Abstract
The aim of the study was to determine whether an elevated IgA level at the time of the diagnosis of IgA nephropathy has an effect on the severity of kidney biopsy findings and long-term outcomes in children. We retrospectively studied 89 children with IgA nephropathy who were stratified into Group 1- elevated serum IgA and Group 2 – normal serum IgA at baseline. The level of IgA, proteinuria, hematuria, glomerular filtration rate (GFR) and hypertension (HTN) were compared at baseline and after the end of the follow-up period of 4.0 ± 3.1 years. Kidney biopsy findings were evaluated using the Oxford classification. The evaluation of treatment included immunosuppressive therapy and renoprotection with angiotensin converting-enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB), or no treatment. The elevated serum IgA was found in 46 (52 %) patients and normal serum IgA level was found in 43 (48 %) patients. No differences were found between the two groups regarding the mean age of patients, proteinuria, and the number of patients with reduced GFR or HTN at baseline. In kidney biopsy, mesangial proliferation and segmental sclerosis were significantly more common in Group 1 compared with Group 2 (p < 0.05). Immunosuppressive therapy was used in 67 % children in Group 1 and 75 % children in Group 2. The Kaplan-Meier survival curves for renal function (with normal GFR) and persistent proteinuria did not differ significantly depending on the serum IgA level at baseline. We conclude that in IgA nephropathy the elevated serum IgA at baseline may be associated with mesangial proliferation and segmental sclerosis contribute to glomerulosclerosis, but has no effect on the presence of proteinuria or on the worsening of kidney function during several years of disease course.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
IgA nephropathy (IgAN) is a form of chronic glomerulonephritis with predominant IgA deposits in kidney biopsy findings. It is a chronic kidney disease that develops in 5–30 % of patients at 10 years of age and in 25–50 % of patients at 20 years of age (Coppo and D’Amico 2005; Yoshikava et al. 2001). Complete remission of IgAN has been reported in only 3–30 % of cases (Coppo and D’Amico 2005).
IgA may be present in a monomeric form or may polymerise (pIgA) due to the J-chain. In healthy subjects, most pIgA is produced by the mucosal immune system, while patients with IgAN show reduced mucosal pIgA1 production and increased pIgA1 production in the bone marrow (Van der Boog et al. 2005). Different studies show that pIgA1 is the major component of glomerular deposits. IgA glomerular deposits sustain over a long-term follow-up in patients with persisting urinary changes and disappear in the course of clinical remission.
IgA1 in patients with IgAN shows an increased ability to form larger conglomerates as a result of abnormal galactosilation within the O-glycosylation hinge region. A defect of β1,3-galactose transferase, responsible for IgA galactosilation, has been found in B cells of patients with IgAN. Normally, IgA1 is catabolised in the liver by binding with an asialoglycoprotein receptor (ASGPR), and O-glycosylation results in a significant decrease of hepatic IgA1 clearance. IgA1 O-glycoforms that are deficient in galactose have an increased immunogenic potential attributable to hinge region O-glycans protecting IgA1 from the immunerecognition (Suzuki et al. 2009). IgA1 molecules with reduced galactose content form immunologic complexes that attach to mesangial fibronectin, laminin, and collagen type IV, and activate C3 further promoting nonspecific inflammatory responses within the glomeruli (Silva and Hogg 1989). Japanese studies have shown that an elevated serum IgA level is observed in 50–70 % of the adults and 16 % of children with IgAN (Yoshikava et al. 2001).
The aim of the present study was to determine whether an elevated IgA level at the time of the diagnosis of IgAN in children is related to the severity of kidney biopsy findings and long-term treatment outcomes.
2 Methods
We retrospectively studied 89 children from 8 pediatric nephrology centers, including 56 (63 %) boys and 33 (37 %) girls aged 11 ± 4 years, in whom IgAN was diagnosed based on kidney biopsy findings. The patients were divided into two groups based on the baseline serum IgA level: Group 1 included patients with elevated serum IgA and Group 2 included patients with normal serum IgA level. The analysis consisted of the assessment of the severity of urinary changes at baseline, kidney biopsy findings, applied therapy, and the long-term outcomes in both compared groups.
