the entrainment of circadian natural-killer cell function. Brain Behav Immun 25:101–109
Maestroni GJ (1995) Adrenergic regulation of haematopoiesis. Pharmacol Res 32:249–253
Maestroni GJ, Conti A (1994) Noradrenergic modulation of lymphohematopoiesis. Int J Immunopharmacol 16:117–122
McConkey DJ, Orrenius S, Jondal M (1990) Agents that elevate cAMP stimulate DNA fragmentation in thymocytes. J Immunol 145:1227–1230
Meltzer JC, MacNeil BJ, Sanders V, Pylypas S, Jansen AH, Greenberg AH, Nance DM (2004) Stress-induced suppression of in vivo splenic cytokine production in the rat by neural and hormonal mechanisms. Brain Behav Immun 18:262–273
Molina PE (2001) Noradrenergic inhibition of TNF upregulation in hemorrhagic shock. Neuroimmunomodulation 9:125–133
Morgan IJ, Wigham CG, Perris AD (1984) The promotion of mitosis in cultured thymic lymphocytes by acetylcholine and catecholamines. J Pharm Pharmacol 36:511–515
Rabelink TJ, de Boer HC, de Koning EJ, van Zonneveld AJ (2004) Endothelial progenitor cells: more than an inflammatory response? Arterioscler Thromb Vasc Biol 24:834–838
Radojcic T, Baird S, Darko D, Smith D, Bulloch K (1991) Changes in beta-adrenergic receptor distribution on immunocytes during differentiation: an analysis of T cells and macrophages. J Neurosci Res 30:328–335
Ramer-Quinn DS, Baker RA, Sanders VM (1997) Activated T helper 1 and T helper 2 cells differentially express the beta-2-adrenergic receptor: a mechanism for selective modulation of T helper 1 cell cytokine production. J Immunol 159:4857–4867
Reavey M, Saner H, Paccaud F, Marques-Vidal P (2013) Exploring the periodicity of cardiovascular events in Switzerland: variation in deaths and hospitalizations across seasons, day of the week and hour of the day. Int J Cardiol 168:2195–2200
Sanders VM, Munson AE (1984a) Beta adrenoceptor mediation of the enhancing effect of norepinephrine on the murine primary antibody response in vitro. J Pharmacol Exp Ther 230:183–192
Sanders VM, Munson AE (1984b) Kinetics of the enhancing effect produced by norepinephrine and terbutaline on the murine primary antibody response in vitro. J Pharmacol Exp Ther 231:527–531
Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE (1997) Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol 158:4200–4210
Santisteban MM, Zubcevic J, Baekey DM, Raizada MK (2013) Dysfunctional brain-bone marrow communication: a paradigm shift in the pathophysiology of hypertension. Curr Hypertens Rep 15:377–389
Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS (2012) Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 37:290–301
Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ (2011) Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol 203:289–297
Singh U (1985) Lymphopoiesis in the nude fetal thymus following sympathectomy. Cell Immunol 93:222–228
Singh U, Owen JJ (1975) Studies on the effect of various agents on the maturation of thymus stem cells. Eur J Immunol 5:286–288
Singh U, Owen JJ (1976) Studies on the maturation of thymus stem cells. The effects of catecholamines, histamine and peptide hormones on the expression of T cell alloantigens. Eur J Immunol 6:59–62
Sudo N, Yu XN, Sogawa H, Kubo C (1997) Restraint stress causes tissue-specific changes in the immune cell distribution. Neuroimmunomodulation 4:113–119
Swanson MA, Lee WT, Sanders VM (2001) IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol 166:232–2340
Vischer TL (1976) The differential effect of cyclic AMP on lymphocyte stimulation by T- or B-cell mitogens. Immunology 30:735–739
Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB (2013) Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension 62:118–125
Whisler RL, Beiqing L, Grants IS, Newhouse YG (1992) Cyclic AMP modulation of human B cell proliferative responses: role of cAMP-dependent protein kinases in enhancing B cell responses to phorbol diesters and ionomycin. Cell Immunol 142:398–415
Yu Y, Kang YM, Zhang ZH, Wei SG, Chu Y, Weiss RM, Felder RB (2007) Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure: role of nuclear factor kappaB. Hypertension 49:511–518
Zhu S, Deng S, Ma Q, Zhang T, Jia C, Zhuo D, Yang F, Wei J, Wang L, Dykxhoorn DM, Hare JM, Goldschmidt-Clermont PJ, Dong C (2013) MicroRNA-10A and microRNA-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group A2. Circ Res 112:152–164
Zubcevic J, Waki H, Raizada MK, Paton JF (2011) Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension 57:1026–1033
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Winklewski, P.J., Radkowski, M., Demkow, U. (2015). Relevance of Immune-Sympathetic Nervous System Interplay for the Development of Hypertension. In: Pokorski, M. (eds) Pathophysiology of Respiration. Advances in Experimental Medicine and Biology(), vol 884. Springer, Cham. https://doi.org/10.1007/5584_2015_169
Download citation
DOI: https://doi.org/10.1007/5584_2015_169
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24482-2
Online ISBN: 978-3-319-24484-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)