The baseline evaluation included the degree of proteinuria, hematuria, serum albumin, creatinine and IgA levels, GFR – the estimation using the Schwartz formula (Schwartz et al. 2009), and the blood pressure measurements. Proteinuria was determined using the turbidimetric method of Exton in a 24-h urine collection and expressed in mg/kg/day. The nephrotic proteinuria was recognized if greater than 50 mg/kg/day. The nephrotic syndrome was diagnosed if the nephrotic proteinuria was accompanied by hypoalbuminemia of less than 2.5 g/dl and hyperlipidemia.
Erythrocyturia was defined as more than 5 erythrocytes per field of view at the magnification of 400×. Hematuria was defined as a visible change of urine color. Serum IgA was determined using immunonephelometric or immunoturbidimetric method and related to the age-specific reference ranges in a given center. Blood pressure was measured using the Korotkoff method. Hypertension was diagnosed if blood pressure exceeded the 95th percentile for height, age, and gender in three independent measurements (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents 2005).
Kidney biopsy was performed in all children at 1.4 ± 2.2 years (median 0.5; range 0.1–13 years) since the disease onset. The specimens were evaluated using the Oxford classification (1 – changes present, 0 – absent: M – mesangial proliferation; E – endocapillary hypercellularity; S – segmental sclerosis; T – tubular atrophy/interstitial fibrosis T0: 0–25 %, T1: 26–50 %, and T2: >50 %) (Roberts et al. 2009). In addition, the absence or presence of crescents (c0/c1) was indicated in the kidney biopsy report. Histopathological evaluation was performed locally and verified at the Department of Pathology, Medical University of Warsaw in Poland.
The treatment modalities included supportive therapy with angiotensin converting-enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) agents, IS – immunosuppressive drugs (prednisone, azathioprine, cyclophosphamide, CsA/Tac – cyclosporin A or tacrolimus, MMF – mycophenolate mofetil). The follow-up period ranged from 1 to 14 years (mean 4.0 ± 3.1 years). The severity of proteinuria and erythrocyturia, GFR, and the presence of hypertension were evaluated in the course of the long-term follow-up. Disease remission was defined as resolution of urinary changes with normal GFR and blood pressure values.
Results were expressed as means ± SD, median, and minimum-maximum values. Parameters were compared using the Wilcoxon test and the t-test, and frequencies were compared using a proportion test. Linear regression analysis, chi-squared test, Pearson’s correlation coefficient with the exact Fisher test, and Spearman’s rank correlation test were used to evaluate relations between studied parameters at the end of the follow-up in relation to the baseline serum IgA level. Kaplan-Meier curves were plotted for the two study groups.
P values of <0.05 were considered statistically significant. Statistical analyses were performed using the Statistica ver. 10 software package.
3 Results
In all children, IgAN was diagnosed on the basis of kidney biopsy findings. The nephrotic range of proteinuria with erythrocyturia was present in 19 (21 %) children (including 6 with nephrotic syndrome). Non-nephrotic range of proteinuria was found in 48 (54 %) patients, and isolated erythrocyturia/hematuria in 22 (25 %) patients. 19 (21 %) had intermittent gross hematuria. At baseline, GFR <90 mL/min was noted in 35 (39 %) children and hypertension in 18 (20 %) children. Elevated serum IgA level was found in 46 (52 %) out of the 89 patients (Group 1), and the normal serum IgA level was found in 43 (48 %) patients (Group 2). Patient characteristics at baseline are shown in Table 1. In Group 1, proteinuria was less frequent at baseline, although the difference between the groups was insignificant, while the nephrotic range of proteinuria was significantly more common (p < 0.05) compared with the Group 2. No significant differences were found between both groups regarding the patients’ age, protein loss, decreased GFR, and the presence of hypertension at baseline.
Kidney biopsy was performed after 1.2 ± 2.0 years in Group 1 and after 1.6 ± 2.4 years in Group 2 from the disease onset. The timing of kidney biopsy in relation to initial symptoms of the disease did not differ significantly between the two groups. The most common finding in both groups was mesangial proliferation. Both mesangial proliferation (M1) and segmental sclerosis (S1) were significantly more common in Group 1 compared with Group 2 (89 % vs. 72 % and 41 % vs. 21 %, p < 0.05, respectively), while the rates of endocapillary hypercellularity (E1), tubular atrophy/interstitial fibrosis (T1), and the presence of crescents (c1) did not differ between the groups. The biopsy findings in the two groups are shown in Table 2.
Supportive therapy or watchful waiting was applied in 28 (31 %) children, including 16 (35 %) in Group 1 and 12 (28 %) in Group 2; the difference between the two groups was insignificant. Immunosuppressive therapy with prednisone, azathioprine, cyclophosphamide, cyclosporin A, and mycophenolate mofetil was used as a first-choice treatment in 61 (68 %) children, including 33 (72 %) in Group 1 and 28 (65 %) in Group 2 (insignificant difference). The indications for immunosuppressive therapy included severe urinary changes (mainly nephrotic range of proteinuria), reduced GFR and/or histopathological findings of poor prognosis (M, E, S, and T). In Group 1, azathioprine with prednisone were the most frequent first-choice treatment used significantly more frequently compared with Group 2 (p < 0.05). A repeated course of immunosuppressive therapy due to disease recurrence or persisting proteinuria was used in 16 children (18 %), including 10 in Group 1 and 6 in Group 2; insignificant difference. The duration of a follow-up was similar in both groups and ranged from 1 to 14.3 years (mean 4.0 ± 3.1 years in Group 1 and 3.7 ± 2.6 years in Group 2). Treatment modalities in both examined groups are summarized in Table 3.
Comparison of long-term outcomes in relation to the serum IgA level at baseline is shown in Table 4. The number of patients with reduced GFR (<90 mL/min) and the rate of persisting proteinuria did not differ between the two groups examined. We failed to find any relation between the elevated serum IgA level at baseline and reduced GFR (chi-squared test) and/or persisting proteinuria (Spearman’s rank correlation test) at the end of the follow-up.
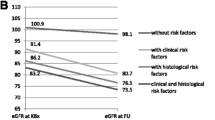
The Kaplan-Meier analysis for survival probability in children with normal GFR and with persisting proteinuria in the two groups did not confirm the presence of a correlation between these parameters and the serum IgA level at baseline (Figs. 1 and 2). However, the baseline GFR <90 mL/min, as compared with that ≥90 mL/min, appeared a poor prognostic indicator of survival (p < 0.001) (Fig. 3).
4 Discussion
A defect underlying IgAN is the production of abnormal, galactose-deficient glycosylated IgA1 that forms circulating immune complexes deposited in the glomeruli (Tanaka et al. 2011). An increase in IgA1 production by bone marrow plasmocytes is visible as an enhanced IgA serum level (Galla 1995), whose prevalence may be as high as 41 % in children (Bulut et al. 2012). In the present study we found an elevated IgA in the serum samples from 46 out of the 89 (52 %) children. In adult patients, IgA >315 mg/dL is considered diagnostic of IgAN, although the IgA level has not been identified as a valuable prognostic factor (Tomino et al. 2000).
Episodes of overt hematuria in the course of IgAN are less frequent in adults compared with children. Such episodes have been found in 43 % (Emancipator et al. 1995) and 18–32 % (Yoshikava et al. 2001) of adult patients. Symptoms may also include chronic erythrocyturia or proteinuria of varying severity, acute nephritic syndrome with hypertension, or acute renal failure with nephrotic syndrome. In children, erythrocyturia or asymptomatic proteinuria has been found in 62 %, hematuria in 26 %, and nephritic syndrome in 12 % of patients (Yoshikava et al. 2001).
In line with those results, in the present study we found hematuria in 21 % of children, non-nephrotic range proteinuria in 54 %, and hypertension in 20 % of patients. Acute kidney injury, observed in 39 % of patients, was frequently associated with nephritic syndrome. The severity of initial symptoms was unrelated to the serum IgA level, and eventfully the nephrotic range of proteinuria was found to be more frequent among children with the normal serum IgA level.
Kidney biopsy showed mesangial cell proliferation and segmental sclerosis to be significantly more common in the group with elevated Serum IgA level, which may be attributable to glomerular injury triggered by circulating immune complexes, in particular those containing aberrantly glycosylated IgA1 (Tanaka et al. 2011). The in vitro studies by Novak et al. (2005) have demonstrated that circulating immune complexes with galactose-deficient IgA1 from the sera of IgAN patients stimulate mesangial cell proliferation more effectively than non-complexed IgA1 or immune complexes isolated from healthy persons.
In view of the lack of standards of IgAN therapy in children, various medical centers use different treatment modalities guided by the severity of urinary changes and GFR values. Supportive therapy was comparable in both groups analysed in the present study. Immunosuppressive treatment was applied more frequently than renoprotection alone in both groups, but the immunosuppression was different in the two groups: azathioprine combined with corticosteroids was used more frequently in the group with elevated Serum IgA level. Pozzi et al. (2010) have failed to demonstrate an additional benefit of low-dose azathioprine added to corticosteroids in patients with IgA nephropathy. However, an important limitation of all studies on the issue of therapy is a short observation period taking into account a slowly progressing nature of this disease (Tanaka et al. 2011).
The mean follow-up time in the present study, amounting to around 4 years, also cannot truly reflect the effects of the long-lasting course of the disease. The Kaplan-Meyer analysis failed to substantiate a relation between a decline in GFR in the follow-up period and a baseline serum IgA level. That, however, does not eliminate the possibility that a pathological IgA contributes to the pathogenesis of the disease, but the association between the renal function and humoral pathology is not straightforward. In contrast, reduced baseline GFR seems a poor prognostic factor, as shown in previous studies (Coppo and D’Amico 2005).
In view of the data suggesting a key role of galactose-deficient IgA in the pathogenesis of IgAN, it seems that the serum level of galactose-deficient IgA1 may be a prognostic factor in children, but the resolution of this issue requires further studies. In conclusion, in children with IgA nephropathy, elevated baseline serum IgA may be associated with mesangial hypercellularity and segmental sclerosis, but has no effect on the presence of proteinuria or decline in renal function in the course of the disease.
References
Bulut IK, Mir S, Sozeri B, Bulut MO, Sen S, Dincel N (2012) Outcome results in children with IgA nephropathy: a single center experience. Int J Nephrol Renovasc Dis 5:23–28
Coppo R, D’Amico G (2005) Factors predicting progression of IgA nephropathies. J Nephrol 18:503–512
Emancipator SN, Gallo GR, Lamm ME (1995) IgA nephropathy: perspective on pathogenesis and classification. Clin Nephrol 24:161–179
Galla JH (1995) Perspectives in clinical nephropathy. Kidney Int 47:377–387
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2005) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents, revised version May 2005. U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, Bethesda
Novak J, Tomana M, Matousovic K, Brown R, Hall S, Novak L, Julian BA, Wyatt RJ, Mestecky J (2005) IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int 67:504–513
Pozzi C, Andruli S, Pani A, Scaini P, Del Vecchio L, Fogazzi G, Vogt B, De Cristofaro V, Allegri L, Cirami L, Procaccini AD, Locatelli F (2010) Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol 21:1783–1790
Roberts ISD, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amigo G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jenette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PKT, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H (2009) The Oxford classification of IgA nephropathy: pathology definitions, correlations and reproducibility. Kidney Int 76:546–556
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Silva FG, Hogg RJ (1989) IgA nephropathy. In: Tisher CC, Brenner BM (eds) Renal pathology with clinical and functional correlations. Lippincott, Philadelphia, pp 434–493
Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J (2009) Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119:1668–1677
Tanaka M, Seki G, Someya T, Nagata M, Fujita T (2011) Aberrantly glycosylated IgA1 as a factor in a pathogenesis of IgA nephropathy. Clin Dev Immunol 2011:470803. doi:10.1155/2011/470803
Tomino Y, Suzuki S, Imai H, Saito T, Kawamura T, Yorioka N, Harada T, Yosumoto Y, Kida H, Kabayashi Y, Endoh M, Sato H, Saito K (2000) Measurement of serum IgA and C3 may predict the diagnosis of patients with IgA nephropathy prior to renal biopsy. J Clin Lab Anal 14:220–223
Van der Boog P, Van Kooten C, De Fijter J (2005) Role of macromolecular IgA in IgA nephropathy. Kidney Int 67:813–821
Yoshikava N, Tanaka R, Iijima K (2001) Pathophysiology and treatment of IgA nephropathy in children. Pediatr Nephrol 16:446–457
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mizerska-Wasiak, M. et al. (2015). Increased Serum IgA in Children with IgA Nephropathy, Severity of Kidney Biopsy Findings and Long-Term Outcomes. In: Pokorski, M. (eds) Ventilatory Disorders. Advances in Experimental Medicine and Biology(), vol 873. Springer, Cham. https://doi.org/10.1007/5584_2015_160
Download citation
DOI: https://doi.org/10.1007/5584_2015_160
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20193-1
Online ISBN: 978-3-319-20194-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)